Abstract
N-glycosylation of E-cadherin has been shown to inhibit cell–cell adhesion. Specifically, our recent studies have provided evidence that the reduction of E-cadherin N-glycosylation promoted the recruitment of stabilizing components, vinculin and serine/threonine protein phosphatase 2A (PP2A), to adherens junctions (AJs) and enhanced the association of AJs with the actin cytoskeleton. Here, we examined the details of how N-glycosylation of E-cadherin affected the molecular organization of AJs and their cytoskeletal interactions. Using the hypoglycosylated E-cadherin variant, V13, we show that V13/β-catenin complexes preferentially interacted with PP2A and with the microtubule motor protein dynein. This correlated with dephosphorylation of the microtubule-associated protein tau, suggesting that increased association of PP2A with V13-containing AJs promoted their tethering to microtubules. On the other hand V13/γ-catenin complexes associated more with vinculin, suggesting that they mediated the interaction of AJs with the actin cytoskeleton. N-glycosylation driven changes in the molecular organization of AJs were physiologically significant because transfection of V13 into A253 cancer cells, lacking both mature AJs and tight junctions (TJs), promoted the formation of stable AJs and enhanced the function of TJs to a greater extent than wild-type E-cadherin. These studies provide the first mechanistic insights into how N-glycosylation of E-cadherin drives changes in AJ composition through the assembly of distinct β-catenin- and γ-catenin-containing scaffolds that impact the interaction with different cytoskeletal components.
Keywords: E-cadherin, N-glycosylation, PP2A, microtubules, actin cytoskeleton
Introduction
Epithelial cell–cell adhesion is predominantly mediated by E-cadherin, a calcium-dependent cell adhesion receptor.1–3 E-cadherin is a transmembrane protein containing five extracellular domains or ectodomains (ECs) that dimerize to form homotypic cell-cell contacts with E-cadherin on adjacent cells.4 The stability of these intercellular contacts is regulated by the ability of E-cadherin’s cytoplasmic domain to organize dynamic multiprotein complexes at the plasma membrane known as adherens junctions (AJs).5 The cytosolic tail of E-cadherin binds catenins that mediate the interaction with the actin cytoskeleton.6 The latter is mediated by α-, β-, γ-catenins, and vinculin, with β-catenin and γ-catenin binding directly to the cytoplasmic tail in a mutually exclusive manner. α-catenin then binds to either β- or γ-catenin where it plays a role in the recruitment of the actin cytoskeleton and the organization of actin filaments at the plasma membrane.6–9
In addition to its well acknowledged interaction with the actin cytoskeleton, E-cadherin has been shown to interact with microtubules (MTs). These cytoskeletal interactions have been found to reinforce AJs and to contribute to their stability and strength.8,10–12 While the connection between E-cadherin and the actin cytoskeleton has been studied extensively,13 less is known about the relationships between E-cadherin and MTs. Nonetheless, β-catenin has been reported to bind to the microtubule-based motor protein dynein, suggesting that E-cadherin complexes and MTs connect physically.14 Moreover, AJs and MTs may cooperate functionally since AJs can recruit MTs and to support local accumulation of E-cadherin at cell-cell contacts.10
E-cadherin can be post-translationally modified by N-glycosylation of EC4 and EC5.15 N-Glycans modify proteins at asparagine residues within the consensus sequence NX(S/T), where X can be any amino acid with the exception of proline.16,17 Not every potential N-glycan addition site on a given N-glycoprotein is modified, and there are frequently variations in the number of sites occupied by N-glycans, their overall sizes and composition. We and others have shown that the presence of complex N-glycans on EC4 interferes with intercellular adhesion and actin cytoskeletal association.15,18 Accordingly, hypoglycosylated E-cadherin is a signature of mature AJs. E-cadherin N-glycosylation is physiologically significant because it is subject to changes with cellular proliferation. Complex N-glycans preferentially modify E-cadherin in proliferating and cancer cells while high mannose/hybrid structures are characteristic of growth-arrested and differentiated cells. Indeed, our recent studies have shown that modification of E-cadherin with complex N-glycans leads to cellular discohesion and increased invasiveness of oral cancer cells in vivo.19
One of the characteristic features of hypoglycosylated E-cadherin is its increased recruitment of γ-catenin, vinculin, and serine/threonine protein phosphatase 2A (PP2A) to AJs.15,19 γ-catenin and vinculin have been shown to play stabilizing roles in AJs.12,15,20–22 The function of PP2A in AJs is unclear, although several observations indicate that PP2A is critical for the maintenance of E-cadherin-mediated cell–cell contacts.23 PP2A plays critical roles in cell growth, signaling, and tumor suppression,24–26 and its dysregulation has been linked to the destabilization of MT populations in numerous cell types.27–29
We have been interested in deciphering how N-glycosylation impacts cytoskeletal association and adhesive function of E-cadherin scaffolds in normal and cancer cells. In the present study, we examined the relationship between the E-cadherin N-glycan-dependent recruitment of PP2A to AJs and microtubule-stabilizing and motor proteins. We provide evidence that reduced N-glycosylation of E-cadherin enhances its association with the MT-associated protein (MAP), dynein, shown to tether MTs to AJs. This interaction involves hypoglycosylated E-cadherin/β-catenin complexes that preferentially associate with PP2A. The association of PP2A with AJs correlates with a decreased phosphorylation of another MAP, tau. On the other hand hypoglycosylated E-cadherin/γ-catenin complexes interact preferentially with vinculin, suggesting that they mediate the association of mature AJs with the actin cytoskeleton. Consistent with its role in promoting cell-cell adhesion, transfection of hypoglycosylated E-cadherin into CHO cells and human epidermoid carcinoma A253 cells enhances transepithelial resistance. Collectively, our studies show for the first time that hypoglycosylated E-cadherin drives intercellular adhesion through the formation of distinct protein complexes that promote the interaction with the two major components of the cytoskeleton, actin microfilaments and MTs.
Materials and methods
Reagents
Monoclonal antibodies to α-catenin, β-catenin, γ-catenin, the cytoplasmic region of human E-cadherin, PP2A, and IgG isotype controls were obtained from BD Biosciences (Franklin Lakes, NJ, USA). Monoclonal antibody to vinculin (clone V284) was from Upstate Biotechnology (Charlottesville, VA, USA). Monoclonal antibody to actin (pan Ab-5, clone ACTN05) was from NeoMarkers (Fremont, CA, USA). Monoclonal antibody to α-tubulin was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibodies to acetylated α-tubulin and Flag were obtained from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal antibody to the GST tag was purchased from Molecular Probes (Carlsbad CA, USA) and that to dynein from GeneTex Corporation (Zeeland MI, USA). Polycolonal antibodies to tau (Ab-231), tau (Phosphor-Ser199) and tau (Phosphor-Thr231) were obtained from GenScript Corporation (Piscataway, NJ, USA). ProFound Co-Immunoprecipitation Kit was purchased from Pierce Biotechnology (Rockford IL, USA). Secondary antibodies for immunostaining included fluorescein isothiocyanate (FITC) and Texas red (Molecular Probes). For Western blot analyses, horseradish peroxidase conjugated secondary antibodies were obtained from Amersham Biosciences (Fairfield CT, USA). Protein G Sepharose and Glutathione Sepharose 4B beads were purchased from GE Healthcare (Piscataway, NJ, USA).
Vector construction and in vitro mutagenesis
Human E-cadherin (GenBankTM accession no. Z13009) was cloned into pCMV5Bmyc vector (Stratagene, La Jolla, CA, USA) and tagged at the C-terminus with either a GST tag or a Flag tag. The E-cadherin N-glycosylation variant V13, lacking site 1 in EC4 and site 3 in EC5, was generated using a QuikChange XL site-directed mutagenesis kit (Stratagene) as described by the manufacturer. The mutations were verified by sequencing.
Cell culture, transfection, and preparation of total cell lysates (TCLs)
CHO and human epidermoid carcinoma A253 cells were grown in minimum essential alpha medium (Invitrogen, Carlsbad, CA, USA) and McCoy’s medium (Invitrogen), respectively, supplemented with 10% fetal calf serum, penicillin and streptomycin in 5% CO2 at 37 °C. CHO and A253 cells were transfected at 50% confluence with E-cadherin and its N-glycosylation variant V13 using Lipofectamine 2000 (Invitrogen). Transfectants were cultured until full confluence in the presence of 0.8 μg/ml G418 (Invitrogen). TCLs were prepared using either Triton/Octylglucoside or RIPA buffers, as described previously.15 Protein concentrations were determined using the BCA protein assay (Pierce Biotechnology).
Western blot
TCLs (10 μg total protein) were fractionated on 7.5% SDS-PAGE and transferred onto PVDF membranes. Nonspecific binding was reduced by blocking with 10% nonfat dry milk in phosphate-buffered saline (PBS) at room temperature (RT) for one hour. The membranes were incubated with primary antibodies in PBS with 1% milk for one hour at RT. Next, membranes were washed four times with 1X PBS with 0.1% Tween 20, followed by incubation with horseradish peroxidase-linked secondary antibody (1:3000) in PBS with 1% milk. Secondary antibodies were detected using the ECL Plus Detection Reagents (Amersham Biosciences). Immunoblots were scanned and densitometric analyses were performed using Kodak 1D software (Eastman Kodak Company, Rochester, NY, USA). Error bars represent standard deviation from three independent experiments; P values were calculated by two-tailed t-test.
Surface biotinylation
CHO cells, transfected with either wild-type E-cadherin or its hypoglycosylated variant V13, were surface biotinylated using Sulfo-NHS-LC-Biotin according to manufacturer’s instructions (Pierce Biotechnology). Biotinylated transfectants were extracted with the lysis buffer, biotin-labeled protein fractions were isolated with streptavidin agarose beads, and surface labeled wild-type and V13 E-cadherins were detected by Western blot using an antibody that recognizes E-cadherin’s cytoplasmic domain.
Immunoprecipitation and GST pulldown
TCLs (300 μg total protein) from CHO cells, transfected with either E-cadherin or V13, were precleared with 100 μl of Sepharose 4B beads and isotype control antibodies. For GST pulldowns, the supernatants were incubated with Glutathione Sepharose 4B beads. For immunoprecipitations of either β- or γ-catenin protein complexes, supernatants were incubated with antibodies to either β- or γ-catenin, followed by protein G beads. The Glutathione Sepharose 4B beads and protein G beads were washed three times with lysis buffer (10 mM Tris HCL pH 7.5, 1 mM EDTA, 1 mM EGTA, and 0.5% Triton X-100). Samples were resuspended in 100 μl LSB, boiled for 5 minutes at 95 °C and analyzed by Western blot. For immunoprecipitations of E-cadherin and V13 from A253 cells transfected with either E-cadherin or V13, a Flag antibody was used.
Double IP
Native, intact E-cadherin protein complexes were isolated from CHO cells transfected with the hypoglycosylated E-cadherin variant V13 using the ProFound Co-Immunoprecipitation Kit. E-cadherin-specific antibody was immobilized by coupling antibody to aldehyde activated gel beads (AminoLink Plus Coupling Gel), as indicated by the manufacturer. TCLs from V13 transfectants, grown in the presence of G418 to confluence, were first precleared with nonactivated beads and then incubated with immobilized antibody at 4 °C for 2 h. V13 complexes were eluted from the column using a nonreducing elution buffer provided by the manufacturer and diluted in Triton buffer. Next, intact V13 complexes lacking the E-cadherin antibody were subjected to a second immunoprecipitation using antibody to either β-catenin or γ-catenin. The immunoprecipitates were then analyzed for associated proteins by Western blot.
Microscopy and immunofluorescence
A253 cells were fixed in 3.7% paraformaldehyde and permeabilized with 0.1% Triton X-100. The samples were blocked with goat serum, rinsed with PBS, and incubated with primary antibodies overnight at 4 °C. Cells were then incubated with FITC- and Texas red-tagged secondary antibodies and rhodamine-tagged phalloidin. Next, cells were rinsed four times with PBS and mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA). The immunostained samples were analyzed with a Zeiss confocal laser scanning microscope LSM510 META (Fluar 5X/0.25, magnification Plan-Apochromat 40X/1.3 oil DIC). For visualization of individual optical sections and for generation of Z-stacks with optical slices of 0.74 μm, LSM 510-expert mode acquisition software was used. To ensure valid comparison of fluorescence intensities between samples, settings were fixed to the most highly stained sample and all other images were acquired at those settings.
Transepithelial resistance
The effects of the hypoglycosylated V13 variant on transepithelial resistance (TER) were measured in CHO and A253 cells seeded onto Transwells (polycarbonate membrane, 12-mm diameter, 0.4 μm pore size; Corning Costar) at 5 × 104 cells/cm2, grown to 50% confluence and transfected with either wild-type E-cadherin or its hypoglycosylated variant, V13. Transfectants were grown to high density and TER was measured directly in culture media using an epithelial voltohmmeter (World Precision Instruments, Sarasota, FL, USA). Values were calculated after subtracting background readings from blank Transwells with the media that were cultured in parallel. Statistical analysis of the data was by ANOVA.
Results
Hypoglycosylated E-cadherin is targeted to the cell surface in CHO cells
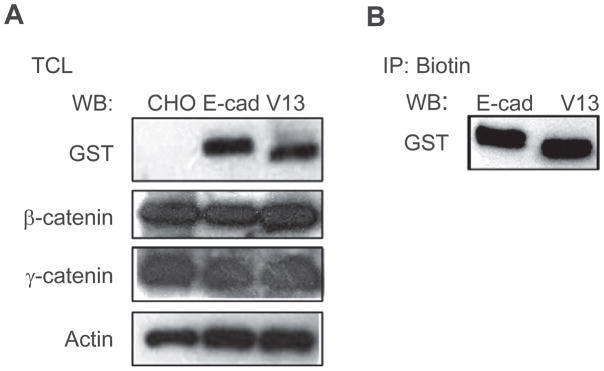
Although CHO cells do not express endogenous E-cadherin, they have been shown to organize E-cadherin-containing AJs at the cell surface upon transfection with exogenous E-cadherin.4,30 Previously, we reported that the hypoglycosylated E-cadherin variant V13 organized AJs with more stabilizing proteins and in a greater association with the actin cytoskeleton compared to wild-type E-cadherin.15 Since N-glycosylation has been shown to influence protein folding and secretion, we examined whether the removal of E-cadherin’s two N-glycans in variant V13 affected its expression and targeting to the cell surface in CHO cells. Total cell lysates (TCLs) from cells transfected with either wild-type GST-E-cadherin or GST-V13 displayed similar levels of E-cadherin expression after normalization to actin, with the expected increased migration of V13 due to its hypoglycosylated status (Figure 1A). No GST-specific signal was detected in untransfected CHO cells (Figure 1A). Comparison of the abundance of E-cadherin’s immediate binding partners, β- and γ-catenins, did not reveal significant differences among untransfected cells and either of the transfectants (Figure 1A). To determine if the targeting of V13 to the cell membrane was influenced by its reduced N-glycosylation status, we labeled the surface pools of E-cadherin in GST-E-cadherin- and GST-V13-transfected CHO cells with biotin and analyzed them by Western blot. Figure 1B shows that biotinylated pools of GST-E-cadherin and GST-V13 were comparable in abundance, with biotinylated GST-V13 displaying an expected mobility shift. Hence, reduced N-glycosylation did not interfere with the targeting of V13 to the cell surface in CHO cells.
Figure 1.
Hypoglycosylated E-cadherin V13 localizes to the cell surface. (A) Western blot (WB) of wild-type E-cadherin (E-cad) and its hypoglycosylated variant V13 (V13), and of associated catenins, expression levels. (B) E-cad- and V13-transfected cells were biotinylated and equal amounts of biotin immunoprecipitates were analyzed for E-cadherin expression by WB. Biotinylated V13 is expressed at levels similar to E-cad.
β-catenin complexes preferentially associate with PP2A in V13-transfected CHO cells
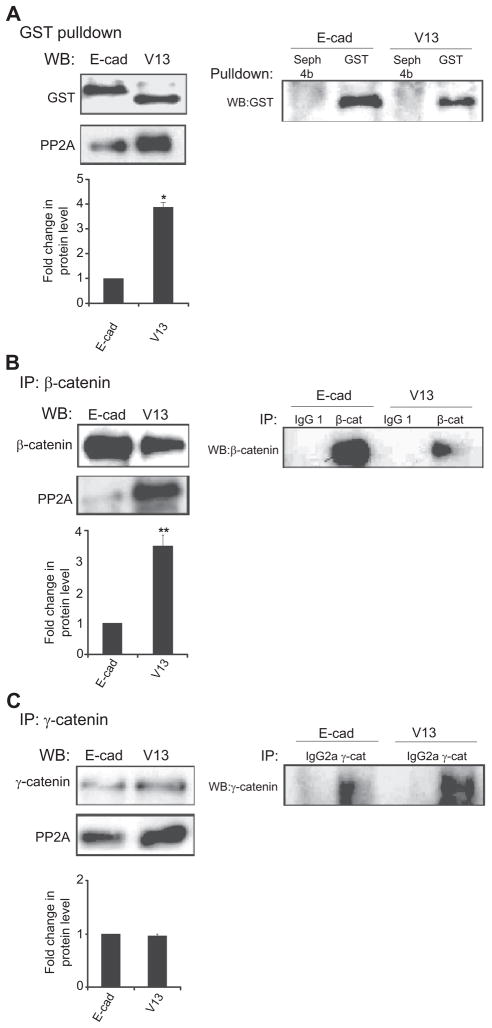
Association of AJs with PP2A has been shown to stabilize intercellular adhesion.23,31,32 Our recent studies have provided evidence that this is likely dependent on the N-glycosylation status of E-cadherin, because excessive N-glycosylation of E-cadherin in oral cancer cells and tissues is associated with diminished recruitment of PP2A to AJs.19 To examine whether E-cadherin N-glycosylation also impacted the association of PP2A with AJs in CHO cells transfected with either GST-E-cadherin or GST-V13, we assessed the abundance of PP2A in GST pulldowns. Results showed a four-fold increase in the amount of PP2A associated with V13-protein complexes compared to E-cadherin (Figure 2A, left panel). The specificity of the association was confirmed in control experiments with Sepharose beads alone (Figure 2A, right panel).
Figure 2.
PP2A preferentially associates with β-catenin in V13-transfected cells. (A) The hypoglycosylated variant V13 (V13) recruits more PP2A than wild-type E-cadherin (E-cad). Left panel, GST pulldown samples from CHO cells transfected with either E-cad or V13 were assessed for association with PP2A by Western blot (WB). Bargraph, Fold change of PP2A protein level from V13 cells was determined in comparison with E-cad cells after normalization to GST (*P < 0.05). Right panel, Controls for specificity of GST pulldown. (B) PP2A preferentially binds to β-catenin complexes. Left panel, WB of PP2A in β-catenin immunoprecipitates. Bar graph, Fold change in PP2A levels in V13 cells was determined in comparison with the E-cad cells in β-catenin immunoprecipitates after normalization to β-catenin (**P < 0.01). Right panel, Isotype controls for specificity of β-catenin immunoprecipitates. (C) N-glycosylation of E-cadherin does not affect the binding of PP2A to γ-catenin complexes. Left panel, WB of PP2A in γ-catenin immunoprecipitates. Bargraph, Fold change in PP2A levels in V13 cells was determined in comparison with the E-cad cells in γ-catenin immunoprecipitates after normalization to γ-catenin. No significant change in its association with the γ-catenin complexes was detected. Right panel, Isotype controls for specificity of γ-catenin immunoprecipitates. Error bars represent standard deviation from three independent experiments; P values were calculated by two-tailed t-test.
Although the recruitment of PP2A to AJs is known to stabilize cell-cell adhesion, its biological significance has not been determined. To gain insights into the function of PP2A in AJs, we first asked whether the increased association of PP2A in V13-expressing cells was mediated by either β-catenin or γ-catenin. Examination of the abundance of PP2A in β-catenin immunoprecipitates from E-cadherin- and V13-transfected CHO cells revealed an increased (2.8-fold) association in V13-expressing cells (Figure 2B, left panel). In contrast, no significant change was detected in the recruitment of PP2A to γ-catenin complexes from E-cadherin and V13-transfected cells (Figure 2C, left panel). The specificity of interactions of PP2A with β- and γ-catenins was confirmed with isotype controls (Figures 2B and C, right panels). These data indicate that PP2A is preferentially recruited to β-catenin in V13 expressing cells.
Diminished N-glycosylation of E-cadherin is linked to dephosphorylation of tau and to association of β-catenin with dynein
One important function of PP2A resides in the stabilization and growth of microtubules (MTs) through dephosphorylation of two microtubule associated proteins (MAPs), tau and dynein.33–36 Tau regulates the growth and stabilization of MTs while dynein is a motor protein that regulates MT dynamics. Both MAPs are active in their dephosphorylated state and both are substrates of PP2A.37–39 Importantly, dynein has also been shown to tether MTs to AJs by binding β-catenin.14,40 Thus, it was possible that the increased binding of β-catenin complexes to PP2A played a role in the association with MTs. To test this hypothesis, we examined the effects of E-cadherin N-glycosylation on the phosphorylation state of tau and on the interaction of dynein with β-catenin.
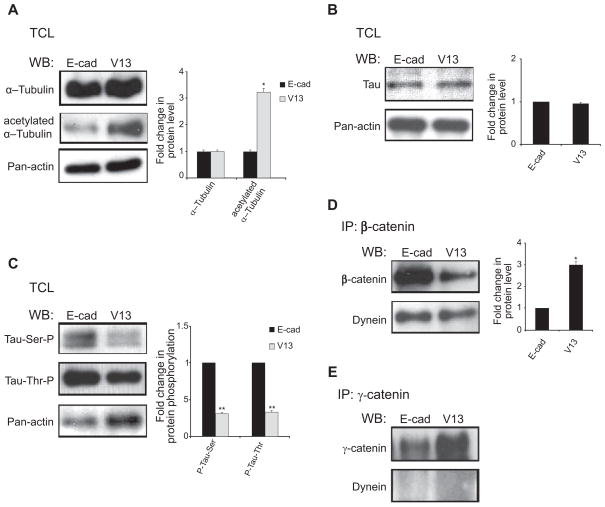
Cell lysates from E-cadherin- and V13-expressing cells did not display significant changes in the levels of α-tubulin, although the abundance of its acetylated form was increased in V13 cells (Figure 3A). Acetylated tubulin has been shown to contribute to the stability of MTs,41,42 although the relationship between the acetylation of tubulin and PP2A is unclear. While total expression of tau was not markedly different in TCLs from E-cadherin- and V13-transfected cells (Figure 3B), its phosphorylation status was significantly diminished in V13 cells (Figure 3C). This was the case for both serine and threonine phosphorylation (Figure 3C). These data suggest that increased recruitment of PP2A to β-catenin complexes plays a role in the stabilization of MTs through the dephosphorylation of tau.
Figure 3.
N-glycosylation status of E-cadherin affects microtubule associated proteins (MAPs), tau and dynein. (A) effects of N-glycosylation of E-cadherin on α-tubulin expression and acetylation. WB of α-tubulin expression in total cell lysates (TCLs) from E-cad- and V13-transfected cells. While no significant difference in tubulin expression was detected, more acetylated α–tubulin was found in V13 cells. Bargraph, Fold changes in α-tubulin and its acetylated pool from V13 cells were determined in comparison to E-cad cells after normalization to actin (*P < 0.05). (B) effects of N-glycosylation of E-cadheirn on tau expression. WB of tau steady-state levels in TCLs from E-cad- and V13-transfected cells. No significant differences were detected between E-cad and V13 cells. (C) V13 cells have lower levels of phosphorylated tau. TCLs from E-cad and V13 cells were analyzed for ser199 and Thr231 phosphorylation of tau. Levels of P-tau were significantly lower in V13 compared to E-cad cells. Bargraph, Fold changes in P-tau levels in V13 cells were determined in comparison to E-cad cells after normalization to actin (**P < 0.01). (D) Recruitment of dynein to β-catenin complexes in E-cad and V13 cells. β-catenin immunoprecipitates from E-cad and V13 cells were assessed for association with dynein by WB. Bargraph, Fold change of dynein levels in γ-catenin immunoprecipitates from V13 cells was determined in comparison with E-cad cells after normalization to γ-catenin (*P < 0.05). (E) Association of dynein with γ-catenin complexes is not affected by N-glycosylation of E-cadherin. Bargraph Fold change in dynein levels in γ-catenin immunoprecipitates from V13 cells were determined in comparison with E-cad cells after normalization to γ-catenin. No significant differences were detected between E-cad and V13 cells. Error bars reflect standard deviation from at least three independent studies, and P-values were calculated by two-tailed t-test.
Dynein has been shown to interact with E-cadherin scaffolds through a complex with β-catenin.14,40 Examination of GST pulldowns from E-cadherin- and V13-tranfected cells showed preferential binding (2–2.5-fold increase) of dynein to β-catenin in V13-transfectants (Figure 3D). No association was detected between dynein and γ-catenin (Figure 3E), confirming that dynein interacted specifically with β-catenin.
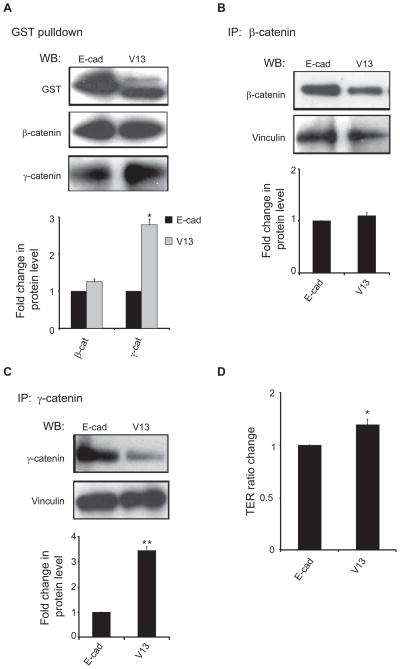
CHO cells expressing V13 exhibit increased association of γ-catenin with vinculin
The stability of AJs has been shown to depend on their interaction with the actin cytoskeleton (8). Our previous studies have shown that in MDCK cells, V13 exhibited an increased association with γ-catenin and vinculin compared to wild-type E-cadherin.15 Vinculin is a cytoskeletal protein known to promote the interaction of AJs with the actin cytoskeleton.22 Analysis of GST pulldowns from E-cadherin and V13-transfected cells confirmed that in CHO cells, V13 showed enhanced association with γ-catenin (Figure 4A). Since β-catenin complexes exhibited greater interaction with PP2A and dynein, we examined whether γ-catenin complexes displayed increased interaction with vinculin. Analysis of vinculin in β-catenin immunoprecipitates showed no significant change between E-cadherin- and V13-transfectants (Figure 4B). In contrast, the abundance of vinculin in γ-catenin immunoprecipitates was significantly increased (Figure 4C). Therefore, the observed increased interaction of γ-catenin with vinculin is likely to reflect increased association of these complexes with the actin cytoskeleton in V13-transfected CHO cells.
Figure 4.
The hypoglycosylated E-cadherin variant, V13, promotes the interaction of γ-catenin complexes with vinculin. (A) V13 exhibits increased interaction with γ-catenin. E-cadherin complexes were purified by GST pulldown and analyzed for association with β- and γ-catenins by WB. More γ-catenin was found in V13 complexes compared to E-cad, while no significant change was detected in the interaction between either E-cad or V13 and β-catenin. Bargraph, Fold changes in β- and γ-catenin levels from V13 cells were determined in comparison with E-cad cells after normalization to E-cad (*P < 0.05). (B) N-glycosylation of E-cadherin does not impact the association of vinculin with β-catenin complexes. β-catenin immunoprecipitates from E-cad and V13 cells were assessed for association with vinculin by WB. Bargraph, Fold change of vinculin levels in β-catenin immunoprecipitates from V13 cells was determined in comparison with E-cad cells after normalization to β-catenin. No significant change was detected. (C) V13 cells recruit more vinculin to γ-catenin complexes. γ-catenin immunoprecipitates from E-cad and V13 cells were assessed for association with vinculin by WB. Bargraph, Fold change of vinculin levels in γ-catenin immunoprecipitates from V13 cells was determined in comparison with E-cad cells after normalization to γ-catenin (**P < 0.01). (D) V13 cells display increased TER. TER measurements were carried out with confluent monolayers with duplicate samples. The value of TER for E-cad cells was defined as 1.0. The results were obtained from three independent experiments (*P < 0.05). Error bars were obtained as standard deviation from three independent experiments. P values were calculated by two-tailed t-test.
Since stabilization of AJs has been shown to be required for the mainteneance of tight junctions, we measured the effect of E-cadherin N-glycosylation on CHO cell TER, a direct indicator of the tightness of the paracellular seal and a measure of paracellular permeability. Results showed that confluent V13 cell monolayers formed TJs that displayed increased steady-state TER compared to E-cadherin-bearing cells (Figure 4D).
Enhanced interactions of PP2A with β-catenin and vinculin with γ-catenin occur at V13 scaffolds
Our data showing that in V13-expressing CHO cells, β-catenin bound more PP2A while γ-catenin bound more vinculin, strongly suggested that these interactions were mediated through a direct association with V13. It also reflected the presence of distinct V13/β- and V13/γ-catenin complexes. To prove that V13 formed two junctional complexes mediated by either β- or γ-catenin, we used the recently developed double immunoprecipitation technique.43 This procedure allowed us to isolate V13 complexes linked to either β- or γ-catenin and then to identify whether they displayed preferential association with either PP2A or vinculin. E-cadherin antibody was immobilized on a column (Profound Co-immunoprecipitation Kit; Pierce Biotechnology) and intact V13 complexes that were free from antibody were isolated using nonreducing elution buffer. This made it possible to carry out another set of immunoprecipitations for the members of V13 complexes, β-catenin and γ-catenin. Therefore, the elution sample with antibody-free V13 complexes was divided in two: one part was used to immunoprecipitate β-catenin and the other to immunoprecipitate γ-catenin. This led to the isolation of V13/β-catenin and V13/γ-catenin complexes that could then be probed for association with PP2A and vinculin by Western blot. Results showed that significantly more PP2A was associated with V13/β-catenin, while vinculin was exclusively associated with V13/γ-catenin (Figure 5). In control studies, only trace amounts of γ-catenin were detected in the isolated V13/β-catenin complexes, while no β-catenin was apparent in V13/γ-catenin complexes (Figure 5). These results provided the first evidence that the hypoglycosylated E-cadherin variant V13 promoted increased association with PP2A and vinculin via distinct complexes mediated by β-catenin and γ-catenin, respectively.
Figure 5.
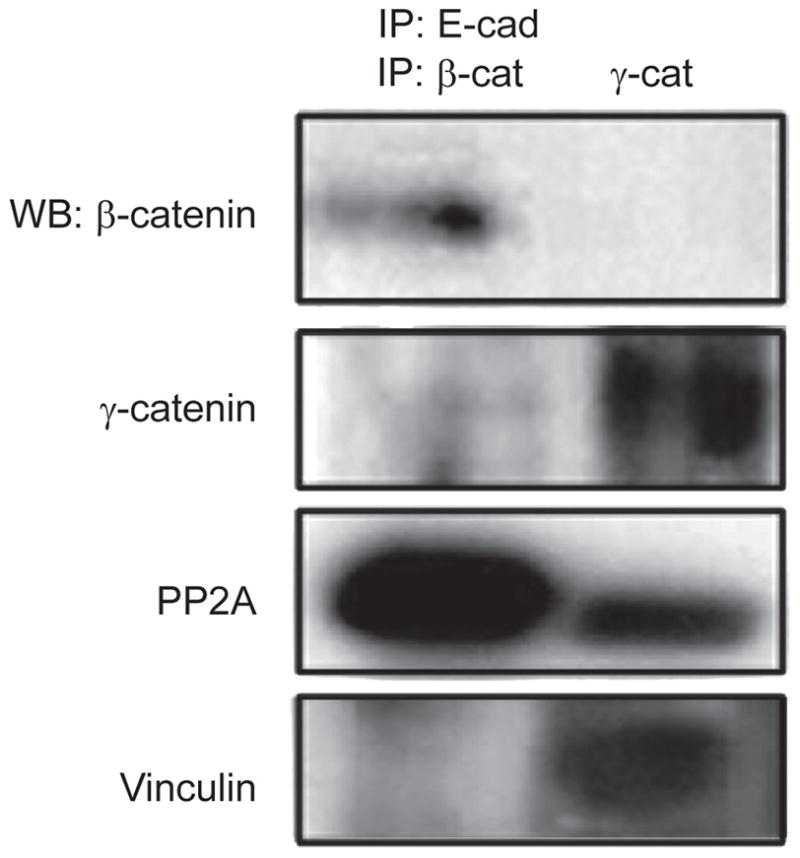
V13 promotes the recruitment of PP2A and vinculin to AJs via distinct β- and γ-catenin scaffolds. A double immunoprecipitation approach was used to separate V13/β-catenin and V13/γ-catenin complexes from V13-tranfected CHO cells (described in Materials and methods). V13-containing complexes, devoid of E-cadherin antibody, were isolated and subjected to a second round of immunoprecipitation using antibodies to either β-catenin or γ-catenin. The immunoprecipitates were then analyzed for β-catenin, γ-catenin, PP2A and vinculin by WB. Results are representative of two independent experiments.
Hypoglycosylated E-cadherin enhances intercellular adhesion in A253 cancer cells
Recently, we have shown that reduction of cellular N-glycosylation in A253 cancer cells resulted in a diminished N-glycosylation of E-cadherin, and that this resulted in enhanced intercellular adhesion and the reversion of cancer phenotype to an epithelial morphology19 Since V13 drives the association of AJs with dynein and vinculin, we examined if tranfection of V13 alone into A253 cells could promote the formation of mature AJs and enhance intercellular adhesion.
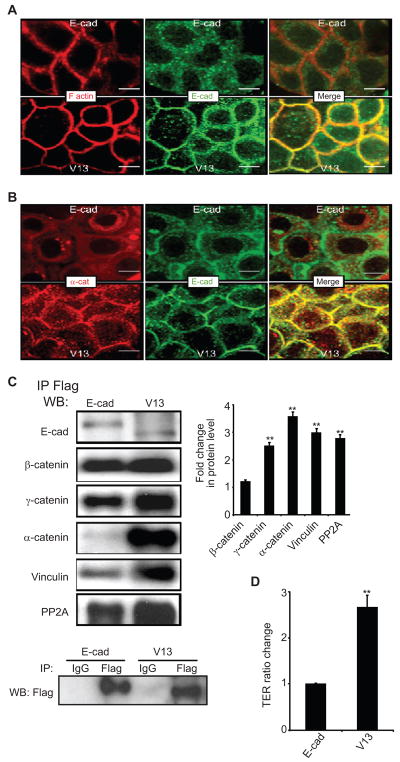
We have previously reported that A253 cancer cells do not form mature AJs and fail to assemble functional TJs due to excessive N-glycosylation of endogenous E-cadherin(19). Immunofluorescence localization of E-cadherin in A253 cells transfected with Flag-tagged wild-type E-cadherin showed diffuse, primarily cytoplasmic distribution, similar to that of endogenous E-cadherin (Figure 6A). In contrast, A253 cells transfected with Flag-V13 displayed well-organized E-cadherin at cell-cell interfaces (Figure 6A). The well-localized continuous staining of E-cadherin in Flag-V13-expressing cells correlated with intense F-actin staining, suggesting co-localization (Figure 6A). Furthermore, in V13 cells, α-catenin was better organized at cell-cell borders and showed more co-localization with E-cadherin than in wild-type E-cadherin-expressing cells (Figure 6B). Biochemical analyses of exogenous Flag-E-cadherin- and Flag-V13 scaffolds were then carried out with Flag immunoprecipitates from Flag-E-cadherin and Flag-V13-transfected A253 cells. Results showed that V13 recruited more stabilizing proteins to AJs, including γ-catenin, α-catenin, vinculin, and PP2A (Figure 6C). The specificity of Flag immunoprecipitates was confirmed using an isotype control (Figure 6C, IgG).
Figure 6.
Transfection of V13 into A253 cancer cells enhances intercellular adhesion. (A), Immunofluorescence localization of E-cadherin and F-actin in A253 cells following transfection with either E-cad or V13. E-cadherin from V13 cells exhibit increased co-localization with F-actin at cell-cell contact sites. E-cad and V13 cells were grown to confluence and processed for indirect immunofluorescence staining using an antibody to the cytoplasmic region of E-cadherin. Cells were counterstained for F-actin with rhodamine-phalloidin. Shown are 0.74 μm confocal x–y sections. Size bars, 10 μm. (B) Immunofluorescence localization of E-cadherin and α-catenin in A253 cells transfected with E-cad and V13. Enhanced lateral colocalization of E-cadherin and α-catenin was detected in V13 cells compared to E-cad cells. Shown are 0.74 μm confocal x–y section. Size bars, 10 μm (C) A253 cells transfected with V13 remodel AJs with increased amounts of stabilizing proteins. E-cadherin complexes were immunoprecipitated from A253 cells transfected with E-cad and V13 using Flag antibody. Samples were analyzed for association with β-catenin, γ-catenin, α-catenin, vinculin and PP2A by WB. Bargraph, Fold changes in protein levels associated with E-cadherin from V13 cells were determined in comparison to E-cad after normalization to Flag (*P < 0.05). (D) Hypoglycosylated E-cadherin variant, V13 enhances TER in A253 cells. The TER for nontransfected A253 cells was defined as 1.0. Error bars reflect standard deviation from three independent studies; P values were calculated by two-tailed t-test.
The hypoglycosylated variant V13 also enhanced the integrity of TJs in A253 cells. When plated on Transwell filters and grown to high density, A253 cells transfected with V13 displayed a TER value higher than cells transfected with wild-type E-cadherin (Figure 6D). These results indicate that AJs with hypoglycosylated E-cadherin enhance cell-cell adhesion in A253 cancer cells.
Discussion
E-cadherin plays pivotal roles in the maintenance of adult tissue architecture and in the prevention of tumor spread. The ability of E-cadherin to perform these functions depends on the formation of mature AJs that require interactions with cytoskeletal components, the actin cytoskeleton and most likely with MTs.8,40 Our present studies provide evidence that interactions of E-cadherin scaffolds with these cytoskeletal components are affected by the N-glycosylation status of E-cadherin. Moreover, they show for the first time that the association of AJs with these two cytoskeletal components is enhanced through the assembly of distinct hypoglycosylated E-cadherin-containing scaffolds mediated by either β- or γ-catenins.
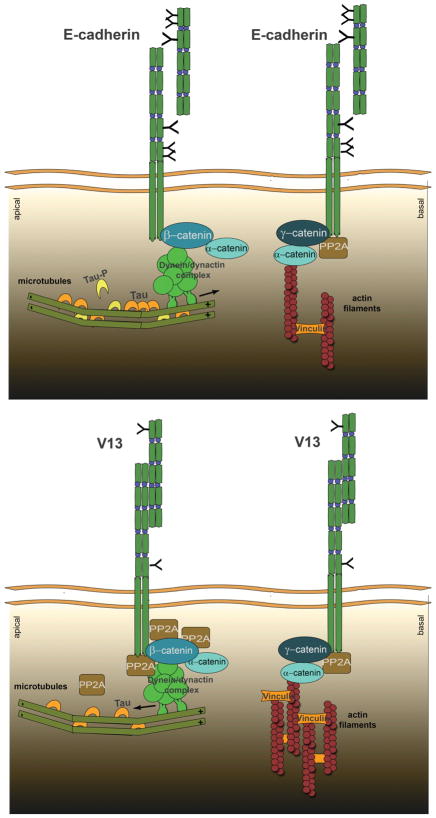
Our data show that diminished N-glycosylation of E-cadherin in CHO cells does not interfere with its targeting to the cell surface (Figure 1B) but rather leads to the remodeling of AJs (Figures 2–3). Both wild-type and hypoglycosylated E-cadherins formed more than one type of complex, either with different components or with different stoichiometries of association. This is not surprising in light of the diverse functions of E-cadherin junctions. The novelty of our finding, however, stems from the identification of two distinct β- and γ-catenin- containing scaffolds assembled by the hypoglycosylated E-cadherin variant, V13, that drive the association with different cytoskeletal components. These scaffolds display increased affinity for dynein, which has been shown to mediate the association of AJs with MTs, and vinculin, which mediates association of AJs with the actin cytoskeleton (Figure 7).
Figure 7.
Schematic representation of how N-glycosylation of E-cadherin affects cytoskeletal dynamics. Extensively N-glycosylated E-cadherin/β-catenin complexes associate with less PP2A and dynein compared to hypoglycosylated V13/β-catenin AJs. V13/β-catenin complexes recruit more PP2A, which correlates with dephosphorylation of tau, and most likely dynein, leading to stabilization of MTs. Also, V13/γ-catenin complexes recruit more vinculin, most likely via α-catenin, which promotes the interaction of AJs with the actin filaments.
We have previously shown that extensively N-glycosylated E-cadherin inhibited the formation of mature AJs and TJs by interfering with the recruitment of PP2A to E-cadherin scaffolds.19 Although to date, several studies have shown that PP2A is required for the integrity of AJs, its role(s) in E-cadherin junctions has not been elucidated. Here, our findings provide the first molecular explanation for the recruitment of PP2A to AJs. They show that hypoglycosylated E-cadherin/β-catenin complexes preferentially bind PP2A and dynein, which are likely to enhance the tethering of MTs to AJs. Since such binding of MTs to the sites of AJs has been shown to enhance the clustering of AJs,10 increased interaction of hypoglycosylated E-cadherin-containing AJs with MTs is consistent with the diminished-N-glycosylation-driven maturation of AJs.
MTs form a well-organized network and run principally parallel to cell-cell contacts with their (+) ends oriented towards the basal aspects of cells.44 In epithelial cells, a sub-population of MTs extends into E-cadherin-based cell-cell contacts with their (+) ends oriented towards the adhesion sites.10 MT dynamics are tightly regulated both spatially and temporally. PP2A has emerged as a key regulator of MT dynamics by dephosphorylating several major MAPs, such as tau, which is critical for MT assembly and stabilization,27 and dynein, which enhances this MT-motor protein’s sliding activity33,45
Increased recruitment of PP2A to V13-containing AJs correlated with a three-fold greater abundance of dynein in β-catenin complexes. Dynein has been shown to bind β-catenin and to localize at AJs where it tethers MTs to AJs.14 It is possible that upon increased recruitment to the hypoglycosylated E-cadherin/β-catenin complex, PP2A can dephosphorylate dynein and enhance its sliding activity. Cytoplasmic dyneins are a family of motor proteins that drive movement towards the (−) ends of MTs and facilitate the transport of vesicles to the apical cell surface in epithelial cells.46,47 Since hypoglycosylated E-cadherin-containing AJs drive intercellular adhesion, their preferential interaction with MTs may also explain the recently reported role for N-glycosylation in cell polarity.
Another role of PP2A in AJs is likely to involve the dephosphorylation of tau, thereby enhancing its stabilizing function and maintaining the normal cellular tubulin: tau ratio. Tau is known to promote MT nucleation and assembly, with a cellular tubulin:tau ratio usually being low at 40:1.48 At this concentration, tau becomes distributed in a proximal to distal gradient to stabilize MTs and assist in organelle transport.49,50 Tau hyperphosphorylation is believed to be the triggering event in the tau aggregation cascade.51 Clusters of tau on the MTs interfere with the function of motor proteins and the transport of cytoplasmic material along MTs. Dynein complexes encountering high concentrations of tau will reverse direction and fail to transport vesicles to the (−) end of MTs or to the apical part of the cell.46,47 Indeed, inhibition of PP2A by okadaic acid has been shown to increase the concentration of P-tau.29 Thus, increased presence of PP2A in the hypoglycosylated E-cadherin-containing AJs in close proximity to MTs may confer stability to the overall MT network and promote its interaction with AJs. Accordingly, our data show that V13- transfected cells had less P-tau, which coincides with more stable MT dynamics.
While phosphorylation of β-catenin at tyrosine residues has been shown to induce the disassembly of E-cadherin-catenin complexes from the actin filament network, inhibition of PP2A by okadaic acid has been reported to lead to β-catenin’s hyperphosphorylation and AJ disruption.52 Therefore, the increased recruitment of PP2A to hypoglycosylated E-cadherin-containing AJs is also likely to confer stability to the E-cadherin/β-catenin complex.
Previously, we reported that hypoglycosylated E-cadherin-containing AJs exhibited increased interaction with γ-catenin, α-catenin, and vinculin.15 Our studies now extend these observations and show that it is through a preferential recruitment of γ-catenin that hypoglycosylated E-cadherin complexes interact with vinculin. Because α-catenin dimers regulate the bundling of the actin flaments,8 and because the interaction of vinculin with F-actin has been suggested to collaborate with α-catenin to promote vinculin activation and formation of actin bundles, our studies suggest hypoglycosylated E-cadherin/γ-catenin/vinculin complexes preferentially mediate the interaction with the actin cytoskeleton.
It is unclear how the removal of N-glycans induces changes in the composition of E-cadherin scaffolds. One possibility is that the extracellular domains of hypoglycosylated E-cadherin form stronger bonds in trans that lead to a conformational change in cis of the cytoplasmic domain. Since E-cadherin-mediated adhesion is known to be regulated by its cytoplasmic domain, V13-driven conformational change may result in an increased affinity of AJs for stabilizing proteins.53 Here, we show that hypoglycosylated E-cadherin organizes two distinct β- and γ-catenin-mediated scaffolds with increased stoichiometries of stabilizing proteins.
Collectively, our studies reveal novel insights into the roles of N-glycosylation in AJ remodeling and cytoskeletal interactions. We show that hypoglycosylated E-cadherin drives the formation of mature AJs through the organization of two distinct junctional complexes that are likely to promote the interaction of AJs with either the actin cytoskeleton or MTs (Figure 7). Hypoglycosylated E-cadherin/β-catenin complexes preferentially recruit PP2A and dynein. The presence of PP2A in close proximity to dynein suggests that PP2A can dephosphorylate dynein, enhance its motor activity, and promote the tethering of MTs to AJs. Moreover, increased association of PP2A with AJs tethered to MTs is likely to maintain tau in a dephosphorylated state, providing strength and support to the MT network. In addition to promoting AJ clustering, the tethering of MTs to AJs may serve to coordinate the maturation of AJs with the MT-directed transport of polarity proteins to the apical domain and with the establishment of cell polarity. On the other hand, hypoglycosylated E-cadherin/γ-catenin complexes preferentially mediate the interaction with the actin cytoskeleton through the recruitment of vinculin. Thus, protein N-glycosylation has now emerged as one of the key regulators of intercellular adhesion and cytoskeletal dynamics, highlighting the cross talk between cellular metabolism and cell structure and behavior.
Footnotes
Disclosure
This work was supported by grant sponsor, National Institutes of Health. Grant number: DE010183; DE015304.
References
- 1.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell-adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107(4):1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 3.Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987;105(6 Pt 1):2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: A fundamental determinant of cadherin function. Curr Biol. 1997;7(5):308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112(4):535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 6.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4(4):E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 7.Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5(8):614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stehbens SJ, Paterson AD, Crampton MS, et al. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006;119(9):1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- 11.Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18(5):541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Leonard M, Chan Y, Menko AS. Identification of a novel intermediate filament-linked N-cadherin/gamma-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Dev Biol. 2008;319(2):298–308. doi: 10.1016/j.ydbio.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligon LA, Karki S, Tokito M, Holzbaur ELF. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3(10):913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 15.Liwosz A, Lei TL, Kukuruzinska MA. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem. 2006;281(32):23138–23149. doi: 10.1074/jbc.M512621200. [DOI] [PubMed] [Google Scholar]
- 16.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 17.Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- 18.Vagin O, Tokhtaeva E, Yakubov I, Shevchenko E, Sachs G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia – Contribution of the Na,K-ATPase beta(1) subunit. J Biol Chem. 2008;283(4):2192–2202. doi: 10.1074/jbc.M704713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nita-Lazar M, Noonan V, Rebustini I, Walker J, Menko AS, Kukuruzinska MA. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009;69(14):5673–5680. doi: 10.1158/0008-5472.CAN-08-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JL, Menko AS, Khalil S, Rebustini I, Hoffman MP, Kreidberg JA, et al. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Dev Dyn. 2008;237(11):3128–3141. doi: 10.1002/dvdy.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palovuori R, Eskelinen S. Role of vinculin in the maintenance of cell-cell contacts in kidney epithelial MDBK cells. Eur J Cell Biol. 2000;79(12):961–974. doi: 10.1078/0171-9335-00120. [DOI] [PubMed] [Google Scholar]
- 22.Watabe-Hchida M, Uchida N, Imamura Y, et al. Alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sontag JM, Sontag E. Regulation of cell adhesion by PP2A and SV40 small tumor antigen: An important link to cell transformation. Cell Mol Life Sci. 2006;63(24):2979–2991. doi: 10.1007/s00018-006-6300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353(Pt 3):417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13(1):7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 26.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158(5):967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurland G, Gundersen GG. Protein phosphatase inhibitors induce the selective breakdown of stable microtubules in fibroblasts and epithelial-cells. Proc Natl Acad Sci U S A. 1993;90(19):8827–8831. doi: 10.1073/pnas.90.19.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merrick SE, Trojanowski JQ, Lee VMY. Selective destruction of stable microtubules and axons by inhibitors of protein serine/threonine phosphatases in cultured human neurons (NT2N cells) J Neurosci. 1997;17(15):5726–5737. doi: 10.1523/JNEUROSCI.17-15-05726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tar K, Birukova AA, Csortos C, Bako E, Garcia JGN, Verin AD. Phosphatase 2A is involved in endothelial cell microtubule remodeling and barrier regulation. J Cell Biochem. 2004;92(3):534–546. doi: 10.1002/jcb.20036. [DOI] [PubMed] [Google Scholar]
- 30.Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17(3):1085–1095. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Nakajima E, Suzuki K. Involvement of protein phosphatase 2A in the maintenance of E-cadherin-mediated cell-cell adhesion through recruitment of IQGAP1. J Cell Physiol. 2006;206:814–820. doi: 10.1002/jcp.20524. [DOI] [PubMed] [Google Scholar]
- 32.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan V. Motor proteins: A dynamic duo. Curr Biol. 1996;6(6):630–633. doi: 10.1016/s0960-9822(09)00434-5. [DOI] [PubMed] [Google Scholar]
- 34.Yang PF, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275(25):18905–18912. doi: 10.1074/jbc.M002134200. [DOI] [PubMed] [Google Scholar]
- 35.Tar K, Csortos C, Czikora I, et al. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem. 2006;98(4):931–953. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 36.Sontag E, NunbhakdiCraig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2A. Neuron. 1996;17(6):1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 37.Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain – Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275(8):5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 38.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2A and-1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485(1):87–93. doi: 10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- 39.Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton. 2002;52(1):33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- 40.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8(7):808–819. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 41.Piperno G, Fuller MT. Monoclonal-antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sale WS, Besharse JC, Piperno G. Distribution of acetylated alpha-tubulin in retina and in vitro-assembled microtubules. Cell Motil Cytoskeleton. 1988;9(3):243–253. doi: 10.1002/cm.970090306. [DOI] [PubMed] [Google Scholar]
- 43.Leonard M, Chan Y, Menko AS. Identification of a novel intermediate filament-linked N-cadherin/gamma-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Dev Biol. 2008;319(2):298–308. doi: 10.1016/j.ydbio.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert T, Lebivic A, Quaroni A, Rodriguezboulan E. Microtubular organization and its involvement in the biogenetic pathways of plasmamembrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991;113(2):275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burkhardt JK. The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim Biophys Acta. 1998;1404(1–2):113–126. doi: 10.1016/s0167-4889(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 46.Dixit R, Ross JL, Goldman YE, Holzbaur ELF. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319(5866):1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuko K, Shoichiro T. Cell polarity, microtubule growing end-binding proteins and cell polarity. Cell Technology. 2000;19(12):1774–1780. [Google Scholar]
- 48.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84(3):335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 49.Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament- dependent manner. J Neurosci. 1996;16(18):5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Black MM, Slaughter T, Moshiach S, Obrocka M, Fischer I. Tau is enriched on dynamic microtubules in the distal region of growing axons. J Neurosci. 1996;16(11):3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goryunov D, Liem RKH. CHIP-ping away at tau. J Clin Invest. 2007;117(3):590–592. doi: 10.1172/JCI31505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serres M, Grangeasse C, Haftek M, Durocher Y, Duclos B, Schmitt D. Hyperphosphorylation of beta-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exper Cell Res. 1997;231(1):163–172. doi: 10.1006/excr.1996.3443. [DOI] [PubMed] [Google Scholar]
- 53.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]