Abstract
Voluntary eye movements and covert shifts of visual attention activate the same brain regions. Specifically, the intra parietal sulcus and the frontal eye fields (FEF) appear to be involved both with generating voluntary saccades as well with attending to a peripheral spatial location. Furthermore, these regions appear to be required by both tasks – functional disruption of these regions impairs both tasks. Therefore, it appears that the targeting system that allows us to plan saccades is the same system that allows us to covertly track peripheral visual information. Recent neuroimaging studies suggest that these brain regions are also activated when participants engage in auditory spatial attention tasks. However, it remains unclear whether these regions are required by these tasks. We used repetitive Transcranial Magnetic Stimulation (rTMS) to disrupt the FEF while participants performed an auditory localisation task. On each trial, a visual cue directed attention to the probable laterality of the auditory target, and the participant decided whether the subsequent target sound came from an upper or lower speaker. In the absence of TMS, individuals were faster to respond to targets that occurred on the cued side (valid trials) than when the target appears contralaterally to the cued side (invalid side). TMS interfered with this effect, such that the costs associated with ipsilateral invalidly cued targets were substantially reduced. These results suggest that the eye-movement system is needed for normal auditory attention.
Keywords: Frontal eye fields (FEF), endogenous attention, reaction times, humans, audition
1. Introduction
The most effective way of directing visual attention is to point the eyes at the object of interest, so that its image lands on the fovea. These overt shifts of perception result in enhanced performance, with individuals being faster and more accurate at identifying foveated items than items that appear in peripheral vision. However, individuals are also able to covertly attend to a region of their peripheral visual field. People are faster and more accurate at identifying attended peripheral information than similar stimuli presented at an unattended peripheral location. The neural mechanisms underlying covert visual attention have been intensely studied. Many argue that covert visual attention is mediated by the same targeting circuits used for overt eye movements (Rizzolatti et al., 1994). Evidence for this “premotor” model of attention comes from studies that show that the same regions of the brain are involved with both executing eye movements and covert shifts of visual attention (de Haan et al., 2008). However, this model remains controversial (Juan et al., 2004).
There is a growing body of evidence that visual attention is closely coupled with the brain regions involved with eye movement programming. Here we focus on evidence regarding the Frontal Eye Fields (FEF). The FEF plays a clear role in eye movement generation. For example, patients with injury to the FEF have difficulty generating voluntary contralateral saccades as well as suppressing ipsilateral reflexive saccades (Henik et al., 1994). Furthermore, neuroimaging has revealed that this region is involved during both eye movements as well as peripheral attention tasks (for review, see Corbetta et al., 1998). In previous work we have demonstrated a causal relationship between the FEF and voluntary attention (Smith et al., 2005). This work complements that of other researchers who have briefly disrupted the FEF using Transcranial Magnetic Stimulation (TMS) to reveal an association between FEF and visual selection (Muggleton et al., 2003), voluntary visual attention (Grosbras & Paus, 2002) encoding spatial position (Campana et al., 2007) and the inhibitory consequences of reflexive attention (IOR: where a peripheral cue initially benefits visual processing, but over time begins to exert a significant cost on the processing of the stimuli, compared to uncued locations, see Ro et al., 2003). Furthermore, TMS applied to the FEF can abolish the perceptual enhancement effect that is normally observed for visual stimuli that appear near the location of a saccade target (Neggers et al., 2007). In addition, TMS applied to the FEF appears to modulate the electrophysiological signals generated by the posterior visual brain, influencing signals evoked by both the anticipation of visual stimuli as well as those generated in response to the subsequent onset of the visual stimuli (Taylor et al., 2007). Therefore, it appears that the FEF are required for both normal eye movement execution and visual attention.
Curiously, while the relationship between eye-movements and visual attention has been extensively investigated, relatively little interest has been shown in the relationship between saccades and auditory attention. This is interesting, as some of the classic psychological studies of spatial attention have focused on audition, for example the ‘Cocktail Party Effect’ of attending to a single voice in a noisy room filled with many voices (Cherry, 1953; Treisman, 1969). However, many of these auditory attentional effects may rely on non-spatial attributes (e.g. attending to a specific tone, or anticipated words). Spence and Driver (1996) have developed a technique that demonstrates robust effects for auditory spatial attention – participants are visually cued (either by a central strategic cue or a peripheral reflexive cue) to attend to either the left or right side, and then asked to judge whether a subsequent sound occurred slightly above or below the cued location. Spence and Driver found that participants were much faster to detect target sounds at cued locations than at uncued locations. Note that the target sounds were defined purely by spatial location, so the observed benefits could not be due to non-spatial attributes (i.e. the left versus right cue does not predict the up/down motoric response). This paradigm can easily be adapted to explore whether auditory spatial attention involves the eye movement system. In one of the few recent studies to address this issue, Rorden & Driver, (1999) demonstrated faster auditory discrimination in the vicinity of an upcoming saccade, a result that is consistent with the premotor theory (analogous to improved visual performance observed at the location of an upcoming saccade, for a review see Montagnini and Castet, 2007). Further evidence that eye gaze can influence audition comes from Pavani and colleagues (2005), who note that patients with neglect (who are slow to respond to stimuli on their left side following right hemisphere injury) showed improved performance for auditory targets on their left side when they were looking toward the left. Furthermore functional imaging of crossmodal visual/auditory orienting reveals a network of active areas, including the IPS and premotor cortex, potentially including FEF (Lewis et al., 2000). However, note that although both of these studies suggest that eye movements may influence auditory spatial attention, they do not reveal whether the eye movement system is required by these tasks. Disruption studies (such as the visual studies conducted using neurological patients or transient disruption using transcranial magnetic stimulation) could reveal the extent to which the eye movement system plays a causal role in auditory spatial attention.
It should be noted that it is logically possible that auditory spatial attention is completely independent of eye-movement control. After all, the raw sound information received at the ears is not influenced by changes in eye position. It is entirely possible that eye movements do not influence other modalities in the way that they modulate visual perception. For example, visual attention may have evolved by commandeering the eye movement targeting system, while auditory spatial selection works on an independent system. In support of this view, Mondor and colleagues (2000) suggest that eye movement planning did not influence the inhibition of return effect in the auditory modality. However, there is some circumstantial evidence that spatial selection may be independent of modality, with the rapid and spatially accurate eye movement system being an ideal candidate for crossmodal spatial selection. For example, brain imaging studies indicate that the activation of the frontal eye fields is modulated during auditory attention tasks (Mayer et al., 2006; Wu et al., 2007). Of course, as with all activation studies, these findings illustrate that the FEF is involved with auditory orienting, but not whether it is required for normal performance.
The lack of research into the effects of eye-movements on auditory attention is surprising as links between saccades and auditory attention have the potential to offer new insights into the relationship between eye-movements and crossmodal attention. If auditory/visual crossmodal cueing effects are dependent on activity in the eye-movement system the prediction would be that interfering with the functioning of the eye-movement system should interfere with the crossmodal cueing effects demonstrated by Rorden and Driver (1999). On the other hand, if auditory attention is independent of oculomotor control (as suggested by Mondor et al., 2000) disrupting the planning of eye-movements should have no effect on auditory attention. In this study a visual predictive cue is used to orient attention to the left or right. We propose to disrupt the oculomotor system by stimulating the FEF in the period around the onset of a predictive cue. If crossmodal visual/auditory attentional effects are dependent on the eye-movement system, stimulation over FEF during the presentation of the cue should interfere with the ability to orient auditory attention.
2. Methods
2.1. Participants
Fifteen participants (6 female) were recruited from the undergraduate community of the University of Nottingham. Ages ranged from 20 to 34 with a mean age of 22 years. Four participants were left-handed writers. All participants had normal or corrected to normal vision. Participants were screened for risk factors including epilepsy according to the safety guidelines for rTMS (Wassermann, 1998). Four participants did not exhibit robust cueing effects in the control condition (i.e. they were not faster to respond to validly cued targets, collapsed across all conditions) and were excluded from the analysis.
2.2. Apparatus and Stimuli
Participants were stimulated using a Magstim Rapid TMS system (www.magstim.com) that delivered TMS pulses through a 96mm diameter figure of eight coil. The rTMS stimulation was triggered through E-Prime software (www.pstnet.com) running on a desktop Windows-based computer. This software was also used to present the fixation point, visual cue stimuli and auditory probe stimuli. The fixation cross and visual cue were displayed on a 17-inch CRT monitor with a refresh rate of 70Hz. Four Hi-Tex CP-55 computer speakers were used to present auditory stimuli – each mounted to one of the corners of the monitor. A single Panasonic portable CD player (SL-S320)was used to present the auditory target, continuously playing pre-recorded pink noise, with computer controlled reed relays used to switch an individual speaker on and off to present a target sound. Responses were collected with a two button mouse held in the right hand, rotated such that it was horizontal (so that the buttons were oriented up and down instead of left and right). Throughout the experimental sessions the position of the participants’ heads was stabilised by a chin-rest.
2.3. Procedure
Subjects were seated 57cm from the computer screen. Therefore, each speaker was 30° from the central fixation point. Each trial began with presentation of a black fixation point in the centre of the otherwise white monitor. After 1500ms the fixation point was replaced by a small central black arrow pointing left or right. The arrow was displayed for 45ms. The target was presented from one of the four speakers 240ms after the onset of the directional cue. The target sound consisted of four 15ms bursts of pink noise, each separated by 15ms of silence. Participants were instructed to press the top mouse button if the sound occurred from one of the upper speakers or the bottom button if the sound was played over one of the lower speakers. Participants were instructed to maintain fixation on the fixation point throughout the entire experiment. The directional cue correctly predicted the side of presentation on 70% of trials. Each participant completed 240 trials (168 valid, 72 invalid, with precisely half the targets presented on the left and the remainder on the right side, further half of the targets were presented from the upper speakers, and half from the lower speakers). Participants responded with the hand ipsilateral to the stimulated hemisphere. This manipulation prevented reaction times being contaminated by accidental activations of motor cortex.
2.4. rTMS Site Localisation
TMS localization followed an identical procedure as described by Smith et al. (2005), and therefore is only described briefly here (though note that here we targeted the right instead of left FEF). Localisation of the FEF was achieved using structural T1-weighted MRI scans (1.5T Siemens Vision scanner, 3D MPRAGE sequence with a flip-angle = 12°, TR = 9.7ms, TE = 4.0ms, 1×1×1mm). Images were processed using the normalization command of SPM2, but constrained for affine only transforms (as the subsequent registration only creates an affine transform). All of the subjects’ MRI scans were co-registered using software we wrote and now freely distribute (www.mricro.com/mrireg.html), and scalp coordinates were measured using an Ascension minibird magnetic tracking system. The right hand motor area of M1 was identified for each individual, and the appropriate area was marked on the scalp. Single pulse TMS was used to confirm the location of the site, as stimulation of the right hand motor cortex produces contractions in the left hand. Once the most anterior part of the right hand motor area had been localised, a mark was placed a further 20mm anterior along the horizontal axis. This location should place the wand in the vicinity of the FEF (Ro et al., 1999; Paus, 1996) and was used for subsequent stimulation.
2.5. rTMS Procedure
The motor threshold for each subject was determined by reducing stimulation amplitude until no visible movement was seen in the left hand. TMS output was then set to 10% above each individual’s threshold. Participants were stimulated for 176ms at 28Hz (5 pulses), and stimulation began 32 ms before the onset of the central cue. During FEF stimulation the TMS coil was held tangentially to the skull, with the axis of the coil angled approximately 90° from the mid-sagittal axis. During sham stimulations the coil was rotated through 90° such that the edge of the coil was located over FEF stimulation site. This manipulation ensured that participants experienced the auditory alerting effects of TMS, but were not subject to neural stimulation. Throughout the experiment rTMS trials were randomly interleaved with no TMS control trials. All testing was conducted in a single session, with the order of TMS or Sham-TMS counterbalanced across participants.
3. Results
Response accuracy was in the range of 92–93% across all conditions. Trials in which participants made the incorrect response were removed prior to analysis of the reaction time data.
Separate analyses were conducted on the median reaction time data for the FEF and Sham conditions. The data from the FEF condition was subjected to a 2 × 2 × 2 repeated measures ANOVA with factors of Stimulation (TMS/No TMS), Validity (Valid/Invalid) and Side of Target (Left/Right). The ANOVA revealed a significant main effect of Validity (F1,10=20.70, P<0.001), such that responses were faster to validly cued targets than invalidly cued targets, and a significant two-way Validity × TMS interaction (F1,10=5.86, P<0.05).
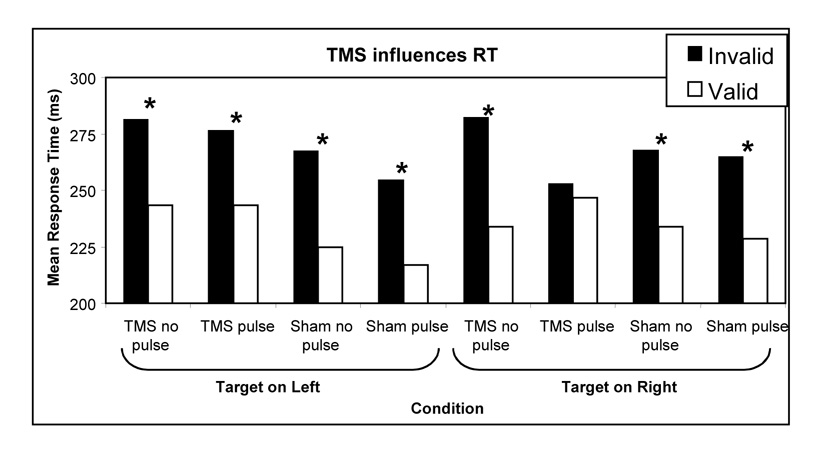
Based on the results of our findings with visual attention (Smith et al. 2005), we predicted any effects of TMS on attention would be observable principally in the contralateral side of space. In order to test this hypothesis planned comparisons were performed on the response times to valid and invalidly cued targets in the left and right VF during right FEF stimulation (Figure 1). Contrary to our hypothesis there was a significant facilitation effect for valid targets in the left (contralateral) side of space (t10=2.28, P<0.05) but not in the right (ipsilateral) side of space (t10=0.46, P=0.659)
Figure 1. Response times to valid and invalidly cued targets.
The vertical axis shows the mean time to respond to targets. The horizontal axis displays the different conditions: half the trials coincided with the sounds of a TMS burst (‘pulse’), while the remaining trials were presented without TMS (‘no pulse’). In addition, the TMS coil was either positioned to stimulate the cortex (‘TMS’), or rotated 90° to mimic the sound of TMS without disrupting brain function (Sham). The auditory target could appear on the left side of the participant (left half of figure) or the right side of the participant (right side of figure). 70% of the trials were valid, with the visual cue accurately predicting the location of the subsequent stimuli, while a few trials were presented on the unexpected side (invalid). TMS appears to eliminate the cost for an invalidly cued auditory target occurring on the right side (e.g. trials where the participant expected a target to appear on the left, but the target was presented on the right). Note that for all but one condition (target on right, TMS pulse) the valid trials were responded to significantly faster than the invalid trials (p<0.05 with a one-tailed t-test, indicated by an asterix).
Data from the sham condition was also subjected to a 2 × 2 × 2 repeated measures ANOVA with factors of Stimulation (TMS/No TMS), Validity (Valid/Invalid) and Side of Target (Left/Right). The ANOVA revealed a significant main of Validity (F1,10=5.88, P<0.05) and no significant interactions.
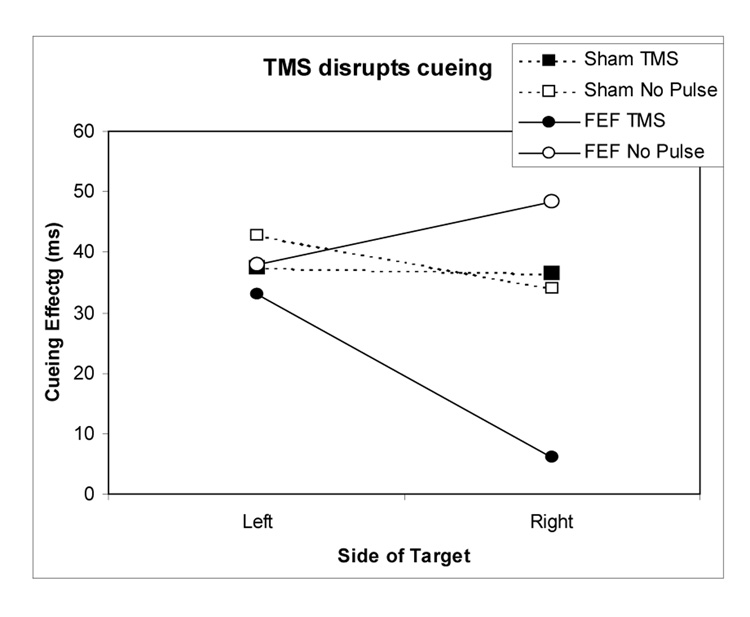
To better illustrate the differing effects of TMS on auditory attention, Figure 2 shows the magnitude of the cueing effect (i.e. the difference in reaction time to valid and invalidly cued targets) in each of the conditions. As is clear form the figure, the only condition in which rTMS impacts on auditory discrimination is when TMS is delivered over right FEF and the target occurs on the right side of space.
Figure 2. Magnitude of cueing effects (ms) in each experimental condition.
The cuing effect is calculated as the benefit for valid cues versus invalid cues. Note that there was a positive cuing effect for all conditions (faster responses when the cue accurately predicted the location of the subsequent target), however TMS over right FEF dramatically reduces the magnitude of the cueing effect for targets occurring in the right hemispace.
4. Discussion
The key observation of this study was that stimulation of the right FEF (a cortical region involved in the control of eye-movements and visual attention) interfered with the processing of auditory information arising from the right side of space. This result offers the first evidence that crossmodal links between visual and auditory attention are mediated by structures involved in the control of eye-movements, such as the frontal eye fields.
However, before drawing any conclusions regarding the role of the FEF in orienting auditory attention, it is necessary to discount artifactual explanations that could account for these data. For example, it is theoretically possible that the loud right-lateralised cracking sound generated by TMS could influence responses to the auditory stimuli (e.g. reflexively cuing the right side of space). According to this model, the cracking sound of the TMS wand on the right side might make one faster to respond to targets occurring on the right side, and this effect might be larger for invalid trials as the valid response times are influenced by a floor effect. However, our sham-TMS condition induced similar auditory stimulation but did not influence the magnitude of the observed cuing effect. This strongly suggests that this effect reflects brain stimulation, rather than a reflexive auditory cue. Nevertheless, we acknowledge that the TMS and sham condition have slightly different sounds, and that the TMS condition also elicits a twitch in scalp muscles that could act as an exogenous cue. Future studies might address this by using a sham condition that mimics both the auditory and tactile properties of the TMS trials. For example employing a Transcutaneous Electrical Nerve Stimulation system can emulate the cutaneous feeling of a TMS pulse (Borckardt et al., 2008).
An additional possibility is that TMS over FEF actually affected the visual processing of the central visual cue, rather than the auditory target. If the processing of the cue were disrupted, there would be no shift of attention and therefore no benefit of a valid cue or cost of an invalid cue. This explanation is unlikely, as it cannot account for the lateralised nature of our result. If TMS interfered with cue processing, it would have disrupted auditory processing on both the left and right sides (recall that the cue is presented at fixation), yet our data clearly show the effects of TMS are restricted to the ipsilateral side of space. Furthermore, it is worth noting that our design is an auditory analogue of the visual study conducted by Smith et al. (2005), yet our results are dramatically different, this suggests that processing of the visual cue alone is unlikely to explain our results (as the use of a visual cue was common to both studies, though note that the earlier study targeted the left FEF). Therefore, we suggest that the TMS did not significantly interfere with the early visual processing of the cue, but rather disrupted the attentional shift signalled by the cue.
One explanation for these data is that FEF stimulation interfered with the programming of a saccadic eye-movement in response to the visual cue, and this interference disrupted the orienting of auditory attention. Indeed, Rorden and Driver (1999) have previously demonstrated that planning an eye-movement triggers a shift of attention to the saccade goal. This explanation would be consistent with premotor accounts of attention that hold that covert attention is the product of planned but unexecuted movements (e.g. Rizzolatti et al., 1994). However, there is now compelling evidence from studies of primate neurophysiology that the visual selective and motor functions of the FEF can be dissociated (Juan et al., 2004; Moore et al., 2003; Moore & Fallah, 2001; Murthy et al., 2001; Sato & Schall, 2003; Schall, 2002; Schall, 2004; Thompson et al., 1997). The spatial resolution of TMS is such that it is impossible to dissociate the effects of TMS on the motor and visual functions of the FEF. Therefore, although these data are consistent with the predictions of the premotor theory, they cannot speak to the key hypothesis of the theory, that planning a saccade is necessary for a shift of attention.
An alternative explanation for these data may relate to the effects of TMS over FEF on “saliency maps”. It has been proposed that the FEF maintains a salience map which represents the spatial locations of stimuli which are behaviourally relevant and to which a saccade might be required (Schall, 2002; Schall, 2004; Moore, Armstrong, & Fallah, 2003; Moore & Fallah, 2001; Thompson, Bichot, & Sato, 2005). Changes in neural activity at specific locations in this map are hypothesised to modulate visual processing through a series of reciprocal connections to extrastriate visual cortex. This modulation corresponds to the allocation of spatial attention. Furthermore, the activity of neurons in the FEF is not specific to visual processing. Some FEF neurons have auditory receptive fields while others have motor fields which discharge prior to saccades to auditory targets (Russo & Bruce, 1994), indicating that the FEF is also involved in orienting towards auditory stimuli. It is therefore plausible that auditory stimulation contributes to activity in the salience map, and that activity in the salience map may influence both visual and auditory processing. TMS delivered over the FEF could interfere with this salience map thus interfering with the allocation of both visual and auditory attention (either adding noise to the map and rendering all locations less salient, or by raising the level of activity in the salience map and therefore facilitating stimulus detection, c.f Grosbras & Paus 2002; 2003),
Although these results demonstrate an effect of TMS over FEF interferes with auditory attention, the effect was only observed in the ipsilateral hemifield. On first inspection this seems a somewhat curious result, given that previous studies of the effects of FEF TMS on visual perception indicate that right FEF stimulation has bilateral disruptive effects on visual search (Muggleton et al., 2003; O'Shea et al., 2004), and bilateral facilitatory affects on visual detection (Grosbras & Paus 2002;2003). However, it should be noted that the disruptive effect of TMS was most apparent for invalid cues - where attention was initially directed into the contralateral, left hemispace and the target appeared in the ipsilateral right hemispace. One speculative but parsimonious explanation for these results is that auditory spatial cuing relies heavily on the superior colliculus (where there are layers that directly code auditory stimulation in register with visual stimuli). Henik et al. (1994) report that patients with frontal eye field injury are slow to make voluntary contralesional eye movements, while at the same time being slow to make reflexive ipsilesional eye movements. This effect is thought to reflect the fact that FEF lesions primarily impact the opposite superior colliculus (Rafal, 2006). Therefore, one could speculate that by stimulating the right FEF we were in effect heavily modulating the output of the left superior colliculus.
It should be noted that TMS can have both excitatory as well as inhibitory effects (for a review, see Paulus 2005), with the presence or absence of stimuli influencing the neural response (e.g. stimulation prior to stimuli onset can elicit different effects than stimulation after onset, see Silvanto and Muggleton, 2008). Furthermore, combined fMRI-TMS studies suggest that stimulation of the frontal eye fields increases activation in early visual cortex regardless of whether a stimulus is present, with increasing TMS intensity increasing activity of early visual cortex that processes the periphery while decreasing activity in regions which represent the central visual field (Ruff et al., 2008). These findings were distinctly different than the pattern observed for stimulation of the parietal cortex. Therefore, our effects could in principal be to either increased or decreased neural activity. However, the important aspect with information processing is the disruption of normal firing sequences.
It is clear from our data that disruption of the FEF has an impact on crossmodal attentional effects. However, it should be noted that this does not necessarily mean that FEF are required for auditory attention per-se. TMS over FEF interfered with the auditory consequences of a visually cued shift of attention. This effect may not be present for tasks that require shifts of attention that are purely auditory. In other words, activity in the oculomotor system related to visual attention may have a beneficial effect on auditory attention, but my not be necessary for auditory attention. Further studies using only auditory cues and targets are required to resolve this issue.
In summary, our work supports a causal connection between the functioning of the eye-movement system and crossmodal visual/auditory attention. This result is consistent with theories of attention that propose a tight coupling between the neural systems used to control movements and the neural systems used to control covert attention, and suggests a role for the FEF in crossmodal attentional orienting. However, further investigation is required to ascertain the precise role of the oculomotor system in orienting auditory attention.
Acknowledgements
All stages of this study were done with support from James Stewart and Robin Birt, who assisted this research as part of their undergraduate projects. We also wish to thank the participants who were involved with this study. CR was supported by the National Institutes of Health (R01 NS054266).
References
- Borckardt JJ, et al. Development and evaluation of a portable sham transcranial magnetic stimulation system. Brain Stimulation. 2008;1:52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana G, Cowey A, Casco C, Oudsen I, Walsh V. Left frontal eye field remembers "where" but not "what". Neuropsychologia. 2007;45:2340–2345. doi: 10.1016/j.neuropsychologia.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Cherry EC. Some experiments upon the recognition of speech with one or two ears. Journal of the Acoustical Society of America. 1953;25:975–979. [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21(4):761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- de Haan B, Morgan PS, Rorden C. Covert orienting of attention and overt eye movements activate identical brain regions. Brain Res. 2008 doi: 10.1016/j.brainres.2008.01.105. PMID: 183296332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras M-H, Paus T. Transcranial Magnetic Stimulation of the Human Frontal Eye Field: Effects on Visual Perception and Attention. Journal of Cognitive Neuroscience. 2002;14(7):1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Grosbras M-H, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci. 2003;18(11):3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- Henik A, Rafal R, Rhodes D. Endogenously Generated and Visually Guided Saccades after Lesions of the Human Frontal Eye Fields. Journal of Cognitive Neuroscience. 1994;6(4):400–411. doi: 10.1162/jocn.1994.6.4.400. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(43):15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, DeYoe EA. A comparison of visual and auditory motion processing in human cerebral cortex. Cerebral Cortex. 2000;10(9):873–888. doi: 10.1093/cercor/10.9.873. [DOI] [PubMed] [Google Scholar]
- Mondor TA, Terrio NA, Hurlburt J. On the role of eye movements and saccade preparation in generating auditory inhibition of return. Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale. 2000;54(4):326–338. doi: 10.1037/h0087348. [DOI] [PubMed] [Google Scholar]
- Montagnini A, Castet E. Spatiotemporal dynamics of visual attention during saccade preparation: Independence and coupling between attention and movement planning. J Vis. 2007;7 doi: 10.1167/7.14.8. 8.1-16. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40(4):671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah T. Control of eye movements and spatial attention. Procedings of the National Academy of Science. 2001;98(3):1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey A, Walsh V. Human frontal eye fields and visual search. Journal of Neurophysiology. 2003;89(6):3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Murthy A, Thompson KG, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. Journal of Neurophysiology. 2001;86(5):2634–2637. doi: 10.1152/jn.2001.86.5.2634. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Huijbers W, Vrijlandt CM, Vlaskamp BN, Schutter DJ, Kenemans JL. TMS pulses on the frontal eye fields break coupling between visuospatial attention and eye movements. J Neurophysiol. 2007;98:2765–2778. doi: 10.1152/jn.00357.2007. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. Journal of Cognitive Neuroscience. 2004;16(6):1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34(6):475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Paulus W. Toward establishing a therapeutic window for rTMS by theta burst stimulation. Neuron. 2005 Jan 20;45(2):201–206. doi: 10.1016/j.neuron.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Rafal RD. Oculomotor functions of the parietal lobe: Effects of chronic lesions in humans. Cortex. 2006;42:730–739. doi: 10.1016/s0010-9452(08)70411-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Sheliga BM. Attention and Performance Xv. Vol. 15. 1994. Space and Selective Attention; pp. 231–265. [Google Scholar]
- Ro T, Cheifet S, Ingle H, Shoup R, Rafal R. Localization of the human frontal eye fields and motor hand area with transcranial magnetic stimulation and magnetic resonance imaging. Neuropsychologia. 1999;37(2):225–231. doi: 10.1016/s0028-3932(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Ro T, Farne F, Chang E. Inhibition of return and the frontal eye fields. Experimental Brain Research. 2003;150:290–296. doi: 10.1007/s00221-003-1470-0. [DOI] [PubMed] [Google Scholar]
- Rorden C, Driver J. Does auditory attention shift in the direction of an upcoming saccade? Neuropsychologia. 1999;37(3):357–377. doi: 10.1016/s0028-3932(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38(4):637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye fields. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2002;357:1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Research. 2004;44(12):1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton MG. New light through old windows: Moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. NeuroImage. 2008;39:549–552. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Smith DT, Rorden C, Jackson SR. Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia. 2005;43(9) doi: 10.1016/j.neuropsychologia.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Spence C, Driver J. Audiovisual links in endogenous covert spatial attention. Journal of Experimental Psychology-Human Perception and Performance. 1996;22(4):1005–1030. doi: 10.1037//0096-1523.22.4.1005. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. Journal of Neurophysiology. 2005;93(1):337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. Journal of Neurophysiology. 1997;77(2):1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wu CT, Weissman DH, Roberts KC, Woldorff MG. The neural circuitry underlying the executive control of auditory spatial attention. Brain Res. 2007;1134(1):187–198. doi: 10.1016/j.brainres.2006.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]