Abstract
Individuals infected with HIV experience numerous comorbidities caused by the disease progression and medications, lack of (or inability to perform) physical activity, malnutrition, or a combination of these causes. Common symptoms include loss of muscle mass, fatigue, lypodystrophy, lypoatrophy, and decreases in strength, functional capacity, and overall quality of life. Studies have shown that exercise is a potential treatment of many of these symptoms. Research suggests that exercise may produce beneficial physiological changes in the HIV-infected population such as improved body composition and increases in both strength and endurance. In addition, psychological conditions such as depression and anxiety have been shown to be positively affected by exercise. The purpose of this review is to examine the literature regarding effects of aerobic, resistance, and combined aerobic and resistance exercise training on HIV-infected individuals.
Keywords: physical activity, AIDS, weight lifting, training, body composition
The emergence of highly active anti-retroviral therapy (HAART) has allowed those infected with HIV to live longer and more productive lives. However, there are a number of HIV-related side effects that are induced by the virus, HAART, and other associated therapies (growth hormone, testosterone) associated with the disease. With the advent socioeconomic classes, there became a larger population of people living with HIV who have personal and environmental factors that predispose to high visceral fat mass and obesity.2 Health care professionals are currently seeking alternative ways to prevent and manage these complications. One nonpharmacological method that is emerging is moderate-of HAART, patients are beginning therapy with higher body fat mass and body mass index (BMI) than reported in previous years, as well as reduced strength and muscle mass.1 As the demographics of the epidemic moved into minority and lower intensity aerobic and resistance exercise.3 Moderate-intensity exercise has been shown to improve total cholesterol, body composition, and psychological well-being and to increase functional capacity, muscular strength, overall health, and quality of life. The purpose of this article is to examine the effects that aerobic, resistance, and combined aerobic and resistance exercise training have on HIV-infected individuals.
The methods used for this review included a search of journal articles using a combination of major electronic databases (ie, PubMed, Medline). Specific keywords (or combinations of) used in the search included the following: HIV, AIDS, exercise, aerobic, resistance, and training. The date of publication was not used as a limit to the literature search.
The included articles are those that focused on aerobic exercise training, resistance exercise training, or a combination of both as a primary intervention to treat any symptom and/or side effect of HIV and/or HIV-related pharmacological side effects. HIV-associated conditions and symptoms have been shown to be quite different in adults compared with children. Therefore, studies that focused on patients younger than 18 years of age were excluded. New medications could possibly hinder the effects of exercise training; thus, any study that introduced new medications to any of the subjects at the time of the research investigation were excluded as well.
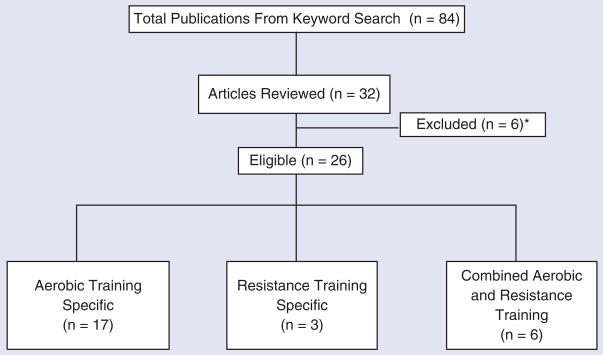
Using the keywords stated previously, a total of 84 results were yielded. However, 52 of those were not reviewed because the purpose of the investigation was not exercise related. The literature search found 32 relevant research publications pertaining to the purpose of this review. Of those 32, 6 were excluded, leaving a total of 26 articles used in this review. Reasons for the exclusion of articles were as follows: 2 included children younger than 18 years, 2 looked solely at the use of hormonal therapy without exercise, and 2 introduced new medications. Of the 26 articles reviewed, 17 looked solely at the effects of aerobic exercise training, 3 were resistance training specific, and 6 incorporated a combined treatment of aerobic and resistance training (Figure 1).
Figure 1.
Flow chart of the literature search. *Reasons for exclusion: subjects <18 years of age, introduction of new medications, nonexercise related.
Aerobic Exercise and HIV Symptomatology
In populations with chronic illness, infection, or disease, there is a growing body of evidence showing that health benefits can be obtained by incorporating structured aerobic exercise into the individual’s recovery and/or treatment plan.4–6 For those stricken with conditions such as HIV, cancer, and type 2 diabetes, aerobic exercise has been shown to have positive effects in alleviating symptoms and side effects of the disease.7–9
Individuals with HIV often experience disease- and/or treatment-related symptoms and side effects. Fatigue, a major contributor to physical inactivity, is one of the most commonly reported symptoms of people infected with HIV.10 Consequently, this population usually has lower levels of physical activity, leading to a reduced functional capacity and health-related quality of life.11 This sedentary lifestyle also has the potential to exacerbate current HIV-related symptoms as well as to accelerate the rate of disease progression. Numerous investigations12–17 have shown significant benefits of aerobic exercise in HIV-infected persons in the areas of cardiorespiratory capacity, immune status, and metabolic activity as well as psychological variables such as a reduction in symptoms of depression and anxiety. These benefits have been observed in as little as 6 weeks of training; however, long-term benefits have not been fully investigated.
Physiological and Psychological Effects of Aerobic Exercise
Most research examining the health benefits of exercise in the HIV-infected population have used some form of aerobic exercise, such as treadmill or cycle ergometry. Both Schlenzig et al12 and LaPerriere et al14 examined the effects of aerobic exercise on psychological components, such as depression and anxiety. Schlenzig et al demonstrated a significant reduction of anxiety and depression in 15 subjects after 8 weeks of moderate-intensity aerobic exercise (~50%–60% of VO2max), twice per week for 60 minutes.12 Similarly, LaPerriere et al showed that after 5 weeks of aerobic exercise training (45 minutes a day, 3 days a week), subjects exhibited reduced anxiety and depression scores upon learning of their HIV seropositive status.14 See Table 1 for a list of the exercise interventions, population descriptions, and physiological and psychological outcomes of various HIV and exercise studies.
Table 1.
Physiological and Psychological Outcomes of Exercise Interventions in HIV-Infected Populations
| Author | Subjects | Intervention | Outcomes/Conclusions |
|---|---|---|---|
| Schlenzig et al (1989)12 | Total: 28 men EX (n = 15) CON (n = 13) |
8 wk of aerobic exercise 2 times per wk for 60 min at moderate intensity | Significant reduction in anxiety and depression |
| LaPerriere et al (1990)14 | Total: 23 men | 45 min of aerobic exercise on a stationary bicycle at 80% age-predicted max HR | Significant reduction in anxiety and depression scores on the POMSa |
| MacArthur et al (1993)9 | Total: 25; 6 finished at 24 wk High intensity (n = 3) Low intensity (n = 3) |
24 wk of aerobic exercise 3 times per wk for 40 min at 50%–60% VO2max (low) or 24 min at 75%–85% VO2max (high) | Significant increases in VO2max, minute ventilation, and oxygen pulse for those compliant; significant reduction in perceived stress (both groups for each) |
| Stringer et al (1998)19 | Total: 34; 4 women, 30 men High intensity (n = 9) Moderate (n = 9) CON (n = 8) |
6 wk of aerobic exercise 3 times per wk for 60 min at 60% VO2max (moderate) or 40 min at 75% VO2max (high) | Significant improvements in quality of life and aerobic fitness for both exercise groups |
| Perna et al (1999)20 | Total: 28 EX (n = 18) CON (n = 10) |
12 wk of aerobic exercise 3 times per wk for 45 min at 70%–80% HRmax | Significant increases in VO2max, oxygen pulse, and max tidal volume |
| Terry et al (1999)21 | Total: 21; 17 women, 4 men High intensity (n = 11) Moderate intensity (n = 10) |
12 weeks of aerobic exercise 3 times per week for 30 minutes at 60% HRmax (moderate) or 84% HRmax (high) | Significant improvements in functional capacity in both groups |
| Smith et al (2001)22 | Total: 49; 8 females, 41 males EX (n = 19) CON (n = 30) |
12-wk supervised aerobic exercise program; 3 d/wk for 30 min at 60%–80% VO2max | EX subjects increased time to fatigue, lost weight, and decreased BMI, subcutaneous fat, and abdominal girth |
| Thoni et al (2002)15 | Total: 17; 5 women, 12 men All exercised, no control group |
4-mo aerobic exercise twice a wk for 45 min at a light intensity | Significant increases in VO2max and HDL; significant decreases in total abdominal adipose tissue, total cholesterol, and triglycerides |
| Neidig et al (2003)18 | Total: 30; 8 women, 22 men EX (n = 30) CON (n = 30) |
12 wk of aerobic exercise 3 times per wk for 60 min at 60%–80% VO2max | Significant reduction in depressive symptoms and depressed mood |
| Terry et al (2006)16 | Total: 30; 10 women, 20 men EX (n = 15) CON (n = 15) |
12-wk aerobic exercise program; 3 d/wk for 30 min at 70%–85% HRmax | Significant increases in VO2max and decreased BMI, waist-to-hip ratio, body density, and body fat |
Abbreviations: BMI, body mass index; CON, control group; EX, exercise group; HDL, high-density lipoprotein; HR, heart rate; POMS, Profile of Mood States.
POMS is a 64-item questionnaire that measures 6 mood states: tension/anxiety, depression/dejection, anger, vigor, fatigue, confusion.
Over the past 15 years, a handful of investigations using aerobic exercise as the preferred intervention recorded identical results to those of the Schlenzig et al12 and LaPerriere et al9,18,19 studies. The time frames of most of these interventions ranged from 6 to 12 weeks. A common methodology used by the researchers was relative moderate- intensity exercise for 3 weekly sessions for a total of 180 minutes of exercise per week. Like those investigations previously discussed, reductions in depressive symptoms and/or significant improvements in quality of life were also shown.9,18,19 These findings suggest that as little as 180 minutes of aerobic exercise per week can be used as a beneficial method for improving the negative affect (depression, anxiety, quality of life) experienced by HIV-infected individuals. This is important because these patients often experience social isolation and depression after learning of their positive status.
MacArthur et al9 looked at the effects of aerobic exercise on maximal oxygen consumption (VO2max) and perceived stress in a sample of 25 HIV-infected men. This 24-week intervention consisted of a lower- intensity group that exercised 3 times a week at 50% to 60% of their VO2max for 40 minutes and a higher-intensity group that exercised 3 times a week at 75% to 85% of their VO2max for 24 minutes. There was a significant increase in VO2max and a significant reduction in perceived stress found in both exercise groups. A major limitation to this study, however, was the high attrition rate of 76%, possibly due to the total length of the study. A similar study conducted by Terry et al16 randomized subjects into either a higher intensity or moderate-intensity aerobic exercise group. The higher intensity group exercised at 84% of their maximal heart rate, whereas the moderate-intensity group exercised at 60% of their maximal heart rate. Both groups exercised for a total of 30 minutes, 3 days a week for 12 weeks. There was a significant improvement in functional capacity, measured by time to fatigue, in both groups. The higher- intensity intervention resulted in greater increases in functional capacity compared with the moderate-intensity group (680 ± 81 vs 750 ± 151 seconds for the moderate-intensity group and 651 ± 122 vs 841 ± 158 seconds for the high-intensity group). Recent work by Hand et al17 indicates that even very moderate doses of physical activity (moderate-intensity aerobic and resistance training for 1 hour twice per week) can enhance functional aerobic capacity in HIV-infected individuals with significant impairment. These results suggest that HIV-infected persons can experience sufficient gains in aerobic capacity at both high- and moderate-intensity levels, but greater gains are experienced when working at a higher intensity. However, the comparison of results is confounded by the significantly higher dose (intensity × time) of exercise in the higher intensity group as compared with the moderate-intensity group.
Other physiological variables measured in aerobic exercise interventions in HIV-infected populations include body composition and high-density lipoprotein (HDL) cholesterol. Thoni et al15 conducted a 16-week intervention, composed of men and women, that consisted of a lower intensity aerobic exercise regimen (~40% VO2max), twice a week for 45 minutes. There were significant increases in VO2max and HDL cholesterol, as well as significant decreases in total abdominal adipose tissue, total cholesterol, and triglycerides. Similarly, Terry et al reported a significant increase in VO2max as well as decreases in BMI, waist-to-hip ratio, body density, and body fat after a 12-week moderate-intensity aerobic exercise intervention (~60% of HRmax) performed for 30 minutes a day, 3 days a week.
Perna and colleagues20 found significant increases postintervention in VO2max, oxygen pulse, and maximum tidal volume in 18 HIV-infected men. The exercise intervention consisted of 12 weeks of moderate-intensity (70%–80% HRmax) aerobic exercise, 3 times a week for 45 minutes.
These investigations suggest that the HIV-infected individual can benefit physically and psychologically in as little as 6 weeks from aerobic activity at any intensity if performed at least 2 to 3 sessions per week. However, a comparison across intensities is difficult, as most of the studies did not equalize the dosage of exercise across the intensity range.
Effects of Aerobic Exercise on Immunological Variables
Research has also looked at the effects of aerobic exercise on immunological variables in the HIV-infected population. In addition to their findings on physiological and psychological variables, MacArthur et al9 reported no significant changes in CD4+ count in both the higher and moderate-intensity groups. Similarly, Stringer et al19 reported no significant changes in CD4+ cell count or viral load in the moderate- or higher intensity exercise groups after 6 weeks of training or in the control group. Terry et al21 found no significant changes from baseline (12- week aerobic exercise program) in CD4+ cell count, CD8+ cell count, leukocytes, or lymphocytes in the moderate- intensity exercise group, their higher intensity group, or the control group. Recently, Terry et al16 repeated their protocol in 30 healthy HIV-positive subjects with dyslipidemia and lipodystrophy. No significant changes in immunological variables were detected in any groups after completing 12 weeks of aerobic exercise, 3 days a week for 30 minutes at a 70% to 85% maximal heart rate.
The only group to date to report significant increases in CD4+ cell counts and other immunological measures in HIV-infected men was LaPerriere and colleagues.13 Their investigations used a 12-week interval training method on a stationary bicycle for 45 minutes a session, 3 days a week at 70% to 80% of maximal heart rate. They reported similar results in which they found a significant increase in CD4+ cell counts, along with increases in CD2+, CD8+, CD45RA+CD4+, and CD20+ cell counts. However, it was proposed by Dudgeon et al3 in a previous review article that this observed increase may actually be the measurement of the mobilization of cells from the lymphoid tissue rather than an actual increase systemically in cell quantity.
Although most studies do not support a beneficial effect of exercise on immune function in HIV-infected individuals, it is important to note that aerobic exercise performed at low, moderate, or high intensity does not negatively affect immune function or disease progression in this population. In fact, no study to date has shown an exercise-induced reduction in immune cell count or function at any exercise intensity. Table 2 provides further information regarding the impact of aerobic exercise interventions in HIV-infected populations.
Table 2.
Outcomes of Combined Aerobic and Resistance Exercise Interventions in HIV-Infected Populations
| Author | Subjects | Intervention | Outcomes/Conclusions |
|---|---|---|---|
| Fairfield et al (2001)26 | Total: 43 men Testosterone, no exercise (n = 10) Testosterone, exercise (n = 11) Placebo, no exercise (n = 12) Placebo, exercise (n = 10) |
Progressive strength training: 3×/wk for 12 wk Wk 1–2: 2 sets at 60% 1-RM Wk 3–6: 2 sets at 70% 1-RM Wk 7–9: 2 sets at 70% and 1 set at 80% 1-RM Wk 10–12: 3 sets at 80% 1-RM Aerobic: 30 min 3×/wk at 60%-70% HRmax |
↑ Muscle attenuation with testosterone and training (P = .03) |
| Grinspoon et al (2000)27 | Total: 43 men Testosterone, no exercise (n = 10) Testosterone, exercise (n = 11) Placebo, no exercise (n = 12) Placebo, exercise (n = 10) |
Progressive strength training: 3×/wk for 12 wk Wk 1–2: 2 sets at 60% 1-RM Wk 3–6: 2 sets at 70% 1-RM Wk 7–9: 2 sets at 70% and 1 set at 80% 1-RM Wk 10–12: 3 sets at 80% 1-RM Aerobic: 20 min 3×/wk at 60%-70% HRmax |
↑ Cross-section muscle area in trained vs nontrained and testosterone (TEST) vs placebo: arm and leg ↓ HDL: TEST vs placebo ↑ HDL: Trained vs nontrained |
| Hand et al (2008)17 | Total: 40 men Experimental (n = 20) Control (n = 20) |
Progressive strength training: 2×/wk for 6 wk at 60% 3-RM Aerobic: 30 min 2×/wk at approximately 60% VO2 peak |
↑ VO2max, endurance ↓ HR at submaximal absolute workload ↑ Strength: knee extension, bench press, knee flexion, and elbow flexion ↑ Total muscle mass and reduced fat mass |
| Dolan et al (2006)29 | Total: 40 women | 16-wk home-based combo regimen: Aerobic: 20 min, 60% 1–2 wk and 30 min, 75% 3–16 wk Resist: knee-hip extension, bench, knee flex, lat raise, stand calf, arm curl: 1–2 wk: 60%, 3 s/10R 3–4: 70% and 5–16: 80% 1-RM, 4 s/8R on each muscle |
↑ VO2max, endurance ↑ Strength: knee extension, bench press, knee flexion, shoulder abduction, ankle plantar flexion, and elbow flexion ↑ Total muscle area and attenuation |
| Fillipas et al (2006)30 | Total: 40 men Experimental (n = 20) Control (n = 20) |
Aerobic: 2×/wk 20 min; 60%–75% HRmax Resistance: lats, arm curl, shoulder elevation, knee extension, knee flexion, calf press, and abs; 3 s/10R; 60% to 80% 1-RM |
↑ Self-efficacy ↑ Cardiovascular fitness by reducing HR ↑ Overall health ↑ Cognitive function |
| Engelson et al (2006)31 | Total: 18 women | Diet: 5024-kJ hypoenergetic 90 min 3×/wk supervised: Aerobic: 30 min 70%–80% HRmax Resistance: 7 major muscle groups: 3 s/8–10R: 10 exercises |
↓ Daily food intake and body weight (95.5% fat) ↓ Resting energy expenditure ↑ Strength, fitness, and QOL ↔ CD4, viral load, fasting glucose, insulin, insulin sensitivity, fasting lipids, TPA, PAI-1 |
| Robinson et al (2007)32 | Total: 5 men and women | 16 wk preceded by 2-wk phase-in period Aerobic: 20 min, 3×/wk, 70%–80% VO2max Resistance: 2×/wk, 1 s/8–10R at 80% 1-RM: lat, seated row, shoulder press, bench press, leg press, calf press, seated leg curl |
↓ Total and trunk fat mass |
Abbreviations: HR, heart rate; QOL, quality of life.
Resistance Exercise and HIV Symptomatology
Only a small number of studies have examined the effect of resistance training, independent of aerobic exercise, on the health and fitness of HIV-infected individuals. These few investigations focused mainly on levels of strength and muscle mass rather than overall fitness or immune function. See Table 3 for a complete list of resistance exercise interventions conducted in HIV-infected populations.
Table 3.
Immunologic Outcomes of Exercise Interventions in HIV-Infected Populations
| Author | Subjects | Intervention | Outcomes/Conclusions |
|---|---|---|---|
| LaPerriere et al (1990)14 | Total: 23 men Exercise (n = 10) Control (n = 13) |
5 wk of interval training on stationary bike at 70%–80% max HR, 3 times per week for 45 min per session | Control: 61 cells/mm3 decrease in CD4+ count Exercise: 38 cells/mm3 increase in CD4+ count |
| LaPerriere et al (1991) | Total: 39 men Exercise (n = 23) Control (n = 16) |
5 wk of interval training on stationary bike at 70%–80% max HR, 3 times per wk for 45 min per session | Significant increases in exercise group CD4+ cells and CD45RA+CD4+ cells |
| MacArthur et al (1993)9 | Total: 25 men 6 finished at 24 wk High intensity (n = 3) Low intensity (n = 3) |
24 wk of aerobic exercise 3 times per week for 40 min at 50%–60% VO2max (low) or 24 min at 75%–85% VO2max (high) | No significant change in CD4+ cells |
| LaPerriere et al (1994) | Total: 14 men Exercise (n = 7) Control (n = 7) |
10 wk of aerobic exercise on stationary bike 3 times per wk for 45 min at 70%–80% HRmax | Significant increases in CD2+, CD4+, CD8+, CD45RA+CD4+, and CD20+ cells |
| Stringer et al (1998)19 | Total: 34; 4 women, 30 men High intensity (n = 9) Moderate (n = 9) CON (n = 8) |
6 wk of aerobic exercise 3 times per wk for 60 min at 60% VO2max (moderate) or 40 min at 75% VO2max (high) | No significant changes in viral load or CD4+ cells in all groups |
| Terry et al (1999)21 | Total: 21; 17 women, 4 men High intensity (n = 11) Moderate intensity (n = 10) |
12 wk of aerobic exercise 3 times per wk for 30 min at 60% HRmax (moderate) or 84% HRmax (high) | No significant changes in CD4+ cells, CD8+ cells, leukocytes, or lymphocytes in all 3 groups |
| Terry et al (2006)16 | Total: 30; 10 women, 20 men EX (n = 15) CON (n = 15) |
12-wk aerobic exercise program, 3 days a wk for 30 min at 70%–85% HRmax | No significant changes in CD4+ cells or viral load in either group |
Abbreviations: CON, control group; EX, exercise group; HR, heart rate.
Yarasheski et al23 examined the effects of a 16-week resistance exercise–training program in 18 asymptomatic HIV-infected men. The regimen consisted of 3 upper-body and 4 lower-body exercises, completed 4 times a week, with each session lasting approximately 1 to 1.5 hours. Subjects progressed from lower intensity (50%–65% of 1 RM), high-repetition (10 or more repetitions per exercise) work to higher intensity (75%–85% of 1-RM), low-repetition (5–8 repetitions per exercise) work by week 16. The training regimen resulted in increased whole-body lean tissue mass and thigh muscle cross-sectional area. Performance was also improved as strength on all exercises increased by 23% to 38%.
In a similar training study, Roubenoff and Wilson24 compared the effects of resistance exercise on self-reported physical functioning in those infected with HIV. Subjects were divided into groups of participants with body mass wasting (BMI <20 kg/m2 or 10% unintentional weight loss during previous 12 months) or without wasting. The exercise regimen consisted of 3 sessions per week for 8 weeks, followed by 8 weeks of usual activity, for a total length of 16 weeks. Each session involved 3 sets of 8 repetitions using compound, large-muscle exercises. The first session was performed at 50% 1-RM, the second session at 60% 1-RM, and the remaining sessions at 75% to 80% of 1-RM. Those who completed the exercise program showed increases in both 1-RM average for all exercises performed (wasted = 44% and non-wasted = 60%; P < .05) and in lean body mass (5.3% wasted and 2.3% nonwasted; P < .05). Physical function improved in the wasting subjects to the degree that the wasting subjects had a statistically higher level of physical function by week 16 than the nonwasting subjects. The data also indicate that increases in both lean body mass and strength were independently associated with increases in physical function.
In addition to resistance training, testosterone replacement has been studied in an effort to find more significant improvements in strength and body composition. Bhasin and colleagues25 compared among HIV-infected individuals the effects of testosterone replacement with and without resistance exercise. Sixty-one hypogonadal (serum total testosterone levels less than 12.1 nmol/L) subjects who had involuntary weight loss of at least 5% in the past 6 months were randomly assigned to 1 of 4 groups: placebo, no exercise (n = 14); testosterone enanthate (weekly 100-mg intramuscular injections), no exercise (n = 17); placebo and exercise (n = 15); or testosterone enanthate and exercise (n = 15). The resistance exercise began with lower intensity (60% 1-RM), higher volume exercise (3 sets of 12–15 repetitions), 3 times a week for the first 4 weeks. During weeks 5 to 10, subjects were progressed to higher intensity (90% of 1-RM on heavy days, 80% of 1-RM on medium days, and 70% of 1-RM on light days), lower volume exercise (4 sets of 4–6 repetitions), 3 times a week. Finally, subjects finished weeks 11 to 16 with a lower volume (5 sets of 4–6 repetitions) and an increase in resistance of 7% in the upper-body exercises and 12% in the lower-body exercises.
Results indicated an increase in body weight in the exercise-only group and in the testosterone-only group. Increases in maximal voluntary strength were seen in most of the resistance exercises for those in the exercise-only, testosterone-only, and testosterone and exercise groups. Thus, these data suggest that a 16-week progressive resistance exercise training program in hypogonadal individuals with weight loss can improve muscular strength and increase body weight; however, it appears testosterone supplementation is needed to produce beneficial changes in lean tissue mass.
In conclusion, the literature indicates that resistance training can increase strength, lean body mass, and functional status in HIV-infected individuals with wasting syndrome. However, further research is still needed because of the small number of studies in this area.
Combined Aerobic and Resistance Training
A relatively strong body of literature illustrates the beneficial effects of combined aerobic and resistance exercise training for those infected with HIV. The advantage of combined exercise training is enhanced cardiorespiratory function in a population that is typically deconditioned with the strength and muscle mass gains of resistance training. A summary of the studies combining aerobic and resistance training in persons with HIV can be found in Table 4.
Table 4.
Outcomes of Resistance Exercise Interventions in HIV-Infected Populations
| Author | Subjects | Intervention | Outcomes/Conclusions |
|---|---|---|---|
| Yarasheski et al (2001)23 | Total: 18 men | Resistance exercise: 3 upper-body/4 lower-body done 1–1.5 h/d, 4×/wk for 64 sessions Exercises not clearly stated |
↑ Whole-body lean mass (1.4 kg) ↑ Thigh cross-sectional area (5–7 cm2) ↑ Strength (23%–38%) all exercises ↓ Fasting serum triglycerides (281–204 mg/dL) |
| Bhasin et al (2000)25 | Total: 61 men Placebo, no exercise (n = 14) Testosterone, no exercise (n = 17) Placebo, exercise (n = 15) Testosterone, exercise (n = 15) |
Resistance exercise 1–4 wk: 3 sets/12–15 reps: 60% 1-RM 3×/wk 5–10 wk: 4 sets/4–6 reps: 90%, 80%, 70% 1-RM: 3×/wk 11–16 wk: 5 sets/4–6 reps: ↑7% Upper body and 12% Lower body |
↑ Body weight 2.2 kg exercise (EX) and 2.6 kg testosterone (TEST) ↑ Max leg press, leg curl, bench, lats for EX, TEST, and EX+TEST ↑ Thigh muscle vol: TEST and EX ↑ LBM: TEST and EX+TEST |
| Roubenoff et al (2001)24 | Total: 25 men and women Wasting (n = 6) Nonwasting (n = 19) |
Resistance: 3×/wk for 8 wk: 50% 1-RM first session, 60% second session, and 75%–80% for remainder of sessions 3 sets/8 reps: double leg press, leg extension, seated chest press, seated row |
↑ 1-RM average for 4 machines: 44% nonwasted and 60% wasting ↑ Lean body mass: 2.3% nonwasting and 5.3 wasting ↑ Physical function: 6 patients wasting (at 16 wk wasting = higher function level than nonwasting) |
Fairfield and colleagues26 randomized 54 patients with AIDS-associated wasting (<90% of ideal body weight or weight loss >10% of baseline weight) to a 12 week 2 × 2 factorial designed study. The groups consisted of subjects receiving either testosterone (200 mg/wk intramuscular injections) or placebo and resistance training or no training. Subjects in the 12-week training group performed thrice weekly (6–8 repetitions per set) resistance exercises that included leg extension, leg curl, leg press, latissimus dorsi pull down, arm curl, and triceps extensions. Weeks 1 to 2 consisted of 2 sets at 60% 1-RM; weeks 3 to 6, 2 sets at 70% 1-RM; weeks 7 to 9, 2 sets at 70% and 1 set at 80% 1-RM; and weeks 10 to 12, 3 sets at 80% 1-RM. In addition, all patients who were randomized into the training groups performed 30 minutes of aerobic exercise on a stationary bike 3 times per week at 60% to 70% HRmax. The results indicate that muscle mass increased in both the testosterone and the training groups to the same degree.
A study using a similar dose of testosterone and progressive resistance training with eugonadal men with HIV wasting was conducted by Grinspoon et al.27 Patients were stratified as either weighing <90% or ≥90% ideal body weight. Subjects were randomized in a 12-week 2 × 2 factorial design to receive testosterone enanthate (200 mg/wk intramuscularly), receive placebo and progressive resistance training, or receive no intervention. Patients randomized into the training group began each exercise session by performing 20 minutes of aerobic exercise on a stationary bike, 3 times per week at 60% to 70% HRmax, followed by a series of resistance exercises that included major muscle groups throughout the body. Patients completed 6 to 8 repetitions of each set 3 times per week. The intensity was increased from 2 sets at 60% 1-RM in the first week to 3 sets at 80% 1-RM in the final 2 weeks. Results showed increases in cross-sectional muscle area in the trained group when compared with the nontrained group and in the testosterone group compared with the placebo group.
Recently, the effects of combined moderate-intensity resistance and aerobic exercise was examined in a group of HIV-infected men in various disease stages.17 HIV-infected individuals were randomized to an exercise group, which completed 6 weeks of moderate-intensity exercise training, or to a control group, which attended all training sessions but did not receive the exercise intervention. Twice weekly, the exercise group completed 30 minutes of moderate-intensity aerobic training (60%–65% of their age predicted maximum heart rate) followed by moderate-intensity resistance training (8 exercises targeting the chest, upper back, triceps, upper anterior and posterior legs, and lower legs were performed on resistance-training machines, while movements targeting the biceps brachii and deltoids were performed with free weights). Prior to and following the intervention, body composition for each subject was determined via dual-energy x-ray absorptiometry (DXA), and peak strength was determined on 4 exercises (leg extension, leg curl, lat pull down, and chest press).
Data demonstrate that the exercise group (n = 31) had a significant increase in lean tissue mass (LTM) following the intervention, whereas the controls (n = 27) had no significant changes in body composition. The exercise group was then divided at the median body fat (BF) percentage of 20%. Analysis of the exercise group then revealed that those below 20% BF increased LTM only, whereas those above 20% BF decreased fat mass, total body fat percentage, and percentage trunk fat, while also increasing LTM. Peak strength increases between 14% and 28% were observed on all exercises.28 These results suggest that combined aerobic and resistance exercise training may have different beneficial effects on LTM and BF in HIV-infected persons based on body composition.
The effects of a supervised, home-based combined aerobic and resistance training program has also been examined in an HIV-infected population.29 Forty HIV-infected women with self-reported changes in fat distribution were randomized into either a 16-week training regimen or a nonintervention control group. Subjects completed 2-hour supervised in-house exercise sessions 3 times per week, on alternating days of the week. The aerobic exercise component consisted of stationary cycling for 20 minutes at 60% HRmax for the first 2 weeks and 30 minutes at 75% HRmax for the remaining 14 weeks. The resistance exercise component included alternating upper- and lower-body exercises, with the intensity for the first 2 weeks set at 60% 1-RM for 3 sets of 10 repetitions. During subsequent weeks, the intensity was increased to 4 sets of 8 repetitions at 80% 1-RM. Baseline data indicated that the cardiorespiratory fitness (VO2max) was lower than that of healthy women (15.4 mL/kg/min vs 26–35 mL/kg/min). Following the intervention, there was an increase in VO2max (P < .001) and endurance (P < .001) along with increases in strength (P < .001) across all muscle groups. The data also showed increases in total muscle area (P = .02) for the exercise group as compared with the control group. These data indicate that a home-based exercise program can be successful at improving functional aerobic capacity and body composition in HIV-infected women.
In the longest study to date, Fillipas et al30 examined the effects of a 6-month combination aerobic and resistance training regimen on the self-efficacy of HIV-infected men. Forty HIV-infected men were randomized into either an experimental group (supervised aerobic and resistance program) or a control group (unsupervised walking program). The experimental group performed two 60-minute exercise sessions per week consisting of 20 minutes of aerobic exercise at 60% HRmax and progressing to 75% HRmax. The resistance exercise component targeted upper- and lower- body muscle groups. Each resistance exercise was initially set at 60% 1-RM and progressed to 80% 1-RM with 3 sets of 10 repetitions. The control group’s intervention consisted of walking 2 times per week, starting at 60% HRmax and progressing to 75% HRmax. The control group’s attendance was monitored by having them walk at a local exercise forum. Self-efficacy and cognitive function were measured. Overall health was determined based on CD4 counts, viral loads, and individual body weight. Results showed that the experimental group improved their self- efficacy by almost a standard deviation, cardiovascular fitness as determined by heart rate at a given submaximal exercise intensity, overall health, and cognitive function more so than did the control group.
Although there have been a few studies that have looked at the effects of exercise training in the HIV population, only 1 study has incorporated a dietary component. Engelson and colleagues31 examined the influence of diet and exercise training on the body composition and biomarkers of metabolic function in 18 obese (BMI between 30 kg/m2 and 38 kg/m2) HIV-infected women. All subjects had been on a stable antiretroviral drug regimen for at least 4 weeks prior to beginning the study. Subjects were put on a 5024-kJ hypoenergetic diet and exercise regimen for 12 weeks. Thrice-weekly exercise sessions consisted of 30 minutes of moderate-intensity exercise on a treadmill at 70% to 80% estimated HRmax followed by 3 sets of 8 to 10 repetitions on 10 different exercises performed on a multigym. Resting metabolic rate was determined via indirect calorimetry. Quality of life (QOL) was measured using 5 established measures including the Medical Outcomes Survey 36-item Short-Form Health Survey, the Brief Symptom Inventory, the 5-item Satisfaction With Life scale, the 13-item Sense of Coherence scale, and the 18-item Life Distress Inventory. Food intake was assessed via Food Processor software (ESHA Research, Salem, Oregon) based on 3-day food diaries. Following the intervention, daily food intake, resting metabolic rate, and total body weight decreased as compared with baseline measurements. In addition, strength, fitness, and QOL metrics all improved from baseline measurements. No changes were seen in CD4 count or viral load, which is consistent with most other studies showing no effect of exercise on viral activity or immune status.
Most of the studies already discussed in this review used moderate-intensity levels of activity; however, Robinson et al32 investigated the effects of a 16-week, high-intensity aerobic and resistance exercise regimen on HIV metabolic abnormalities in 5 HIV-infected subjects. Subjects participated in a program consisting of 3 sessions per week for 16 weeks that was preceded by a 2-week phase-in period. The 2-week phase-in period was performed so participants could reach the desired prescription before starting the main regimen. The aerobic component included 20 minutes of treadmill activity at an intensity of 70% to 80% VO2max followed by 7 resistance exercises that included all major muscle groups performed at 80% 1-RM for 1 set of 8 to 10 repetitions. Trunk and total fat masses were determined using whole-body DXA. There was a decrease in total fat mass and trunk fat mass along with an increase in the mean sum of 1-RM for all 7 exercises.
In conclusion, the studies reviewed indicate that a combined aerobic and resistance training regimen can provide beneficial health outcomes in HIV-infected individuals. These changes include decreases in trunk and total fat mass and increases in muscle area and strength. The results imply that overall health and QOL are enhanced. However, as with programs that include only aerobic or resistance training, it does not appear that combination programs counteract immunosuppression.
Summary
Results to date indicate that moderate- to high-intensity aerobic, resistance, and combined aerobic and resistance exercise regimens can be safe and elicit favorable and beneficial changes in the HIV-infected population. These benefits can include changes in body composition, functional capacity, muscular strength, total and HDL cholesterol, cognitive function, depression and anxiety, overall health, and QOL. Most studies indicate no beneficial effect of exercise training on HIV status, viral load, or immune function. However, aerobic exercise has shown no negative effect on immunity or disease progression. The effects of resistance or combined exercise programs on immunity have yet to be determined. Further research is needed to determine the minimal and optimal duration, frequency, and intensity of exercise needed to produce beneficial changes in the HIV-infected population. A confounding factor in most studies has been the lifestyle choices of the participants, including severe deconditioning, which is often confused with HIV-associated wasting or other symptoms evoked by poor diet and exercise participation. Furthermore, most studies have not approached the exercise program from the perspective of validating commonly used prescriptions for physical activity or from a dose-response perspective. Both approaches are critical for a full understanding of the effects of training on the HIV-infected population.
Acknowledgments
This work was supported by NIH/NCMHD (1 P20 MD001770-030003) and the American College of Sports Medicine Foundation.
Footnotes
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- 1.Kotler DP, Rosenbaum K, Wang J, Pierson RN. Studies of body composition and fat distribution in HIV-infected and control subjects. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(3):228–237. doi: 10.1097/00042560-199903010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Sidney S, Lewis CE, Hill JO, et al. Association of total and central adiposity measures with fasting insulin in a biracial population of young adults with normal glucose tolerance: the CARDIA study. Obes Res. 1999;7(3):265–272. doi: 10.1002/j.1550-8528.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 3.Dudgeon WD, Phillips KD, Bopp CM, Hand GA. Physiological and psychological effects of exercise interventions in HIV disease. AIDS Patient Care STDS. 2004;18(2):81–98. doi: 10.1089/108729104322802515. [DOI] [PubMed] [Google Scholar]
- 4.Bergasa NV, Mehlman J, Bir K. Aerobic exercise: a potential therapeutic intervention for patients with liver disease. Med Hypotheses. 2004;62(6):935–941. doi: 10.1016/j.mehy.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 6.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116(10):693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 8.Dimeo FC, Thomas F, Raabe-Menssen C, Propper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery: a randomised controlled trial. Support Care Cancer. 2004;12(11):774–779. doi: 10.1007/s00520-004-0676-4. [DOI] [PubMed] [Google Scholar]
- 9.MacArthur RD, Levine SD, Birk TJ. Supervised exercise training improves cardiopulmonary fitness in HIV-infected persons. Med Sci Sports Exerc. 1993;25(6):684–688. [PubMed] [Google Scholar]
- 10.Justice AC, Rabeneck L, Hays RD, Wu AW, Bozzette SA. Sensitivity, specificity, reliability, and clinical validity of provider-reported symptoms: a comparison with self-reported symptoms. Outcomes Committee of the AIDS Clinical Trials Group. J Acquir Immune Defic Syndr. 1999;21(2):126–133. [PubMed] [Google Scholar]
- 11.Paton NI, Elia M, Jebb SA, Jennings G, Macallan DC, Griffin GE. Total energy expenditure and physical activity measured with the bicarbonate-urea method in patients with human immunodeficiency virus infection. Clin Sci (Lond) 1996;91(2):241–245. doi: 10.1042/cs0910241. [DOI] [PubMed] [Google Scholar]
- 12.Schlenzig C, Jager H, Rieder H. Supervised physical exercise leads to psychological and immunological improvement in pre-AIDS patients. Proceedings of the 5th International AIDS Conference; June 1989; Montreal, Canada. [Google Scholar]
- 13.LaPerriere A, Klimas N, Fletcher MA, et al. Change in CD4+ cell enumeration following aerobic exercise training in HIV-1 disease: possible mechanisms and practical applications. Int J Sports Med. 1997;18:S56–S61. doi: 10.1055/s-2007-972700. [DOI] [PubMed] [Google Scholar]
- 14.LaPerriere AR, Antoni MH, Schneiderman N, et al. Exercise intervention attenuates emotional distress and natural-killer-cell decrements following notification of positive serologic status for HIV-1. Biofeedback Self Regul. 1990;15(3):229–242. doi: 10.1007/BF01011107. [DOI] [PubMed] [Google Scholar]
- 15.Thoni GJ, Fedou C, Brun JF, et al. Reduction of fat accumulation and lipid disorders by individualized light aerobic training in human immunodeficiency virus infected patients with lipodystrophy and/or dyslipidemia. Diabetes Metab. 2002;28(5):397–404. [PubMed] [Google Scholar]
- 16.Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy 5. Med Sci Sports Exerc. 2006;38(3):411–417. doi: 10.1249/01.mss.0000191347.73848.80. [DOI] [PubMed] [Google Scholar]
- 17.Hand GA, Phillips KD, Dudgeon WD, Lyerly GW, Durstine JL, Burgess SE. Moderate intensity exercise training reverses functional aerobic impairment in HIV-infected individuals. AIDS Care. 2008;20(9):1066–1074. doi: 10.1080/09540120701796900. [DOI] [PubMed] [Google Scholar]
- 18.Neidig JL, Smith BA, Brashers DE. Aerobic exercise training for depressive symptom management in adults living with HIV infection. J Assoc Nurses AIDS Care. 2003;14(2):30–40. doi: 10.1177/1055329002250992. [DOI] [PubMed] [Google Scholar]
- 19.Stringer WW, Berezovskaya M, O’Brien WA, Beck CK, Casaburi R. The effect of exercise training on aerobic fitness, immune indices, and quality of life in HIV+ patients. Med Sci Sports Exerc. 1998;30(1):11–16. doi: 10.1097/00005768-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Perna FM, Laperriere A, Klimas N, et al. Cardiopulmonary and CD4 cell changes in response to exercise training in early symptomatic HIV infection. Med Sci Sports Exerc. 1999;31(7):973–979. doi: 10.1097/00005768-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Terry L, Sprinz E, Ribeiro JP. Moderate and high intensity exercise training in HIV-1 seropositive individuals: a randomized trial. Int J Sports Med. 1999;20(2):142–146. doi: 10.1055/s-2007-971108. [DOI] [PubMed] [Google Scholar]
- 22.Smith BA, Neidig JL, Nickel JT, et al. Aerobic exercise: effects on parameters related to fatigue, dyspnea, weight and body composition in HIV-infected adults. AIDS. 2001;15:693–701. doi: 10.1097/00002030-200104130-00004. [DOI] [PubMed] [Google Scholar]
- 23.Yarasheski KE, Tebas P, Stanerson B, et al. Resistance exercise training reduces hypertriglyceridemia in HIV-infected men treated with antiviral therapy. J Appl Physiol. 2001;90(1):133–138. doi: 10.1152/jappl.2001.90.1.133. [DOI] [PubMed] [Google Scholar]
- 24.Roubenoff R, Wilson IB. Effect of resistance training on self-reported physical functioning in HIV infection. Med Sci Sports Exerc. 2001;33(11):1811–1817. doi: 10.1097/00005768-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin S, Storer TW, Javanbakht M, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000;9;283(6):763–770. doi: 10.1001/jama.283.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairfield WP, Treat M, Rosenthal DI, et al. Effects of testosterone and exercise on muscle leanness in eugonadal men with AIDS wasting. J Appl Physiol. 2001;90(6):2166–2171. doi: 10.1152/jappl.2001.90.6.2166. [DOI] [PubMed] [Google Scholar]
- 27.Grinspoon S, Corcoran C, Parlman K, et al. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting: a randomized, controlled trial. Ann Intern Med. 2000;133(5):348–355. doi: 10.7326/0003-4819-133-5-200009050-00010. [DOI] [PubMed] [Google Scholar]
- 28.Lyerly GW, Phillips KD, Dudgeon WD, et al. Effects of combined resistance and aerobic exercise training in HIV-infected men. Med Sci Sports Exerc. 2007;39(5 suppl):S443. [Google Scholar]
- 29.Dolan SE, Frontera W, Librizzi J, et al. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med. 2006;166(11):1225–1231. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL. A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immunodeficiency virus: a randomised controlled trial. Aust J Physiother. 2006;52(3):185–190. doi: 10.1016/s0004-9514(06)70027-7. [DOI] [PubMed] [Google Scholar]
- 31.Engelson ES, Agin D, Kenya S, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55(10):1327–1336. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Robinson FP, Quinn LT, Rimmer JH. Effects of high-intensity endurance and resistance exercise on HIV metabolic abnormalities: a pilot study. Biol Res Nurs. 2007;8(3):177–185. doi: 10.1177/1099800406295520. [DOI] [PubMed] [Google Scholar]