Abstract
The first cell lineage specification in mouse embryo development is the formation of trophectoderm (TE) and inner cell mass (ICM) of the blastocyst. This article is to review and discuss the current knowledge on the cellular and molecular mechanisms of this particular event. Several transcription factors have been identified as the critical regulators of the formation or maintenance of the two cell lineages. The establishment of TE manifests as the formation of epithelium, and is dependent on many structural and regulatory components that are commonly found and that function in many epithelial tissues. Distinct epithelial features start to emerge at the late 8-cell stage, but the fates of blastomeres are not fixed as TE or ICM until around 32-cell stage. The location of blastomeres at this stage, i.e., external or internal of the embryo, in effect defines the commitment towards the TE or ICM lineage, respectively. Some studies implicate the presence of a developmental bias among blastomeres at 2- or 4-cell stage, although it is unlikely to play a decisive role in the establishment of TE and ICM. The unique mode of cell lineage specification in the mouse embryo is further discussed in comparison with the formation of initial cell lineages, namely the three germ layers, in non-mammalian embryos.
1. Introduction
Animal development is initiated by fertilization of the egg with sperm, which is immediately followed by mitotic cell divisions, or cleavages, to generate blastomeres. In most animals, the first step of cell type diversification is the creation of the primary germ layers, namely endoderm, mesoderm and ectoderm. In general, endoderm is the precursor of the gastrointestinal tract, which is essential for nutrient absorption; mesoderm gives rise to muscle and blood cells, which are involved in locomotion and cardiovascular circulation, respectively; and ectoderm develops into epidermis and neurons, which are critical for protection from and sensing of the environment, respectively. Thus, the formation of the three germ layers lays the groundwork for generating various tissues that are essential for animal life, and is an evolutionarily conserved event that takes place at the beginning of animal development.
The situation, however, is slightly different for the development of mammals, specifically eutherians, such as the mouse and human. The first cell differentiation event in mammalian development is not the formation of the three germ layers, but is the establishment of two distinct cell lineages: the trophectoderm (TE) and the inner cell mass (ICM) (Fig. 1). TE engages in implantation by directly interacting with the mother’s uterus, and gives rise to tissues in the placenta. It is only after implantation that the three germ layers form from the ICM, which ultimately generates all the tissues in the animal body. For most non-mammalian animals, early development is dependent on the nutritional reserves stored in the egg yolk until the embryo develops a mouth and digestive system to intake nutrients from the environment. In this sense, the formation of the three germ layers is crucial as the first cell differentiation event for embryos to construct tissues and organs essential for feeding. By contrast, the eggs of mammals are generally yolk-poor, and the embryonic development is largely dependent on the nutrients derived from the mother through the placenta. Thus, during the course of evolution towards mammals, a unique developmental stage was inserted at the very beginning of embryo development to generate TE, which is a vital tissue to interact with the mother’s uterus and to create the placenta.
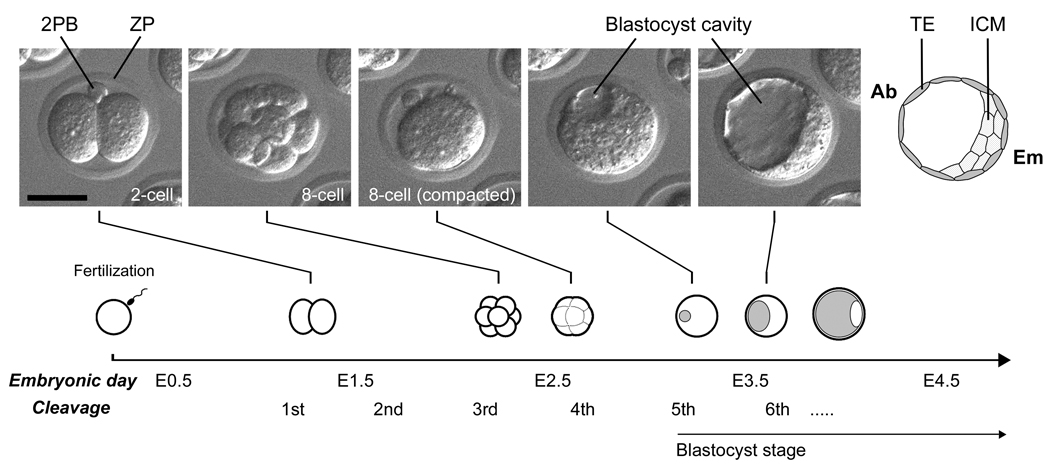
Fig. 1.
Morphological transformation of mouse embryo during the first three days after fertilization. Embryonic day is often used in literatures to describe the developmental stage of embryos, which correspond to days after the mating of parents (or days post coitum). The timing of developmental progression in this diagram is based on embryos that are cultured in vitro. 2PB, second polar body; ZP, zona pellucida; TE, trophectoderm; ICM, inner cell mass; Ab, abembryonic side; Em, embryonic side. Scale bar, 50 µm.
The objective of this article is to review and discuss the current knowledge on the mechanisms of TE and ICM formation, specifically in the mouse embryo. Understanding of such mechanisms is important not only from the perspective of animal evolution as mentioned above, but also from a clinical point of view, particularly for human artificial reproductive technology (ART). An ideal practice at fertility clinics is to raise embryos under an optimal culture condition after in vitro fertilization, and to transfer a highest-quality embryo to the mother’s uterus to achieve successful implantation and fetal development. Significant advancements in human ART have been made in the past couple of decades, many of which were fundamentally dependent on the prior knowledge obtained from the experimental studies using mouse embryos. Examples are the development of better embryo culture media, embryo cryopreservation technology, intracytoplasmic sperm injection (ICSI) as a method for efficient in vitro fertilization, and also blastomere biopsy for preimplantation genetic diagnosis as a way to exclude embryos carrying undesired genetic abnormalities. Even so, the current ART may still not be perfect in a sense that clinical pregnancy rate per transfer of a single embryo is 40–60% in many cases (Jain et al., 2004; Stern et al., 2007; Leniaud et al., 2008; Stillman et al., 2009). Thus, the elucidation of the mechanisms of TE and ICM formation would help possibly in improving culture conditions to raise human embryos to a better-quality state, in which the development of TE and ICM is enhanced for robust implantation and fetal development.
2. Morphological overview of the first three days of mouse development
This section briefly describes the major morphological events during the first three days of mouse development, the pre-implantation stage, which results in the generation of TE and ICM (Fig. 1). The pre-implantation stage of development normally takes place within the oviduct of the mother, but it can also be recapitulated in vitro in a chemically defined culture medium without adversely affecting the developmental potential of embryos (Summers and Biggers, 2003). The unfertilized egg is arrested at metaphase II of meiosis, and the site of the egg nucleus is defined as the animal pole. After fertilization, the egg resumes and completes meiosis with the emission of the second polar body (Fig. 1). In many cases, the second polar body remains attached to the egg through an intercellular bridge (Gardner, 1997), so that its location often serves as a landmark of the animal pole during later development, although how long this attachment stably persists may vary among embryos. The first cleavage occurs at about 16–20 hours after fertilization, and the following cleavages take place at an interval of roughly 12 hours. Notably, the cell cycle length is considerably variable not only among different embryos but also among blastomeres within the same embryo. Thus, the total blastomere number of an embryo at a given time point is sometimes other than 2n. The details of asynchronous cell division cycles are particularly evident in the recent studies using time-lapse cinematography and three-dimensional visualization techniques of developing embryos (Kurotaki et al., 2007; Bischoff et al., 2008; Fujimori et al., 2009).
The first three rounds of cleavages yield a total of eight blastomeres that are morphologically similar to each other (Fig. 1). Before the fourth cleavages, compaction occurs, which converts an embryo with eight clearly demarcated blastomeres into a ball of highly packed cells where the outlines of blastomeres are not readily distinguishable (Fig. 1). Upon compaction, an apical-basal polarity emerges in all eight blastomeres that are manifested as polarized distributions of various membrane and cytoplasmic components along the axis from the surface (apical) to the centre (basal) of the embryo (Handyside, 1980; Johnson and Ziomek, 1981a; Reeve, 1981; Reeve and Ziomek, 1981; Fleming and Pickering, 1985; Maro et al., 1985; Johnson and McConnell, 2004). The fourth cleavages generate a total of 16 blastomeres, some of which are positioned on the surface of the embryo, whereas others are entirely surrounded by neighboring blastomeres. The number of external and internal blastomeres at the 16-cell stage varies considerably among embryos. In some studies, they are estimated to be 6–7 internal and 9–10 external blastomeres on average (Handyside, 1981; Johnson and Ziomek, 1981b; Fleming, 1987; Suwińska et al., 2008). External blastomeres retain an apical-basal polarity, whereas internal blastomeres are apparently devoid of polarity. The fifth cleavages generate more external and internal cells, and a 32-cell stage embryo is estimated to contain 12–13 internal and 19–20 external blastomeres on average (Handyside, 1978, 1981; Fleming, 1987; Suwińska et al., 2008). The above estimates on the external and internal cell numbers at 16- and 32-cell stages are based on the retention of surface labels after blastomere dissociation (e.g., labeled ones as external; Handyside, 1981; Fleming, 1987; Suwińska et al., 2008) or on the shape and size of dissociated blastomeres (e.g., polarized and larger ones as external; Johnson and Ziomek, 1981b). However, it is of note that other methodologies, particularly those that are based on fluorescent-labeling of cell cortex in fixed whole embryos, have yielded significantly different estimates: only 1–2 internal blastomeres on average are present in a 16-cell stage embryo (Dietrich and Hiiragi, 2007; Fujimori et al., 2009). The cause of such discrepancy is currently unknown. However, because the size of area that is exposed to the surface is considerably variable among individual external blastomeres, it is possible that some blastomeres, especially those with a lesser surface exposure (Fig. 2A), may not be detected (or defined) as external blastomeres under certain methodologies.
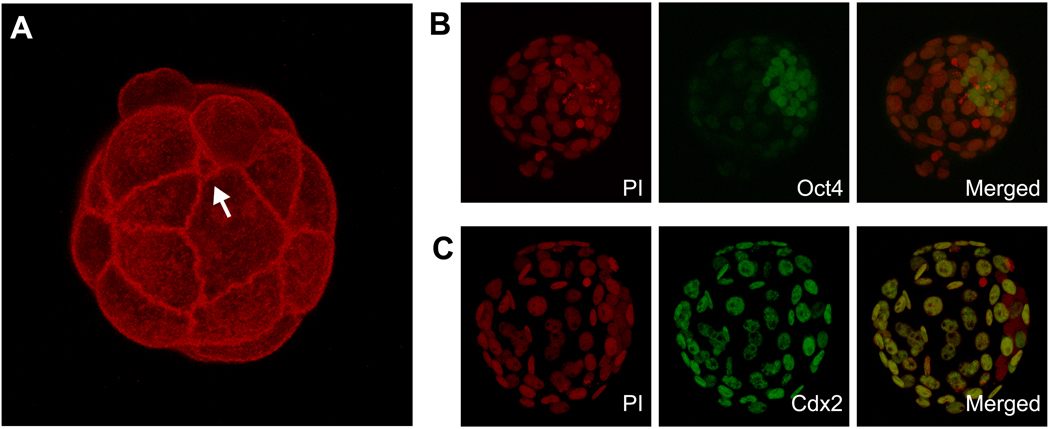
Fig. 2.
(A) A 3D projection of confocal images of an embryo that is stained for actin filament using fluorescently labeled phalloidin. Belt-like intense staining of actin filament represents adherens junctions around the apical edge of cell-cell boundaries. The image is to highlight considerable variations among blastomeres in terms of the size of area that is exposed to the surface. Particularly, the one that is pointed out with an arrow has an extremely small surface exposed. This blastomere may be identified as either external or internal depending on methodologies used. (B) A 3D projection of confocal images of a late blastocyst that is stained for nuclei with propidium iodide (PI, red color) and for Oct4 protein with a specific antibody (green color). Note that Oct4 is most strongly expressed in the inner cell mass. (C) A 3D projection of confocal images of a late blastocyst that is stained for nuclei with PI and for Cdx2 protein with a specific antibody (green color). Note that Cdx2 is exclusively expressed in the trophectoderm.
After the fifth cleavages, one or more small cavities start to form between blastomeres. These initial intercellular cavities are apparently derived from intracellular vesicles, or vacuoles, that are secreted from external blastomeres by exocytosis (Aziz and Alexandre, 1991). Cavities then continually expand and fuse with each other to form a single large cavity. At this point, the embryo is specifically called the “blastocyst”, in which external cells (now defined as TE) adopt the epithelial morphology to surround the entire embryo, whereas internal cells (now defined as ICM) aggregate as a single mass that is attached to the basal surface of TE (Fig. 1). Due to the eccentric localization of ICM, the blastocyst displays a distinct morphological axis: the side where ICM is located is referred to as the embryonic (Em) pole, and the opposite side where the cavity is situated is referred to as the abembryonic (Ab) pole (Fig. 1). Importantly, because of such polarized association, TE and ICM further diversify during later development. For example, the region of TE that is in contact with ICM gives rise to the extraembryonic ectoderm in response to the paracrine signal, namely Fgf4, that is produced by ICM (Gardner, 1983; Nichols et al., 1998). Also, a population of ICM cells gives rise to the primitive endoderm on the surface that is facing the blastocyst cavity. While the exposure to the surface can trigger the differentiation of ICM to the primitive endoderm, recent studies also suggest that the precursor cells for primitive endoderm are present within ICM in a mosaic manner at the blastocyst stage, which later migrate towards the surface (Chazaud et al., 2006; Plusa et al., 2008). Thus, the embryonic-abembryonic (Em-Ab) axis of the blastocyst is closely linked to the organization of later stages of embryos.
3. TE formation and epithelialization
TE, which is the first cell type to differentiate in mouse development, possesses the characteristics of epithelium. The formation of epithelium by embryonic cells is a common feature of early development in many animals. Epithelium formation is also accompanied by the creation of a large cavity at the center of the embryo in a wide range of animals, including sea anemone, nematode, sea urchin, and frog (Shook and Keller, 2003; Schierenberg, 2005; Magie et al., 2007; Wu et al., 2007). The cavity, which is specifically called the blastocoel, is generated by the epithelium that engages in polarized transport of ions and water. In these animals, the blastocoel provides a space for mesoderm and endoderm to move into through the morphogenesis of gastrulation. In many literatures, the cavity in the mammalian blastocyst is also referred to as the blastocoel. However, it is important to note that the blastocyst cavity is not developmentally or phylogenetically equivalent to the blastocoel of other animals, as the former is not the space for mesoderm or endoderm to move into.
In the mouse embryo, epithelium formation starts as compaction at the late 8-cell stage and continues for the next 24 hours to complete at around the 32-cell stage. During compaction, intercellular adhesion is markedly enhanced primarily through the activity of Ca2+-dependent adhesion molecule E-cadherin (Shirayoshi et al., 1983; Johnson et al., 1986; De Vries et al., 2004). E-cadherin is a major component of adherens junctions (AJ), which are protein complexes that are located at cell-cell junctions in most epithelial tissues (Niessen and Gottardi, 2008). In mouse embryos, the lack of E-cadherin expression leads to defective AJ, which in turn interferes with the formation of another junctional complex of epithelium, tight junction (TJ) (Larue et al., 1994; Ohsugi et al., 1997). In a typical epithelium, cell membranes are tightly connected between apposing cells through TJ, which is composed of distinct proteins, such as claudin, occludin and zonula occludens (ZO) proteins, and which creates a barrier that is virtually impermeable to fluid (Tsukita et al., 2008). In mouse embryos, some of the TJ components start to localize near the apical side of cell-cell boundaries at the time of compaction, and the assembly of mature TJ is completed by around 32-cell stage, which is capable of sealing the blastocyst cavity (Johnson and McConnell, 2004; Eckert and Fleming, 2008). Various experimental studies have demonstrated that many of the TJ components play critical roles in the formation or maintenance of the blastocyst cavity. Those TJ components include ZO-1, ZO-2 and claudin 4/6, whose knockdown or inhibition results in defective or delayed formation of the cavity (Moriwaki et al., 2007; Sheth et al., 2008; Wang et al., 2008). However, it is of note that those phenotypes caused by a loss-of-function of one TJ component are far less severe than those of the E-cadherin knockout, possibly due to functional redundancy among various TJ components (Katsuno et al., 2008; Tsukita et al., 2008; Xu et al., 2008).
Furthermore, in most epithelial tissues, the establishment of cell polarity and junctional complexes is dependent on the PAR-aPKC system. The PAR-aPKC system is composed of a set of evolutionarily conserved proteins, including partition-defective (PAR) homologs and atypical protein kinase C (aPKC), whose distributions and/or activities are distinctly localized along the apical-basal axis of the epithelium (Suzuki and Ohno, 2006; Ebnet et al., 2008; Assémat et al., 2008). The PAR-aPKC system may also play critical roles in the formation of TE in mouse embryos. For example, some of the components become localized asymmetrically along the apical-basal axis of cells after compaction, namely Pard3, Pard6b, and aPKCζ (Prkcz) proteins to the apical side, and Par1 homolog Emk1 (Mark2) to the basal domain (Pauken and Capco, 2000; Eckert et al., 2004; Thomas et al., 2004; Plusa et al., 2005; Vinot et al., 2005). Also, the interference with Pard3 or aPKC leads to a decreased tendency to develop into TE (Plusa et al., 2005).
In order for the blastocyst cavity to form and expand, TE needs not only to create a tightly sealed layer with junctional complexes but also to pump fluid into the cavity. The major driving force for water influx from the apical side to the basal side of TE is the osmotic pressure generated by an increased concentration of sodium ions on the basal side of the epithelium. Transport of sodium ions into the basal side, where a nascent blastocyst cavity forms, is mediated by the actions of several carriers, including Na+/H+ exchanger for Na+ influx at the apical membrane and Na+/K+-ATPase for Na+ efflux at the basal membrane (Wiley, 1984; Manejwala et al., 1989; Watson, 1992; Barr et al., 1998; Barcroft et al., 2004; Kawagishi et al., 2004). Interestingly, the knockdown of Na+/K+-ATPase β1 subunit not only interferes with the formation of the blastocyst cavity but also results in an aberrant distribution of TJ components ZO-1 and occludin (Madan et al., 2007). This implicates that proper establishment of Na+ gradient across the TE layer is essential for the assembly of mature TJ, which may be a potential link between two distinct functional aspects of epithelium, i.e., tight sealing and fluid pumping.
How does an embryo decide when to initiate the epithelium formation? The mechanism of such regulation of timing is currently not well-understood. Compaction typically starts at the late 8-cell stage. However, this timing is unlikely to be controlled by the number of cell divisions, because the inhibition of cytokinesis or DNA replication does not delay the initiation of compaction (Smith and McLaren, 1977; Smith and Johnson, 1985). Interestingly, compaction and cavity formation are accelerated when embryos are manipulated in such a way that a portion of cytoplasm has been removed at the 1-cell stage or replaced with cytoplasm from later stages of embryos (Feng and Gordon, 1997; Lee et al., 2001). A possible explanation for this is that certain suppressive factors are expressed in embryos from 1-cell stage to prevent compaction, but those factors gradually disappear during development, so that compaction is no longer suppressed at the late 8-cell stage. The notion of the presence of suppressive factors is consistent with the observation that compaction occurs precociously when embryos are treated with a protein synthesis inhibitor (Levy et al., 1986). While the molecular nature and mode of actions of such hypothetical factors are unknown, several studies also suggest that the activation of protein kinase C and the redistribution of E-cadherin to the basolateral membrane are involved in the regulation of timing of compaction (Winkel et al., 1990; Ohsugi et al., 1997; Pauken and Capco, 1999; Kawai et al., 2002).
4. Roles of transcription factors in TE and ICM formation
Transcription factors are critical regulators of cell fate determination during animal development. A few transcription factors have been identified that are essential to generate TE and ICM correctly in the mouse blastocyst. Oct4 (Pou5f1) is a POU-domain transcription factor, and is strongly expressed in ICM (Okamoto et al, 1990; Rosner et al., 1990; Schöler et al., 1990; Fig. 2B). Oct4-null embryos can give rise to blastocysts that appear morphologically normal, i.e., epithelial TE surrounding a large cavity and an internal cell aggregate that is reminiscent of ICM. However, the internal cell aggregates of the Oct4-null blastocyst express TE-specific intermediate filaments (Nichols et al., 1998). Also, the inactivation of Oct4 in embryonic stem (ES) cells, which are ICM-derived cell lines, results in the differentiation of TE (Niwa et al., 2000). Thus, Oct4 is essential to prevent ICM from diverting towards the TE lineage. Transcriptional targets of Oct4 have been vigorously investigated, especially in light of its role in the molecular regulation of pluripotency in ES cells (Niwa, 2007). One of the well-defined Oct4 targets is the Fgf4 gene, which encodes a ligand of the fibroblast growth factor signaling pathway. Fgf4 is secreted from ICM and induces proliferation in adjacent polar TE (Yuan et al., 1995; Nichols et al., 1998). Another well-defined target is the Nanog gene, which encodes a homeodomain transcription factor. Nanog is also essential for the maintenance of pluripotency in ICM and ES cells, although, unlike Oct4, it prevents ES cells from giving rise to the primitive endoderm lineage (Chambers et al., 2003; Mitsui et al., 2003; Kuroda et al., 2005; Rodda et al., 2005).
Cdx2 is a caudal-type homeodomain transcription factor, and is specifically expressed in TE (Beck et al., 1995; Fig. 2C). In Cdx2-null embryos, the initial phase of epithelialization, including compaction, blastomere polarization, and cavity formation, takes place normally (Strumpf et al., 2005; Ralston and Rossant, 2008). However, by late blastocyst stage, Cdx2-null embryos lose the epithelial integrity of TE, as evidenced by disturbed AJ and TJ, and fail to maintain the blastocyst cavity. Cdx2-null embryos also exhibit an increased incidence of apoptosis at embryonic day E4.5, which may contribute to the collapse of the cavity (Strumpf et al., 2005). Notably, Oct4 is strongly expressed in the external cells of Cdx2-null embryos, indicating that Cdx2 is necessary to repress the expression of Oct4 in TE (Strumpf et al., 2005; Niwa et al., 2005). ES cells can be derived from Cdx2-null embryos (Chawengsaksophak et al., 2004), whereas no derivatives of TE, including trophoblast giant cells and trophoblast stem (TS) cell lines, can be obtained from them (Strumpf et al., 2005). Thus, Cdx2 is essential for the maintenance of TE, but is dispensable for the formation and maintenance of ICM. One of the potential transcriptional targets of Cdx2 is the Eomes gene, which encodes a T-box transcription factor and is expressed in the TE lineage (Ciruna and Rossant, 1999; Russ et al., 2000). The expression level of Eomes is markedly decreased in Cdx2-null embryos at E4.5 (Strumpf et al., 2005; Ralston and Rossant, 2008). Unlike Cdx2-null embryos, Eomes-null embryos form morphologically normal blastocysts with an expanded cavity and the correct expression patterns of Oct4 and Cdx2 (Strumpf et al., 2005). However, TE of Eomes-null embryos is unable to generate trophoblast giant cells and TS cell lines (Russ et al., 2000; Strumpf et al., 2005).
Tead4 is a TEA domain transcription factor. Although Tead4 is ubiquitously expressed in the blastocyst without an apparent localization to ICM or TE (Nishioka et al., 2008), Tead4-null embryos exhibit defects specifically in TE. Compaction occurs normally at the 8-cell stage, but the blastocyst cavity never forms in Tead4-null embryos (Yagi et al., 2007; Nishioka et al., 2008). E-cadherin appears to be expressed normally in Tead4-null embryos, suggestive of intact AJ (Yagi et al., 2007; Nishioka et al., 2008), although the integrity of TJ in Tead4-null embryos is currently unknown. Similar to Cdx2-null embryos, ES cells, but not TS cells, can be derived from Tead4-null embryos, indicating that Tead4 is essential for TE but dispensable for ICM. Notably, the expression of Cdx2 is virtually absent in Tead4-null embryos, whereas Oct4 and Nanog are ectopically expressed in external cells at late blastocyst stage (Yagi et al., 2007; Nishioka et al., 2008). A recent study suggests that the TE-specific action of Tead4 is mediated by the localized stabilization of the Yap protein in external blastomeres, which binds to Tead4 and acts as a transcriptional activator (Nishioka et al., 2009). The Cdx2 gene may be one of the transcriptional targets of Tead4. However, the phenotype of Tead4-null embryos is more severe than that of Cdx2-null embryos, as the latter can still form a cavity initially. Thus, other target genes of Tead4 may play essential roles in epithelialization and cavity formation.
5. Timing of cell fate determination
After the fourth cleavages, internal blastomeres are distinguishable from external blastomeres not only geometrically but also morphologically, as the former are non-polarized and the latter are polarized. However, in terms of developmental fate, when do internal and external blastomeres become committed to giving rise to ICM and TE, respectively? Various experimental studies using micromanipulation techniques, such as blastomere isolation, aggregation, and transplantation, have led to a general conclusion that the fates of blastomeres are not determined until the fifth cleavages. For example, external blastomeres isolated from the 16-cell stage embryo can give rise to ICM when experimentally replaced internally, whereas internal blastomeres can give rise to TE when replaced externally (Rossant and Vijh, 1980; Ziomek and Johnson, 1982; Ziomek et al., 1982; Johnson and Ziomek, 1983). This notion is further elaborated in a recent study (Suwińska et al., 2008). The surface of 16- or 32-cell stage embryos is labeled with fluorescent microbeads or dyes, and then the embryos are dissociated into individual blastomeres. This allows the identification and separation of external (labeled) blastomeres from internal (non-labeled) blastomeres. When 16 of internal or external blastomeres isolated from 16-cell stage embryos are aggregated as a single mass, both types of aggregates are capable of not only forming expanded blastocysts with the correct expressions of Oct4 and Cdx2, but also in giving rise to healthy adult mice when transferred into surrogate mothers. On the contrary, the situation is different when 32 of internal or external blastomeres isolated from 32-cell stage embryos are aggregated. Most aggregates made of external blastomeres generate TE but are essentially devoid of ICM. Aggregates of internal blastomeres form a cavity in a delayed manner, but are unable to implant in surrogate mothers (Suwińska et al., 2008). These experiments suggest that significant changes occur in the developmental potential of blastomeres at around the time of the fifth cleavages.
Consistent with the above notion, internal blastomeres that are isolated from early cavitating blastocysts can still regenerate a TE layer with a cavity. In many experimental studies, internal blastomeres and ICM are obtained from various stages of embryos by immunosurgery, which is a convenient and effective method to isolate pure populations of internal cells by destroying all the surface cells using antibodies and complements (Solter and Knowles, 1975). When exposed to the surface after isolation, the originally non-polarized internal blastomeres exhibit morphological changes to acquire a distinct apical-basal polarity, which is followed by epithelialization and the formation of a new blastocyst cavity (Handyside, 1978; Spindle, 1978; Johnson, 1979; Chisholm et al., 1985; Louvet-Vallée et al., 2001; Eckert et al., 2004). Isolated internal blastomeres from early cavitating blastocysts (hereafter called “isolated ICM”) can also implant in the uterus when transferred into surrogate mothers, indicating that regenerated TE is functional (Rossant and Lis, 1979). The process of polarization and epithelialization in isolated ICM is relatively rapid, as the cavity formation is observed in about 6 hours without cell division (Louvet-Vallée et al., 2001). This is quite a contrast to the normal course of epithelialization, which starts at late 8-cell stage and matures at around 32-cell stage, taking about 20 hours over two rounds of cell divisions. Interestingly, the polarization of isolated ICM occurs in the presence of a protein synthesis inhibitor (Louvet-Vallée et al., 2001). This suggests that internal blastomeres of early cavitating blastocysts already express sufficient components to establish an apical-basal polarity, but they are unable to do so until exposed to the surface. Thus, the regeneration of TE in isolated ICM is unlikely to be a process of “trans-differentiation”, which typically requires cell divisions and the expression of a new set of gene products (Thowfeequ et al., 2007; Zhou and Melton, 2008).
Molecular mechanisms that are responsible for the dramatic change in cell fate determination at around the fifth cleavages are currently unknown. However, the change appears to be associated with an alteration in the expression pattern of Cdx2 protein, a transcription factor essential for normal TE development. At 8- to 16-cell stages, Cdx2 is detectable at various levels in the nuclei of most blastomeres, regardless of their external or internal location. However, during the transition from 16- to 32-cell stage, the level of Cdx2 becomes stronger in the external blastomeres and weaker in the internal blastomeres, eventually establishing the TE-specific expression at the blastocyst stage (Dietrich and Hiiragi, 2007; Ralston and Rossant, 2008). Thus, the disappearance of Cdx2 expression in internal blastomeres appears to coincide with the decrease in their potential to form TE. It is important to note, however, that the lack of Cdx2 expression itself is not the cause of inability to develop TE, because Cdx2-null embryos can initially form a blastocyst cavity (Strumpf et al., 2005). Hence, certain cellular events that are triggered by the exposure to the surface are likely to direct the elevation and maintenance of the Cdx2 expression as well as the commitment towards the TE lineage in external blastomeres at around 32-cell stage.
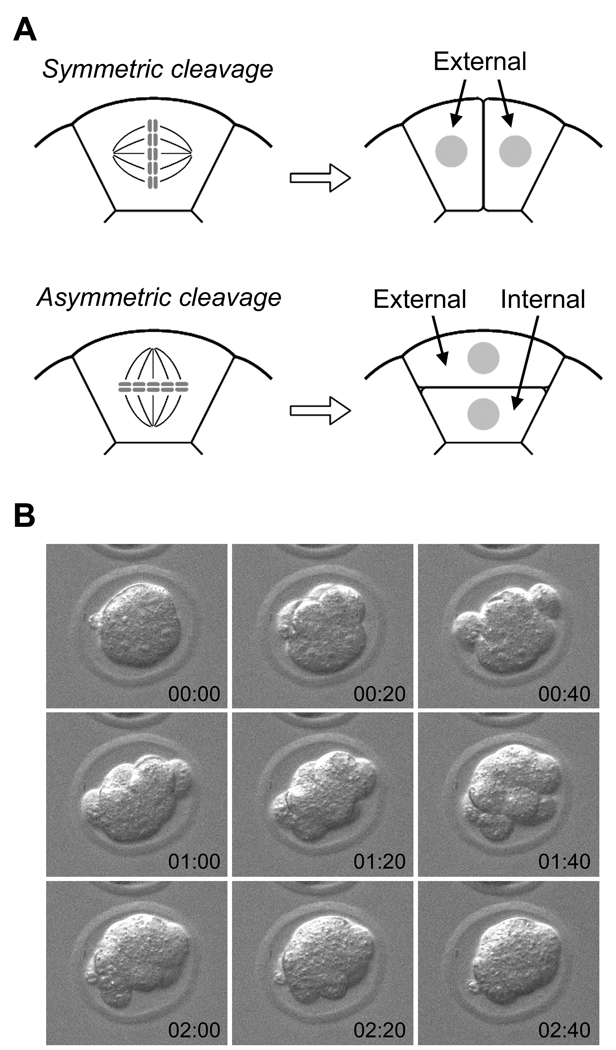
Because the location of blastomeres (i.e., external or internal) at around 32-cell stage is tightly linked to the commitment towards TE or ICM lineage, the patterns of the fourth and fifth cleavages in each blastomere could impact on how many cells would contribute to TE or ICM. Conceptually, cleavage patterns of blastomeres of 8-cell stage embryos and of external blastomeres of 16-cell stage embryos can be categorized into two groups: symmetric and asymmetric. Symmetric cleavage, in which the plane of cell division is parallel to the apical-basal axis, yields two external blastomeres, whereas asymmetric cleavage, in which the plane of cell division runs perpendicular to the apical-basal axis, yields one external and one internal blastomere (Fig. 3A). Thus, in theory, the ratio of TE to ICM can be determined by how many of symmetric or asymmetric cleavages are executed in individual blastomeres. The question is, does the embryo actually employ such a mechanism of controlling the cleavage pattern to regulate the formation of TE and ICM? Examination of fourth cleavages in developing embryos using time-lapse cinematography has revealed that the planes of cell divisions occur at various angles relative to the apical-basal axis (Sutherland et al., 1990), suggesting that the orientations of cleavage planes are not strictly regulated in a bimodal fashion (i.e., parallel or perpendicular to the apical-basal axis). Also revealed by time-lapse cinematography is that relative positions of blastomeres change dynamically during the fourth and fifth cleavages: a blastomere first becomes spherical and uncompacted during mitosis, and divides into two round blastomeres, which then flatten and merge with the rest of the embryo (Kurotaki et al., 2007; Fig 3B). Thus, it appears unlikely that the numbers of TE and ICM cells in the blastocyst are determined by a strict regulation of cleavage orientations between symmetric and asymmetric patterns at the fourth and fifth cleavages. Nonetheless, several studies have put forward a model that the cleavage orientations are actively regulated by specific molecular machineries, which ultimately determine how a blastomere contributes to TE and ICM (Plusa et al., 2005; Bischoff et al., 2008). Particularly, Cdx2 has been proposed to be the key regulator of such machineries, whose elevated expression in blastomeres promotes symmetric cleavages to generate TE preferentially (Jedrusik et al., 2008). However, another recent study has shown that in chimeric embryos made of normal and Cdx2-null embryos, Cdx2-null blastomeres appear to contribute to both TE and ICM as efficiently as normal blastomeres (Ralston and Rossant, 2008). Thus, the role of Cdx2 in the regulation of cleavage patterns is currently unclear.
Fig. 3.
(A) A schematic diagram depicting two types of cleavage patterns. Symmetric cleavage divides a blastomere along the apical-basal axis to generate two external blastomeres, whereas asymmetric cleavage divides perpendicular to the axis to generate one external and one internal blastomere. (B) Dynamic behaviors of blastomeres during cleavages can be seen by time-lapse cinematography. Images were taken every 20 minutes, starting at the beginning of the fourth cleavages (time is given in hours:minutes in each frame). Note that blastomeres during cleavages uncompact and then compact again, resulting in a dynamic change in the overall shape of the embryo.
6. Does a developmental bias exist among blastomeres at 2- to 4-cell stages?
Evidently, cell fates are not fixed until around 32-cell stage. However, this does not preclude the possibility that developmental potentials are unequal among blastomeres at earlier stages. For example, a particular blastomere in the 4-cell or even 2-cell stage embryo may already be biased to give rise preferentially to a particular type of cells or to contribute to a specific region in the blastocyst, although such bias may be erased or modified by experimental manipulations. In line with this notion, several studies have put forward a model that one blastomere at the 4-cell stage is biased to contribute exclusively to TE on the Ab side (Piotrowska-Nitsche and Zernicka-Goetz, 2005; Piotrowska-Nitsche et al., 2005; Torres-Padilla et al., 2007; Bischoff et al., 2008; Jedrusik et al., 2008). A particular group of 4-cell stage embryos (named ME-pattern) arise from specific asynchronous cleavages of the 2-cell stage blastomeres: the earlier cleavage occurs meridional (M) or parallel to the animal-vegetal axis, and the later cleavage occurs equatorial (E) or perpendicular to the animal-vegetal axis. In these ME-pattern 4-cell stage embryos, which are found in roughly half of the embryo population, the descendants of the vegetal-most blastomere almost exclusively contribute to the abembryonic pole of the blastocyst, or specifically to polar TE. Also, aggregates that are made of 3 or 4 of the vegetal-most blastomeres isolated from ME-pattern embryos do not develop as efficiently as those that are made of other types of blastomeres, suggesting that the developmental ability of the vegetal-most blastomere is limited. Based on these observations, a model has been proposed that one of the blastomeres at the 4-cell stage, which can be identified as the vegetal-most blastomere in ME-pattern embryos, already possesses a distinct developmental potential that is biased toward TE of the abembryonic side (Piotrowska-Nitsche and Zernicka-Goetz, 2005; Piotrowska-Nitsche et al., 2005). Furthermore, it has been shown that the descendants of the vegetal-most blastomere in ME-pattern embryos tend to execute symmetric types of cleavages, which yield more external blastomeres during fourth and fifth cleavages (Bischoff et al., 2008), that the Cdx2 gene is expressed preferentially in the vegetal-most blastomere to promote symmetric cleavages (Jedrusik et al., 2008), and that the nucleus of the vegetal-most blastomere carries a unique epigenetic feature, namely a reduced level of histone methylation H3R26me, which may interfere with the expression of ICM-specific genes (Torres-Padilla et al., 2007). These observations further augment the above model with potential cellular and molecular mechanisms that are responsible for the biased developmental potential of one of the 4-cell stage blastomeres.
Nevertheless, the notion of biased developmental potential at the 4-cell stage is currently of profound controversy for various reasons. First, a developmental bias that is similar to the vegetal-most blastomere in ME-pattern embryos has not been demonstrated in other types of embryos, namely EM-, MM-, and EE-patterns. These types of embryos altogether constitute more than half of embryo populations, and are capable of full-term development as efficiently as ME-pattern embryos (Piotrowska-Nitsche and Zernicka-Goetz, 2005). This raises a question regarding the universality of the biased potential at the 4-cell stage as a critical means of embryo patterning. Second, when an isolated vegetal blastomere of ME-pattern embryo is aggregated with four of randomly isolated blastomeres, it can contribute to the derivatives of ICM, such as epiblast, as effectively as other blastomeres (Piotrowska-Nitsche et al., 2005), indicating that the developmental bias of the vegetal-most blastomere towards TE does not manifest under such experimental situation. However, it is unlikely that experimental manipulations, namely isolation and aggregation, would erase the developmental bias, because aggregates that are made entirely of isolated vegetal-most blastomeres still exhibit defective development (Piotrowska-Nitsche et al., 2005). Third, despite that the vegetal-most blastomere of ME-pattern embryos preferentially contribute to TE in whole embryos, aggregates made of the vegetal-most blastomeres develop less effectively to expanded blastocysts than aggregates made of other blastomeres (Table 2 in Piotrowska-Nitsche et al., 2005). If the ineffective development of a blastocyst cavity is indicative of defective TE formation, then the notion that the vegetal-most blastomeres are biased towards TE lineage appears contradictory. Fourth, the notion that one of the 4-cell stage blastomeres almost exclusively contribute to the abembryonic side is inconsistent with a recent cell lineage study, which has shown that an exclusive contribution of one of the 4-cell stage blastomeres to the Ab region occurs only in about 8% of the cases (i.e., in about 92% of the cases, 4-cell stage blastomeres contributes to ICM) (Kurotaki et al., 2007). Lastly, another recent cell lineage study has shown that the vegetal-most blastomere of ME-pattern embryos contributes to the Em side as effectively as to the Ab side (Alarcón and Marikawa, 2008).
Another model of biased developmental potential has also been proposed: the two blastomeres at the 2-cell stages are already different from each other such that one blastomere contributes mainly to the Em half and the other blastomere to the Ab half of the blastocyst (Fig. 4A). This model is based on two types of observations. One is that the boundary between the Em and Ab halves of the early blastocyst tends to align with the plane of the first cleavage, which is estimated by the location of the second polar body or traced by marking of the surrounding egg shell, called the zona pellucida (ZP) (Gardner, 1997; 2001). The other type of observation is that the descendants of each of the 2-cell stage blastomeres, when traced up to the early blastocyst stage using a fluorescent label, tend to occupy either the Em or Ab half (Piotrowska et al., 2001). Importantly, as stipulated in the model, the segregation of 2-cell stage blastomeres into the Em and Ab halves occurs only as a tendency: it is observed in 60–70% of embryo populations (Gardner, 2001; Piotrowska et al., 2001). Furthermore, the model is in accord only with normal and undisturbed development, as any experimental manipulations, such as separation of blastomeres, may obliterate the developmental bias or activate certain regulatory mechanisms to compensate for the disturbance.
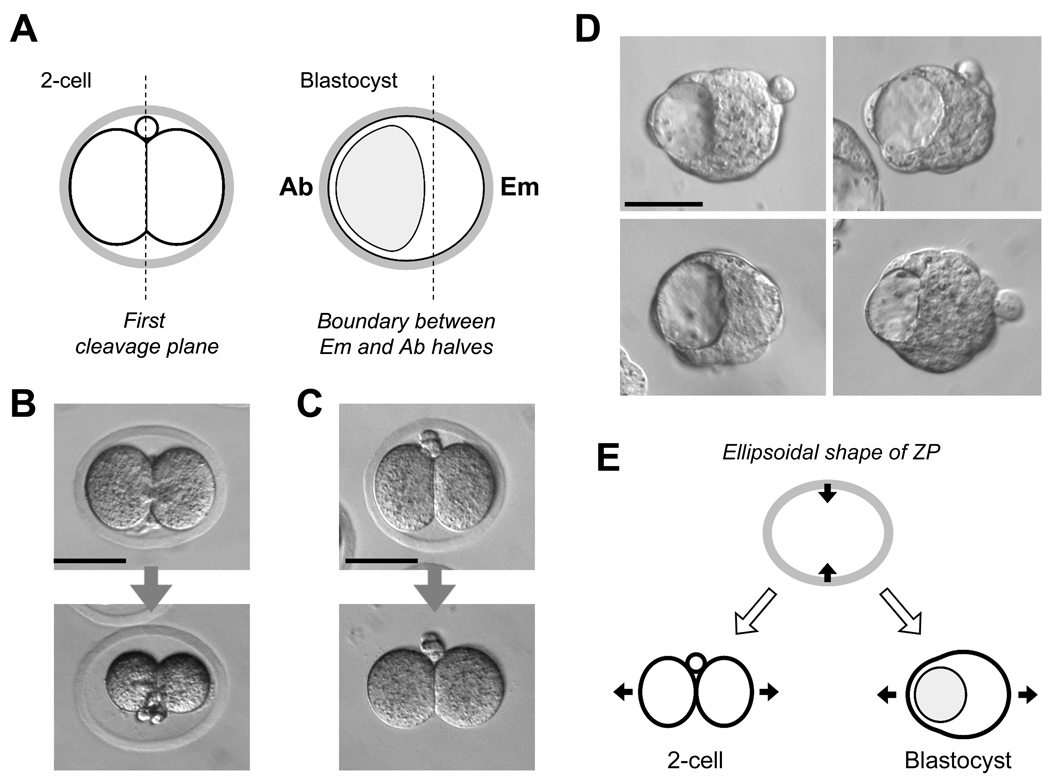
Fig. 4.
(A) A schematic diagram depicting the relationship between the first cleavage plane at the 2-cell stage and the boundary between the embryonic and abembryonic halves at the blastocyst stage. (B) The ellipsoidal shape of zona pellucida (ZP) is stable and not dependent on the tension from the 2-cell stage blastomeres. When placed in a medium of high salt concentration, the embryo markedly shrunk, while the ZP maintained its elongated shape. (C) The ZP is firm and constricts an embryo within. The ZP was digested with pronase, which relieved an embryo from constriction. (D) Images of early blastocysts that were cultured without the ZP from the 8-cell stage. Note that embryos are slightly elongated along the Em-Ab axis likely due to the expansion of the blastocyst cavity. (E) A schematic diagram depicting how a mechanical constraint posed by an ellipsoidal ZP may affect the orientation of an elongated embryo at the 2-cell and blastocyst stages.
However, this model also encounters various objections. Several independent cell lineage studies have shown that the descendants of 2-cell stage blastomeres do not segregate between Em and Ab halves at an appreciable frequency (Alarcón and Marikawa, 2003; 2005; Chróścicka et al., 2004; Motosugi et al., 2005; Kurotaki et al., 2007). In addition, an alternative explanation has been put forward in several studies to account for the apparent correlation between the first cleavage plane and the boundary between the Em and Ab halves (Motosugi et al., 2005; Kurotaki et al., 2007; Alarcón and Marikawa, 2008). The shape of ZP is often not spherical but rather ellipsoidal. In that case, a two-cell stage embryo would be positioned within the ZP most fittingly when the two blastomeres are lined up along the longest diameter of the ZP (Fig. 4B,C). Also, a blastocyst is likely to adopt a slightly elongated shape when an eccentrically located cavity expands (Fig. 4D). In that case, a blastocyst would be positioned most fittingly within the ZP when a cavity is situated at one end of the longest diameter. As a result of these two types of physical restraints imposed by an ellipsoidal ZP, the boundary between Em and Ab halves of the blastocyst would be likely to align along the first cleavage plane (Fig. 4E). Such influence of embryo shape on the position of the blastocyst cavity (therefore, the Em-Ab axis) has been demonstrated by various experiments, such as mechanical flattening of embryos (Motosugi et al., 2005), embedding of embryos in an alginate gel (Kurotaki et al., 2007; Alarcón and Marikawa, 2008), and removing of ZP (Kurotaki et al., 2007), and also by computer simulations using mathematical models (Honda et al., 2008). These studies further support that an apparent correlation of the two-cell stage blastomeres with the Em-Ab axis is merely a consequence of mechanical constraint from the ZP, rather than due to unequal developmental potential between the two blastomeres.
Even so, the notion of biased developmental potential in the two-cell stage embryo has not been abandoned, as exemplified by a recent study that opposes the involvement of mechanical constraint from the ZP (Gardner, 2007). Nonetheless, it is imperative to underscore the following two points with respect to developmental bias at the 2-cell or 4-cell stage. (1) In spite of persistent and painstaking attempts in various studies, a developmental bias at these stages is not consistently or reproducibly observed. Thus, even if a bias does exist, it is likely to be extremely fragile or manifests only under a certain restricted condition. (2) Numerous experimental studies have indisputably demonstrated that mouse embryos are perfectly capable of full-term development even without any developmental bias at the 2-cell or 4-cell stage. This fact does not a priori exclude the existence of a bias. However, because it is dispensable for normal development, such bias, and the molecular and genetic mechanisms behind it, would less likely be conserved during evolution.
7. Concluding remark
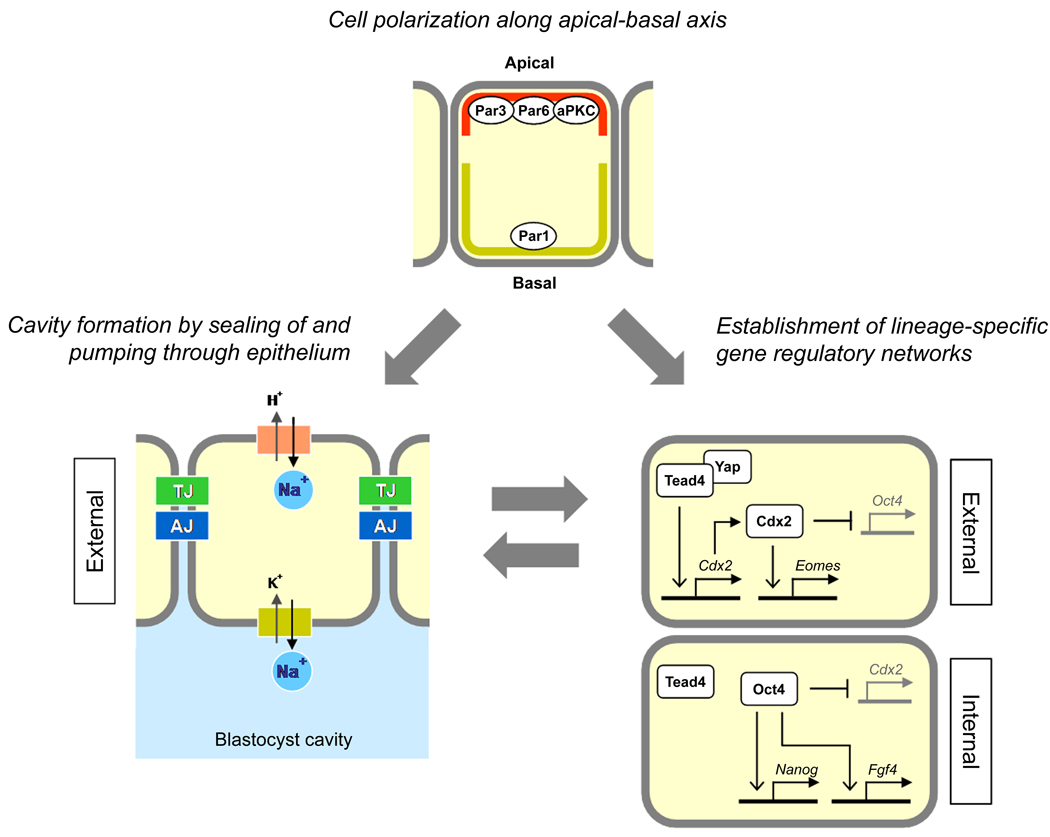
In the past several decades, numerous experimental studies have clearly demonstrated that the establishment of the first two cell lineages, TE and ICM, is regulated by the location of blastomeres in mouse embryos. However, the major question still remains to be answered: what is the molecular nature of such mechanisms? The external location of blastomeres, i.e., the exposure to the surface, is likely to be a structural precondition for cellular polarization, which is linked to the generation of epithelial features, such as the formation of TJ and the localization of Na+/K+-ATPase (Fig. 5). But, how do such structural and physiological properties of the external blastomere promote the TE-specific nuclear activity, namely the up-regulation of the Cdx2 gene expression? Or, is the gene expression in external blastomeres controlled independently from the morphological process of epithelialization? A similar question can also be posed for the unique phenotype of Tead4-null embryos, in which external blastomeres are unable to form epithelium and to up-regulate Cdx2 (Yagi et al., 2007; Nishioka et al., 2008). Does Tead4 regulate both epithelialization and the Cdx2 expression independently, or does it control epithelialization, which in turn affects the Cdx2 expression? These questions may be answered through future studies that take advantage of various modern technologies, such as targeted knockout/knockdown of specific gene products, three-dimensional visualization of fluorescently tagged gene products, and time-lapse cinematography of development in normal as well as manipulated embryos.
Fig. 5.
Summary diagrams depicting the roles of the molecules in the formation of TE and ICM that are discussed in this review article.
In most non-mammalian animals, the establishment of the first cell lineages, i.e., the three germ layers, is governed by the factors stored in the egg that are often localized asymmetrically along the animal-vegetal axis. These localized factors are partitioned unequally among blastomeres during the initial rounds of cleavages, generating distinct cell populations in the early embryo. Several localized factors have been identified in chordates, such as ascidians, fish and frogs, that regulate the establishment of germ layers, many of which are mRNA encoding transcription factors or signaling molecules (Zhang et al., 1998; Nishida and Sawada, 2001; Bjornson et al., 2005; Birsoy et al., 2006). In these animals, the formation of the three germ layers is a rapid event, as it is typically completed within a day after fertilization. Such rapidity may be prerequisite to employ localized factors in the egg as key regulators of cell lineage specification, especially when the factors are relatively fragile or have high turnover rate, possibly as in the case for the above molecules. On the contrary, as reviewed in this article, the first cell lineage specification in mammalian development, i.e., the formation of TE and ICM, is a slow event, and it takes about three days after fertilization. Interestingly, the timing of blastocyst implantation is not determined by how fast embryos can reach the blastocyst stage, but rather by when the mother’s uterus becomes ready to accept blastocysts. This situation is exemplified by the phenomenon called diapause or delayed implantation in certain mammals, including the mouse, where fully developed blastocysts can be suspended alive in the uterus without implantation. The diapause may last for up to several weeks until the mother overcomes certain sub-optimal conditions for reproduction, such as unfavorable climate or limited maternal nutrients due to the presence of a suckling litter (Mantalenakis and Ketchel, 1966; Yoshinaga and Adams, 1966; Surani and Fishel, 1981; Lopes et al., 2004). Thus, a rapid mode of initial cell lineage specification may not necessarily be beneficial for mammalian embryos as compared to non-mammalian embryos, in which various tissues need to be generated in a timely manner, especially for feeding. Furthermore, because of considerable slowness of pre-implantation development, the use of localized factors in the egg, like those molecules used in other chordates, may not be effective as the principal regulators of initial cell lineage specification in mammalian embryos. Consistent with this notion, mouse embryos use the location of blastomeres, i.e., external or internal, as the major cue to establish two distinct cell types. Such mechanisms of initial lineage establishment are certainly different from those used in non-mammalian embryos, and might have been invented during the course of evolution towards mammals. In that sense, although fertilization is regarded as the start point of development, what follows immediately afterwards may be fundamentally different between mammals and non-mammals.
Lastly, for those readers who are interested in mouse embryo development beyond the blastocyst stage, particularly regarding the molecular regulations of germ layer formation, we recommend several recent review articles (Tam and Loebel, 2007; Arnold and Robertson, 2009; Rossant and Tam, 2009).
Acknowledgements
We sincerely apologize to all the authors whose extensive and careful work, while relevant to the topic of the article, were not cited or discussed due to space constraints. We thank Dana Ann A. Tamashiro for help with preparing the manuscript, and Kanako Kono and Katherine Gelber for producing the images in Figure 2. Figures used in this article were obtained through the authors’ research, which is supported by grant NIH P20RR024206.
References
- Alarcón VB, Marikawa Y. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol Reprod. 2003;69:1208–1212. doi: 10.1095/biolreprod.103.018283. [DOI] [PubMed] [Google Scholar]
- Alarcón VB, Marikawa Y. Unbiased contribution of the first two blastomeres to mouse blastocyst development. Mol Reprod Dev. 2005;72:354–361. doi: 10.1002/mrd.20353. [DOI] [PubMed] [Google Scholar]
- Alarcón VB, Marikawa Y. Spatial alignment of the mouse blastocyst axis across the first cleavage plane is caused by mechanical constraint rather than developmental bias among blastomeres. Mol Reprod Dev. 2008;75:1143–1153. doi: 10.1002/mrd.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Aziz H, Alexandre H. The origin of the nascent blastocoele in preimplantation mouse embryos: ultrastructural cytochemistry and effect of chloroquine. Roux’s Arch Dev Biol. 1991;200:77–85. doi: 10.1007/BF00637187. [DOI] [PubMed] [Google Scholar]
- Barcroft LC, Moseley AE, Lingrel JB, Watson AJ. Deletion of the Na/K-ATPase alpha1-subunit gene (Atp1a1) does not prevent cavitation of the preimplantation mouse embryo. Mech Dev. 2004;121:417–426. doi: 10.1016/j.mod.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Barr KJ, Garrill A, Jones DH, Orlowski J, Kidder GM. Contributions of Na+/H+ exchanger isoforms to preimplantation development of the mouse. Mol Reprod Dev. 1998;50:146–153. doi: 10.1002/(SICI)1098-2795(199806)50:2<146::AID-MRD4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. Vg 1 is an essential signaling molecule in Xenopus development. Development. 2006;133:15–20. doi: 10.1242/dev.02144. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Parfitt DE, Zernicka-Goetz M. Formation of the embryonic-abembryonic axis of the mouse blastocyst: relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Development. 2008;135:953–962. doi: 10.1242/dev.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CR, Griffin KJ, Farr GH, 3rd, Terashima A, Himeda C, Kikuchi Y, Kimelman D. Eomesodermin is a localized maternal determinant required for endoderm induction in zebrafish. Dev Cell. 2005;9:523–533. doi: 10.1016/j.devcel.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci USA. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Chisholm JC, Johnson MH, Warren PD, Fleming TP, Pickering SJ. Developmental variability within and between mouse expanding blastocysts and their ICMs. J Embryol Exp Morphol. 1985;86:311–336. [PubMed] [Google Scholar]
- Chróścicka A, Komorowski S, Maleszewski M. Both blastomeres of the mouse 2-cell embryo contribute to the embryonic portion of the blastocyst. Mol Reprod Dev. 2004;68:308–312. doi: 10.1002/mrd.20081. [DOI] [PubMed] [Google Scholar]
- Ciruna BG, Rossant J. Expression of the T-box gene Eomesodermin during early mouse development. Mech Dev. 1999;81:199–203. doi: 10.1016/s0925-4773(98)00243-3. [DOI] [PubMed] [Google Scholar]
- De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Iden S, Gerke V, Suzuki A. Regulation of epithelial and endothelial junctions by PAR proteins. Front Biosci. 2008;13:6520–6536. doi: 10.2741/3172. [DOI] [PubMed] [Google Scholar]
- Eckert JJ, Fleming TP. Tight junction biogenesis during early development. Biochim Biophys Acta. 2008;1778:717–728. doi: 10.1016/j.bbamem.2007.09.031. [DOI] [PubMed] [Google Scholar]
- Eckert JJ, McCallum A, Mears A, Rumsby MG, Cameron IT, Fleming TP. PKC signalling regulates tight junction membrane assembly in the pre-implantation mouse embryo. Reproduction. 2004;127:653–667. doi: 10.1530/rep.1.00150. [DOI] [PubMed] [Google Scholar]
- Feng YL, Gordon JW. Removal of cytoplasm from one-celled mouse embryos induces early blastocyst formation. J Exp Zool. 1997;277:345–352. doi: 10.1002/(sici)1097-010x(19970301)277:4<345::aid-jez8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Fleming TP. A quantitative analysis of cell allocation to trophectoderm and inner cell mass in the mouse blastocyst. Dev Biol. 1987;119:520–531. doi: 10.1016/0012-1606(87)90055-8. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Pickering SJ. Maturation and polarization of the endocytotic system in outside blastomeres during mouse preimplantation development. J Embryol Exp Morphol. 1985;89:175–208. [PubMed] [Google Scholar]
- Fujimori T, Kurotaki Y, Komatsu K, Nabeshima Y. Morphological organization of the mouse preimplantation embryo. Reprod Sci. 2009;16:171–177. doi: 10.1177/1933719108331120. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Origin and differentiation of extra-embryonic tissues in the mouse. Int. Rev Exp Pathol. 1983;24:63–133. [PubMed] [Google Scholar]
- Gardner RL. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal-vegetal axis of the zygote in the mouse. Development. 1997;124:289–301. doi: 10.1242/dev.124.2.289. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Specification of embryonic axes begins before cleavage in normal mouse development. Development. 2001;128:839–847. doi: 10.1242/dev.128.6.839. [DOI] [PubMed] [Google Scholar]
- Gardner RL. The axis of polarity of the mouse blastocyst is specified before blastulation and independently of the zona pellucida. Hum Reprod. 2007;22:798–806. doi: 10.1093/humrep/del424. [DOI] [PubMed] [Google Scholar]
- Handyside AH. Time of commitment of inside cells isolated from preimplantation mouse embryos. J Embryol Exp Morphol. 1978;45:37–53. [PubMed] [Google Scholar]
- Handyside AH. Distribution of antibody- and lectin-binding sites on dissociated blastomeres from mouse morulae: evidence for polarization at compaction. J Embryol Exp Morphol. 1980;60:99–116. [PubMed] [Google Scholar]
- Handyside AH. Immunofluorescence techniques for determining the numbers of inner and outer blastomeres in mouse morulae. J Reprod Immunol. 1981;2:339–350. doi: 10.1016/0165-0378(81)90004-8. [DOI] [PubMed] [Google Scholar]
- Honda H, Motosugi N, Nagai T, Tanemura M, Hiiragi T. Computer simulation of emerging asymmetry in the mouse blastocyst. Development. 2008;135:1407–1414. doi: 10.1242/dev.014555. [DOI] [PubMed] [Google Scholar]
- Jain T, Missmer SA, Hornstein MD. Trends in embryo-transfer practice and in outcomes of the use of assisted reproductive technology in the United States. N Engl J Med. 2004;350:1639–1645. doi: 10.1056/NEJMsa032073. [DOI] [PubMed] [Google Scholar]
- Jedrusik A, Parfitt DE, Guo G, Skamagki M, Grabarek JB, Johnson MH, Robson P, Zernicka-Goetz M. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22:2692–2706. doi: 10.1101/gad.486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Molecular differentiation of inside cells and inner cell masses isolated from the preimplantation mouse embryo. J Embryol Exp Morphol. 1979;53:335–344. [PubMed] [Google Scholar]
- Johnson MH, Maro B, Takeichi M. The role of cell adhesion in the synchronization and orientation of polarization in 8-cell mouse blastomeres. J Embryol Exp Morphol. 1986;93:239–255. [PubMed] [Google Scholar]
- Johnson MH, McConnell JM. Lineage allocation and cell polarity during mouse embryogenesis. Semin Cell Dev Biol. 2004;15:583–597. doi: 10.1016/j.semcdb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Ziomek CA. Induction of polarity in mouse 8-cell blastomeres: specificity, geometry, and stability. J Cell Biol. 1981a;91:303–308. doi: 10.1083/jcb.91.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981b;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Ziomek CA. Cell interactions influence the fate of mouse blastomeres undergoing the transition from the 16- to the 32-cell stage. Dev Biol. 1983;95:211–218. doi: 10.1016/0012-1606(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagishi R, Tahara M, Sawada K, Morishige K, Sakata M, Tasaka K, Murata Y. Na+/H+ exchanger-3 is involved in mouse blastocyst formation. J Exp Zoolog A Comp Exp Biol. 2004;301:767–775. doi: 10.1002/jez.a.90. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Yamaguchi T, Yoden T, Hanada M, Miyake M. Effect of protein phosphatase inhibitors on the development of mouse embryos: protein phosphorylation is involved in the E-cadherin distribution in mouse two-cell embryos. Biol Pharm Bull. 2002;25:179–183. doi: 10.1248/bpb.25.179. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki Y, Hatta K, Nakao K, Nabeshima Y, Fujimori T. Blastocyst axis is specified independently of early cell lineage but aligns with the ZP shape. Science. 2007;316:719–723. doi: 10.1126/science.1138591. [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DR, Lee JE, Yoon HS, Roh SI, Kim MK. Compaction in preimplantation mouse embryos is regulated by a cytoplasmic regulatory factor that alters between 1- and 2-cell stages in a concentration-dependent manner. J Exp Zool. 2001;290:61–71. doi: 10.1002/jez.1036. [DOI] [PubMed] [Google Scholar]
- Leniaud L, Poncelet C, Porcher R, Martin-Pont B, Cédrin-Durnerin I, Hugues JN, Wolf JP, Sifer C. Prospective evaluation of elective single-embryo transfer versus double-embryo transfer following in vitro fertilization: a two-year French hospital experience. Gynecol Obstet Fertil. 2008;36:159–165. doi: 10.1016/j.gyobfe.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Levy JB, Johnson MH, Goodall H, Maro B. The timing of compaction: control of a major developmental transition in mouse early embryogenesis. J Embryol Exp Morphol. 1986;95:213–237. [PubMed] [Google Scholar]
- Lopes FL, Desmarais JA, Murphy BD. Embryonic diapause and its regulation. Reproduction. 2004;128:669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- Louvet-Vallée S, Dard N, Santa-Maria A, Aghion J, Maro B. A major posttranslational modification of ezrin takes place during epithelial differentiation in the early mouse embryo. Dev Biol. 2001;231:190–200. doi: 10.1006/dbio.2000.0147. [DOI] [PubMed] [Google Scholar]
- Madan P, Rose K, Watson AJ. Na/K-ATPase beta1 subunit expression is required for blastocyst formation and normal assembly of trophectoderm tight junction-associated proteins. J Biol Chem. 2007;282:12127–12134. doi: 10.1074/jbc.M700696200. [DOI] [PubMed] [Google Scholar]
- Magie CR, Daly M, Martindale MQ. Gastrulation in the cnidarian Nematostella vectensis occurs via invagination not ingression. Dev Biol. 2007;305:483–497. doi: 10.1016/j.ydbio.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Manejwala FM, Cragoe EJ, Jr, Schultz RM. Blastocoel expansion in the preimplantation mouse embryo: role of extracellular sodium and chloride and possible apical routes of their entry. Dev Biol. 1989;133:210–220. doi: 10.1016/0012-1606(89)90312-6. [DOI] [PubMed] [Google Scholar]
- Mantalenakis SJ, Ketchel MM. Frequency and extent of delayed implantation in lactating rats and mice. J Reprod Fertil. 1966;12:391–394. doi: 10.1530/jrf.0.0120391. [DOI] [PubMed] [Google Scholar]
- Maro B, Johnson MH, Pickering SJ, Louvard D. Changes in the distribution of membranous organelles during mouse early development. J Embryol Exp Morphol. 1985;90:287–309. [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Moriwaki K, Tsukita S, Furuse M. Tight junctions containing claudin 4 and 6 are essential for blastocyst formation in preimplantation mouse embryos. Dev Biol. 2007;312:509–522. doi: 10.1016/j.ydbio.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Motosugi N, Bauer T, Polanski Z, Solter D, Hiiragi T. Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev. 2005;19:1081–1092. doi: 10.1101/gad.1304805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Sawada K. macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature. 2001;409:724–729. doi: 10.1038/35055568. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev. 2008;125:270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Ohsugi M, Larue L, Schwarz H, Kemler R. Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev Biol. 1997;185:261–271. doi: 10.1006/dbio.1997.8560. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Pauken CM, Capco DG. Regulation of cell adhesion during embryonic compaction of mammalian embryos: roles for PKC and beta-catenin. Mol Reprod Dev. 1999;54:135–144. doi: 10.1002/(SICI)1098-2795(199910)54:2<135::AID-MRD5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pauken CM, Capco DG. The expression and stage-specific localization of protein kinase C isotypes during mouse preimplantation development. Dev Biol. 2000;223:411–421. doi: 10.1006/dbio.2000.9763. [DOI] [PubMed] [Google Scholar]
- Piotrowska K, Wianny F, Pedersen RA, Zernicka-Goetz M. Blastomeres arising from the first cleavage division have distinguishable fates in normal mouse development. Development. 2001;128:3739–3748. doi: 10.1242/dev.128.19.3739. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005;132:479–490. doi: 10.1242/dev.01602. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche K, Zernicka-Goetz M. Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev. 2005;122:487–500. doi: 10.1016/j.mod.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka-Goetz M. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci. 2005;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A, Rossant J. Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev Biol. 2008;313:614–629. doi: 10.1016/j.ydbio.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Reeve WJ. Cytoplasmic polarity develops at compaction in rat and mouse embryos. J Embryol Exp Morphol. 1981;62:351–367. [PubMed] [Google Scholar]
- Reeve WJ, Ziomek CA. Distribution of microvilli on dissociated blastomeres from mouse embryos: evidence for surface polarization at compaction. J Embryol Exp Morphol. 1981;62:339–350. [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Rossant J, Lis WT. Potential of isolated mouse inner cell masses to form trophectoderm derivatives in vivo. Dev Biol. 1979;70:255–261. doi: 10.1016/0012-1606(79)90022-8. [DOI] [PubMed] [Google Scholar]
- Rossant J, Vijh KM. Ability of outside cells from preimplantation mouse embryos to form inner cell mass derivatives. Dev Biol. 1980;76:475–482. doi: 10.1016/0012-1606(80)90395-4. [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- Schierenberg E. Unusual cleavage and gastrulation in a freshwater nematode: developmental and phylogenetic implications. Dev Genes Evol. 2005;215:103–108. doi: 10.1007/s00427-004-0454-9. [DOI] [PubMed] [Google Scholar]
- Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- Sheth B, Nowak RL, Anderson R, Kwong WY, Papenbrock T, Fleming TP. Tight junction protein ZO-2 expression and relative function of ZO-1 and ZO-2 during mouse blastocyst formation. Exp Cell Res. 2008;314:3356–3368. doi: 10.1016/j.yexcr.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Shirayoshi Y, Okada TS, Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983;35:631–638. doi: 10.1016/0092-8674(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Smith R, McLaren A. Factors affecting the time of formation of the mouse blastocoele. J Embryol Exp Morphol. 1977;41:79–92. [PubMed] [Google Scholar]
- Smith RK, Johnson MH. DNA replication and compaction in the cleaving embryo of the mouse. J Embryol Exp Morphol. 1985;89:133–148. [PubMed] [Google Scholar]
- Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci USA7. 1975;2:5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle AI. Trophoblast regeneration by inner cell masses isolated from cultured mouse embryos. J Exp Zool. 1978;203:483–489. doi: 10.1002/jez.1402030315. [DOI] [PubMed] [Google Scholar]
- Stern JE, Cedars MI, Jain T, Klein NA, Beaird CM, Grainger DA, Gibbons WE. Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275–282. doi: 10.1016/j.fertnstert.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Stillman RJ, Richter KS, Banks NK, Graham JR. Elective single embryo transfer: A 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2008.09.023. (doi:10.1016/j.fertnstert.2008.09.023) [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9:557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- Surani MA, Fishel SB. Embryonic and uterine factors in delayed implantation in rodents. J Reprod Fertil Suppl. 1981;29:159–172. [PubMed] [Google Scholar]
- Sutherland AE, Speed TP, Calarco PG. Inner cell allocation in the mouse morula: the role of oriented division during fourth cleavage. Dev Biol. 1990;137:13–25. doi: 10.1016/0012-1606(90)90003-2. [DOI] [PubMed] [Google Scholar]
- Suwińska A, Czołowska R, Ozdzeński W, Tarkowski AK. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev Biol. 2008;322:133–144. doi: 10.1016/j.ydbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Thomas FC, Sheth B, Eckert JJ, Bazzoni G, Dejana E, Fleming TP. Contribution of JAM-1 to epithelial differentiation and tight-junction biogenesis in the mouse preimplantation embryo. J Cell Sci. 2004;117:5599–5608. doi: 10.1242/jcs.01424. [DOI] [PubMed] [Google Scholar]
- Thowfeequ S, Myatt EJ, Tosh D. Transdifferentiation in developmental biology, disease, and in therapy. Dev Dyn. 2007;236:3208–3217. doi: 10.1002/dvdy.21336. [DOI] [PubMed] [Google Scholar]
- Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007;445:214–218. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Ohno S, Pawson T, Maro B, Louvet-Vallée S. Asymmetric distribution of PAR proteins in the mouse embryo begins at the 8-cell stage during compaction. Dev Biol. 2005;282:307–319. doi: 10.1016/j.ydbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wang H, Ding T, Brown N, Yamamoto Y, Prince LS, Reese J, Paria BC. Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse. Dev Biol. 2008;318:112–125. doi: 10.1016/j.ydbio.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33:492–504. doi: 10.1002/mrd.1080330417. [DOI] [PubMed] [Google Scholar]
- Wiley LM. Cavitation in the mouse preimplantation embryo: Na/K-ATPase and the origin of nascent blastocoele fluid. Dev Biol. 1984;105:330–342. doi: 10.1016/0012-1606(84)90290-2. [DOI] [PubMed] [Google Scholar]
- Winkel GK, Ferguson JE, Takeichi M, Nuccitelli R. Activation of protein kinase C triggers premature compaction in the four-cell stage mouse embryo. Dev Biol. 1990;138:1–15. doi: 10.1016/0012-1606(90)90171-e. [DOI] [PubMed] [Google Scholar]
- Wu SY, Ferkowicz M, McClay DR. Ingression of primary mesenchyme cells of the sea urchin embryo: a precisely timed epithelial mesenchymal transition. Birth Defects Res C Embryo Today. 2007;81:241–252. doi: 10.1002/bdrc.20113. [DOI] [PubMed] [Google Scholar]
- Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669–1678. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3:382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Ziomek CA, Johnson MH. The roles of phenotype and position in guiding the fate of 16-cell mouse blastomeres. Dev Biol. 1982;91:440–447. doi: 10.1016/0012-1606(82)90050-1. [DOI] [PubMed] [Google Scholar]
- Ziomek CA, Johnson MH, Handyside AH. The developmental potential of mouse 16-cell blastomeres. J Exp Zool. 1982;221:345–355. doi: 10.1002/jez.1402210310. [DOI] [PubMed] [Google Scholar]