Abstract
Antioxidants mitigate radiation-induced lethality when started soon after radiation exposure, a delivery time that may not be practical due to difficulties in distribution and because the oral administration of such agents may require a delay beyond the prodromal stage of the radiation syndrome. We report the unexpected finding that antioxidant supplementation starting 24 h after total-body irradiation resulted in better survival than antioxidant supplementation started soon after the irradiation. The antioxidant dietary supplement was l-selenomethionine, sodium ascorbate, N-acetyl cysteine, α-lipoic acid, α-tocopherol succinate, and co-enzyme Q10. Total-body irradiation with 8 Gy in the absence of antioxidant supplementation was lethal by day 16. When antioxidant supplementation was started soon after irradiation, four of 14 mice survived. In contrast, 14 of 18 mice receiving antioxidant supplementation starting 24 h after irradiation were alive and well 30 days later. The numbers of spleen colonies and blood cells were higher in mice receiving antioxidant supplementation starting 24 h after irradiation than in mice receiving radiation alone. A diet supplemented with antioxidants administered starting 24 h after total-body irradiation improved bone marrow cell survival and mitigated lethality, with a radiation protection factor of approximately 1.18.
INTRODUCTION
Dietary supplementation with antioxidants has the potential to increase the probability of survival after an otherwise lethal total-body irradiation (TBI). Recently, Wambi et al. demonstrated that an antioxidant diet started 1 week before the radiation exposure improved the percentage of mice that survived TBI compared with groups of mice that received a control diet (1). Furthermore, at 30 days after TBI, the same percentage of mice survived TBI when mice either switched from the antioxidant diet to a control diet hours before TBI or when mice switched to an antioxidant diet from a control diet 2 h after TBI. The commonality for improved survival under each condition appears to be the presence of antioxidants in the diet at about the time of the radiation exposure plus or minus a few hours. An improved survival as a result of an antioxidant diet has been attributed to a reduction in radiation-induced oxidative stress and apoptosis of the bone marrow cell population, minimizing the bone marrow syndrome (1). An antioxidant diet is a readily available and translatable countermeasure for human use. Unfortunately, antioxidant supplements may have limited potential usefulness in a practical situation unless they are effective approximately 1 day after TBI.
The optimum timing of administration of an anti-oxidant diet as a radiation countermeasure has not been determined. To allow for delivery and distribution of a countermeasure agent, it is likely that a minimum of 1 day is needed. Also, after a significant total-body radiation exposure (1 to 7 Gy), individuals may experience nausea, vomiting and diarrhea, preventing effective administration of the diet. After this prodromal phase, symptoms of radiation exposure subside and victims of radiation exposure could be given an antioxidant diet. The current studies were designed to determine whether an antioxidant diet is an efficacious countermeasure when started 24 h after TBI.
The advantages of an antioxidant diet over other countermeasures requiring an injection, such as growth factors and cytokines, are threefold. First, an antioxidant diet can be made readily available without the government stockpiling and distribution that would be needed for growth factors and cytokines. Second, an antioxidant diet can be given orally, unlike injectables that usually require trained personnel for administration. Third, an antioxidant diet is considered safe even with prolonged use, which may not be the case for growth factors and cytokines (2–4).
In this communication we present the previously unreported observation that the survival of mice improves when the beginning of administration of the antioxidant diet is delayed for 24 h after the radiation exposure compared to antioxidant diet supplementation started a few hours after radiation exposure.
METHODS AND MATERIALS
Animal studies were performed in an AAALAC-accredited facility at Henry Ford Hospital and were reviewed and approved by the IACUC at Henry Ford Hospital. Groups of C57BL/6 mice, 7 to 8 weeks old, were exposed to radiation alone or in combination with antioxidants as described below. Mice were acclimated for 1 week before irradiation, were housed in a temperature-controlled, HEPA-filtered environment, and were offered food and acidified water ad libitum. Food was either AIN-93G rodent chow (Land O'Lakes Purina Feed, Lansing, MI) or the same diet supplemented with antioxidants.
Antioxidant
The antioxidant-supplemented AIN-93G rodent chow was prepared under our direction by Land O'Lakes Purina Feed (Lansing, MI). Antioxidant diet supplementation was started at a fixed time, as indicated, after the radiation exposure. Once started, the diet was continued for the duration of the experiment. The antioxidant supplements per gram of diet were: 0.12 μg l-selenomethionine, 19 μg sodium ascorbate, 51 μg N-acetyl cysteine, 100 μg α-lipoic acid, 8.6 μg α-tocopherol succinate and 51 μg co-enzyme Q10; the antioxidant formula was designed to be identical to the “Diet A” regimen in the study of Guan et al. (5), but some rounding errors occurred in its preparation resulting in minor deviations.
Lethality End Point
Survival was measured in C57BL/6 mice after TBI alone or in combination with the antioxidant diet. The radiation was delivered to unanesthetized mice four at a time using a 5000-Ci (185-TBq) 137Cs source (Mark I, J. L. Shepherd and Associates, San Fernando, CA). Mice were killed humanely 30 days after irradiation because mice that survive 30 days after a TBI have normal blood counts and, if allowed, generally have a normal life span (6). Survival was defined as the time from radiation exposure to the time of euthanasia. Mice that were assessed to be moribund were euthanized based on criteria that relied on changes in weight, behavior and appearance. Mice were weighed at least three times a week with care taken to minimize potential distress (e.g., a slow, even motion was used when transporting mice between the cage and the weighing boat). When two consecutive measurements indicated that mice were losing weight, they were weighed daily. Mice that exhibited a weight loss of 20% or more were scrutinized more closely with respect to their behavior and appearance. Mice were observed for changes in response to external stimuli (e.g., lack of response when the animal was touched gently) and for changes in such nonspecific behaviors as a decrease in the frequency of grooming, eating (assessed from fecal material in cage), and drinking. Changes in appearance indicating a loss of normal body condition included posture changes (e.g. prolonged hunched posture) and sunken eyes and/or skin upon pinching that did not return quickly to the normal position, indicative of advanced dehydration. Mice were observed for weakness and/or inability to obtain food and water (e.g., inability or reluctance to stand), inability to ambulate that prevented the animal's access to food and/or water, and inability of the animal to maintain itself in an upright position. Using these criteria, the institution's staff veterinarian made a determination of moribundity, the requirement for euthanasia.
Reactive Oxygen Species (ROS) in Cells
ROS in vitro or in vivo were measured by the oxidation of dihydroethidium (DHE). WI-38 human embryonic fibroblasts obtained from the American Type Culture Collection were maintained in Eagle's minimum essential medium with 10% fetal bovine serum. Approximately 50% confluent WI-38 cells were γ-irradiated (using the 137Cs source described above) or were sham-irradiated. Immediately after irradiation or sham irradiation, fresh cell culture medium with or without an antioxidant supplement was added and the cells were returned to their incubators until the next day. The antioxidant supplement was 50 μM ascorbic acid, 50 μM α-lipoic acid, 10 μM l-selenomethionine, 10 μg/ml co-enzyme Q10, 50 μM vitamin E succinate, and 0.1% (vol/vol) ethanol (solvent). DHE staining was performed 24 h after irradiation as described below for tissue sections.
ROS in Tissue
The effect of the antioxidant diet on the ROS in skin was assessed in mice that received TBI with or without the diet given starting 24 h later. Two weeks after sham irradiation or irradiation (i.e. after 13 days of the antioxidant diet), mice were injected with DHE (27 mg/kg, i.p.); 4 h later, mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Then skin was excised, frozen at –80°C, and cryosectioned for subsequent fluorescence microscopy. DHE powder was dissolved in dimethyl sulfoxide to create a DHE stock solution (10 mg/ml). The DHE injectate (200 ml final, 27 mg/kg) was produced by adding DHE stock solution to PBS maintained at 40uC. Quick and Dugan (7) noted that temperatures lower than 37°C resulted in precipitation of the DHE.
Spleen Colony-Forming Unit (CFU) Assay
The relative number of bone marrow cells surviving TBI was quantified by the endogenous spleen CFU assay as described previously (6). The number of spleen colony-forming units was measured to assess the in vivo effect of the antioxidant diet on bone marrow cell survival. Groups of C57BL/6 mice were exposed to 7.0 or 7.5 Gy alone or in combination with the antioxidant diet (started 24 h after radiation exposure). Twelve days after TBI, the spleens of the mice were excised and immersed in Bouin's solution for at least 1 day. Then the colonies were counted using a dissecting microscope.
Peripheral Blood Count
At the selected times after TBI, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) for blood collection. Blood (0.5 ml) obtained by cardiac puncture with a 25 gauge needle was placed into heparinized anticoagulant tubes. Complete blood counts were measured using an Advia 120 hematology analyzer (Siemens Diagnostics) by Antech Diagnostics (Detroit, MI).
RESULTS
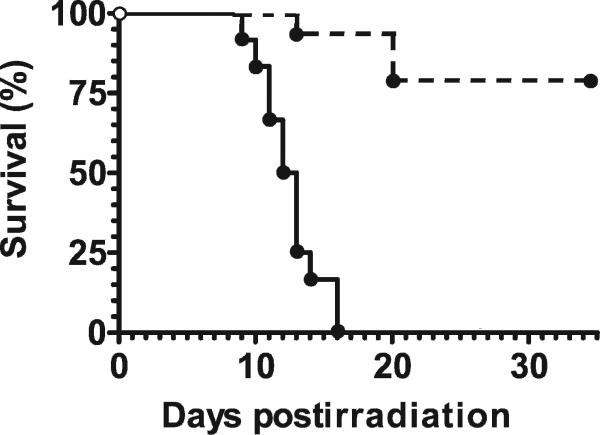
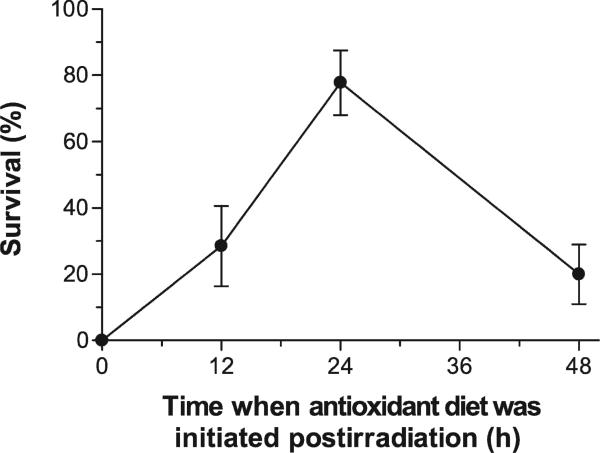
The majority (78% ± 10%) of a group of 18 C57BL/6 mice survived an otherwise lethal dose of radiation when their diet was supplemented with antioxidants. Figure 1 illustrates that the antioxidant diet given starting 24 h after TBI provided significant mitigation from radiation-induced lethality (Kaplan-Meier test, P < 0.005). Similar results were obtained for TBI with a dose of 7.5 Gy (results not shown). Four of eight mice receiving TBI alone at this dose died, while all mice receiving TBI plus the antioxidant diet survived. The benefit of the antioxidants depended strongly on the time of their administration (Fig. 2). All mice died within 30 days of irradiation when they were supplied a diet supplemented with antioxidants started immediately after TBI. The diet given starting 24 h after 8 Gy TBI provided significant mitigation compared with the antioxidant diet started either immediately after TBI, 12 h after TBI, or 48 h after TBI (logrank test, P < 0.005).
FIG. 1.
Antioxidants given starting 24 h after TBI enhanced the survival of C57BL/6 mice. Data are shown for 8.0 Gy with (dashed line) and without antioxidants (solid line).
FIG. 2.
The effectiveness of antioxidants in reducing radiation-induced lethality was greatest when the start of the antioxidant diet followed irradiation by 24 h. Groups of 14–20 mice received 8 Gy TBI and were then started on the antioxidant diet immediately or 12–48 h later.
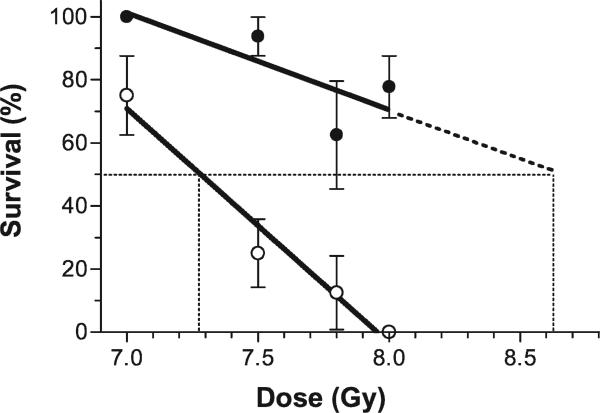
A quantitative assessment of the magnitude of mitigation was made when the antioxidant diet was started 24 h after irradiation. Figure 3 shows that with increasing radiation dose, survival decreased approximately linearly with dose over the range of 7.0 to 8.0 Gy (correlation coefficients, r2, for radiation alone and radiation plus antioxidant diet were 0.97 and 0.63, respectively). The slope of the line for mice that received radiation alone was found to be significantly (P = 0.02) different from zero with a slope of -74 ± 10 (% survival/Gy), whereas the deviation from zero was not significant (P = 0.2) for the line for the antioxidant diet started 24 h after irradiation, which had a slope of –31 ± 17. Over the range of radiation doses studied, the addition of the antioxidant diet made a significant difference in survival. The radiation protection factor was approximately 1.18, calculated as the ratio of the extrapolated estimate of the LD50 from the curve for radiation plus antioxidant diet and the estimated LD50 for radiation alone.
FIG. 3.
The survival of mice at 30 days (n = 8–18/group) decreased with increasing radiation dose; the slope for mice without antioxidants in their diet (open symbols) was steeper than that for mice receiving the antioxidant diet (solid symbols). Lines were fitted and LD50's were calculated as described in the text.
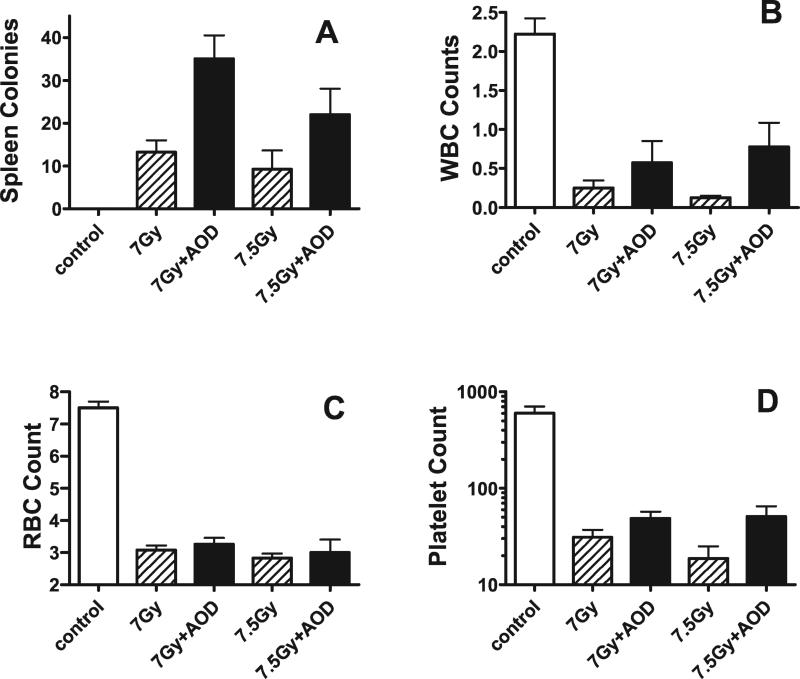
The effect of the antioxidant diet given starting 24 h after TBI on bone marrow stem cells was measured using the endogenous S-CFU assay (Fig. 4). Twelve days after sublethal irradiation plus antioxidant diet (started 24 h after irradiation), the numbers of spleen colonies were two- to threefold higher (P < 0.01, Student's t test) than in the group of mice exposed to either 7.0 Gy or 7.5 Gy radiation alone. Spleen weights were less in mice that received TBI. Spleen weights were significantly greater in mice receiving the antioxidant diet starting 24 h after irradiation, although they were still below the spleen weights of unirradiated control mice (data not shown).
FIG. 4.
Number of endogenous spleen colonies and blood counts in mice that after sublethal TBI. Data are shown for 7.0 and 7.5 Gy with and without antioxidants (n = 4 mice per group; the same mice were used for both blood counts and spleen colony assays). Spleen colonies are actual counts/spleen. WBC counts are 103 per μl of blood. RBC counts are 106 per μl of blood. Platelet counts are 103 per μl of blood.
Peripheral blood counts are shown in Fig. 4 for blood collected 12 days after sublethal TBI alone (7.0 Gy or 7.5 Gy) or TBI followed by antioxidant diet supplementation starting 24 h later. Leukocyte, erythrocyte, platelet and neutrophil counts were all significantly lower in irradiated mice than in unirradiated control mice (n = 4; P < 0.05, Student's t test). In mice that received the antioxidant diet for 11 days starting 1 day after irradiation, there was a trend toward higher numbers of cells compared with mice that did not receive the antioxidant diet (n = 4 per group); the elevated blood counts reached significance for leukocytes in mice receiving 7.5 Gy (P = 0.05, Student's t test), erythrocytes in mice that received 7.0 Gy (P = 0.05, Student's t test), and platelets in mice that received either 7.0 Gy (P < 0.005 Student's t test) or 7.5 Gy (P < 0.05 Student's t test). Peripheral neutrophil counts were not changed. Despite the lack of benefit on neutrophil numbers, the overall results indicate that the antioxidant diet mitigated bone marrow radiation injury, increased the number of blood cells, and increased an animal's chance of survival after a potentially lethal radiation exposure.
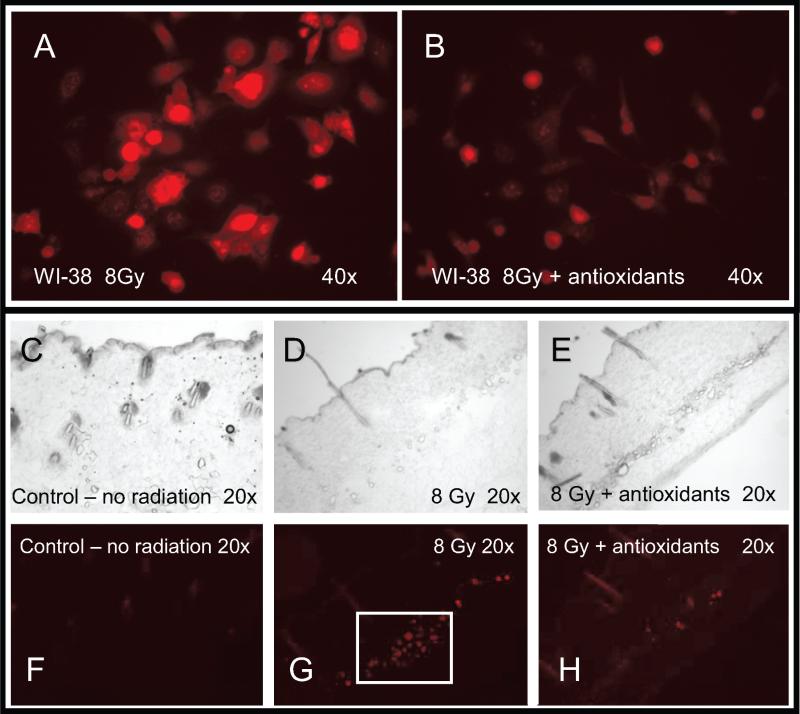
In an attempt to confirm that the antioxidants produced their beneficial effect by reducing reactive oxygen species, as proposed by others (8), we measured ROS in cells and mouse skin after irradiation with or without antioxidant supplementation. WI-38 human embryonic lung fibroblasts were exposed to 8 Gy and culture medium was immediately replaced with medium containing or not including an antioxidant supplement. Cells were assessed 24 h later for ROS by DHE fluorescence staining. Figure 5A and B shows that cells treated with antioxidants contained less ROS. The results were confirmed by studies of skin samples from mice previously receiving radiation alone or radiation followed 24 h later by the start of a diet supplemented with antioxidants for about 2 weeks. As with cultured cells, irradiated tissue demonstrated an increase in ROS. The increase in ROS was mitigated by antioxidant diet supplementation starting after irradiation (Fig. 5C–H). Consequently, both in vitro and in vivo, ROS were increased days (in cell culture) to weeks (in animals) after irradiation, and the effect was mitigated by antioxidant supplementation.
FIG. 5.
Radiation-induced reactive oxygen species were reduced by antioxidants in vitro and in vivo. Panel A: WI-38 cells were exposed to 8 Gy and ROS was assayed 24 h later by fluorescence from oxidized dihydroethidium (DHE). Panel B: DHE fluorescence in WI-38 cells treated with antioxidants for 24 h starting immediately after the radiation exposure. Panels C–H: Skin from mice 2 weeks after 8 Gy TBI. Panels C, D and E are light microscope images of skin sections after sham irradiation, 8 Gy TBI alone, and TBI plus antioxidants, respectively. Panels F, G and H are fluorescence images of oxidized DHE in tissue slices adjacent to panels C, D and E, respectively. Original magnifications are shown in individual panels.
DISCUSSION AND CONCLUSIONS
Ionizing radiation initiates cellular damage directly by ionization and indirectly by producing free radicals. Approximately two-thirds of radiation-induced damage is caused by the free radicals that are generated during exposure. In addition to short-lived free radicals produced during exposure, free radicals are generated after the radiation exposure; ROS and pro-inflammatory cytokines induce a multitude of biological injuries long after the radiation exposure has ended. One of the approaches to counter oxidative stress caused by free radicals and ROS is to use antioxidants such as α-tocopherol succinate, ascorbic acid, β-carotene, vitamin A, α-lipoic acid, N-acetylcysteine, selenium or an SH compound (e.g. amifostine) (9, 10).
The rationale for using a combination of antioxidants is based on a number of observations. Individual antioxidants can act as pro-oxidants when they themselves are oxidized; therefore, individual antioxidants could enhance the progression of postirradiation damage to tissues and organs. In addition, humans have a pool of antioxidants, both endogenous antioxidants that are constitutively synthesized by cells and antioxidants that are consumed in the diet. Individual antioxidants function by different mechanisms and have different affinities for various free radicals. For example, α-tocopherol is more effective as a quencher of free radicals in a reduced oxygen environment, vitamin E has little effect on oxidants derived from nitric oxide, and vitamin A is most effective under higher atmospheric pressures. Ascorbic acid is needed to protect cellular components in aqueous environments, whereas carotenoids, vitamins A and E protect cellular components in non-aqueous environments. Vitamin C recycles oxidized vitamin E to an active form (11). Vitamins E and C combined inhibit apoptosis in human endothelial cells more effectively than each alone, increasing Bcl-2 and down-regulating the pro-apoptotic Bax (12).
Other observations affected our choice of antioxidant mixture. The form and type of vitamin E are important in determining its functional abilities. For example, various organs of rats selectively absorb the natural form of vitamin E and α-tocopherol succinate, the most effective form of vitamin E, for inhibiting cancer growth and a potent radioprotector when given prior to TBI (13). Selenium is a co-factor of glutathione peroxidase, and Se-glutathione peroxidase acts as an antioxidant. Glutathione cannot be used orally to increase intracellular levels of glutathione, because it is completely hydrolyzed in the gut. In contrast, an oral administration of N-acetylcysteine (NAC) and α-lipoic acid, another endogenous antioxidant, can increase the intracellular levels of glutathione by different mechanisms and can be used in place of oral glutathione to reduce the radiation injury. Co-enzyme Q10, a weak endogenous antioxidant, scavenges peroxy radicals at a faster rate than α-tocopherol and, like vitamin C, can regenerate vitamin E in a redox cycle. The foregoing discussion suggests that a combination of antioxidants may be more effective in reducing the radiation-induced injury than any individual antioxidant alone. Guan et al. showed that diet supplement with a combination of antioxidants completely prevented the reduction in the plasma levels of total antioxidant status in mice and rats exposed to proton or HZE-particle radiation (5). Recent studies with 225 kVp X rays demonstrated marked protection from radiation injury, but generally it is believed that antioxidants need to be present during the irradiation or up to 2 h after irradiation to have a significant protecting effect (1).
The data presented here show that an antioxidant-supplemented diet started 24 h after an otherwise lethal radiation exposure effectively mitigated death (Fig. 1) mediated by a sparing of bone marrow cells (Fig. 4), perhaps due to a reduction in reactive oxygen species (Fig. 5). The effect of 8 Gy on the gastrointestinal system warrants discussion. Recent evidence suggests that the mechanisms governing the bone marrow syndrome and the gastrointestinal syndrome after TBI evolve concomitantly (14). Consequently, the possible implications of radiation damage for the uptake of antioxidants need be considered. One might expect an even greater mitigating effect if the biodistribution of antioxidants were compromised by gastrointestinal injury.
The connection between ROS and hematopoiesis is being elucidated on a molecular level. Growth factors that stimulate hematopoiesis such as IL3 and GM-CSF have been shown to cause an increase in intracellular ROS levels (15, 16). The generation of ROS in response to hematopoietic growth factors contributes to downstream signaling events involving tyrosine phosphorylation such as cell proliferation (15) and apoptosis (17). Iiyama et al. (17) implicated ROS in hematopoietic cytokine-induced cell cycle progression from G1 to S phase through inducing expression of c-Myc, cyclin D2 and cyclin E and reducing expression of p27. Iiyama et al. (16) also showed that ROS play a role in cytokine activation of Jak2 with downstream signaling of proapoptosis pathways including MEK/ERK. Treatment with antioxidants inhibits the increase in ROS, reduces tyrosine phosphorylation, reduces proliferation induced by GM-CSF (15, 16), and reduces apoptosis (1).
Our data are the first to show that a delay in antioxidant administration after cellular stress can be beneficial to cell and animal survival. The kinetics of ROS generation by hematopoietic cytokines as well as the mechanisms by which ROS are involved in cytokine receptor signaling to regulate proliferation and apoptosis of hematopoietic cells was studied by Iiyama et al. (16). They demonstrated that hematopoietic cytokines IL3 and Epo induce a rapid and transient increase in ROS that peaked at 30 min followed by a slow progressive increase in ROS 24 h after the cytokine administration. It would appear that ROS pathways controlling proliferation and apoptosis of hematopoietic cells involve two separate increases in ROS, a transient increase at 30 min and a prolonged increase that continues for at least 24 h.
Mitigation of radiation lethality by antioxidants administered soon after radiation exposure has been attributed to a reduction in apoptosis (1). Our experience with C57BL/6 mice is not inconsistent with these results, as shown in Fig. 2, which also illustrates the added benefit of waiting to start administering a diet supplemented with antioxidants until 24 h after irradiation. It would appear that the first transient wave of ROS has some beneficial effect on survival since minimizing ROS early has a detrimental effect on bone marrow cell survival.
In addition to inhibiting apoptosis, reducing ROS by antioxidants soon after the radiation exposure inhibits the progression of cells from G1 to S (18), the phase of the cell cycle in which repair of DNA damage is most efficient (19). Repair of DNA damage has a half-time of 1 to 2 h (20, 21). Consequently arresting cells before S phase too soon after a radiation exposure may decrease the ability of the cells to completely repair the damaged DNA. One explanation for the increased animal survival when the antioxidant diet is given starting 24 h after irradiation is that delaying the start of the antioxidant diet allows for the most efficient repair of radiation injury and the largest increase in the survival of bone marrow cells. Further studies are needed to confirm or refute this hypothesis.
In conclusion, our results extend the work of others to show that a diet supplemented with antioxidants is effective at mitigating radiation lethality when it is started 24 h after the radiation exposure and is more effective than if given soon after the exposure. Our results support the value of antioxidants as countermeasures against radiological terrorism, especially in the practical scenario of starting a diet supplemented with antioxidants 24 h after the exposure.
ACKNOWLEDGMENT
These studies were supported by U19AI067734-020005 (Director: Jae Ho Kim), a grant from NIH/NIAID that is part of the Center for Medical Countermeasures against Radiation injury (CMCR) at the Medical College of Wisconsin (PI: John E. Moulder).
REFERENCES
- 1.Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko Y-H, Sayers CM, Baran M, Ware JH, Kennedy AR. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat. Res. 2008;169:384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farese AM, Herodin F, McKearn JP, Baum C, Burton E, MacVittie TJ. Acceleration of hematopoietic reconstitution with a synthetic cytokine (SC-55494) after radiation-induced bone marrow aplasia. Blood. 1996;87:581–591. [PubMed] [Google Scholar]
- 3.Herodin F, Bourin P, Mayol JF, Lataillade JJ, Drouet M. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma-irradiation promotes survival. Blood. 2003;101:2609–2616. doi: 10.1182/blood-2002-06-1634. [DOI] [PubMed] [Google Scholar]
- 4.Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp. Hematol. 2005;33:1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Guan J, Stewart J, Ware JH, Zhou Z, Donahue JJ, Kennedy AR. Effects of dietary supplements on space radiation-induced induction in total antioxidant status in CBA mice. Radiat. Res. 2006;165:373–378. doi: 10.1667/rr3523.1. [DOI] [PubMed] [Google Scholar]
- 6.Brown SL, Kolozsvary A, Liu J, Ryu S, Kim JH. Histone deacetylase inhibitors protect against and mitigate the lethality of total-body irradiation in mice. Radiat. Res. 2008;169:474–478. doi: 10.1667/RR1245.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quick KL, Dugan LL. Superoxide stress identifies neurons at risk in a model of ataxia-telangiectasia. Ann. Neurol. 2001;49:627–635. [PubMed] [Google Scholar]
- 8.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br. J. Radiol. 2007;80:S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 9.Prasad KN, Cole W, Hovland P. Cancer prevention studies: past, present and future direction. Nutrition. 1998;14:197–210. doi: 10.1016/s0899-9007(97)00443-7. [DOI] [PubMed] [Google Scholar]
- 10.Kumar KS, Srinivasan V, Toles R, Jobe L, Seed TM. Nutritional approaches to radioprotection: Vitamin E. Mil. Med. 2002;167:57–59. [PubMed] [Google Scholar]
- 11.Guo Q, Parker L. Ascorbate-dependent recycling of the vitamin E homologue Trolox by dihyrolipoate and glutathione in murine skin homogenates. Free Radic. Biol. Med. 2000;29:368–374. doi: 10.1016/s0891-5849(00)00309-9. [DOI] [PubMed] [Google Scholar]
- 12.Haendeler J, Zeiher AM, Dimmler S. Vitamin C and vitamin E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulating Bcl-2 and Bax. Eur. J. Pharmacol. 1996;17:407–411. doi: 10.1016/s0014-2999(96)00759-5. [DOI] [PubMed] [Google Scholar]
- 13.Prasad KN, Kumar B, Yan XD, Hanson AJ, Cole WC. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J. Am. Coll. Nutr. 2003;22:108–117. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 14.Rotolo JA, Kolesnick R, Fuks Z. Timing of lethality from gastointestinal syndrome in mice revisited. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:6–8. doi: 10.1016/j.ijrobp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, Griffin JD. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93:2928–2935. [PubMed] [Google Scholar]
- 16.Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal. 2006;18:174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Yousefi S, Green DR, Blaser K, Simon HU. Protein-tyrosine phosphorylation regulates apoptosis in human eosinophils and neutrophils. Proc. Natl. Acad. Sci. USA. 1994;91:10868–10872. doi: 10.1073/pnas.91.23.10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havens CG, Ho A, Yoshioka N, Dowdy SF. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol. Cell Biol. 2006;26:4701–4711. doi: 10.1128/MCB.00303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinclair WK. Cyclic x-ray responses in mammalian cells in vitro. Radiat. Res. 1968;33:620–643. [PubMed] [Google Scholar]
- 20.Thames HD, Jr., Withers HR, Peters LJ. Tissue repair capacity and repair kinetics deduced from multifractionated or continuous irradiation regimens with incomplete repair. Br. J. Cancer Suppl. 1984;6:263–269. [PMC free article] [PubMed] [Google Scholar]
- 21.Millar WT, Hendry JH. Haemopoietic injury after irradiation: analysis of dose responses and repair using a target-cell model. Int. J. Radiat. Biol. 1997;72:561–573. doi: 10.1080/095530097143068. [DOI] [PubMed] [Google Scholar]