Abstract
Since the beginning of the highly active antiretroviral therapy (HAART) era, epidemiological evidence indicates an increasing incidence of Alzheimer's (AD)-like brain pathology in aging HIV patients. Emerging evidence warns of potential convergent mechanisms underlying HIV- and Aβ-mediated neurodegeneration. We found that HIV-1 Tat and gp 120 promote the secretion of Aβ 1–42 in primary rat fetal hippocampal cell cultures. Our results demonstrate that the variant of Tat expressed by the neurotropic subtype of HIV-1 virus (HIV-1 clade B) specifically induces both the release of amyloidogenic Aβ 1–42 and the accumulation of cell-bound amyloid aggregates. The results of the research rationalize testing of the ability of β-amyloid aggregation inhibitors to attenuate HIV protein-mediated cognitive deficits in animal models of NeuroAIDS. The long-term goal of the study is to evaluate the potential benefits of anti-amyloidogenic therapies for management of cognitive dysfunction in aging HIV-1 patients.
Keywords: Cell Culture, HIV Tat, HIV gp120, Amyloid Peptide, Neurotoxicity
Introduction
The development of persistent cognitive deficits in aging HIV patients with suppressed viral replication is now recognized as a serious and growing medical problem [25]. HAART medication has shifted neuropathology from a subacute encephalitic condition to a more subtle neurodegenerative process involving synaptic and dendritic degeneration, particularly of hippocampal neurons [30]. Epidemiologic evidence indicates a growing incidence of AD-like brain pathology in aging HIV patients. Clinical studies reveal similar changes in Aβ 1–42 levels in CSF from cognitively impaired HIV-1 patients and in patients with mild dementia of the AD-type [9]. These alarming facts suggest that HIV-associated pathology is evolving [7]. Neurotoxic regulatory and core viral proteins, such as Tat and gp120, are thought to contribute to the development of HIV- associated cognitive dysfunction. Although the role of viral proteins in HIV-associated memory impairment has been documented, the mechanism of this dysfunction is poorly defined. Recent reports warn that Aβ biogenesis and clearance venues may be influenced by HIV-1 proteins [19,30].
Adverse effects of HIV-1 Tat and gp120 on neuronal function [6,11,29] resemble alterations elicited by soluble Aβ oligomers [28]. Existing evidence of strikingly similar characteristics of beta-amyloid- and HIV protein-mediated neurodegeneration rationalizes the idea that viral proteins, which are known to induce deficits in learning and memory, may facilitate accumulation of misfolded Aβ in hippocampal cells. Nevertheless, the potential ability of Tat or gp120 to induce formation of amyloidal aggregates has not been systematically investigated.
In this study, we investigate the connection between Tat or gp120 -mediated cell injury and amyloid formation in primary cultures of rat fetal hippocampal neurons.
Materials and Methods
Purified proteins. Recombinant original Tat 1–86 and (Cys22→Gly22) –substituted Tat 1–86 (LAI/Bru strain of HIV-1 clade B, GenBank accession no. K02013) were purchased from Diatheva (Italy). Tat 1-101 clade C was purchased from Prospec (Israel). The recombinant gp120 was purchased from Protein Sciences, (Meriden, CT). Synthetic Aβ 1–42 was purchased from Anaspec (Fremont, CA).
Primary hippocampal cell cultures were prepared from 18-day-old Sprague-Dawley rat fetuses as previously described [2]. Cultures were used for experiments after 14 days in culture and were >85–90% neuronal as determined by anti-MAP-2/anti-GFAP/Hoechst fluorescent staining.
The treatment of hippocampal cell cultures was carried out by the addition of freshly-prepared stock solutions of the recombinant gp120, Tat polypeptides, or pre-aggregated stock solutions of Aβ 1–42 into the cell culture growth medium. An equal volume of the vehicle was added to control cell cultures. The preparation of pre-aggregated Aβ 1–42 was carried out as previously described [1].
Hippocampal cell viability was assessed using the microplate reader-formatted [2,3,4] variant of the Live/Dead assay (Molecular Probes, Inc., Eugene, OR). Fluorescence was measured using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT).
Direct ELISA measurements of the Aβ 1–42 immunoreactivity in cell-conditioned medium (CM) samples were performed using the rabbit polyclonal anti-Aβ 1–42 antibody (Abcam Inc, Cambridge, MA,1:500). Specificity of anti-Aβ antibodies was tested using samples of fresh cell culture medium with and without recombinant HIV-1 proteins (negative controls). Serial dilutions of freshly prepared stocks of the synthetic rat Aβ 1–42 (0–2500 nM) with cell culture medium were used for calibration.
The formation of cell-bound amyloid β-sheet aggregates in living hippocampal cells was analyzed by fluorescent/DIC microscopy following the Congo Red staining (20 µM Congo Red for 30 min). The Congo Red specific binding (543 nM excitation/560–615 nm emission) was visualized using a 20× objective of the inverted fluorescent microscope (Nikon Eclipse TE2000-E). Congo Red-staining and DIC images were captured using a CCD camera. Merged images of the specific Congo Red binding and DIC were produced and analyzed by the NIS Elements imaging software (Nikon). Parts of merged Congo Red/DIC images were magnified by placing a selection box over the area of interest and saving the selection as a new image with higher resolution.
Numbers of Congo Red-positive cells were counted using the Object counting option of the NIS Elements imaging software package in 20× images of 4 random fields of vision. For each field of vision total numbers of hippocampal cells were determined using Hoechst fluorescent staining of cell nuclei.
Statistical comparisons were made using ANOVA and planned comparisons were used to determine specific treatment effects. Significant differences were set at P< 0.05.
Results
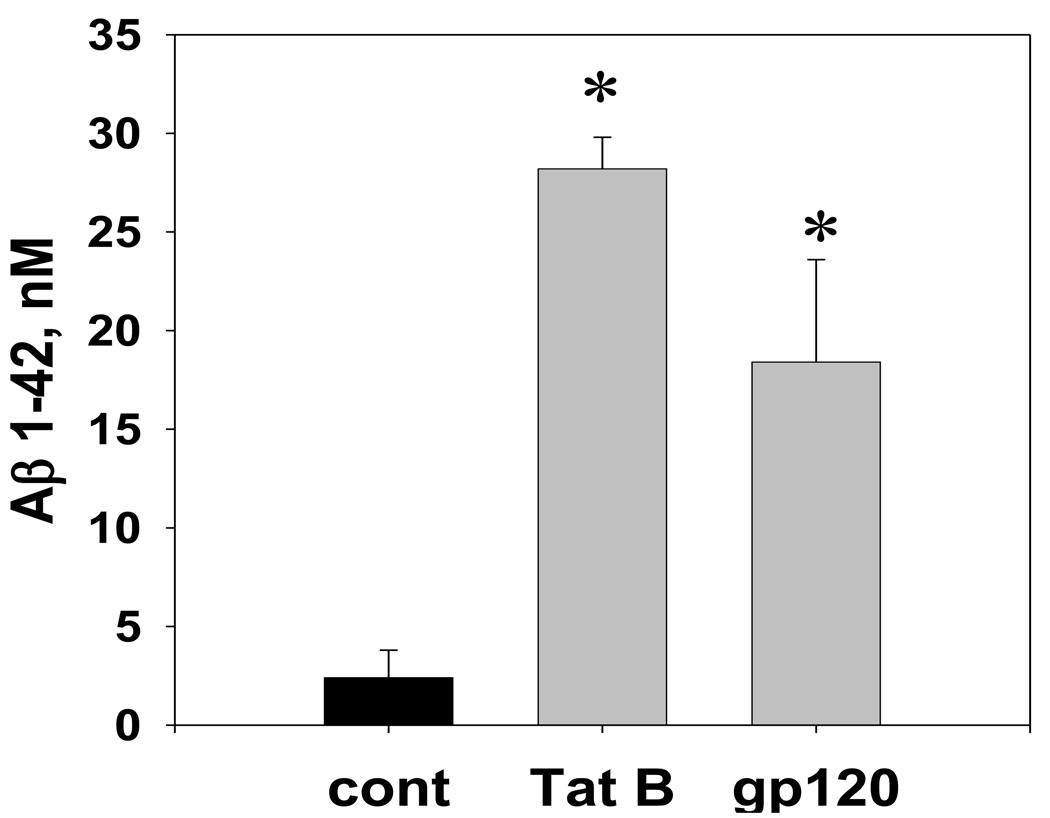
Results of the anti-Aβ 1–42 ELISA analysis of conditioned medium (CM) samples from hippocampal cultures exposed for 3 days to a toxic dose (100 nM) of Tat or gp120 revealed a significant increase in the Aβ 1–42 production (Figure 1). At the age of 14 days in vitro (DIV), approximately 20–25% of neurons in hippocampal cultures express amyloid protein precursor (APP) isoforms and 1.5–3.0 nM of extracellular Aβ 1–42 immunoreactivity can be detected in the cell culture medium. According to the anti-Aβ 1–42 ELISA measurements, CM samples from cultures treated with Tat; gp120; or vehicle contained subsequently 28.2 ± 1.6 nM; 18.4 ± 5.2 nM; or 2.4 ± 1.4 nM of the immunoreactive Aβ 1–42.
Figure 1. The Aβ 1–42 immunoreactivity in the conditioned medium from hippocampal cell cultures exposed to HIV-1 Tat or gp120.
The graph represents results of direct anti-Aβ 1–42 ELISA measurements in the CM samples collected from hippocampal cultures (n=8 per group) treated with either 100 nM Tat 1–86 B, 100 nM gp120, or the equal volume of vehicle. The freshly prepared Aβ 1–42 stock was serially diluted with cell culture growth medium to obtain ELISA calibration curves. Data presented as mean values ± SEM. *- marks significant (P<0.05) differences in extracellular Aβ 1–42 immunoreactivity.
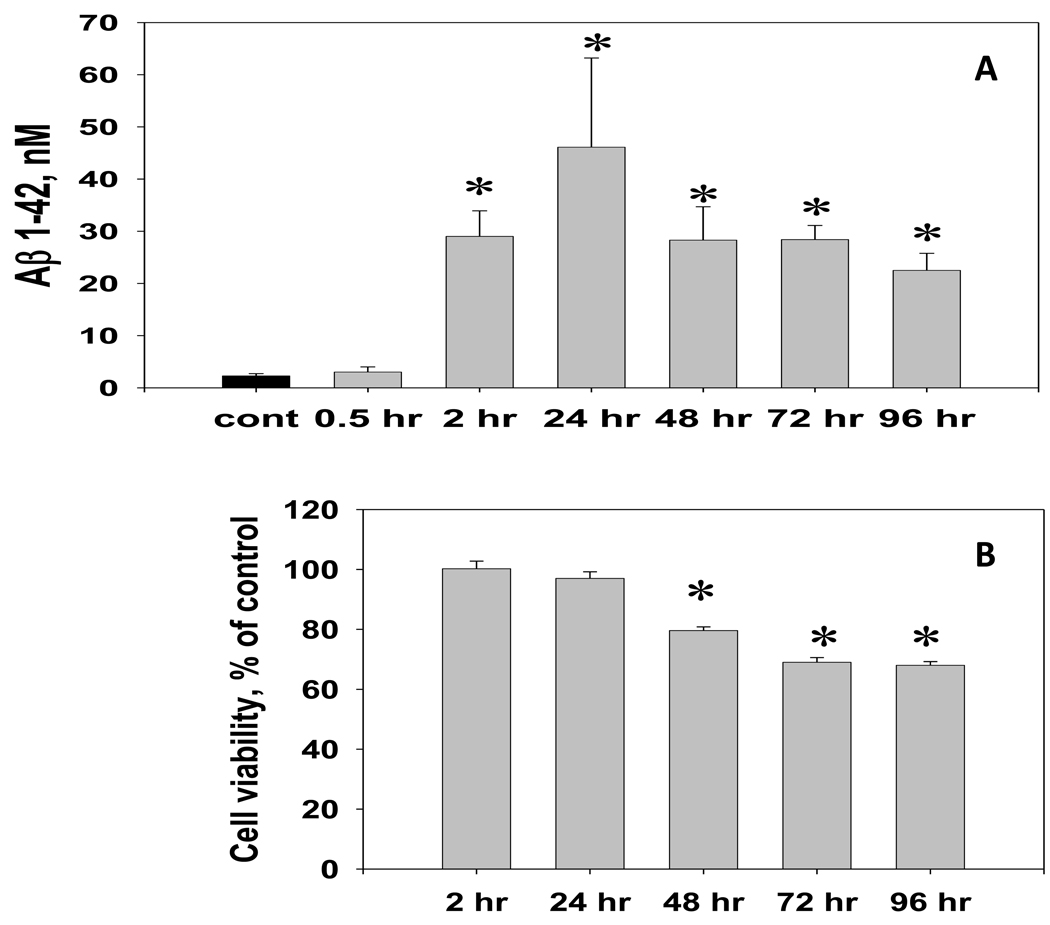
The analysis of Aβ 1–42 immunoreactivity in hippocampal cell cultures treated with 50 nM dose of Tat 1–86 B for different periods of time (typical protocol for the Tat cytotoxicity analysis described in [3]) revealed that the increase of soluble Aβ 1–42 in CM from hippocampal cell cultures becomes evident early after the start of the exposure and precedes the cell viability decline (Figure 2A,B). The maximum level of specific Aβ1–42 immunoreactivity in the CM occurred after 24-hour Tat exposure and was 46.1 ± 17.1 nM.
Figure 2. The temporal relationship between the Aβ 1–42 release and cell viability changes in hippocampal cell cultures exposed to a toxic dose of Tat 1–86 B.
A. The Aβ 1–42 immunoreactivity was measured in CM samples collected from hippocampal cultures during the continuous exposure (0.5 – 96 hours) to 50 nM Tat 1–86 or vehicle (n=5 per group). Data are presented as mean values ± SEM. *- marks significant (P<0.05) differences in extracellular Aβ 1–42 immunoreactivity between Tat-treated and vehicle-treated cell cultures.
B. Cell viability in hippocampal cell cultures (treated side-by-side, n=8 per group) was determined using Live/Dead assay. Data are presented as mean % of control values ± SEM. *- marks incubation time points for significant (P<0.05) differences in Live/Dead ratios between cultures treated with 50 nM Tat 1–86 and vehicle-treated controls have been observed.
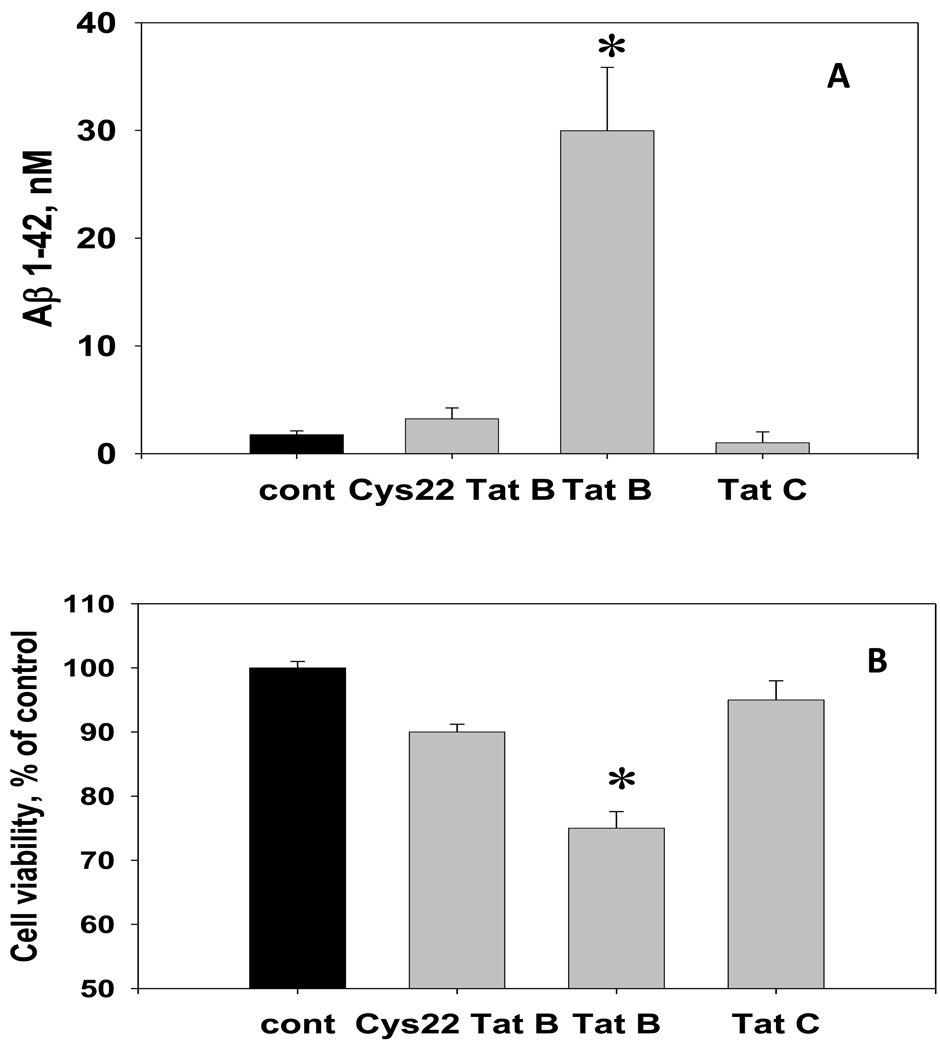
Mutations of single cysteine residues at the position 22 or 31 within the conservative cysteine-rich domain of Tat dramatically decrease the ability of Tat to cause apoptosis [3,17]. Unlike with 100 nM dose of Tat 1–86 B, the 48-hour exposure to the same dose of either HIV-1 B Tat 1–86 with cys22→gly22 substitution (Cys22 Tat B) or the HIV-1 C Tat 1–101 in which the cysteine 31 is mutated, did not induce cytotoxicity and did not increase Aβ 1–42 production in hippocampal cell cultures (Figure 3A,B).
Figure 3. The effect of non-neurotoxic variants of HIV-1 Tat (Cys22 Tat 1–86 B and Tat 1–101 C) on the Aβ 1–42 production in hippocampal cell cultures.
The graph represents results of direct anti-Aβ 1–42 ELISA measurements in the CM samples collected from individual cultures (n=8 per group) treated with 100 nM Tat 1–86 B, Cys22 Tat 1–86 B, Tat 1–101 C, or the equal volume of vehicle for 72 hours. Data are presented as mean values ± SEM. Cell viability in hippocampal cell cultures was determined using Live/Dead assay. *- marks significant (P<0.05) differences in hippocampal cell viability or extracellular Aβ 1–42 immunoreactivity.
Staining with Congo Red is a classical method of the detection of amyloids. Compared to other amyloid-binding dyes, Congo Red exhibits very low non-specific binding and cannot be internalized by the cells probably because of the presence of two hydrophilic sulfonic groups. We used the Congo Red staining to determine the potential ability of Tat or gp120 to promote accumulation of amyloidal aggregates in cultured hippocampal cells.
In young mature (14 DIV) hippocampal cell cultures no more than 1% of cells were Congo Red-positive. Consistent with previous studies carried out using this procedure [27], we observed that the exposure to pre-aggregated Aβ 1–42 increased the Congo Red staining of cultured hippocampal cells. Approximately 50% of all cells were positively stained with Congo Red following 24-hour incubation with 25 µM of pre-aggregated Aβ 1–42. The exposure to 50 nM Tat 1–86 B also caused the increase in Congo Red staining suggesting the accumulation of amyloidal aggregates in hippocampal cells (Figure 4). Increased numbers of Congo Red-positive cells (17.2 ± 3.5%, P<0.05) were detected in cultures exposed to Tat 1–86 B for 4 hours. After 24 hours of treatment, 26 ± 3% of the hippocampal cells were positively stained with Congo Red. In cultures treated with 50 nM Cys22 Tat 1–86 B or Tat 1–101 C, which do not induce Aβ production, the results of Congo Red staining were the same as in vehicle-treated controls (data not shown).
Figure 4. The detection of cell-bound β-amyloid aggregates by the Congo Red staining in living hippocampal cells exposed to Aβ 1–42 or HIV-1 Tat 1–86 B.
Representative images show the Congo Red binding to hippocampal cell cultures exposed to a toxic dose of Aβ 1–42 (40-hour exposure), Tat 1–86 B (24-hour exposure), or vehicle. Results of the Congo Red staining in cultures treated with 100 nM gp120 for 72 hours, 100 nM Cys22 Tat or Tat 1–101 C for 24 hours were not different from the vehicle-treated controls and are not shown in the Figure. Colored boxes mark areas of Congo Red/DIC images, which are shown as new magnified images with greater resolution.
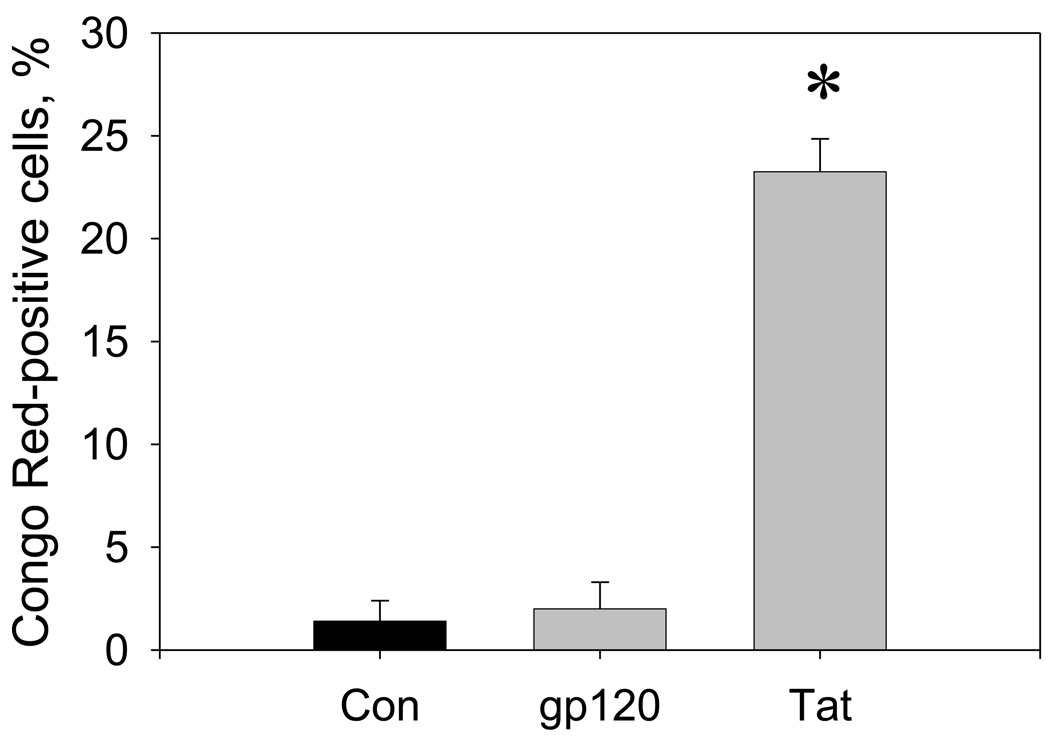
In hippocampal cultures treated for 3 days with 100 nM Tat 1–86 B, 23.3 ± 1.6% of the remaining cells were positively stained with Congo Red. Relative numbers of Congo Red-positive cells in cultures exposed to 100 nM gp120 (2.0 ± 1.3%) were not significantly different from vehicle-treated controls (1.4 ± 1.0%) (Figure 5).
Figure 5. Changes in numbers of Congo Red-positive cells in hippocampal cell cultures exposed to HIV-1 Tat or gp120.
The graph represents relative numbers (% of total cells) of Congo Red-positive cells in hippocampal cell cultures after 3 days of treatment with vehicle (control), gp120 (100 nM), or Tat 1–86 B (100 nM). For each individual cell culture in a group (n=4), Congo Red-positive hippocampal cells were counted in 4 random fields of vision. For each field of vision (0.5×0.37 mm) results were normalized to the subsequent total cell number determined using Hoechst staining of cell nuclei (typically 70–95 cells per field). Data are presented as mean values ± SEM. *- marks significant (P<0.05) differences in relative numbers of Congo Red-positive cells.
Discussion
Several recent studies [10,21] have reported that HIV-1 Tat can increase the level of Aβ 1–40 in neuronal cell cultures. Consistently, we present the evidence of the increased secretion of highly amylodogenic variant of Aβ peptide, Aβ 1–42, in hippocampal cell cultures exposed to Tat 1–86 clade B. Our results also show that the biogenesis of Aβ in hippocampal cell cultures may be influenced not only by Tat, but by gp120 as well. Hence, changes in the APP/Aβ metabolism may be a shared molecular event of the neuronal response to injury incited by two different HIV proteins that are thought to contribute to the development of cognitive deficits associated with chronic HIV-1 infection in vivo.
The increased Aβ production can be associated with various types of neurotoxic insults [14,15]. Notably, the stimulation of NMDA receptors, which is a common component of HIV-1 Tat and gp120-mediated toxicity mechanisms [16], is known to increase Aβ release in hippocampal neurons [15]. Therefore, it is not surprising that Tat and gp120 can share the ability to affect Aβ production.
Supportive to the idea that the release of soluble Aβ peptides is linked to the HIV protein-mediated neurotoxicity mechanisms are our experiments demonstrating that the Tat variants with attenuated pro-apoptotic potential were unable to increase the Aβ 1–42 secretion. Interestingly, patients infected with the subtype C of HIV-1 virus have been shown by other researchers to have decreased incidence of cognitive deficits [20].
The Aβ derivatives of APP are known to have dualistic effects on neuronal function and viability [12,18]. Non-aggregated Aβ monomers have a role in the support of neuronal repair, but their conversion into oligomers with anti-parallel β-sheet structure leads to neurotoxicity [18,24]. The irreversible, spontaneous insertion of amyloidal protein aggregates into neuronal cell membranes impairs synaptic transmission and eventually can cause death of affected neurons. Strong evidence suggests that the first essential elements in the vast majority of neurodegenerative processes are misfolded and aggregated proteins [26]. Most amyloid peptides interact strongly with cell membranes and this interaction is enhanced by conditions which favor β-sheet formation. For the first time our study presents the direct evidence that Tat 1–86 B not only increases Aβ release, but also causes the accumulation of amyloidal protein aggregates in hippocampal cells.
Unlike with Tat, gp120-induced Aβ 1–42 production was not associated with the increase in numbers of Congo Red-positive cells in hippocampal cultures. This result indicates that the ability to simultaneously increase Aβ secrection and facilitate the accumulation of amyloidal aggregates in neuronal membranes may be the unique property of Tat. Inibitory effects of HIV-1 Tat on proteolytic enzymes involved in degradation of damaged and misfolded proteins [5,10, 19,23] as well as Tat interaction with the low density lipoprotein receptor-related protein (LRP) involved in re-uptake and clearance of amyloid peptides [13] may underlie the observed ability of HIV-1 Tat B to promote deposition of cell-bound amyloids in hippocampal cell cultures. The recently obtained in vivo evidence of increased deposition of aggregated Aβ in the brain of Tat/PS1/APP transgenic mice [13] is in line with our cell culture findings.
The conditions for culturing of hippocampal cells isolated from the hippocampus of E18 rat fetuses are designed to restrict glial proliferation. Primary fetal hippocampal cell cultures do not contain microglia and include no more than 10–20% (depending on the cell culture age) of astrocytes [8]. Neurotoxic effects of gp120 are largely mediated through interactions with glial cells and it is possible that indirect mechanisms involving microglia/macrophages or macroglia may be essential to facilitate aggregation of extracellular Aβ produced in response to gp120. Hence, despite the lack of ability to increase the number of Congo Red-positive hippocampal cells in vitro, gp120-mediated changes in Aβ production may lead to the formation of neurotoxic amyloidal oligomers in vivo.
As indicated by this study, Tat and gp120, which are known to cause hippocampal-dependent memory deficits, demonstrate the common ability to induce production of Aβ 1–42 in vitro, in hippocampal cell culture. The reported results draw attention to the specific connection between the cytotoxic potential of the HIV-1 protein (Tat) and the increased production of Aβ. Further, new evidence is presented indicating that Tat B may possess a unique property to directly stimulate both Aβ biogenesis and accumulation of cell-bound β-amyloids. Via the ability to trigger innate immunity defense mechanisms, amyloid-β oligomers formed with theparticipation of neurotoxic HIV-1 proteins may contribute to the setup of a vicious cycle of neuronal apoptosis, inflammation, and immune dysregulation [22] that results in the progressive decline of memory performance in the HIV-infected brain. In a broader sense, our study supports the idea that the HIV-associated cognitive dysfunction may be a variant of so-called "amyloid pore disease" [26] and rationalizes testing of the potential ability of anti-amyloidogenic compounds to attenuate HIV protein-induced neuronal injury.
Acknowlegements
This work is supported by NIH grants DA 11337, DA 13137, HD 43680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aksenov MY, Aksenova MV, Carney JM, Butterfield DA. Alpha 1-antichymotrypsin interaction with A beta (1–42) does not inhibit fibril formation but attenuates the peptide toxicity. Neurosci Lett. 1996;217:117–120. [PubMed] [Google Scholar]
- 2.Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. Attenuated neurotoxicity of the transactivation-defective HIV-1 Tat protein in hippocampal cell cultures. Exp Neurol. 2009;219:586–590. doi: 10.1016/j.expneurol.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksenova MV, Aksenov MY, Adams SM, Mactutus CF, Booze RM. Neuronal survival and resistance to HIV-1 Tat toxicity in the primary culture of rat fetal neurons. Exp Neurol. 2009;215:253–263. doi: 10.1016/j.expneurol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apcher GS, Heink S, Zantopf D, Kloetzel PM, Schmid HP, Mayer RJ, Krüger E. Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal alpha and beta subunits. FEBS Lett. 2003;553:200–204. doi: 10.1016/s0014-5793(03)01025-1. [DOI] [PubMed] [Google Scholar]
- 6.Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18 Suppl 1:75–78. Review. [PubMed] [Google Scholar]
- 8.Brewer GJ, Lim A, Capps NG, Torricelli JR. Age-related calcium changes, oxyradical damage, caspase activation and nuclear condensation in response to glutamate and beta-amyloid. Exp. Gerontol. 2005;40:426–437. doi: 10.1016/j.exger.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JS. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J Neurovirol. 2006;12:153–160. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- 11.Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006;83:489–496. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- 12.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giunta B, Hou H, Zhu Y, Rrapo E, Tian J, Takashi M, Commins D, Singer E, He J, Fernandez F, Tan J. HIV-1 Tat Contributes to Alzheimer's Disease-like Pathology in PSAPP Mice. Int J Clin Exp Pathol. 2009;2:433–443. [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata A, Chen XH, McIntosh TK, Browne KD, Smith DH. Long-term accumulation of amyloid-beta in axons following brain trauma without persistent upregulation of amyloid precursor protein genes. J Neuropathol Exp Neurol. 2002;61:1056–1068. doi: 10.1093/jnen/61.12.1056. [DOI] [PubMed] [Google Scholar]
- 15.Lesné S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verracchia C, Vivien D, Buisson A. NMDA receptor activation inhibits α-secretase and promotes neuronal amyloid-β production. J Neurosci. 2005;12:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- 17.Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol. 2008;63:366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- 18.Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulliam L. HIV regulation of amyloid beta production. J Neuroimmune Pharmacol. 2009;4:213–217. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- 20.Ranga U, Shankarappa R, Siddappa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78:2586–2590. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 22.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: amyloid-beta oligomers trigger innate immunity defense via pattern recognition receptors. Prog. Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. Review. [DOI] [PubMed] [Google Scholar]
- 23.Seeger M, Ferrell K, Frank R, Dubiel W. HIV-1 tat inhibits the 20 S proteasome and its 11 S regulator-mediated activation. J Biol Chem. 1997;272:8145–8148. doi: 10.1074/jbc.272.13.8145. [DOI] [PubMed] [Google Scholar]
- 24.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uversky VN, Kabanov AV, Lyubchenko YL. Nanotools for megaproblems: probing protein misfolding diseases using nanomedicine modus operandi. J Proteome Res. 2006;5:2505–2522. doi: 10.1021/pr0603349. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakabayashi M, Matsuzaki K. Formation of amyloids by Abeta-(1–42) on NGF-differentiated PC12 cells: roles of gangliosides and cholesterol. J Mol Biol. 2007;371:924–933. doi: 10.1016/j.jmb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 29.Xiong H, Zeng YC, Zheng J, Thylin M, Gendelman HE. Soluble HIV-1 infected macrophage secretory products mediate blockade of long-term potentiation: a mechanism for cognitive dysfunction in HIV-1-associated dementia. J Neurovirol. 1999;5:519–528. doi: 10.3109/13550289909045381. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]