1.0. Introduction
Molecular imaging is the visualization, characterization and measurement of biological processes at the molecular and cellular levels in humans and other living systems. Molecular imaging agents are probes used to visualize, characterize and measure biological processes in living systems. These two definitions were put forth by the Sociey of Nuclear Medicine (SNM) in 2007 as a way to capture the interdisciplinary nature of this relatively new field. The emergence of molecular imaging as a scientific discipline is a result of advances in chemistry, biology, physics and engineering, and the application of imaging probes and technologies has reshaped the philosophy of drug discovery in the pharmaceutical sciences by providing more cost effective ways to evaluate the efficacy of a drug candidate and allowing pharmaceutical companies to reduce the time it takes to introduce new therapeutics to the marketplace. Finally the impact of molecular imaging on clinical medicine has been extensive since it allows a physician to diagnose a patient’s illness, prescribe treatment and monitor the efficacy of that treatment non-invasively.
Single Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET) were the first molecular imaging modalities used clinically. SPECT requires the use of a contrast agent labeled with a gamma emitting radionuclide, which should have an ideal gamma energy of 100-250 keV. These gamma rays are recorded by the detectors of a dedicated gamma camera or SPECT instrument and after signal processing can be converted into an image indentifying the localization of the radiotracer. PET requires the injected radiopharmaceutical to be labeled with a positron emitting radionuclide. As the radionuclide decays it ejects a positron from its nucleus which travels a short distance before being annihilated with an electron to release two 511 keV gamma rays 180° apart that are detected by the PET scanner (Figure 1). After sufficient acquisition time the data are reconstructed using computer based algorithms to yield images of the radiotracer’s location within the organism. When compared to SPECT, PET has greater advantages with respect to sensitivity and resolution and has been gaining in clinical popularity, with the number of PET-based studies expected to reach 3.2 million by 2010.1 While SPECT and PET technology has been around for decades, its use remained limited because of the limited availability of relevant isotopes which had to be produced in nuclear reactors or particle accelerators. However, the introduction of the small biomedical cyclotron, the self-contained radionuclide generator and the dedicated small animal or clinical SPECT and PET scanners to hospitals and research facilities has increased the demand for SPECT and PET isotopes.
Figure 1.
Cartoon depicting the fundamental principle of Positron Emission Tomography (PET). As the targeting group interacts with the cell surface receptor, the positron emitting radio-metal decays by ejecting β+ particles from its nucleus. After traveling a short distance in the electron rich tissue, the positron recombines with an electron in a process called annihilation. During annihilation, the mass of the positron and electron are converted into two high energy photons (511 keV gamma rays), which are released approximately 180° apart to ensure that energy and momentum are conserved. Although attenuation is possible, these two gamma rays are usually energetic enough to escape the organism and be collected by the detectors of a PET scanner.
Traditional PET isotopes such as 18F, 15O, 13N and 11C have been developed for incorporation into small molecules, but due to their often lengthy radio-syntheses, short half-lives and rapid clearance, only early time points were available for imaging, leaving the investigation of biological processes, which occur over the duration of hours or days, difficult to explore. With the continuing development of biological targeting agents such as proteins, peptides, antibodies and nanoparticles, which demonstrate a range of biological half-lives, a need arose to produce new radionuclides with half-lives complementary with their biological properties. As a result, the production and radiochemistry of radiometals such as Zr, Y, In, Ga and Cu have been investigated as radionuclide labels for biomolecules since they have the potential to combine their favorable decay characteristics with the biological characteristics of the targeting molecule to become a useful radiopharmaceutical (Tables 1 and 2).2
Table 1. Gamma- and Beta-Emitting Radiometals.
| Isotope | T1/2 (h) | Production Methods |
Decay Mode |
Eγ (keV) | Eβ− (keV) | Reference |
|---|---|---|---|---|---|---|
| 67Cu | 62.01 | accelerator 67Zn(n,p) |
β− (100%) | 91, 93, 185 | 577, 484,395 |
578 |
| 67Ga | 78.26 | cyclotron | EC (100%) |
91, 93, 185, 296, 388 |
578 | |
| 90Y | 64.06 | 90Sr/90Y generator | β− (72%) | 2288 | 578 | |
| 111In | 67.9 | cyclotron, 111Cd(p,n)111n |
EC (100%) |
245, 172 | 578 |
Table 2. Positron-Emitting Radiometals.
| Isotope | T1/2 (h) | Methods of Production |
Decay Mode | Eβ+ (keV) | Reference |
|---|---|---|---|---|---|
| 60Cu | 0.4 | cyclotron, 60Ni(p,n)60Cu |
β+ (93%) EC (7%) |
3920, 3000 2000 |
578 |
| 61Cu | 3.3 | cyclotron, 61Ni(p,n)61Cu |
β+ (62%) EC (38%) |
1220, 1150 940, 560 |
578 |
| 62Cu | 0.16 |
62Zn/62Cu generator |
β+ (98%) EC (2%) |
2910 | 578 |
| 64Cu | 12.7 | cyclotron, 64Ni(p,n)64Cu |
β+ 19(%) EC (41%) β− (40%) |
656 | 578 |
| 66Ga | 9.5 | cyclotron, 63Cu(α,nγ)66Ga |
β+ (56%) EC (44%) |
4150, 935 | 578 |
| 68Ga | 1.1 |
68Ge/68Ga generator |
β+ (90%) EC (10%) |
1880, 770 | 578 |
| 86Y | 14.7 | cyclotron, 86Sr(p,n)86Y |
β+ (33%) EC (66%) |
2335, 2019 1603, 1248 1043 |
578 |
| 89Zr | 78.5 | 89Y(p,n)89Zr | β+ (22.7%) EC (77%) |
897 909, 1675, 1713, 1744 |
208,578 |
The number of papers published describing the production or use of these radiometals continues to expand rapidly, and in recognition of this fact, the authors have attempted to present a comprehensive review of this literature as it relates to the production, ligand development and radiopharmaceutical applications of radiometals (excluding 99mTc) since 1999. While numerous reviews have appeared describing certain aspects of the production, coordination chemistry or application of these radiometals,2-18 very few exhaustive reviews have been published.10,12 Additionally, this review has been written to be used as an individual resource or as a companion resource to the review written by Anderson and Welch in 1999.12 Together, they provide a literature survey spanning 50 years of scientific discovery. To accomplish this goal, this review has been organized into three sections: the first section discusses the coordination chemistry of the metal ions Zr, Y, In, Ga and Cu and their chelators in the context of radiopharmaceutical development; the second section describes the methods used to produce Zr, Y, In, Ga and Cu radioisotopes; and the final section describes the application of these radiometals in diagnostic imaging and radiotherapy.
2.0. The Coordination Chemistry of Cu, Ga, Y, In and Zr
2.1. General Considerations
The development of metal-based radiopharmaceuticals represents a dynamic and rapidly growing research area which requires an intimate knowledge of metal coordination chemistry and ligand design. This section of the review covers general considerations regarding the parameters that are important in developing stable, kinetically inert radiometal complexes that can be incorporated into radiopharmaceuticals. Additionally, the aqueous coordination chemistry of these metals and their coordination complexes which are most relevant to radiopharmaceutical development are discussed below.
Relevant properties in aqueous solution of the five metal cations covered in this review are presented in Table 3. The acidic cations Ga(III), In(III), and especially Zr(IV) present precipitation problems at neutral pH in the absence of suitable complex formation. In terms of plausible aqueous redox processes relevant to radiopharmaceutical applications, only Cu(II) and its complexes are susceptible to reduction chemistry, although the possibility of an ascorbic acid reduction of a 89Zr(IV) complex has been postulated.19 Based on Pearson’s Hard-soft Acid-base theory, the tetravalent Zr(IV) is an extremely hard acidic cation, followed by Y(III), Ga(III), and In(III). The Cu(II) cation is considered a borderline acid.
Table 3. Properties of Relevant Metal Cations.
| Cation/Electron Configuration |
Ionic Radius,a (CN) | pKab | kexchange s−1c | Eredd V (acid) |
Hardness Classification (IA)e |
|---|---|---|---|---|---|
| Cu(II)/[Ar]3d9 | 57 (4) 65 (5) 73 (6) |
7.53 | 2 × 108 | +0.34 (Cu0) +0.16 (CuI) |
Borderline (2.68) |
| Ga(III)/[Ar]3d10 | 47 (4) 55 (5) 62 (6) |
2.6 | 7.6 × 102 | −0.56 (Ga0) −0.65 (GaII) |
Hard (7.07) |
| In(III)/[Kr]4d10 | 62 (4) 80 (6) 92 (8) |
4.0 | 4.0 × 104 | −0.34 (In0) −0.49 (InII) |
Hard (6.30) |
| Y(III)/[Kr] |
90 (6) 102 (8) 108 (9) |
7.7 | 1.3 × 107 | −2.37 (Y0) | Hard (10.64) |
| Zr(IV)/[Kr] | 59 (4) 72 (6) 84 (8) 89 (9) |
0.22 | −1.54 (Zr0) | Hard |
Since the preponderance of radiometal complexes of note feature at least tetradentate ligands, we have restricted our discussion here to ligands with four or more donor sites coordinating the cation of interest. Rather than exhaustive coverage of all chelators of potential interest, we will discuss only selected representatives of the most-frequently reported ligands, especially those with more complete data of relevance. For the chosen representative chelators of each cation, we have listed available pertinent data on their denticity, coordination geometry, and thermodynamic stability. Where X-ray structural data are available, geometrical data on the coordination mode can provide useful insight into the “goodness of fit” for a specific cation-chelator pairing, the caveat being that actual solution structures or indeed number of species may be distinct from solid-state observations. For the four diamagnetic cations, solution NMR spectroscopic studies can be used to supplement X-ray data. Despite the difficulty of comparing stability constants of complex formation between ligands of different basicity and denticity, the listed log KML’s provide a convenient gauge of their relative affinities for a specific metal.
For in vivo applications, kinetic inertness of metal-chelator complexes or conjugates can be more relevant than thermodynamic stability.12,20,21 In general, acyclic chelator complexes are less kinetically inert than macrocyclic complexes of comparable stability.22-26 By the same token, acyclic chelators typically have faster metal-binding kinetics compared to their macrocyclic analogues, which can be a significant advantage for shorter-lived radiometals.27-30 There have been efforts to enhance the binding rate of macrocycles by incorporation of an acyclic polydentate pendant.31
A variety of in vitro assays of metal-chelator complex integrity can be found in the literature.32-35 A popular assay of aqueous kinetic inertness is acid decomplexation. This has some relevance in biological environments that are relatively acidic such as in hypoxic tissues and certain cell vesicles. However, the extremely high acidities, e.g. 1-5 M HCl, often required to decompose relatively inert complexes clearly have no parallel to any in vivo conditions. Nor can such data be relied upon, without considerations of other factors, as the sole predictor of biological behavior.36 Typically, the decomplexation of Cu(II) complexes is readily monitored through their electronic spectra. Demetallation of the diamagnetic Ga(III), In(III), Y(III), and Zr(IV) complexes can usually be followed by proton and 13C NMR spectroscopy in acidified D2O solutions. Where practicable, 71Ga, 115Ga, In, and 89Y NMR studies can also be undertaken.37-39 Although detailed mechanistic investigations are sometimes reported, more commonly only pseudo-first order half-lives are reported which should only be used to rank inertness qualitatively. Nonetheless, such data remain useful as a preliminary indicator of the in vivo viability of specific metal-based radiopharmaceuticals.
Competition or challenge assays of complexes of interest with excess biometals and biochelators are relevant since their typical concentrations are orders of magnitude higher than the radiolabeled complex’s, requiring high chelator selectivity for the radiometal. For example, copper homeostasis is tightly regulated in biology,40 and as a result a variety of copper-binding biomolecules are present in extracellular (e.g. serum albumin, ceruloplasmin, transcuprin, etc.) as well as intracellular (e.g. transporters, chaperones, metallothioneins, superoxide dismutase, cytochrome c oxidase, etc.) environments.41-43 A viable Cu(II) chelator should therefore be both thermodynamically stable and kinetically inert to transchelation challenges by these species. Highly-charged cations like Y(III) and Zr(IV) may also have high affinity for bone tissues while the avid Ga(III) binding of transferrin is well-established.44-46 Serum stability studies using radiometal-labeled chelator complexes or their bioconjugates are routinely used in inertness assays. These are readily monitored by radio-TLC, HPLC and LC-MS techniques.47-49 In vitro uptake studies using specific cell lines have also been carried out in many assays. While simulating extracellular environments to an extent, these studies cannot always accurately forecast in vivo behavior. Ultimately studies of animal biodistribution and bioclearance using radiometal-labeled complexes or bioconjugates need to be carried out to obtain realistic data on their in vivo performance.
The following discussion of pertinent acyclic and macrocyclic ligands and their specific metal coordination chemistry is organized according to their denticity. Most of these ligands have been designed to provide a minimum of four donor atoms, usually also incorporating anionic sites for charge balance (See Figures 2 and 3.). While all are given numerical “L(number)” designations, many have been labeled additionally with their respective acronyms. Published X-ray crystal structures of Cu, In, Ga, Y and Zr coordination complexes involving these ligands are also provided where appropriate. They were prepared from published CIF files using CrystalMaker 8.2 for Mac (CrystalMaker Software Ltd., Centre for Innovation & Enterprise, Oxford University Begbroke Science Park, Sandy Lane, Yarnton, Oxfordshire, OX5 1PF, UK; http://www.crystalmaker.com). Each atomic sphere is scaled to 0.4 times the covalent atomic radius, using the recently updated radii of Alvarez and coworkers.50 In addition to the labeled and uniquely colored metal atoms, common elements are color coded as follows: C = gray, Cl = green, F = light green, N = blue, O = red, P = orange, and S = yellow. Hydrogen atoms have been omitted from the structures for clarity.
Figure 2.
Selected acyclic chelators
Figure 3.
Selected macrocyclic chelators
2.2. Aqueous Copper Coordination Chemistry
While +1 and +3 oxidation states are both accessible for copper in the presence of suitable donors, 3d9 Cu(II) remains the predominant state for radio-copper chemistry in protic media. The aqueous cupric ion was long believed to have a tetragonally distorted hexa-aqua structure until a 2001 report suggested only five-coordination.51 Its water-exchange rate has been found to be very rapid compared to most common first-row transition metal cations and as a result it has relatively facile substitution chemistry despite having some crystal-field stabilization. This is usually ascribed to the Jahn-Teller distortion that elongates one or more of its coordinated ligands. Classified as a cation of borderline hardness, the high affinity of Cu(II) for borderline nitrogen donors is well-established. With a relatively small ionic radius of between 57 to 73 pm for coordination numbers 4 through 6, it is particularly suitable for the formation of 5-membered chelate rings; indeed the chelate effect is epitomized in its ethylenediamine family of complexes.52 The popular use of polyazamacrocycles, especially cyclen and cyclam, for strong binding of Cu(II) is a consequence of the added advantage of the macrocyclic effect,53 as borne out by their extensive coordination literature.54-57
The importance of in vivo redox activation of metallodrugs incorporating Pt(IV), Ru(III), and Co(III) has received increasing attention.58-61 The role of bioreduction in copper radiopharmaceutical efficacy has been intensively studied in their thiosemicarbazone complexes, especially Cu-ATSM (L9).62-64 Convincing evidence for the formation and selective retention/decomplexation of Cu(I)-intermediates from Cu(II) precursors in hypoxic tissues has been presented.65,66 Whether Cu(II)/Cu(I) bioreduction is also a viable pathway for irreversible in vivo radio-copper loss from other chelator complexes and their bioconjugates is an intriguing possibility. There is some compelling evidence for the deteriorated in vivo performance of related Cu(II) complexes differing only in their reduction propensities. Specifically, the “long arm” dicarboxyethyl pendant-armed Cu(II) complex of cross-bridged cyclam has an Ered almost 400 mV higher (or more positive) than that its carboxymethyl-armed analogue, Cu-CB-TE2A (L57).67 The former has been found to exhibit significantly inferior bioclearance behavior despite very similar coordination geometry and acid-inertness. More structure-activity studies, including the consequence of protonation on reduction feasibility, are warranted. Most polyazamacrocyclic complexes of Cu(II), however, have rather negative reduction potentials that are well below the estimated −0.40 V (NHE) threshold for typical bioreductants. It should be further noted that an appropriate in vivo donor able to alter the first or, perhaps even second coordination sphere around a metal cation can dramatically facilitate its redox processes. The relevance of this tuning of redox-active metal lability during biological iron transfer has been substantiated.68,69 Whether such ternary interactions can play a role in the reductive demetallation of thermodynamically-stable Cu(II) complexes in vivo has not been explored.
2.3. Copper(II) Complexes of Selected Chelators
The plasticity of the Cu(II) coordination geometry can be gleaned from a literature survey of 89 of its complexes with cyclen and cyclam derivatives.70 Coordination numbers (CN) ranging from 4 through 6 were found with geometries approximating square planar, square pyramidal, trigonal bipyramidal, as well as octahedral. Tetradentate chelators are usually designed to cater to Cu(II)’s strong affinity for ligands favoring a square-planar geometry. Common donor sets include two amino or imino nitrogens combined with two charge-neutralizing anionic amido, oxo, or thiolato sites. These include numerous Schiff-base or amino-acid derived chelators. Full envelopment of Cu(II) in its maximum six-coordinate mode is much sought after. As a result, hexadentate chelators have become the most investigated in radio-copper chemistry. Popular scaffolds include triaza- or tetraazamacrocycles, especially TACN (L26), cyclen (L38), and cyclam (L48). Methodologies for selective attachment of appropriate pendant arms to their secondary amine nitrogen sites as well as to the carbon backbone have been developed.71-79 Resulting donor sets usually incorporate anionic carboxylate or thiolate sites to provide a medley of charge-neutralizing N3O3, N3S3, or N4O2 coordination spheres. Data for selected Cu(II)-chelator complexes are listed in Table 4.
Table 4. Data on Selected Cu(II)-Chelator Complexes.
| Chelator | Donor Set (Total CN) |
Cation Coordination Geometry |
logKMLa | Ered or Epb,c | Acid Inertness, t1/2 (Conditions) |
Reference |
|---|---|---|---|---|---|---|
| L6 | N2S2 (4) |
distorted square planar | 80 | |||
| L9, ATSM | N2S2 (4) |
distorted square planar |
−0.40 (q-rev) | 81,82 | ||
| L10, EDTA | N2O4 (6) |
18.8 19.2 |
587,588 | |||
| L12, DTPA | N3O3 (6) |
21.4 | 589 | |||
| L17 | N6 (6) |
distorted octahedron | 16.3 | 0.08 (q-rev) | 590 | |
|
L19,
TACHPYR |
N6 (6) |
distorted octahedron | 88 | |||
|
L29,
NOTA |
N3O3 (6) |
distorted trigonal prsim |
19.8 21.6 |
~−0.70 (irrev) |
< 3 min (5M HCl, 30°) |
586,591,592 |
|
L38,
CYCLEN |
N4 (5) |
square pyramid | 24.6 | < 3 min (5M HCl, 30°) |
112,593 | |
| L34 | N4 | distorted square pyramid |
8.3 | ~−1.60 b(irrev) |
99,100 | |
| L36 | (5) | |||||
|
L39,
DOTA |
N4O2 (6) |
distorted octahedron | 22.2 22.7 |
~−0.74 (irrev) |
< 3 min (5M HCl, 90°)112 |
145,594,595 |
| L46, DO2P | N4O2 (6?) |
28.7 | 103 | |||
|
L48,
CYCLAM |
N4 (5-6) |
square pyramid, tetragonally elongated octahedral |
27.2 | ~−0.48 (irrev) |
3.8 min (5M HCl, 90°)112 |
593 |
| L49, TETA | N4O2 (6) |
distorted octahedron | 21.1 21.9 |
~−0.98 (irrev) |
< 3 min (5M HCl, 90°)112 |
145,594,595 |
| L54, TE2P | N4O2 (6) |
tetragonally-distorted octahedron |
26.5 | ~−0.45 (irrev) |
1.7 h (1M HCl, 60°) |
114 |
|
L56,
CB- CYCLAM |
N4 (5) |
distorted square pyramid |
27.1 | −0.32 (q-rev) | 11.8 min (1M HCl, 90°) |
111,112,596 |
|
L37,
CB-DO2A |
N4O2 | distorted octahedron | ~−0.72 (irrev) |
4.0 h (1M HCl, 30°) |
21,112 | |
|
L57,
CB-TE2A |
N4O2 (6) |
distorted octahedron | −0.88 (q-rev) | 154 h (5M HCl, 90°) |
111,112 | |
|
L62,
DIAMSAR |
N6 (6) |
distorted octahedron or trigonal prism |
~−0.90 (irrev)13 | 40 h (5M HCl, 90°)13 |
118 |
KML = [ML]/[M][L].
(q-rev) = quasi-reversible; Ered = reduction potential; (irrev) = irreversible reduction, Ep = peak potential only.
vs. NHE.
2.3.1. Acyclic Tetradentate Chelators
A dimethyl ester of N,N’-ethylenediamine-di-L-cysteinato EC (L5) was reacted with Cu(II) and the resulting complex structurally characterized and found to be substantially twisted (21°) from a square planar geometry (Figure 4).80
Figure 4.

Cu-L6
Bis(thiosemicarbazonato) complexes of cold and radio-Cu(II) have been intensely investigated for hypoxia imaging (vide infra).62,63,81 A series of X-ray structures of these complexes have been determined and near square-planar geometries were typically observed (e.g. Cu-GTS (Cu-L7) and Cu-PTSM (Cu-L8) in Figures 5 and 6). Alkylation at the backbone C atoms was found to increase the backbone C-C bond length and allow the metal to fit better into the ligand cavity with shorter Cu-S bonds.82 Their Cu(II)/Cu(I) reduction potentials have been shown to have significant bearing on their in vivo biological behavior.62,63,66
Figure 5.

Cu-GTS (L7)
Figure 6.

Cu-PTSM (L8)
2.3.2. Acyclic Hexadentate Chelators
The Cu(II)-EDTA (L10) structure has been reported to be a tetragonally-distorted N2O4 octahedron along one O-Cu-O axis (Figure 7).83 A DTPA (L12) analogue also features a hexadentate chelator but with an N3O3 coordination environment (Figure 8).84
Figure 7.

Cu-EDTA (L10)
Figure 8.

Cu-DTPA (L12)
Rigid BISPIDINE (3,7-diazabicyclo[3.3.1]nonane) derivatives with two appended pyridyl functions have been shown to be tetradentate in five-coordinate Cu(II) complexes (Cu-L17, Figure 9).85 Variations with four pyridyl as well as two non-coordinating carboxylate groups for charge neutralization are hexadentate chelators which were found to bind Cu(II) rapidly. An X-ray structure revealed a distorted octahedral N6 coordination mode (Cu-L18, Figure 10). This chelator was conjugated to bombesin, radiolabeled with 64Cu and studied in rats.86
Figure 9.

Cu-L17
Figure 10.

Cu-L18
Hexadentate ligands based on the 1,3,5-triaminocyclohexane backbone appended with three methylpyridines (TACHPYR, L19) have been investigated as radio-copper chelators.87,88 These form tetragonally-distorted octahedral Cu(II) complexes (Figure 11).
Figure 11.

Cu-TACHPYR (L19)
2.3.3. Macrocyclic Chelators
The 14-membered N2S2 macrocycle L24 was found to form the most inert Cu(II) complex compared to other ring sizes.89 New N2S2 macrocycles with two appended carboxymethyl arms (L25) have been synthesized and complexed with both Cu(II) and 64Cu(II).90 Molecular modeling suggested that only the 13-membered macrocycle can form a six-coordinate complex while the 14-membered analogue can only coordinate with its O2S2 donors.
Derivatives of TACN with either two (NO2A, L28) or three (NOTA, L29) carboxymethyl pendant arms both complex Cu(II) with good affinity. Their structures (Figure 12 and 13) reflect the ability of the cation to adopt either 5 or 6-coordination modes. The former has an N3O2 square pyramidal geometry with one N axial.91 The latter N3O3 donor set forms a distorted trigonal prismatic geometry.92 A TACN derivative with three oxime arms (L31) yielded a 5-coordinate Cu(II) complex with one oxime arm uncoordinated but held by a strong OH3⋯O hydrogen bond to a coordinated oxime (Figure 14).93
Figure 12.

Cu-NO2A (L28)
Figure 13.

Cu-NOTA (L29)
Figure 14.

Cu-NOTAM (L30)
Monocationic diimine dioxime (L32) complexes of Cu(II) were labeled with 64Cu and their biodistributions studied.94-96 The X-ray structure of a Cu(II) complex of a pyridyl-tethered derivative revealed a tetradentate ligand (L33) forming the base of a square pyramid with an axial water but no pyridyl coordination (Figure 15).97 Propylenediamine dioxime ligands have also been examined for their Cu(II) complexation in order to model radiopharmaceuticals.98 The cation was again found to adopt a square pyramidal coordination geometry featuring an apical water.
Figure 15.

Cu-L33
Dioxotetraazamacrocyclic Cu(II) complexes have been investigated for their radio-copper chelation potential.99 Of the chelators with varying ring sizes studied, dioxocyclam (L34) was shown to form the most stable complex. A methylquinoline pendant-armed dioxocyclam (L35) was found to doubly deprotonate at its amide N’s upon Cu(II) binding. Its structure has a distorted square-planar coordination geometry (Figure 16).82 Recently, a benzo-annelated dioxocyclam (L36) was synthesized and its Cu(II) complexation and 64Cu radiolabeling investigated.100 A four-coordinate distorted square planar structure was found. This complex has an extremely low reduction potential but can be reversibly oxidized.
Figure 16.

Cu-L35
Carboxymethyl pendant-armed derivatives of cyclen including DOTA(L39) and DO3A(L42) have been investigated for their Cu(II) binding. An X-ray structure of Cu-DOTA (Cu-L39) shows the expected pseudo-octahedral geometry with all cyclen N’s and two carboxymethyl arms from non-adjacent N’s coordinating cis to each other (Figure 17).101 An analogous geometry was found for the Cu-DO3A(L42) structure.102 The dicarboxymethyl armed cross-bridged cyclen (CB-DO2A, L37) was shown to envelop the Cu(II) in a very distorted octahedral geometry with the carboxylate donor sites cis to each other (Figure 18).21 It was pointed out that the metal center is significantly distended from the chelator cavity, which may account for its significantly lower inertness compared to its cyclam analogue Cu-CB-TE2A (Cu-L57) (vide infra).
Figure 17.

Cu-DOTA (L39)
Figure 18.

Cu-CB-DO2A (L37)
Cyclen appended with methanephosphonate pendant arms such as DO2P (L46) and DOTP (L47) have been synthesized and chelated to Cu(II) and 64Cu.103 Mixed phosphonate- and acetate-armed cyclen (e.g. DO2A2P, L45) have also been studied with the highest log KML found for Cu-DO2P (Cu-L46).104
Due to the good cation/cyclam match, numerous Cu(II) complexes of cyclam and its derivatives with at least one carboxymethyl pendant arm on N have been prepared and studied. As expected, the singly-armed chelator C-hexamethyl-TE1A (Me6-L50) yielded a square-pyramidal Cu(II) structure (Figure 19).105 Several structures of the doubly-armed ligand complexes have been determined. The first features carboxylates on adjacent ring N’s (1,4-TE2A, L51, Figure 20),106 while the latter on non-adjacent N’s (1,8-TE2A, L52, Figure 21).107 Two diprotonated Cu-TETA (L49) structures were shown to have their axial-elongations either along the acetate O’s or across two cyclam ring N’s (Figure 22).108 A C-functionalized TETA Cu(II) complex, Cu-L55 also has a pseudo-octahedral metal coordination mode (Figure 23).109
Figure 19.

Cu-TE1A (L50)
Figure 20.

Cu-L51
Figure 21.
Cu-L52
Figure 22.

Cu-H2TETA (L49)
Figure 23.

Cu-L55
Two cross-bridged cyclam chelators, one with two carboxymethyl arms (CB-TE2A, L57), the other with one carboxymethyl and one acetamide arm (CB-TEAMA, L58) have been prepared to compare the in vivo behavior of their 64Cu complexes.110 The former structure revealed full envelopment of the Cu(II) in a pseudo-octahedral geometry with elongation along one N-Cu-O axis (Figure 24).111 While the latter has a very similar geometry, a weaker amide O-coordination is observed (Figure 25).110 The Cu-CB-TE2A complex has been shown to have remarkable kinetic inertness towards acid decomplexation, with a half-life of almost a week in 5 M HCl, even at 90°.112 A derivative of this chelator with a C-functionalized p-isothiocyanatobenzyl group has been prepared and successfully conjugated to biotin.113
Figure 24.

Cu-CB-TE2A (L57)
Figure 25.

Cu-CB-TEAMA (L58)
The diphosphonate pendant-armed cyclam TE2P (L54) has been reported to form a very stable Cu(II) complex with a tetragonally-distorted octahedral geometry (Figure 26).114 This complex also has a respectable acid inertness compared to typical cyclam copper complexes.
Figure 26.
Cu-TE2P (L54)
Pendant-armed derivatives of adjacent or side-bridged cyclam (L58, L59) have been prepared and complexed with Cu(II).108 These chelators strongly favor square planar coordination of the cation by the macrocycle with axial coordination by a pendant arm to give square pyramidal geometries. A recent novel side-bridged cyclam with one acetate and one phosphonate pendant arm (L60) has been synthesized, labeled with 64Cu and studied in vivo. It was reasoned that, unlike CB-TE2A, this bifunctional chelator (BFC) will form a charge-neutral Cu(II) complex despite conjugation of one acetate arm.115
The venerable hexaamine cryptand, Sarcophagine, is well known for its strong binding of Cu(II) and inertness of its complexes.116-118 Its derivatives DIAMSAR (L62) and SARAR (L65) have been investigated as ligands for copper radiopharmaceuticals by several research groups.119-124 Diprotonated DIAMSAR (L62) was found to have a Cu(II) coordination mode halfway towards trigonal prismatic but with two elongated trans-Cu-N bonds (Figure 27).118 A related carboxymethyl pendant armed complex (Cu-L63) is closer to an octahedral coordination mode again with two long trans-bonds (Figure 28),125 while a doubly carboxymethylated DIAMSAR Cu(II) complex (Cu-L64) has two elongated cis-bonds (Figure 29).126 Recently the Cu(II) complex of a glutaric acid DIAMSAR derivative suitable for peptide conjugation was synthesized. Its coordination geometry is again distorted octahedral with axial Jahn-Teller elongations.124
Figure 27.

Cu-L62
Figure 28.

Cu-L63
Figure 29.

Cu-L64
2.4. Aqueous Gallium(III) Coordination Chemistry
The prevalent gallium oxidation state in aqueous solution is +3. This small and highly-charged cation of ionic radius 47-62 pm (CN 4-6) is quite acidic with a pKa of 2.6 in its hydrated form. As a result it has low solubility in normal pH media in the absence of suitable donors. Due to its strong affinity for hydroxide, at very high pH it also has a propensity to demetallate from its complexes and form the gallate anion Ga(OH)4−. Among the four water-soluble metal cations discussed in this review, aqueous Ga(III) has the most sluggish water exchange rate due to its small size and high charge.
As a classic hard acidic cation, Ga(III) is strongly bound to ligands featuring multiple anionic oxygen donor sites, although it has also been shown to have good affinity for thiolates. Typically chelators have been developed to sequester Ga(III) up to its maximum coordinate number of 6 in a pseudo-octahedral geometry. A comprehensive review of six-coordinate Ga(III) complexes has appeared recently.127
It is well known that the biological iron transporter transferrin has a strong affinity for Ga(III) (Table 5). 46,128 Radio-gallium chelator complexes must therefore be sufficiently inert to transchelation by this biomolecule to have efficacy for in vivo applications. Data for selected Ga(III)-chelator complexes are presented in Table 5.
Table 5. Data for Selected Ga(III)-Chelator Complexes.
| Chelator | Donor Set (Total CN) |
Cation Coordination Geometry |
logKMLa | Reference |
|---|---|---|---|---|
| L1 | NO3 (6) | distorted octahedron | 19.1 (p-NO2) 25.2 (p-OMe) |
129 |
| L2 | NS3 (4) | distorted tetrahedron | 20.5 | 130 |
| L4, BAT-TM | N2S2 (5) | distorted square pyramid | 132 | |
| Transferrin | NO5 (6) | 20.3 19.8 |
46,128 | |
| L5, EC | N2O2S2 (6) | distorted octahedron | 31.5 | 133,586 |
| L10, EDTA | N2O4 (6) | distorted octahedron | 21.0 22.0 |
588,593 |
| L11, HBED | N2O4 (6) | 37.7 38.5 |
135,172 | |
| L12, DTPA | N3O3 (6?) | 25.5 | 593 | |
| L15, SBAD | N4O2 (6) | distorted octahedron | 28.3 | 139 |
| L16, BAPEN | N4O2 (6) | distorted octahedron | 143,144 | |
| L29, NOTA | N3O3 (6) | distorted octahedron | 31.0 | 145,597 |
| L27, TACN-TM | N3S3 (6) | distorted octahedron | 34.2 | 145 |
| L39, DOTA | N4O2 (6) | distorted octahedron | 21.3 | 145 |
| L49, TETA | N4O2 (6) | distorted octahedron | 19.7 | 145 |
| L37, CB-DO2A | N4O2 (6) | distorted octahedron | 162 | |
| L57, CB-TE2A | N4O2 (6) | distorted octahedron | 163 | |
| L20, DiP-LICAM | O6 (6) | 38.6 | 187 | |
| L66 | O6 (6) | 27.5 | ||
| L21 | 25.6 | 598 | ||
| L22 | 27.3 | |||
| L23, DFO | O6 (6) | 28.6 | 599 |
KML = [ML]/[M][L].
2.4.1. Tetradentate Ligands
Tetradentate o-hydroxybenzyl derivatives of iminodiacetic acid (L1) were designed to provide an NO3 donor set which completes the distorted octahedral coordination around their Ga(III) center together with two cis-coordinated waters (Figure 30).129 In accordance with substituent effects, the p-OMe derivative has the highest stability constant while p-NO2 has the lowest.
Figure 30.

Ga-L1
The tripodal NS3 chelator tris(2-mercaptobenzyl)amine (L2) formed a stable 4-coordinate Ga(III) complex with a distorted tetrahedral structure (Figure 31).130 Under similar preparative conditions, the analogous In(III) complex was found to be 5-coordinate with additional solvent binding.
Figure 31.

Ga-L2
A bis(aminothiolate) N2S2 chelator yielded a distorted square pyramidal GaCl complex with one S donor in the axial site.131 A similar structure was determined for the related GaCl-BAT-TM (GaCl-L4) except that chloride is the axial ligand (Figure 32).132 In aqueous acetonitrile solution, however, NMR spectra revealed the presence of two different N2S2–coordinated species.
Figure 32.

Ga-BAT-TM (L4)
2.4.2. Hexadentate Ligands
An acyclic hexadentate chelator N,N’-ethylene-di-L-cysteine (EC, L5) with N2O2S2 donor sites has been found to form a very stable complex with Ga(III) (Table 5).133 This structure is a distorted octahedron with the two carboxylate O’s in trans arrangement (Figure 33). Additionally, the [Ga-EDTA, L10]− structure has been reported in which the acyclic chelator is fully hexadentate in a distorted octahedral coordination sphere (Figure 34).134
Figure 33.

Ga-EC (L5)
Figure 34.

Ga-EDTA (L10)
The aminophenolate chelator N,N’-bis(2-hydroxybenzyl)ethylenediamine-N,N’-diacetic acid (HBED, L11) has an N2O4 donor set and its Ga(III) complex has a very high stability constant (Table 5).135 As a consequence, it has generated considerable interest as a bifunctional chelator. The derivative N,N’-bis[2-hydroxy-5-carboxyethyl)-benzyl]ethylene diamine-N,N’-diacetic acid (HBED-CC)136 and its tetrafluorophenyl ester derivative have been described to promote facile coupling to both scFV and anti-Ep-CAM diabodies.137 Using this BFC these biomolecules could be labeled with 68Ga to give specific activities of 142 GBq/μmol. Interestingly, a potentially octadentate DTPA (L12) derivative gave a less stable complex likely due to its structural constraints.138 Linear tetraamines end-capped with phenols, like SBAD (L15), have been synthesized and their Ga(III) complexations investigated.139 These were all found to favor Ga(III) over In(III) binding.
Although numerous complexes of both cold and radio-Ga(III) with DTPA (L12) and its derivatives have been prepared and studied, surprisingly no X-ray structure of the parent complex has yet been published. It is noteworthy that both EDTA (L10) and DTPA (L12) bind In(III) more avidly than Ga(III) (Tables 5 and 6). Recently, a variant of DTPA (L12) incorporating the trans-1,2-diaminocyclohexane backbone, CHX-A”-DTPA (L14),140 has been bioconjugated and studied as a chelator for 68Ga, 86Y, and 111In in melanoma imaging.141
Table 6. Data for Selected In(III)-Chelator Complexes.
| Chelator | Donor Set (Total CN) | Cation Coordination Geometry | logKMa | Reference |
|---|---|---|---|---|
| L2 | NS3 (5) | ~trigonal bipyramid | 21.2 | 130,173 |
| L3 | NS3 (5) | ~trigonal bipyramid | ||
| L4, BAT-TM | N2S2 (5) | square pyramid | 132 | |
| Transferrin | NO5 (6) | distorted octahedron(?) | 18.7 | 128 |
| L5, EC | N2O2S2 (6) | distorted octahedron | 33.0 | 133 |
| L11, HBED | N2O4 (6) | 27.9 27.8 |
138,172 | |
| L10, EDTA | N2O4 (7) | ~pentagonal bipyramid | 24.9 25.1 25.3 |
593 588,600 |
| L12, DTPA | N3O4 (7) N3O5 (8) |
~pentagonal bipyramid ~square antiprism |
29.5 29.0 29.0 |
587,588,600 |
| L15, SBAD | N4O2 | 24.5 | 139 | |
| L29, NOTA | N3O3 (6) | distorted octahedron |
26.2 |
37,147 |
|
L27,
TACN-TM |
N3S3 (6) | distorted octahedron | 36.1 | 145 |
| L39, DOTA | N4O2 (8?) | 23.9 | 186 | |
| L49, TETA | N4O2 | 21.89 | 186 | |
| L57, CB-TE2A | N4O2 (6) | distorted octahedron | 155 | |
| L23, DFO | O6 | 21.4 | 599 | |
|
L20,
DiP-LICAM |
O6 | 39.2 | 187 |
KML = [ML]/[M][L].
A family of hexadentate bis(salicylaldimine) chelators, BAPEN (L16), have been developed as carriers for 68Ga radiopharmaceuticals.142-144 These all feature a set of N4O2 donors enveloping the Ga(III) in a pseudo-octahedral coordination sphere. The structure of one of these is shown (Figure 35).
Figure 35.

Ga-BAPEN (L16)
The tris-acetate pendant armed 1,4,7-triazacyclononane (TACN) derivative, NOTA (L29), and its relatives have been found to form highly stable Ga(III) complexes.145 An X-ray structure of Ga-NOTA (Ga-L29) confirmed the distorted octahedral N3O3 envelopment of the cation (Figure 36).37,146 Remarkably, Ga-NOTA (Ga-L29) showed such high acid inertness that it survived 5M HNO3 for over 6 months.37 This is in stark contrast to In-NOTA (In-L29) which demetallated irreversibly within minutes at pD < 0. The tris(2-mercaptoethyl) pendant-armed TACN chelator, TACN-TM (L27), also formed a distorted octahedral structure with Ga(III), again with impressive stability (Figure 37).146 The superb stability of Ga(III)-NOTA (Ga-L29) (log K = 30.1; pM = 26.4) 147 underscores the use of this agent in radiopharmaceutical chemistry. This exceptional stability is believed to arise from the effective encapsulation of the gallium ion, which has an ionic radius of 0.76 Å within the macrocyclic cavity. Additional protection is afforded by the pendant carboxymethyl arms that help to protect the Ga ion from nucleophilic attack, which would cause complex instability and transchelation in vitro or in vivo. Since its synthesis, several NOTA (L29), analogues have been prepared with modified pendant groups for coupling to biological molecules, to influence the charge on the complexes once complexation has occurred or to facilitate coupling during peptide synthesis.148-154
Figure 36.

Ga-NOTA (L29)
Figure 37.

Ga-TACN-TM (L27)
Since the potentially octadentate DOTA (L39) can more than saturate Ga(III)’s usual 6-coordination sphere, both its mono- and diprotonated structures feature a similar distorted octahedral coordination composed of two cis-carboxylates and four macrocyclic N’s (Figure 38).155,156 It is advantageous that a free carboxymethyl arm can be available for conjugation to targeting moieties. A variety of such complexes have appeared in the literature. The Ga-DOTA-D-PheNH2 (Ga-L40) coordination mode is again pseudo-octahedral with cis-carboxylate coordination while the remaining acetate and amide arms are unbound (Figure 39).157 It was therefore postulated that the better kidney clearance of the radiolabeled DOTA0-D-Phe1-Tyr3-octreotide, 67Ga-DOTA(L39)-TOC, compared to its 90Y-labeled analogue was due to their different coordination modes. Whereas the former has uncoordinated amide and carboxylate arms, the latter is likely fully octa-coordinated. When DOTA (L39) was linked to a mitochondrion-targeting triphenylphosphonium moiety to give DO3A-TPP (L44), its Ga(III) complex again retained the 6-coordinate pseudo-octahedral geometry (Figure 40).158 Unfortunately, DOTA (L39) has many potential drawbacks as a BFC for gallium radiometals, which include an overly large cavity size that can lead to lower thermodynamic stability as well as non-selectivity as a metal chelator.159 Additionally, the complexation kinetics are slower, and as a result the complexation reactions often require elevated temperatures and longer reaction times, which are counterproductive given the short half-life of the 68Ga radionuclide. The thermodynamic stability of Ga(III)-DOTA (Ga-L39) is approximately 10 orders of magnitude lower than Ga(III)-NOTA (Ga-L29), with a log K of 21.3 and a pM of 15.2.160 Given the low pM values, it is interesting that radio-gallium DOTA (L39) complexes have shown in vivo stability in agents such as 67/68Ga-DOTA(L39)-TOC. However, there are reports that radio-gallium DOTA(L39)-RGD peptides have demonstrated compromised stability in vivo, with the radio-gallium demonstrating binding to plasma proteins.161
Figure 38.

Ga-DOTA (L39)
Figure 39.

Ga-DOTA-D-PheNH2 (L40)
Figure 40.

Ga-DO3A-TPP (L44)
Related to Ga-DOTA (Ga-L39) is the complex Ga-TETA (Ga-L49) but, no structural data has been reported for it. Further, Ga-TETA (Ga-L49) has a lower stability constant than the former complex (log K = 19.7; pM = 14.1).160 It should be noted that TETA (L49), unlike DOTA (L39), does not have a favorable fac-coordinating conformation.
Both dicarboxymethyl pendant-armed cross-bridged cyclen (CB-DO2A, L37) and cross-bridged cyclam (CB-TE2A, L57) gallium complexes have been synthesized and structurally characterized.162,163 As expected, distortions from an octahedral geometry are much more pronounced in the former (Figures 41 and 42). Of these two, only Ga-CB-TE2A (Ga-L57) showed impressive acid inertness. A sample of it in 5M DCl at 90° was less than 20% demetallated even after 6 months.
Figure 41.
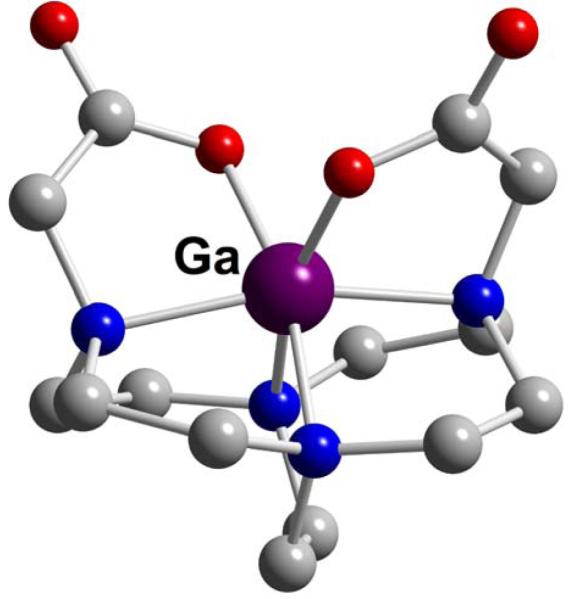
Ga-CB-DO2A (L37)
Figure 42.
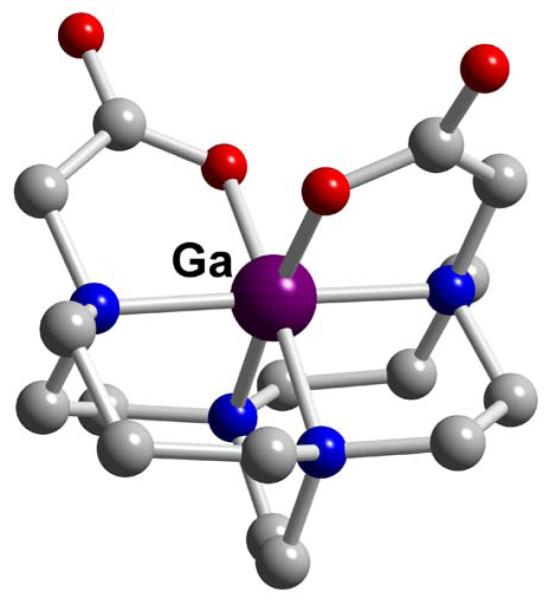
Ga-CB-TE2A (L57)
The high thermodynamic stability of Ga(III) complexes of preorganized O6 trihydroxamate chelators, especially Desferal or Deferrioxamine-B, DFO (L23) (log K = 28.6), has spurred much activity towards their biological and radiopharmaceutical applications.164-169 Early solution NMR spectroscopic studies had revealed the presence of two major isomeric forms of Ga-DFO (Ga-L23).170 An X-ray structure that can model Ga-DFO (Ga-L23) has been obtained in a Ga-tris(benzohydroxamate) complex which has a fac-octahedral coordination sphere (Figure 43).171 The stability constants of Ga(III) complexes of a tris(hydroxamate) cryptate, L63, as well as two acyclic analogues, L21 and L22, were compared with the cryptate complex and found to be the most stable of the three (log Ks = 27.5, 25.6 and 27.3, respectively).172 An Enterobactin model DiP-LICAM (L20) has been reported to bind 67Ga(III) with a very high stability constant (log K = 38.6; Table 5).185
Figure 43.

Ga-tris(benzohydroxamate)
2.5. Aqueous Indium(III) Coordination Chemistry
Like gallium, the only stable aqueous indium oxidation state is +3. The significantly larger size of In(III) at 62-92 pm for CN 4-8, however, results in its attainment of coordination numbers of 7 and even 8 in its complexes. While still a hard acid, its higher pKa of 4.0 and faster water exchange rate also reflect its distinction from Ga(III). A slightly enhanced affinity for softer donor types compared to Ga(III) can be noted in In(III) coordination chemistry. For example, acyclic N4O2 aminophenols as well as the biological iron transporter transferrin bind Ga(III) more avidly than In(III). By contrast, the tripodal NS3 chelators, EDTA (L10), DTPA (L12), and DOTA (L39) all complex In(III) more securely as well as with higher denticity than Ga(III). Such differences in fundamental coordination preferences can have significant consequences in their biological behavior.157 Data for selected complexes are listed in Table 6.
2.5.1. Tetradentate Chelators
A variety of acyclic tetradentate chelators featuring mixed amine and thiolate donor sets have been synthesized for In(III) complexation. The InCl-bis(aminothiolate), InCl-BAT-TM (L4), structure is 5-coordinate and near square pyramidal with an axial chloride (Figure 44).132 While the complex was found to be stable in aqueous acetonitrile at pD 4.6, NMR spectra revealed the presence of two isomeric solution species. Tripodal NS3 chelators such as tris(2-mercaptobenzyl)amine (L2) favor a trigonal bipyramidal coordination including an axial solvent dimethylformamide (DMF) at the metal center (Figure 45).130 This can be contrasted with the ready isolation of a 4-coordinate Ga(III) analogue.130 Both the synthesis and structural determination of a related tris(mercaptoethyl)amine (L3) complex of In(III) have also been reported.173
Figure 44.

InCl-BAT-TM (L4)
Figure 45.

In-L2•DMF
2.5.2. Hexa- to Octadentate Chelators
A hexadentate chelator boasting N2O2S2 donors, N,N’-ethylenedi-L-cysteine (EC, L5), was found to form a distorted octahedral complex of In(III) with both carboxylate donors at axial sites (Figure 46).133 This has an impressively high stability constant and was not demetallated by transferrin. The related N,N’-bis(2-hydroxybenzyl)ethylene-diamine-N,N’-diacetic acid (HBED, L11) has also been found to bind In(III) strongly, though still with much lower affinity when compared to Ga(III) (Tables 5 and 6).138
Figure 46.

In-EC (L5)
Popular acyclic chelators EDTA (L10) and DTPA (L12) form very thermodynamically stable complexes with indium (Table 6). The 7-coordinate In-EDTA (In-L10) structure features a hexadentate chelator and approximates a pentagonal bipyramidal geometry with a single water at an equatorial position (Figure 47).174 In-DTPA (In-L12) structures feature both 7-as well as full 8-coordination by the chelator in distorted pentagonal bipyramidal and square antiprismatic geometries respectively; the latter is shown in Figure 48.175,176 A 13C{1H}NMR spectroscopic study of Na2[In-DTPA] [Na2(In-L12)] in D2O confirmed retention of its square antiprismatic geometry in neutral aqueous solution.176 The N,N”-bis(benzylcarbamoyl-methyl) derivative of DTPA (L12), DTPA-BA2 (L13), was first designed as a model for a doubly-bioconjugated chelator.177 An X-ray structure of its In(III) complex also features an 8-coordinated cation in a distorted square antiprismatic envelopment (Figure 49).178 Unlike In-DTPA (In-L12), however, its solution NMR spectrum revealed the presence of at least three isomeric species. It was proposed that the two amide carbonyls are no longer coordinated in solution, thus rendering this complex more hydrophilic in its HPLC retention behavior compared to its Y(III) analogue. A DTPA derivative with a trans-1,2-diaminocyclohexane backbone, CHX-A”-DTPA (L14), has been conjugated to a targeting peptide and found to be a viable ligand for 68Ga, 86Y, and 111In radiometals for melanoma imaging.141
Figure 47.
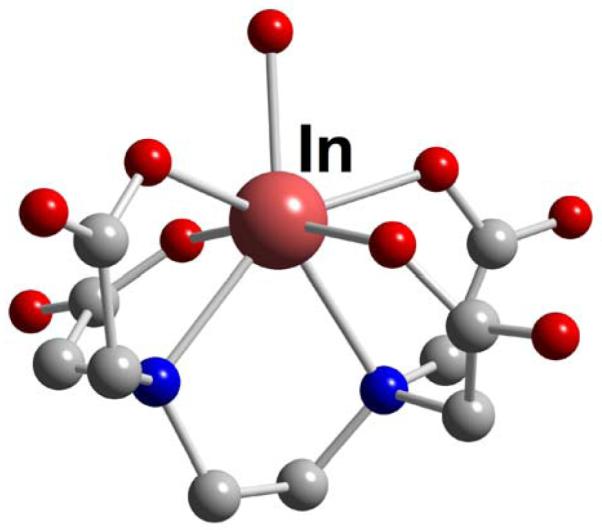
In-EDTA (L9)
Figure 48.

In-DTPA (L12)
Figure 49.
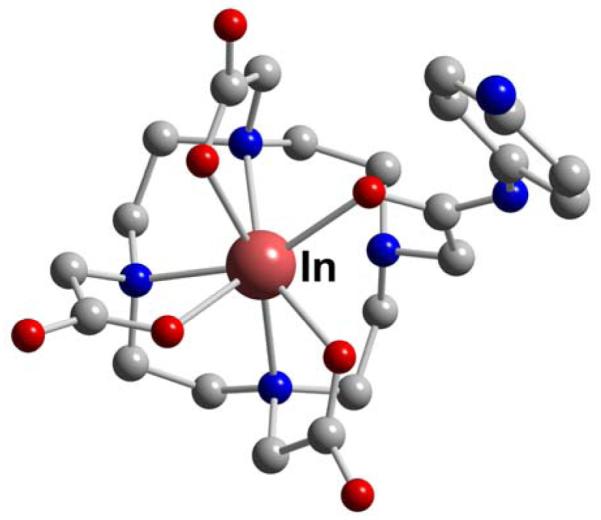
In-DTPA-BA2 (L13)
Linear N4O2 aminophenols such as SBAD (L15) have been prepared and their complexations with Ga(III) and In(III) studied.139 These consistently bind the former cation more tenaciously. Additionally, several In(III) complexes of pendant-armed triazacyclononane (TACN, L26) derivatives have had their solid-state structures determined. An InCl-NOTA (InCl-L29) complex protonated at one carboxylate arm was found to have a near pentagonal bipyramidal geometry with the chelator hexadentate (Figure 50).37,179 In aqueous solution, however, NMR spectral data suggested a time-averaged six-coordinate C3-symmetric species instead. At pD < 0, this complex decomposed irreversibly. The related In-(R)-1,4,7-tris(2′-methylcarboxymethyl)-triazacyclononane structure has a more typical pseudo-octahedral coordination sphere (Figure 51).180 A tris(phenylphosphinate)-armed derivative of TACN (L26),181 as well as the tris(mercaptoethyl)-armed derivative TACN-TM (L27) can both bind In(III) in a similar mode (Figure 52).182
Figure 50.
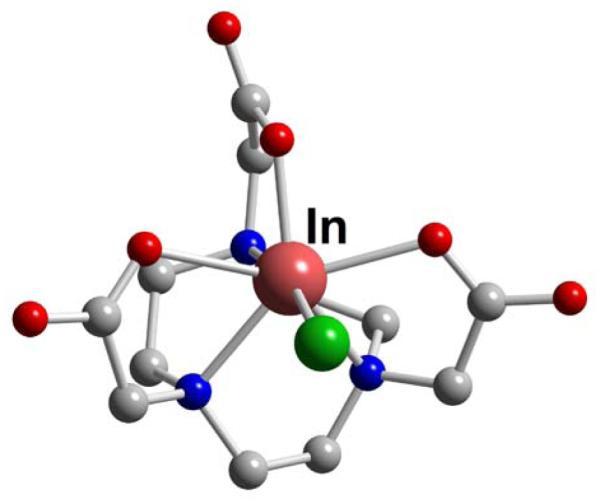
InCl-HNOTA (L29)
Figure 51.

In-TACN (L26)-tris(2′-methylcarboxylmethyl)
Figure 52.

In-TACN-TM (L27)
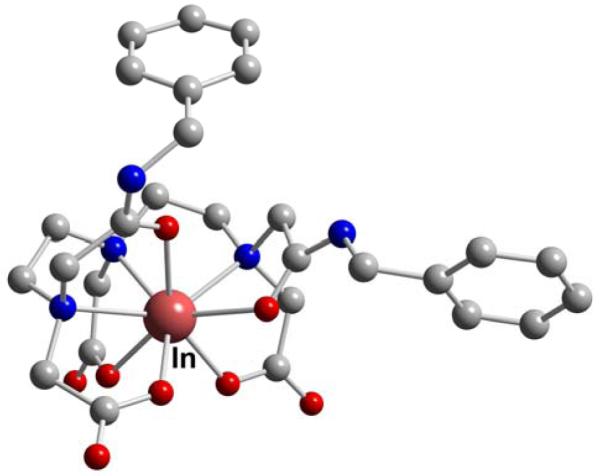
The valuable octadentate chelator DOTA (L39) has also been shown to form a robust complex with In(III) (Table 6).183 Surprisingly, no X-ray structural data are yet available for the parent In-DOTA (In-L39) complex. There are, however, several reported structures of its amide-armed derivatives. The In(III) complex of the p-aminoanilide, DOTA-AA (L41), was found to have a twisted (~28°) square antiprismatic geometry (Figure 53).184 Inside the chelator cavity the cation is approximately 1.2 and 1.3 Å from the O4 and N4 coordination planes respectively. This indicates that its fit may not be as ideal as that for the larger Y(III) cation (vide infra). With one fewer carboxymethyl pendant arm than DOTA (L39), the chelator DO3A (L42) has also been complexed to In(III) and its structure adopts the expected 7-coordinate geometry.185 Biodistribution studies using DOTA (L39) conjugated to D-Phe1-Tyr3-octreotide, DOTATOC (L39-TOC), and labeled with 67Ga, 111In, and 90Y revealed that the Ga-DOTATOC (Ga-L39-TOC) had the best tumor uptake and kidney clearance.157 It was postulated that this may be the result of a hexacoordinate Ga(III) compared to the higher coordination requirements for the In(III) and Y(III) analogues. Another 7-coordinate In(III) geometry, described as monocapped trigonal prismatic, was found for In-DO3A-TPP (L44) which features DOTA (L39) linked to a triphenylphosphonium moiety to target mitochondria (Figure 54).158 Proton NMR data supported retention of this heptadentate chelation mode in aqueous solution.
Figure 53.
In-DOTA-AA (L41)
Figure 54.

In-DOTA-TPP (L44)
A tris(carboxymethyl)-armed cyclam chelator, TE3A (L53) has also been found to complex In(III) in a heptadentate mono-capped trigonal prismatic geometry (Figure 55).185 No structure of In-TETA (In-L49) has been reported though its stability constant has been determined to be 2 orders of magnitude lower than that of In-DOTA (In-L39) due to a poorer cation-chelator match.186
Figure 55.

In-TE3A (L53)
An early report of 111In-labeling of tricatecholamide analogues of Enterobactin (DiP-LICAM (L20) yielded impressive stability constants (Table 4). However biodistribution studies were not performed.187
2.6. Aqueous Yttrium(III) Coordination Chemistry
Aqueous yttrium chemistry is often discussed together with that of the lanthanides because of their common tricationic state and similar ionic radii. Yttrium (III), 90-108 pm (CN 6-9), is significantly larger than the other four metal cations discussed in this review and can readily reach coordination numbers of 8 and 9 in its complexes. With a closed-shell electron configuration, it is also considered a harder acidic cation than Ga(III) or In(III). This can be seen in its higher Drago-Wayland parameter IA which is an indicator of the relative significance of the electrostatic versus covalent component of its coordination interaction.579,580 Data for selected complexes are presented in Table 7.
Table 7. Data for Selected Y(III)-Chelator Complexes.
| Chelator | Donor Set (Total CN) | Cation Coordination Geometry | logKMLa | Reference |
|---|---|---|---|---|
|
L10,
EDTA |
N2O4 (8) | distorted dodecahedron | 18.1 18.5 |
593,601 |
|
L12,
DTPA |
N3O5 (8) | monocapped square antiprism | 21.2 22.0 22.5 |
587,601,602 |
|
L30,
NOTAM |
N3O3 (9) | monocapped square antiprism | 190 | |
|
L39,
DOTA |
N4O4 (8) | square antiprism | 24.3 24.4 24.9 |
37,601,602 |
|
L49,
TETA |
N4O2 (8?) | distorted dodecahedron (?) | 14.8 | 195 |
KML = [ML]/[M][L].
Both EDTA (L10) and DTPA (L12) have been shown to form reasonably stable complexes with Y(III). Several solid-state structures of these have been determined and two selected examples are shown in Figures 56 and 57. The YF2-EDTA (YF2-L10) structure approximates a dodecahedron with a hexadentate EDTA (L10),188 while Y-DTPA (Y-L12) is 9-coordinate with a monocapped anti-prismatic geometry including an octadentate DTPA (L12) and one coordinated water.189 Two related structures are of Y-DTPA-BA2 (L13), a N,N”-bis(benzylcarbamoylmethyl) derivative of DTPA (L12) which served as a model for a dual biomolecule-labeled chelator.177,178 In each case, the 9-coordinate Y(III) adopts a distorted tricapped trigonal prismatic geometry featuring an octadentate chelator plus a coordinated solvent (methanol) molecule (Figure 58). In D2O solution, however, NMR spectroscopy revealed the presence of at least three isomers up to 85 °C. It was suggested that these accessible structural variations may account for the relatively low inertness of typical Y-DTPA (Y-L12) complexes.
Figure 56.

YF2-EDTA (L10)
Figure 57.

Y-DTPA (L12)
Figure 58.

Y-DTPA-BA2 (L13)
The structure of a Y(III) triflate complex of a tris(carbamoylmethyl) derivative of TACN (L26), NOTAM (L30), has been reported.190 This 9-coordinate geometry can be viewed as a capped square anti-prism, although alternative descriptions are also viable (Figure 59). NMR spectroscopic studies showed that this complex is fluxional in acetonitrile solution.
Figure 59.

Y(triflate)2-NOTAM (L30)
A number of yttrium(III) complexes of DOTA (L39) and its derivatives have been prepared. An X-ray structure of [Y-DOTA]− (Y-L39−) is shown in Figure 60.177,191 The 9-coordinate Y(III) center has a monohydrate capped square antiprismatic (SA) coordination sphere (often referred to as the “M” isomeric geometry) featuring an octadentate DOTA (L39) with the cation held significantly closer to the O4 plane than the N4 plane (0.72 versus 1.62 Å respectively). A related structure of a DOTA (L39) derivative with one carboxymethyl arm conjugated to phenylalanine, Y-DOTA-D-PheNH2 (Y-L40), has also appeared (Figure 61). This adopts instead a twisted square anti-prismatic (TSA) geometry (or “m” isomer) with four macrocyclic amine nitrogens, three carboxylate oxygens, and an amide oxygen coordinating.157 As mentioned in the gallium section, octreotide-conjugated 90Y-DOTATOC (90Y-L39-TOC) was found to have inferior biological behavior compared to its 67Ga analogue, possibly as a result of the higher coordination requirement of Y(III). According to solution NMR spectroscopic data, a similar complex with a DOTA (L39) p-aminoanilide was found to retain the chelator’s full N4O4 coordination mode.47 The consequence of substituting a carboxylate pendant arm with a phosphonate in a DOTA (L39) derivative, DO3AP (L43), can be seen in its yttrium complex geometry (Figure 62). A twisted square antiprismatic configuration (TSA twist angle ~ 25°) can be noted while the cation remains closer to the O4 plane (1.04 Å) than the N4 plane (1.52 Å).192
Figure 60.

[Y-DOTA]− (L39)
Figure 61.
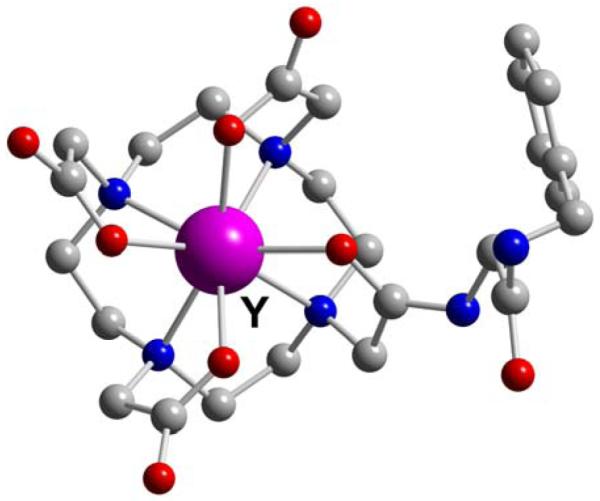
Y-DOTA-D-Phe-NH2 (L40)
Figure 62.

Y-DO3AP (L42)
A very interesting crystal structure of the yttrium(III) complex of a chiral DOTA (L39) derivative bound to the antibody 2D12.5 Fab has been reported,193 but no direct complex-protein interactions were found. Likely as a result of the symmetrical nature of this type of complex, only modest differences in binding affinities were found for enantiomers of opposite helicity. Additionally, C-functionalized DOTA (L39) chelators have recently been investigated as BFC’s for yttrium-86.194 The (S)-p-aminobenzyl derivative’s Y(III) complex was successfully separated into SA (major M) and TSA (minor m) isomeric forms which were assigned by solution 2-D NMR as well as CD spectroscopy.
The cyclam analog, TETA (L49), has been reported to bind Y(III), though with far less affinity than either DOTA (L39) or even the acyclic DTPA (L12) indicating a poorer cation/chelator match (Table 7).195 Both the labeling efficiency and acid inertness of 90Y-TETA (90Y-L49) (half life at pH 3.6 ~ 5 min) have been found to be inferior to that of its DOTA (L39) analogue (no decomplexation after 3 months).196 Although no Y-TETA (Y-L49) structure has been reported to date, it is likely to resemble that of Tb-TETA (Tb-L49) and (Eu-L49). Both of these were described as having a highly-distorted octadentate dodecahedral geometry with the metal cation significantly distended from the cyclam cavity.197,198
Although often used as a surrogate for Y(III) in 90Y-radiopharmaceutical studies, In(III) can exhibit appreciably different coordination chemistry due to its smaller ionic radius and typical coordination numbers of less than 8. Acyclic aminopolycarboxylates like EDTA (L10) and DTPA (L12) form far more stable complexes with In(III) than Y(III). By contrast, the octadentate DOTA (L39) binds Y(III) with more avidity. While Y-DOTA-D-PheNH2 (Y-L40) adopts the twisted square antiprismatic (TSA) solid-state geometry, In-DOTA-D-Phe-NH2 (In-L40) has the square antiprismatic (SA) configuration.157,199 These complexes also have distinct NMR spectral behavior in solution with only the indium complex showing fluxional behavior. Another Y-DOTA (Y-L39) derivative with a single amide pendant arm, Y-DOTA-AA (Y-L41), was observed to retain a rigidly octadentate chelator coordination sphere in solution at ambient temperature, while its In(III) analogue again exhibited dynamic behavior suggestive of an uncoordinated amide pendant arm.184 Consistent with the observation, this and related indium DOTA (L39) complexes were found to be more hydrophilic in their RP-HPLC retention data.184,200 Similar discrepancies were also noted for the corresponding DTPA-RGD (L12-RGD) and DTPA-BA2 (L13) complexes of these cations.178,201 Although potentially significant, no general conclusions about the relevance of these differences to the in vivo properties of these species can yet be made. Finally, the antibody 2D12.5 was reported to bind Y-DOTA (L39) with much higher affinity than In-DOTA (In-L39).193
2.7. Aqueous Zirconium(IV) Coordination Chemistry
With its high charge and small radius (59-89 pm for CN 4-9), hydrated Zr(IV) likely exists only at high dilution in very acidic solutions. Multiple monomeric as well as polynuclear oxo/hydroxy species are believed to be present before onset of precipitation as the pH is raised.202,203
In consequence of its extreme hardness, a strong preference of Zr(IV) for polyanionic hard donor chelators can be expected. This predilection can be seen in the very impressive stability constants of its EDTA (L10) and DTPA (L12) complexes (Table 8). A total coordination number of 8 is typical in their X-ray structures (Figures 63 and 64). The Zr-EDTA (Zr-L10) geometry approximates a dodecahedron including a hexadentate chelator,204 while DTPA (L12) is fully octadentate in its Zr(IV) envelopment.205 The use of ibritumomab tiuxetan, a MX-DTPA (MX-L12)-conjugated monoclonal antibody to chelate 89Zr has been reported. No solid-state structure of Zr-DOTA (Zr-L39) has yet been reported. Although its D2O solution 13C{1H} NMR spectral data were consistent with a time-averaged symmetric C4 species implying full N4O4 chelator coordination, its proton NMR spectrum actually revealed a lower symmetry species in equilibration with a second minor isomer.177
Table 8. Data for Selected Zr(IV)-Chelator Complexes.
| Chelator | Donor Set (Total CN) |
Cation Coordination Geometry | logKMLa | Reference |
|---|---|---|---|---|
|
L10,
EDTA |
N2O4 (8) | ~ dodecahedron | 27.7 29.4 |
589,600 |
|
L12,
DTPA |
N3O5 (8) | ~ dodecahedron | 35.8 36.9 |
587,600 |
|
L39,
DOTA |
N4O4 (8?) | square anti-prism(?) | 177 |
KML = [ML]/[M][L].
Figure 63.

Zr-EDTA (L10)
Figure 64.

Zr-DTPA (L12)
The hydroxamate-based iron chelator Desferal DFO (L23) and its derivatives have been frequently used to bind Zr(IV),19,44,206-210 yet no structural characterization or stability constant data of resulting complexes are available. With the current interest in zirconium-based radiopharmaceuticals, future investigations of other hard metal cation-binding chelators likely to have high affinity for Zr(IV) such as catecholates, and catecholamides should be timely. The family of tripodal hydroxypyridinone (HOPO) chelators developed recently for lanthanide complexation towards MRI applications also merits attention.211
2.8. Summary
Viable chelators for Cu(II) range from tetradentate N2O2, N2S2, and N4 ligands favoring square-planar coordination to fully encapsulating N3O3, N4O2, and N6 hexadentate ligands giving distorted octahedral binding modes. Especially high thermodynamic stabilities as well as acid inertness are realized using polyazamacrocycles and cryptands, often appended with ionizable carboxylate or phosphonate arms. Highly stable Ga(III) complexes have been attained with hexadentate chelators featuring N2O2S2, N2O4, N3O3, N3S3, N4O2, and O6 donor sets, usually in a pseudo-octahedral coordination geometry. Selected macrocyclic N3O3 and N4O2 chelator complexes also have remarkable inertness in strong acids. Although it is possible for the larger In(III) cation to adopt higher coordination numbers of 7 and 8, its most robust complexes are formed by hexadentate chelators similar to those for Ga(III) containing N2O2S2, N2O4, N3O3, N3S3, N4O2, and O6 donor motifs. Due to the higher coordination number requirement of Y(III), the octadentate lanthanide chelator DOTA (L39) provides an almost ideal fit with associated high affinity. Only limited thermodynamic, kinetic, and structural data are available for Zr(IV)-chelator complexes in comparison to the other four metals discussed in this review. These indicate a strong preference for octadentate chelators with hard anionic oxygen donors. Finally, as demonstrated from the examples contained in this section, the affinity between metal and chelator play an integral role in the development of useful radiopharmaceuticals.
3.0. Radioisotope Production
The availability of radiometals that are carrier free and radionuclidically pure is essential to the development of effective radiopharmaceuticals for diagnostic imaging and radiotherapy. While the production of Zr, Y, In, Ga and Cu radiometals require a thorough understanding of the nuclear reactions and decay schemes available, their processing methods rely upon an intimate knowledge of their aqueous solution chemistry. The next section describes these methods.
3.1. Production of Copper Radiometals
The production of copper radiometals has been an area of intense research since they offer a variety of half-lives and decay energies that are applicable to diagnostic imaging and radiotherapeutic applications. Additionally, depending upon the application, copper radionuclides can be utilized by facilities with limited resources.
Copper-62 (t½ = 0.16 h, β+: 98% Eβ+max: 2.19 MeV; EC: 2%) can be produced in a small cyclotron,212 and is the only generator-produced copper radionuclide, resulting from the decay of its parent, 62Zn. Zn-62 is produced by irradiation of an enriched Cu target with protons according to the nuclear reactions 63Cu(p,n)62Zn or 65Cu(p,4n)62Zn.213 Numerous 62Zn/62Cu generator configurations were produced before 1999, using a glycine solution or other aqueous and organic mixtures to elute the 62Cu in a form suitable for radiopharmaceutical preparation.214,215 Haynes and coworkers developed a method for the direct labeling of PTSM (L8) to produce a patient-ready dose using a generator system designed to simultaneously deliver the 62Cu eluate, NaOAc buffer and PTSM (L8) ligand solution into a dedicated syringe.216 While the life span of a 62Cu generator is only 24 h, a clinically useful dose can be prepared every 30 minutes, which can be economical for hospitals that do not have the resources for an onsite cyclotron. Additional improvements in generator preparation by Fukumura et al. have increased the probability of 62Cu use in the clinic.217
Copper 60 (t½ = 0.4 h, β+: 93% Eβ+max: 3.9 MeV and 3.0 MeV; EC: 7%, Eγ max: 2.0 MeV) is another potentially useful copper radionuclide since high quality images can be obtained due to the high percentage of positron decay, despite its high β+ energy and prompt gamma emission.218 Additionally, 61Cu (t½ = 3.3 h, β+: 62% Eβ+ max: 1.2 MeV and 1.15 MeV; EC: 38%, Eγ max: 940 MeV and 960 MeV) also has potential applications in medical imaging since its half-life is suitable for biological applications which occur between 1 and 4 hours after injection.218 Both isotopes can be produced using targetry systems which were designed for the production of 64Cu. Copper-60 can be prepared by the 60Ni(p,n,)60Cu reaction while 61Cu can be prepared by the 61Ni(p,n)61Cu or 61Ni(d,n)61Cu nuclear reactions219 on a biomedical cyclotron using 14.7 MeV protons or 8.1 MeV deuterons respectively and using natural or enriched nickel targets. Approximately 16.7 GBq of 60Cu and up to 3.7 GBq of 61Cu can be produced after the isotopic products are separated from the target material by ion exchange chromatography. However, the need for enriched target materials, which increases production costs, presents a significant limitation to the cost-effective production of these two radiometals and has prompted investigators to develop other production methods using the nuclear reactions 59Co(3He,2n)60Cu and 59Co(3He,n)61Cu, which can be carried out on Co foil using beam energies of 70 MeV.220-222
Copper-67 (t½ = 62.01 h, β−: 100% Eβ−max, 0.577 MeV; Eγ max: 0.185 MeV) decays by β emission. These particles have sufficient energy to penetrate small tumors, and the medium range half-life of 67Cu makes it an attractive radiometal for radioimmunotherapy with intact antibodies. It can be produced on a cyclotron or high energy accelerator using the nuclear reactions 65Zn(p,2p)67Cu and 68Zn(p,2p)67Cu or 65Zn(n,p)67Cu, respectively.223 While several different Zn target materials are used, 68Zn targets are preferred since their irradiation leads to substantial increases in 67Cu yields.224,225 However production yields are relatively low, since numerous contaminants such as 62Zn, 67Ga, 65Zn, 55Co, 58Co and 57Ni have to be removed by numerous ion exchange chromatography steps. Despite the availability of these purification methods, high specific activity 67Cu is still difficult to obtain because of the ubiquity of natural copper and zinc in the environment.224,226 The need for the high energy cyclotron or accelerator, which drives up the cost, has also prevented mass production of this radiometal. Accordingly, interest in the applications of this radiometal remains limited.227-235
The production and preparation of 64Cu (t½ =12.7 h, β+: 19% Eβ+ max, 0.656 MeV; EC: 41%; β−: 40%) has been extensively reviewed and discussed.236-238 Briefly, copper-64 can be prepared in a nuclear reactor by several reactions including 63Cu(n,γ)64Cu, though the product cannot be isolated in a carrier-free state. A second method which can lead to carrier-free 64Cu involves bombarding the target in a reactor with fast neutrons according to the nuclear reaction 64Zn(n,p)64Cu. Two other methods involve the nuclear reaction 64Ni(p,n)64Cu and 64Ni(d,2n)64Cu,239,240 which can be carried out on a biomedical cyclotron to yield carrier-free copper-64, but an enriched nickel target must be irradiated to increase the yield of copper-64,241,242 and has spurred the development of 64NiO targets to enhance 64Ni recovery.243 Recently, Obata and colleagues described the production of 64Cu using 12 MeV proton irradiation via 64Ni(p,n)64Cu.244 Automated processing and purification of the 64Cu by AG1-X8 anion exchange chromatography allowed for the recycling of the 64Ni target material and the generation up to 185 GBq of 64Cu depending upon target thickness.
Avila-Rodriquez et al. were able to simultaneously produce 64Cu and 61Co with a 11.4 MeV proton beam on a biomedical cyclotron using the 64Ni(p,n)64Cu reaction.245 A single chromatography step using an anion exchange column separated the chloride salts of Ni, Cu and Co, providing carrier-free 64Cu with higher specific activities than previously reported.237,244,246
While Van So et al. attempted to produce 64Cu from a 68Zn target,247 others have explored making 64Cu with 64Zn targets using the nuclear reaction 64Zn(d,2p)64Cu, but the production of other radionuclide contaminants has remained problematic.248-250 Recently, Kozempel and colleagues reported the production of no-carrier-added (NCA) 64Cu using the 64Zn(d,2pn)64Cu reaction with a 19.5 MeV deuteron beam.251 After irradiation the radioactive target material is subjected to dual ion exchange chromatography using a strong cation exchange resin to remove any Ga-based radio-impurities and a strong anion exchange column to retain the 64Cu and 64Zn target material, while allowing other impurities such as 24Na and 58Co to flow through the column. The 64Cu is then eluted in 2 M HCl while the Zn fraction is eluted in neutral water. Additionally, Hassanein and coworkers separated 64Cu from the zinc target using a gel matrix consisting of 6-tungstocerate(IV), which is highly insoluble in water and has a mean particle size of 230-464 μm.252 The matrix’s affinity for metal ions such as Zn(II) and Cu(II) is dependent on the hydrogen ion concentration in the column with the distribution of both cations decreasing with decreasing pH. In dilute acid the Cu ions have an increased affinity towards the matrix compared to Zn ions, which can be washed from the column using 1mM HCl while the 64Cu can be eluted in a carrier-free stat using 1 M HCl.252
3.2. Production of Gallium Radiometals
Three gallium radioisotopes have suitable properties to be used in radiopharmaceutical applications. 67Ga is used in SPECT and gamma scintigraphy while 66Ga and 68Ga are used in the development of PET radiopharmaceuticals. This next section will discuss the production and purification of these three radioisotopes.
Gallium-67 (t½ = 78.3 h, γ: 93.3 keV, 37%, γ: 184.6 keV, 20.4%, γ: 300.2 keV, 16.6%) is produced using a cyclotron by bombarding a zinc or copper target with protons using the 67Zn(p,xn)67Ga reaction,253,254 deuterons by the 67Zn(d,2n)67Ga reaction,255 alpha particles by the 64Zn(4He,n)67Ga or 65Cu(4He,2n)67Ga reactions.254,256 Additionally, it can be produced using a tandem NatGe-NatZn target, where separation of the 67Ga from the target material is accomplished by acid dissociation followed by resin-based chromatography on an organic polymer to achieve high radionuclidic purity.256 In some cases however, iron contamination can be problematic, but the use of a reducing agent such as TiCl3 can resolve this issue.257 Recently, 67Ga was produced from the irradiation of a target consisting of natural cobalt foil with 52 MeV 11B4+ and 73 MeV 12C6+ ions through one of the following reactions: 59Co(11B,3n)67Ge or 59Co(12C, 4n)67As.258 The 67Ge and 67As products are short lived radionuclides which decay to 67Ga after a short “cooling” time. Separation of the target material is achieved by liquid-liquid extraction using concentrated acid and trioctylamine (TOA), which is then back-extracted using a basic solution of DTPA or EDTA. Alternatively, 67Ga has been purified from cobalt target materials using alginate biopolymers,259 which are naturally occurring polymers extracted from microalgae, shrimp, crab and fungi that are known to bind strongly to metal ions.260 Upon addition of the radioactive target material, nearly 90% of the 67Ga is retained in the biopolymeric matrix while the cobalt target material is removed from the column using an aqueous solution of NaNO2. After all of the target material is removed, elution of the no-carrier-added 67Ga occurs using 0.1 M HCl, which is suitable for radiopharmaceutical applications. In many medical centers that have PET scanners, the use of 67Ga has been replaced by [18F]FDG-PET.261
Gallium-68 (t½ = 67.71 min, β+: 89%, Eβ+max, 1.9 MeV; EC: 11%, Eγ max: 4.0 MeV) is an important positron emitting radiometal that is produced by electron capture decay of its parent radionuclide 68Ge (t1/2 = 270.95 days),159 and it can be produced from a compact generator system which contains the parent radionuclide. The 68Ge/68Ga generator system provides a continuous source of Ga-based PET radiopharmaceuticals for approximately 1 year, it has been extensively reviewed,262-264 and numerous commercial systems are available. Cyclotron Co. (Obninsk, Russia) has recently produced a popular TiO2-based generator system which can contain 370-18500 MBq 68Ge.159 This system is eluted with 0.1 M HCl to provide the user with ionic 68GaCl3; however, there are drawbacks, including 68Ge breakthrough, large eluate volume, high HCl concentration and the presence of metallic impurities such as Zn2+, Ti4+ and Fe3+, which can lead to difficulties in synthesizing the 68Ga radiopharmaceutical. To circumvent these difficulties research groups have explored purification protocols using anion and cation exchange micro-chromatography264,265 and fractionation,266 while additional work has sought to develop facile synthetic routes to radiopharmaceuticals that complement this generator system including infrared (IR) and microwave supported syntheses.265,267
Gallium-66 (t½ = 9.49 h, β+: 56.5%, Eβ+ max, 4.15 MeV; EC: 43.5%, Eγ max: 4.0 MeV) is another radiometal that is relevant in nuclear medicine and molecular imaging applications. As a positron emitter it can be used in PET imaging, and its longer half-life allows for data collection at later time points, which is not possible with the positron emitting isotope 68Ga. Gallium-66 can be produced and purified using methods similar to those that have been described for 67Ga258 (vide supra). An alternative method for 66Ga production has been accomplished using the 66Zn(p,n)66Ga nuclear reaction on a small biomedical cyclotron.268 This publication also compared purification of the 66Ga by cation exchange chromatography with the traditional purification method involving diisopropyl ether extraction using 20% TiCl3 in 3% HCl. The authors report that using cation exchange chromatography to purify the 66Ga has several advantages. Firstly, 200 mCi of 66Ga can be prepared and purified in this manner. Secondly it can be automated, and the processing time was more than twice as fast when compared to the diisopropyl ether extraction protocol. However a potential limitation lies in the high concentration of metal impurities in the cation exchange column, which will need to be reduced if this process is to be used to regularly prepare 66Ga radiopharmaceuticals. Additionally, although the longer half-life of 66Ga compared to 68Ga provides more flexibility with respect to the types of biomolecules to be investigated and the amount of time required for radiochemistry, the very high energy of the positron (4.15 MeV) as well as a high energy gamma emission (4.0 MeV) provide a prohibitively high absorbed dose to the subject being imaged, as well as to the personnel working with this radiometal. These intrinsic physical properties of this radiometal have severely limited its practical use.
3.3. Production of Indium Radiometals
The production, purification and application of indium radiometals are active areas of research in the nuclear medicine, radiochemistry and molecular imaging communities. While numerous reports exist which involve the use of 110In269,270 110mIn271 and 114mIn272, this review will focus on the production, purification, and radiopharmaceutical applications of 111In, since it is the most widely used and extensively studied indium radiometal.
Indium-111 (t½ = 2.8 d; γ: 171 keV, γ: 245 keV (EC 100%)) is produced commercially by irradiating a natural cadmium target with energetic protons according to the reaction 111Cd(p,n)111In or 112Cd(p,2n)111In, and both remain the most widely used production methods of 111In.273 Indium-111 can be separated from the cadmium target by ion exchange or solvent extraction, with both techniques providing similar yields.270 Alternatively, 111In can also be produced in an accelerator by irradiating a rhodium target with 12C ion beam274 or irradiating silver targets with 55 MeV 11B ions by the 107,109Ag(11B,3pxn)111In nuclear reaction.275 In this process, the 111In is separated from the target material by first dissolving it with HNO3, aliquat-336 (a phase transfer catalyst), and 0.001 M HCl, which are used to separate the silver target and the radiometal. A second extraction step involving 1 M HCl and 0.1 M trioctylamine (TOA) separates the 111In, which remains in the organic phase while impurities such as 116/117Te, and 116/116m/117Sb remain in the aqueous phase. A final extraction using a basic solution of 0.1M EDTA isolates the 111In in a carrier free state.
3.4. Production of Yttrium Radiometals
The most abundant source of yttrium (Y) is found in the naturally occurring isotope 89Y. Additionally there are numerous radioisotopes of Y, which are synthetically produced during the nuclear fission process and have half-lives that range from nanoseconds to months. For example, 88Y (t½ = 106.62 days) is produced by irradiating a strontium target with 16 MeV protons or a rubidium carbonate target with 7.3 MeV deuterons, 19 MeV 3H ions and 12.4 MeV 4He ions.276 This radiometal has been used in place of 90Y for determining biodistribution of radio-yttrium compounds,277 is often considered invaluable as an active component of the 88Y-Be photoneutron source,278 and is important in the characterization of electrical materials. Additionally, 87Y, which serves as the parent radionuclide in a 87Y/87mSr generator, can be prepared by irradiating a rubidium target with alpha particles or by irradiating an enriched 87Sr target with protons.279,280 While a discussion of the production and purification of all of the Y radiometals is beyond the scope of this text, the remainder of this section will be devoted to the production and purification of the most medically relevant yttrium radiometals 90Y and 86Y.
Yttrium-90 (t½ = 64.1 h, β−: 100%, Eβ−max = 2.3 MeV) is currently used as a therapeutic radionuclide in nuclear medicine because it has a half-life of 64.2 h and emits β− radiation (Eβ−max = 2.28 MeV) with no accompanying gamma rays, reducing the received radiation dose to both patients and hospital staff.281 Yttrium-90 can be produced by (n,γ) reactions on yttrium metal or yttrium oxide, but the resulting product is obtained with a low specific activity.281 Additionally, it can be produced by the 90Zr(n,p)90Y reaction in a nuclear reactor.281 Following irradiation of the Zr starting material, it is extracted with HNO3 and mandelic acid leaving a solution containing the 90Y product and the 90 Sr parent, which can be separated from the 90Y product by its retention on a DOWEX cation exchange column. The radiochemical yield of this method is 90±8%.
Since large quantities are needed for nuclear medicine applications, the majority of the 90Y obtained by hospitals is from facilities that manage nuclear materials produced within fission reactors. In this fissile material, 90Y is associated with its parent 90Sr (t½ = 29 years), which must be separated in sufficient purity to meet hospital quality control standards. This has previously been accomplished with the use of co-precipitation282 or solid-supported solvent-extraction chromatography.283
Recently, Happell et al. used nuclear track micro filters (NTMF) as supported liquid membranes by impregnating them with 1:1 bis-(2-ethylhexyl)-phosphate (HDEHP) and tributylphosphate (TBP).284 90Y separation from its parent 90Sr is achieved by its selective transport through the pores of the impregnated NTMF. While initial 90Y recovery was good, the system lacked long term stability and 90Sr breakthrough was problematic.
Although 90Y is becoming more commonly used as a radiotherapeutic, not every hospital has access to 90Y on a routine basis. However, since 90Y and 90Sr have such distinctly different half-lives, they represent a daughter/parent radionuclide combination that is ideal for incorporation into a generator system. Numerous generator systems have been developed where the 90Sr is adsorbed onto a solid support and the 90Y is eluted using lactate,285 methanol and acetate,286 oxalate,287 citrate288 or EDTA.289,290 Recently, Chakravarty et al. developed the Kamadhenu generator.291 This 90Y generator uses a dual electrochemical separation technique to separate 90Y from the 90Sr parent in very high radionuclidic purity by performing both electrolytic steps potentiostatically, and the constant voltage minimizes the level of 90Sr contamination to 30 kBq per 37 GBq 90Y, which is well below 74 kBq, the upper limit of exposure defined by international regulatory agencies. This method offers several advantages over conventional column based generator systems. Firstly, this generator can constantly be supplied by a 90Sr feed solution, and there is an insignificant loss of 90Sr except by natural decay. There is minimal radioactive and non-radioactive waste production, and minimal amounts of chemicals are used in the entire process. Finally, the 90Y is obtained in acetate buffer making it suitable for labeling biological molecules without further modifications.
Yttrium-86 (t½ = 14.7 h, β+: 34%, Eβ+max = 1.2 MeV) has gained attention as a new imaging surrogate for 90Y and as a positron emitting radioisotope for diagnostic PET imaging because of its favorable half-life and decay characteristics. This increased demand for 86Y has led to an intense research effort to develop efficient and high yielding production methods. Several methods for the production and purification of 86Y have been investigated which involve irradiation of germanium targets with accelerator produced heavy ion particles such as 15 MeV 16O+6,292 but most 86Y production protocols have used SrCO3 or SrO as the target material and protons with energies from 8-15 MeV. Separation of the 86Y has been accomplished using co-precipitation and ion exchange chromatography,293 ion exchange chromatography,294,295 a Sr-selective resin,296,297 and electrolysis, which separated the 86Y from the SrCO3 target material resulting in high yields of no-carrier added 86Y in only 2 hours.298
Yoo et al. published a report that described the production of 86Y using either an enriched SrCO3 or SrO target on a small biomedical cyclotron via the 86Sr(p,n)86Y nuclear reaction.299 The authors demonstrated that the use of SrO coated onto a platinum disk target and two electrolytic steps allowed for a more efficient preparation of 86Y with 99% of the available 86Y being recovered. Specific activities with this method were observed to be as high as 282 GBq/μmol, and since no co-precipitates are formed or exchange resins are used in the purification process, the introduction of carrier species is greatly reduced. Furthermore, electrolysis requires fewer steps, is easier to handle, can be adopted for automation and can produce no-carrier-added (NCA) 86Y sufficient for biological studies in less than three hours. Recently, further modifications to the electrolytic procedures have been published which have optimized the electrochemical separation of 86Y, resulting in reliable yields of 91% in only 60 minutes.300
3.5. Production of Zirconium Radiometals
Several isotopes of Zr can be produced on a cyclotron using a variety of nuclear reactions with particle energies between 5-85 MeV, and include 86Zr (t½ =17 h, γ: 100%, Eγ = 241 keV), 88Zr (t½ = 85 d, γ: 100%, Eγ = 390 keV) and 89 Zr (t½ = 78.4 h, β+: 22.8 %, Eβ+max = 901 keV; EC: 77%, Eγ = 909 keV).301 However, for radiopharmaceutical development and radioimmunotherapy applications 89Zr has received considerable attention owing to its favorable decay characteristics and longer half-life, which make it useful in the labeling of antibodies for PET imaging at relatively long time points. A caveat to 89Zr is the high abundance of the 909 keV gamma photons, which contribute greatly to the absorbed dose of 89Zr radiopharmaceuticals, especially in slower clearing agents such as mAbs.
Zirconium-89 was first produced by Link et al. in 1986 by a (p,n) reaction by bombarding 89Y on Y foil with 13 MeV protons.302 After irradiation, 89Zr was purified by a double extraction protocol. The target was first mixed with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione (TTA) in xylene to remove the Zr(IV) from the foil followed by a second extraction step involving HNO3/HF to return the Zr(IV) to the aqueous phase. Anion exchange using 1M HCl/0.01 M oxalate completes the purification procedure to afford 89Zr in an 80% yield and with a purity of 99.99 %. A similar method reported by Dejesus et al. yielded similar results.303
A third method of production involves the irradiation of 89Y pellets, prepared from high purity powder, with 16 MeV deuterons (89Y(d,2n)89Zr reaction).304 Unlike the previous two methods, which relied upon solvent-solvent extraction as a means to purify the 89Zr, this method relied solely upon the differences in the distribution coefficients of these two elements on an anion exchange resin at different HCl concentrations. The authors reported that the overall radionuclidic yield was 80%. The purification protocol is simpler than the procedures proposed by Link, but the precipitation of YCl3 before and after anion-exchange chromatography has been problematic.305
Finally, using a Philips AVF cyclotron, Meijs and coworkers were able to produce 89Zr using the (p,n) reaction, 14 MeV protons and 89Y (300 μmol), which was sputtered onto a natural copper target that was cooled by water during bombardment.306 These simple modifications led to the production of 8140 MBq 89Zr after 2 h. After oxidation of the 89Zr to the M4+ oxidation state using H2O2, it was separated from other metal impurities such as 89Y, 88Y, 65Zr, 48V and 56Co using an anion exchange column chemically modified with hydroxamate groups. Hydroxamate was chosen because of its ability to form complexes with 89Zr under highly acidic conditions, which allows the 89Zr to be retained in the column while the other metal impurities are removed with highly concentrated HCl. The purified 89Zr can then be eluted in 95% yield using 1 M oxalic acid, which is removed by sublimation under vacuum. However, newly established purification and labeling protocols have improved the separation efficiency of the hydroxamate column and rendered this sublimation step obsolete.19
4.0. Applications of Zr, Y, Ga, In and Cu Radiopharmaceuticals
As new production and processing methods of Cu, Ga, In, Y and Zr radiometals become available, their use in radiopharmaceutical development has allowed researchers and physicians to utilize the variable half-lives and emission energies to explore a variety of research problems including imaging multidrug resistance143,307,308 and treating refractory arthritis and synovitis.309,310 However, a thorough discussion of every application is beyond the scope of this text. To make this review more manageable for the reader, only applications involving oncology, gene expression, infection and inflammation and hypoxia and perfusion will be emphasized. A representative summary of applications can be found in Table 9.
Table 9. Biologically Targeted Radiopharmaceuticals Developed with Zr, Y, Ga, In: and Cu Radiometals.
| Metal | Bifunctional Chelator |
Targeting Molecule |
Target | Preparatory Notesa |
|---|---|---|---|---|
| Zr | L23, DFO | antibody | HNSCC19,208 Met expression359 non-hodgkin’s lymphoma45 |
Labeling in oxalate solution requires a strong, indifferent buffer (HEPES (pH 7.2)) required. |
| EGFR44 | ||||
| VEGF210 | Tetrafluorophenyl ester modified DfO facilitates coupling to targeting molecule. |
|||
| Y |
L14, CHX-
A”-DTPA |
peptide antibody |
SSTR433 Lewis Y antigen603 |
acetate buffer (pH 5.5), 25°C |
| L39, DOTA | peptide ODNS |
MC-1R500 GRPR461 gene expression194,509 |
acetate buffer (pH 5.5-7); reaction temperature: 60-90°C (peptides) 25-37°C (antibody) |
|
| DOTA-NHS facilitates coupling to peptides and antibodies. |
||||
| L23, DFO | peptide | folate receptor347 | Tris buffered saline (pH 7.4), 25°C, 24 h is suggested. |
|
| Ga | L11, HBED | peptide antibody |
EGFR137 MUC1136 |
Tetrafluorophenyl ester modified HBED facilitates coupling to targeting molecule. |
| Low final pH (4.1-4.5) is necessary for Ga complexation. |
||||
| L39, DOTA | peptide ODNS |
MC-1R502 GRPR.459,604 K-ras oncogene.511 EGFR479 |
Anion exchange chromatography can be used to concentrate generator eluates.605 |
|
| SSTR 268,439,441-443,605,606 | The use of acetate or HEPES buffer is suggested. |
|||
| The final pH of the reaction solution should be 4-5.5. |
||||
| High reaction temperatures required (85-100°C). |
||||
| Microwave heating reduces reaction time. |
||||
| L29, NOTA | peptide | SSTR154 integrin αvβ3395,401 |
Labeling can occur at 25°C over a pH range of 3.5-6 in less than 30 min. |
|
| L12, DTPA | peptide ODNS/PNA peptidomimetic antibody |
GRPR456 EGFR449,473,474 p21WAF-1CIP-1510 mRNA515 |
Labeling can occur at 25°C with reaction times no longer than 60 min. |
|
| LTB4R.523,607 SSTR9 |
Acetate buffers (pH 5.5-6.5) are recommended. |
|||
| In | L39, DOTA | peptide peptidomimetic |
GRPR457 MC-1R.496 SSTR9,407 |
Final solution pH should be 5.0-7.0. Acetate buffer is recommended. |
| integrin αvβ3 383,386 | ||||
| integrin α4β1 378 | Reaction conditions are 30-60 min. at 65-100°C. |
|||
| L12, DTPA | peptide peptidomimetic |
inflammation | Acetate buffer (pH 5.5) is recommended. |
|
| Final solution pH is 4.5-5.0. | ||||
|
L14, CHX-
A”-DTPA |
antibody | hEGFR-2608 | Acetate buffer is recommended. |
|
| Reaction conditions are 30-60 min. at 25°C. |
||||
| Cu | L39, DOTA | peptide antibody peptidomimetic |
integrin αvβ3 397 αvβ6 376 VEGFR(various)489 490,493 |
Acetate buffer recommended. Final solution pH should be 5.5-8.0. |
| MC-1R.500,501 EGFR480 |
Reaction temperatures are 25- 100°C depending on conjugate. |
|||
| GRPR466 461 464,465 | ||||
| SSTR7,425,609 | PD-10 or Waters SepPak light C18 can be used for antibody or peptide purification, respectively. |
|||
| L49, TETA | peptide | SSTR431,610,611 | Acetate buffer recommended (pH 5.5-6.5). |
|
| Reaction conditions of 60 min. at 25-37 °C are recommended. |
||||
| Gentisic acid (1mg/mL) can be used to counteract radioloysis. |
||||
|
L57, CB-
TE2A |
peptide peptidomimetic |
integrin αvβ3 399,611 integrin αvβ6376 MC-1R504 |
Acetate buffer (pH 7.5-8.5), 95°C; 1-2 h is recommended. |
|
|
L62,
DIAMSAR |
peptide | αvβ3121,399 | Acetate buffer (pH 5.0-8.0) 25°C; 60 min. is recommended. |
|
| L29, NOTA | peptide | GRPR462 467,468,612 | Acetate buffer; final pH 6.5 60 min. at 70 °C is recommended. |
Presented as an initial reference for radiopharmaceutical preparation.
4.1. Oncology
Each year 10.9 million people are diagnosed with cancer worldwide.311 Cancer is caused by the effects of genetic mutation and environmental stress, either individually or in concert. Additionally, the aggressiveness and responsiveness to therapy displayed by each malignancy varies, and while satisfactory progress continues to be made in the successful diagnosis and treatment of some cancers, the overall success rate remains low. While several of the radiometals such as 67Cu227-235 and 90Y312-345 are used in oncology as radiotherapeutics, the remainder of this discussion will focus on the incorporation of In, Cu, Ga, Y, and Zr radiometals into radiopharmaceuticals for the diagnostic imaging of cancer. Moreover, while numerous radiopharmaceuticals have been prepared to detect biomarkers such as the folate receptor,166,346,347 the neurotensin receptor,348,349 oxytocin receptor expressing tumors,350 apoptosis using Annexin-5,351-355 Urokinase-Type Plasminogen Activator Receptor (uPAR),356 matrix metalloproteinases (MMPs),357,358 Met-expression,359 antigens in head-and-neck cancer,208,360-362 lymphoma,363-371 non-Hodgkin’s lymphoma,45 and esophageal and pancreatic cancer,372,373 the remainder of this discussion will be further confined to several biomarkers which have received the most attention and include the integrin αvβ3, the somatostatin receptor, the gastrin-releasing peptide receptor, melanoma and melanocortin-1 receptor, HER-2/neu receptor, VEGF receptor and EGF receptor.
4.1.1. Integrin Imaging
Integrins are a family of cell surface heterodimeric transmembrane glycoproteins that mediate the attachment of cells to the extracellular matrix.374,375 They consist of α and β subunits which are needed for adhesion to the extracellular domain. The cytoplasmic domain of both subunits anchors the cytoskeleton to the plasma membrane and is required to mediate signaling events. While imaging agents that target several different integrins have been reported,376-378the most widely investigated in this superfamily of proteins has been the vitronectin receptor, αvβ3, since it has been observed to be involved in tumor growth, local invasiveness, metastatic potential, and it is highly expressed on endothelial cells undergoing angiogenesis.379,380 While several different protein, peptide and peptidomimetic motifs have been used to image this biomarker,200,381-387 the vast majority of published reports have targeted this receptor using an arginine-glycine-aspartic acid (RGD) peptide motif (Figure 65). For example, Yoshimoto et al. prepared 111In-DOTA(L39)-c(RGDfK)388 and demonstrated that this radiopharmaceutical had rapid tumor uptake and renal clearance in SKOV-3 human ovarian carcinoma cells implanted in female BALB/c nu/nu mice, while van Hagen et al. used 111In-DTPA(L12)-c(RGDyK) to investigate the imaging of neoangiogenesis in CA20948 rat pancreatic tumors.389 Receptor-specific, but dose-dependant accumulation of the tracer was observed in these tumors with more accumulation being observed when the mass of the injected radiopharmaceutical was reduced. Additionally, several 64Cu-DOTA(L39)-RGD based radiopharmaceuticals have been developed to target αvβ3 expression in order to monitor dasatinib therapy390 and image glioma,391 inflammation,392 lung cancer,393 and teratoma.394 Additional research has been conducted to improve αvβ3 positive tumor targeting using RGD multimers,395 enhance the biokinetics of RGD based radiopharmaceuticals using PEG linkers396 and develop multimodal imaging agents for PET/MR397 or PET/NIR applications.398
Figure 65.
Selected RGD analogues used in αvβ3 targeting radiopharmaceuticals.
Since 64Cu-DOTA (L39) complexes were previously shown to be unstable in vivo,21 Wei and colleagues compared the properties of 64Cu-CB-TE2A(L57)-c(RGDyK) and 64Cu-DIAMSAR(L62)-c(RGDfD) in M21 (αvβ3 positive) and M21L (αvβ3 negative) melanoma bearing mice.399 These ligands were developed to form more kinetically inert Cu(II) complexes with the hope of preventing Cu transchelation in vivo. While both radiopharmaceuticals exhibited similar binding affinities for αvβ3 integrin, 64Cu-CB-TE2A(L57)-c(RGDyK) demonstrated better tumor targeting at all time points examined, and demonstrated more rapid liver and blood clearance resulting in lower liver and blood concentrations at 24 h p.i.
In another interesting application using CB-TE2A(L57), Sprague et al. used the radiopharmaceutical 64Cu-CB-TE2A(L57)-c(RGDyK) to image osteoclasts, which express high levels of αvβ3 integrin and are a component of osteolytic bone metastases.400 This radiopharmaceutical was observed to have a 30-fold greater affinity for αvβ3 than αvβ5, and was observed to bind to osteoclasts through αvβ3-mediated interactions. Biodistribution and small animal imaging of a pharmacologically induced mouse model of osteolysis demonstrated increased uptake at the mouse calvarium, which was the site of pharmacological induction (Figure 66). This increased uptake could be blocked with a co-injection of c(RGDyK) demonstrating that imaging of αvβ3-expressing osteoclasts was possible.
Figure 66.
Small-animal PET/CT of PTH-treated mice. Calvarium uptake of 64Cu-CB-TE2A-c(RGDyK) was higher in PTH-treated mice (7.4 MBq [199 mCi],115 ng, SUV 0.53) than in control mice (7.7 MBq [209 mCi], 121 ng, SUV 0.22) (50- to 60-min summed dynamic image). (A) In PTH-treated mice, uptake was reduced in all tissues, including calvarium, after injection of c(RGDyK) (PTH[left]: 159 mCi, 84 ng, SUV 5 0.33; block [right]: 164 mCi, 87 ng, SUV 5 0.18) (static image obtained 60 min after injection, 10-minscan). (B) Arrowheads indicate calvarium of each animal. Fiducials (*) are indicated. Reprinted with permission from reference 400. Copyright 2007 Society of Nuclear Medicine.
Gallium-based imaging agents have been developed for imaging αvβ3. Jeong et al. labeled NOTA(L29)-c(RGDyK) with 68Ga.401 Although some purification was necessary, the purified complex could be obtained with a specific activity of 17.4 GBq/μMol, and biodistribution studies in nude mice bearing human colon cancer (SNUC4) xenografts demonstrated appreciable receptor-mediated tumor uptake. Additionally, small animal PET studies were performed, which demonstrated excellent tumor uptake that was significantly reduced by the coadministration of c(RGDyK); however, quantification of the microPET imaging was not reported. In another report, Li et al. examined the differences between 68Ga-NOTA(L29)-(RGD)4, 68Ga-NOTA(L29)-(RGD)2, and 68Ga-NOTA(L29)-RGD.402 In all cases, nearly quantitative labeling of these conjugates was achieved, and specific activities of 12-17 MBq/nmol were achievable. The 68Ga-NOTA(L29)-(RGD)4 demonstrated the highest receptor affinity and tumor uptake based upon biodistribution and small animal PET studies, but it also demonstrated the highest kidney uptake, which can be problematic since the kidney is a dose limiting organ. Similarly, a recent study by Liu et al. examined the effects PEG and glycine linkers had on the affinity of RGD dimers for αvβ3 positive tumors xenografts.403
4.1.2. Somatostatin Receptor Imaging
Somatostatin (SST), which consists of 14 amino acid (aa) and 28 aa peptides, affects several organ systems including the central nervous system, hypothalamopituitary system, the gastrointestinal tract, the exo- and endocrine pancreas and the immune system.404 Currently five different somatostatin receptor subtypes have been discovered. While many human tumors express these membrane bound receptors, the subtype expression pattern is dependent on the origin and type of tumor.405 This information has led to a continuing flurry of activity, seeking to develop new diagnostic imaging agents for somatostatin receptors and evaluate them in preclinical and clinical settings.
Numerous somatostatin analogues have been prepared, conjugated to various chelators such as DTPA (L12) and DOTA (L39), labeled with 111In and have been evaluated in both laboratory and in the clinic (Figure 67). 9,406-425 de Jong et al. investigated tumor uptake of 111In-DOTA(L39)-Y3-octreotide as a function of injected radiopharmaceutical mass, which is an important factor when seeking to maximize tumor uptake while trying to minimize non-target organ radiotoxicity.426 111In-DOTA(L39)-Tyr3-octreotide was injected into male Lewis rats bearing CA20949 pancreatic carcinoma tumors with the mass of the radiopharmaceutical varying between 0.05-12 μg peptide. Higher specific uptake was observed only in somatostatin receptor subtype 2 (SSTR(2)) positive organs when the mass of the injected radiopharmaceutical was low, illustrating that this parameter is more important in determining radiopharmaceutical uptake in tumor and non-target organs than its specific activity. Another report by Lewis et al. described the evaluation of the somatostatin analogues 64Cu-TETA(L49)-Y3-OC, 64Cu-TETA(L49)-TATE, 64Cu-TETA(L49)-OC and 64Cu-TETA(L49)-Y3-TATE to correlate the in vivo changes in biokinetics of these molecules that occurred when a substitution of tyrosine for phenylalanine is induced at position 3 of the peptide sequence and when the C-terminal alcohol is oxidized to a carboxylic acid functional group.427 The analogue 64Cu-TETA(L49)-Y3-TATE displayed significantly higher affinity to somatostatin receptor positive CA20948 rat pancreatic tumor membranes and demonstrated up to 3.5-fold more tumor retention than the other tracers examined. These findings led to further investigations of this radiopharmaceutical as a diagnostic imaging agent using a non-human primate model428 and has led to preliminary dosimetry studies in a rat model.427 However the experimental evidence of copper transchelation in vivo has led to the use of other chelators that form more kinetically inert copper complexes.20 One such chelator currently being investigated is the cross-bridged chelator CB-TE2A (L57).429 To demonstrate its superiority, Sprague et al. prepared the radiopharmaceuticals 64Cu-CB-TE2A(L57)-Y3-TATE and 64Cu-TETA(L49)-Y3-TATE, which differ only in the copper chelator conjugated to the peptide.430 While both radiotracers had similar binding affinity to SSTR(+) AR42J tumor cell membranes, blood, liver and non-specific uptake was lower for the cross-bridged analog. Additionally at 4 h, the non-cross bridged tracer had 4-fold and 2-fold higher blood and liver uptake while 64Cu-CB-TE2A(L57)-Y3-TATE demonstrated 4-fold greater tumor uptake at the same time point. These interesting properties led Eiblmaier and colleagues to examine the difference in internalization of these two radiopharmaceuticals in A427-7 cells, which were stably transfected for the SSTR(2) to evaluate their radiotherapeutic and cell killing potential.431 Cellular internalization and efflux experiments demonstrated that 64Cu-CB-TE2A(L57)-Y3-TATE internalized more rapidly than 64Cu-CB-TETA(L49)-Y3-TATE, however more 64Cu-TETA(L49)-Y3-TATE localized in the nucleus leading the authors to conclude that the difference in kinetic inertness between the two 64Cu complexes led to the higher localization of activity in the nucleus.
Figure 67.
Selected somatostatin analogues used in somatostatin receptor targeting radiopharmaceuticals.
In 2004 Milenic, et al. reported the development of a DTPA (L12) analogue CHX-A”-DTPA, (L14), which forms stable complexes with a variety of radiometals and is capable of achieving rapid complex formation at room temperature.432 In order to evaluate this bifunctional chelator, it was coupled to octreotide (OC), labeled with 86Y and injected into rats bearing AR42J pancreatic tumors which over express the somatostatin subtype 2 receptor (SSTr2).433 86Y-CHX-A”-DTPA(L14)-OC demonstrated high tumor uptake along with uptake in the kidneys associated with the renal clearance of the agent. Imaging of the tumor was easily visualized using small animal PET at 4 h, but effective clearance from the tumor was demonstrated with only 25% of the activity remaining at 24 h compared to the 4 h time point in biodistribution studies.
Several 68Ga-radiopharmaceuticals have also been developed to the somatostatin receptor on neuroendocrine tumors.434-440 Henze et al. examined the diagnostic utility of 68Ga-DOTA(L39)-D-Phe1-Tyr3-octreotide in patients with meningiomas.441 During the study, rapid blood clearance and high uptake in the meningioma lesions of various sizes could be observed while no tracer accumulation was found in healthy brain tissue allowing for excellent visualization of these lesions. Additionally, Hofmann and co-workers evaluated 68Ga-DOTA(L39)-TOC in patients with somatostatin receptor-positive tumors, and compared their results to those obtained with 111In-DTPA(L12)-octreotide with planar and SPECT imaging.439 Based upon this study, 68Ga-DOTA(L39)-TOC was able to clearly delineate 100% of the lesions predefined with CT or MRI, and an additional 30% more lesions were detected when compared with SPECT using 111In-DOTA(L39)-octreotide. Conversely, only 85% of the lesions previously identified using CT or MRI were correctly identified with the gamma-emitting radiopharmaceutical demonstrating the utility of 68Ga-DOTA(L39)-TOC in PET imaging and preclinical experiments. Kayani et al. demonstrated that PET/CT using 68Ga-DOTA(L39)-TATE was superior to [18F]-FDG for imaging well-differentiated neuroendocrine tumors, but [18F]-FDG was more sensitive in detecting high-grade tumors.442 Additionally, the authors note that the combination use of 68Ga-DOTA(L39)-TATE, [18F]-FDG and PET/CT has the potential for more comprehensive tumor assessment in intermediate and high-grade tumors. Finally, Ugur et al. investigated the potential of 66Ga-DOTA(L39)-D-phe1-Tyr3-octreotide as a potential PET agent and radiotherapeutic for somatostatin receptor-positive tumors in AR42J rat pancreatic tumors implanted in nude mice.443 The authors radiolabeled this radiopharmaceutical precursor with 66Ga, 67Ga and 68Ga. Radiochemical purity of more than 95% was achieved for each radiotracer, and tissue biodistribution demonstrated comparable uptake for the 66Ga, 67Ga and 68Ga complexes. Small animal PET experiments demonstrated efficient tumor retention, but high radioactivity accumulation in the kidney also was observed, suggesting that effective measures to block renal uptake will be needed before therapeutic and imaging protocols using this radiotracer can be implemented.
Targeted radiopharmaceuticals having high specificity and affinity for individual SST subtypes have also been reported. Recently, Ginj et al. compared somatostatin receptor agonists and antagonists as targeting ligands and their specific affinity for the five different subtypes of the somatostatin receptor.444 The somatostatin receptor subtype 2 (SSTR(2)) selective antagonist sst2-ANT was determined to have a high affinity for the SSTR(2), which was not decreased by the presence of conjugated DOTA(L39) or a conjugated natIn-DOTA(L39) complex. Internalization of the SSTR(2) was not observed using immunofluorescence internalization assays even when the concentration of the antagonist exceeded 9 μM. Biodistribution studies using 111In-DOTA(L39)-sst2-ANT revealed high uptake and a slow clearance of the radiopharmaceutical in nude mice bearing tumors comprised of human embryonic kidney (HEK) cells that were stably transfected to only express SSTR(2). Wadas et al. further expanded upon this approach by developing the PET analogue 64Cu-CB-TE2A(L57)-sst2-ANT and comparing its properties as a PET radiopharmaceutical to the agonist 64Cu-CB-TE2A(L57)-Y3-TATE.445 Biodistribution studies revealed that the PET antagonist demonstrated rapid blood clearance but slower clearance from the liver and kidney. Both radiotracers demonstrated substantial uptake in SSTR(+) tissues and AR42J tumors, which was reduced upon the co-injection of blockade indicating that interaction between the radiopharmaceuticals and the tissues are a receptor-mediated process (Figure 68). 64Cu-CB-TE2A(L57)-sst2-ANT demonstrated higher tumor:blood and tumor:muscle ratios at the latest time point of 24 h and excellent tumor visualization during small animal PET imaging experiments, which was believed to reflect the antagonist’s increased chemical stability and increased hydrophobicity. Interestingly, the relative tumor uptake observed by Wadas and colleagues was much lower than what was reported by Ginj et al.444 In the studies by Ginj and coworkers, HEK229 cells that were stably transfected to express SSTR(2) were used, while Wadas and colleagues performed their studies using the SSTR(2) endogenously expressing AR42J rat pancreatic carcinoma cell line. This illustrates the variability in results that can occur when cell lines engineered for receptor expression are used rather than cell lines with a natural phenotype for the receptor of interest.
Figure 68.
(A) Representative small animal PET image at 4 h of rat injected with 64Cu-CB-TE2A-sst2-ANT. Left image is representative slice from small-animal PET/CT fusion image and right image is small-animal PET projection view of same animal. Calculated SUV for the tumor in left hind limb was determined to be 2.7 and SUV for tumor in right hind limb was determined to be 2.8. (B) Representative small-animal PET image at 4 h of rat injected with 64Cu-CB-TE2Asst2-ANT and sst2-ANT as blocking agent. Left image is representative slice from small-animal PET/CT fusion image and right image is small-animal PET projection view of same animal. In animal receiving blockade, SUV for tumor in left hind limb was calculated to be 0.74 and SUV for tumor in right hind limb was calculated to be 0.51. (C) Graphical plot of change in average SUV over time. Even after 24 h, SUV remains high, suggesting enhanced binding of 64Cu-CB-TE2A-sst2-ANT for SST2 receptor. (D) Graphical representation that demonstrates change in observed SUV when excess cold sst2-ANT is coinjected with radiopharmaceutical, indicating that binding of radiopharmaceutical to SSTR-positive tumor is receptor-mediated. Reprinted with permission from reference 445. Copyright 2008 Society of Nuclear Medicine.
4.1.3. HER-2/neu Receptor Imaging
HER-2/neu proto-oncogene, which is also called erbB-2 was first identified by transfection studies, when NIH3T3 cells were transformed with DNA from chemically-induced rat neuroglioblastomas.446 It encodes a protein that has extracellular, transmembrane and intracellular domains, and is over-expressed in 25-30% of human breast cancers. Approximately 90-95% of these cases are the result of uncontrolled gene amplification, which produces a malignant phenotype that correlates with shorter disease free survival and shorter overall survival.447 Several groups have attempted to image this biomarker by developing SPECT imaging agents such as 111In-labeled trastuzimab,140,448,449 or by developing multimodal imaging agents450 which will bind tumor cells that over express the HER-2/neu receptor. However, the slow elimination of intact monoclonal antibodies from the body yields low tumor/blood and tumor/normal tissue ratios and has forced researchers to investigate smaller targeting systems to improve the biokinetics of these imaging agents without sacrificing specificity. For example, Tang et al. prepared 111In-DTPA(L12)-trastuzumab Fab fragments with high radiochemical purity. Although, the measured Kd values for these ligands were 2-fold less than observed for trastuzumab immunoglobulin gamma (IgG), the maximum number of labeled cell sites was 1.5-fold greater.451,452 Moreover, biodistribution studies revealed significant tumor to non-target ratios but high kidney uptake after 72 h, and small animal imaging studies revealed the clear visualization of HER-2/neu positive BT474 human breast cancer xenografts implanted in athymic mice. Similarly, Dennis and colleagues developed 111In-DOTA(L39)-AB.Fab4D5, a Fab fragment derived from trastuzumab, which was designed to bind albumin and HER-2/neu simultaneously.453 Small animal SPECT imaging of MMTV/HER-2 transgenic mice, which develop mammary tumors expressing elevated levels of HER-2, revealed rapid accumulation of 111In-DOTA(L39)-AB.Fab4D5 in these tumors, which was comparable with other 111In-DOTA(L39)-trastuzumab Fab fragments, but had better kidney clearance. Finally, Orlova and co-workers described the radiolabeled affibody molecule 111In-DOTA(L39)-ZHER2:342-pep2.454 High uptake in SKOV-3 tumor bearing mice at 72 h p.i. was observed, but also unusually high kidney retention. Additionally, cell internalization properties of this imaging agent were examined by Wållberg et al., who demonstrated that between 20-40% of 111In-DOTA(L39)-ZHER2:342-pep2 was internalized in several HER-2/neu positive cell lines.455
4.1.4. Gastrin Releasing Peptide Receptor Imaging
Bombesin (BBN), a 14 aa peptide present in amphibian tissues and gastrin releasing peptide (GRP), its human analogue, which consists of 27 amino acids belong to a family of brain gut peptides that share very similar activities and occur primarily in the central nervous system.404 GRP receptors have been observed on a wide variety of human cancers including cancers of the GI tract, lung, prostate, breast and neuroblastomas,404 and represent attractive targets for radiopharmaceutical development. Consequently numerous bombesin analogues have been developed and labeled with In,456-458 Ga,459,460 Y461 and Cu461-466 radiometals in order to evaluate the influence of ligand or chelator structure on the biokinetics of these radiopharmaceuticals (Figure 69). For example, Hoffman et al. examined several DOTA(L39)-X-BBN[7-14]-NH2 peptides where X was a carbon spacer which was either 2, 5, 8 or 11 carbons in length, in order to optimize the pharmacokinetics for specific targeting of human cancers.457 Once synthesized, these compounds were radiolabeled with 111In and evaluated in cellular assays that utilized the human prostate carcinoma cell line (PC-3). Biodistribution studies on the 8 carbon analogue, which were conducted in SCID mice bearing PC-3 tumors, demonstrated rapid blood clearance with high tumor uptake occurring 15 minutes p.i. However, washout of the radiotracer was also rapid with only 20% of the injected activity remaining in the tumor by 24 h p.i. Garrison et al. further evaluated the pharmacokinetic changes induced by aliphatic, aromatic and polyethylene glycol ether functional groups in these analogues.458 Biodistribution studies and small animal SPECT/CT imaging demonstrated that radiopharmaceuticals containing aromatic groups as linkers exhibited the highest percentage of tumor retention 15 minutes p.i., but also exhibited higher uptake in the pancreas and in the GI tract especially at later time points, demonstrating that care needs to be taken when designing radiopharmaceuticals that require chemical linkers between the targeting molecules and the bifunctional chelator.
Figure 69.
Selected bombesin analogues used in targeting the GRP receptor.
Recently, Prasanaphich and colleagues explored the Bombesin-based radiopharmaceutical 64Cu-NOTA(L29)-8-Aoc-BBN(7-14)NH2 for the PET imaging of GRPR-expressing tissues.467 This bioconjugate demonstrated high affinity with an IC50 of 3.1 nM while in vivo biodistribution studies in PC3 tumor bearing mice demonstrated high uptake in tumor tissue with retention evident at 24 h p.i. Small animal PET/CT corroborated biodistribution studies demonstrating excellent image quality and tumor visualization especially when compared to the 64Cu-DOTA(L39) analogue. Additional work by the authors have sought to extend this radiopharmaceutical’s utility by examining its potential as an imaging agent for breast cancer.468
4.1.5. Epidermal Growth Factor Receptor Imaging
The Epidermal Growth Factor Receptor (EGFR) family consists of four related transmembrane receptor tyrosine kinases, which can be activated by a variety of ligands including epidermal growth factor (EGF), causing enhanced cellular proliferation, motility and evasion of apoptosis through downstream pathways.469 Its over-expression has been observed in up to 60% of human breast cancers with the receptor concentration on these cells being 100 times higher than on normal epithelial tissues, and several studies have associated this cellular phenotype with poor long term survival.470 While some groups have attempted to image other malignancies,44,471,472 extensive research has been conducted to target EGFR in an attempt to develop imaging agents for breast cancer. For example, Reilly et al. compared the biodistribution properties of 111In-DTPA(L12)-hEGF, where hEGF is a 53 aa peptide analogue of human epidermal growth factor, and 111In-DTPA(L12) conjugated anti-EGFR monoclonal antibody in cell lines that were known to have low (MCF-7), medium (MDA-MB-231) and high (MDA-MB-468) EGFR expression.473 As expected, both radiopharmaceuticals bound to MDA-MB-468 cells with high affinity, and the peptide-based radiopharmaceutical cleared more rapidly from the blood of the mice that were implanted with MDA-MB-468, MDA-MB-231 and MCF-7 tumors. However, 72 h after radiopharmaceutical injection, no direct correlation between the level of tumor uptake and EGFR receptor expression on the cell surface could be made, while localization of the peptide radiopharamcetical was up to 10-fold lower than that observed with the mAb-based radiopharmaceutical. Small animal imaging demonstrated relatively high accumulation of both radiopharmaceuticals in normal tissue such as liver and kidney and sufficient accumulation in MDA-MB-468 human breast cancer xenografts to visualize the tumor at 72 h p.i. Despite the differences in biokinetics, the mAb based radiopharmaceutical was deemed worthy of future study since its longer circulation time led to high tumor uptake and better tumor visualization at longer time points. Reilly and coworkers have sought to expand upon these interesting results by examining the radiotoxic effect of 111In-DTPA(L12)-hEGF on breast cancer cells over-expressing EGFR,474 and comparing the effects of 111In-DTPA(L12)-hEGF on normal and malignant tissues in EGFR-positive tumor-bearing mice.475 Additional reports from this group have evaluated the preclinical pharmacokinetic, toxicologic and dosimetric properties of 111In-DTPA(L12)-hEGF,476 have correlated the EGFR receptor diversity with nuclear importation,477 and described the development of a GMP compliant kit, which allows for the clinical preparation of 111In-DTPA(L12)-hEGF.478
A 68Ga-labeled analogue, 68Ga-DOTA(L39)-hEGF, was also described by Velikyan and colleagues to image EGFR expression in malignant tumors.479 This radiopharmaceutical demonstrated high affinity for EGFR on A431 cells and biodistribution results established significant uptake during small animal PET imaging; however, liver and kidney uptake were extremely high and may prevent further investigation of this complex as an imaging agent for hepatic metastases.
Investigations to monitor EGFR expression using 64Cu-DOTA(L39)-cetuximab have also been reported.480 Cai et al. reported the evaluation of 64Cu-DOTA(L39)-cetuximab in several tumor-bearing mouse models,481 and using Western blot analysis, a positive correlation was shown to exist between the expression of EGFR and uptake of 64Cu-DOTA(L39)-cetuximab. Li et al. also evaluated 64Cu-DOTA(L39)-cetuximab as a PET imaging agent for EGFR positive tumors.482 Using A431 cells it was determined that this radiopharmaceutical had a kd of 0.28 nm, while biodistribution and small animal imaging studies revealed good tumor uptake. More importantly, tumor-to-blood and tumor-to-muscle ratios were comparable to those values reported by Perk et al. using 89Zr-DFO(L23)-cetuximab44 and were observed to have a better biodistribution profile than an 111In-DTPA(L12) conjugated cetuximab at 48 h p.i.483 Eiblmaier and colleagues correlated EGFR expression with 64Cu-DOTA(L39)-cetuximab affinity and internalization in cervical cancer using cellular assays and microarray analysis of EGFR gene expression.484 EGFR mRNA expression was found to correlate with EGFR cell surface populations in five human cancer cell lines while the amount of internalized radiopharmaceutical correlated with EGFR mRNA expression and observed protein levels. This radiopharmaceutical demonstrated high uptake in Caski cervical cancer tumors 24 h after being injected into nude mice while small animal imaging revealed excellent image quality comparable to other investigations using 64Cu-labeled cetuximab.481
4.1.6. Vascular Endothelial Growth Factor Receptor Imaging
The Vascular Endothelial Growth Factor (VEGF) family of growth factors includes VEGF receptors A, B, C, and D, as well as Placental Growth Factor (PlGF). These ligand/receptor pairs are important for the directed proliferation and migration of endothelial cells that occur during embryonic development and other important biological processes such as wound healing and muscle growth.485 However, they also play a role in the pathological angiogenesis that accompanies the induction and progression of numerous malignancies. More importantly, several VEGF receptors have been shown to play critical roles in tumor progression, metastasis and angiogenesis and offer scientists a valuable target against which to develop molecular imaging agents.486,487 For example, Nagengast and coworkers evaluated 111In-DTPA(L12)-bevacizumab, a monoclonal antibody that recognizes all VEGF isoforms and blocks tumor angiogenesis, in nude mice bearing human SKOV-3 ovarian tumors xenografts. They demonstrated increasing tumor uptake even after 7 days, with appreciable activity still found in the blood, liver and kidney.210 This antibody was also labeled with 89Zr and compared to 89Zr-IGg in ovarian tumor xenografts.210 Tumors were easily visualized at 24 h p.i., and after 168 h p.i. accumulation of the 89Zr-bevacizumab was still observed to be increasing in the tumor (Figure 70). Based upon PET analysis, significantly more activity had accumulated after several days in the tumors of animals that received 89Zr-bevacizumab than those that received 89Zr-IgG. Ex vivo determination of tumor uptake correlated well with the in vivo data, and demonstrated that quantitative measurement of the tracer in the tumor was possible.
Figure 70.
Coronal CT image (A) with clear subcutaneous localization of SKOV-3 tumor (arrow). Fusion of microPET and CT images (B) (168 h after injection) enables adequate quantitative measurement of 89Zr-bevacizumab in the tumor. Reprinted with permission from reference 210. Copyright 2007 Society of Nuclear Medicine.
Others have explored the use of radiolabeled recombinant fusion proteins as imaging agents. Chan and coworkers described a novel recombinant fusion protein, composed of VEGF165 fused to human transferrin (hnTrf), which was used to chelate 111In.488 This radiopharmaceutical accumulated through receptor-mediated processes in highly vascularized U87MG glioblastoma xenografts allowing imaging as early as 24 h p.i. However, since the 111In was bound to hnTrf without the use of a traditional chelator, approximately 65% of the incorporated activity was observed to be transchelated under physiological conditions after 72 h. Additional work has focused on the preparation of 64Cu-DOTA(L39)-VEGF121,489 64Cu-DOTA(L39)-VEGFDEE, (which is specific for VEGFR2)490 and 64Cu-DOTA(L39)-QD-VEGF, which was developed as a multimodal imaging agent combining the PET reporter 64Cu-DOTA(L39) and the optical properties of quantum dots (QDs).491,492 Backer et al. developed a single chain, cysteine-tagged VEGF ligand, which is a fusion protein combining two fragments of human VEGF121 cloned head to tail.493 Upon folding, a single cysteine was engineered to be available for chelator conjugation so that the molecule could be radiolabeled with +2 and +3 metal radionuclides. This molecule was also engineered to contain a PEG moiety to increase its blood circulation time. After attaching the chelator DOTA(L39), the molecule was labeled with 64Cu and biodistribution was determined in female Balb/C mice with 4T1/luc murine mammary carcinoma cells implanted in their mammary fat pads. Data at 2 h p.i. showed prolonged blood circulation due to the addition of the PEG linker and appreciable tumor uptake. Unfortunately, kidney uptake was observed to be extremely high approaching 60% ID/g at this time point.
4.1.7. Melanoma Imaging
Malignant melanoma is the sixth most commonly diagnosed cancer and the most lethal form of skin cancer.494 To improve diagnosis and survival rates, the melanoma cortin-1 (MC-1) receptor has been a very promising melanoma-specific target for the development of imaging probes based upon the alpha melanocyte stimulating hormone (α-MSH), which binds this receptor with nanomolar affinity (Figures 71 and 72).141,495-503 Chen et al. synthesized several DOTA(L39) conjugated α-MSH analogues including DOTA(L39)-[Nle4, D-Phe7]α-MSH (NDP), DOTA(L39)-CCMSH, a linear analogue, DOTA(L39)-CMSH, a disulfide bonded analogue and DOTA(L39)-Re-CCMSH, a rhenium-cyclized analogue in order to optimize the in vivo biological properties through structure modification.496 Of these, 111In-DOTA(L39)-ReCCMSH had significantly higher tumor accumulation and displayed lower radioactivity accumulation in the kidney and other normal tissues when compared to the linear or disulfide cyclized analogues. This led Cheng et al. to expand on this work by examining the relationship between the structure of the rhenium metallopeptide and its biological properties.497-499 One derivative, 111In-DOTA(L39)-ReCCMSH(Arg11), which replaced the lysine at position 11 with arginine demonstrated slightly higher affinity than the 111In-DOTA(L39)-ReCCMSH in vitro, had significantly higher tumor uptake and lower kidney retention at almost every time point investigated when evaluated in a B16/F1 murine melanoma tumor model.
Figure 71.
Selected α-MSH analogues that target the melanocortin-1 (MC-1) receptor.
Figure 72.

Whole-body SPECT/CT, PET/CT and PET images of B16 melanoma tumor-bearing C57 mice 2 h post tail vein injection of radiolabeled CHX-A”-Re(Arg11)CCMSH. (A) SPECT/CT images of tumor-bearing mice injected intravenously with 12.95 MBq (350 μCi) of 111In-CHX-A”-Re(Arg11)CCMSH with (blocked) or without (nonblocked) a 20-μg nonradiolabeled peptide block. PET/CT and PET imaging of melanoma-bearing mice 2 h post tail vein injection of (B) 4.44 MBq (120 μCi) of 86Y-CHX-A”-Re(Arg11)CCMSH with a 20-μg NDP block (blocked) and without block (nonblocked) or (C) 3.7 MBq (100 μCi) of 68Ga-CHX-A”-Re(Arg11)CCMSH with (blocked) and without (nonblocked) a 60-μg NDP block, respectively. Tumor (T), kidney (K) and (BL) bladder locations are highlighted for each mouse. Reprinted with permission from reference 141. Copyright 2009 Elsevier Limited.
McQuade, et al. evaluated the analogous PET radiopharmaceuticals 86Y-DOTA(L39)-ReCCMSH(Arg11) and 64Cu-DOTA(L39)-ReCCMSH(Arg11) using a B16/F1 melanoma model.500 Biodistribution studies demonstrated that uptake in the tumor peaked at 30 minutes and remained stable after 4 h, but by 24 h tumor uptake had decreased by 94% and 68%, respectively. Even with significant clearance from the tumor at 24 h, small animal PET experiments revealed good tumor visualization due to low uptake in non-target tissues.
Wei et al. further modified this radiopharmaceutical by replacing DOTA(L39) with the 64Cu chelator CB-TE2A(L57), whose Cu(II) complex is highly kinetically inert (vide supra).504 While tumor uptake and retention was similar to that observed by McQuade and coworkers, the advantages of the cross-bridged chelator were clearly evident, since it exhibited more rapid renal clearance than either the 64Cu-DOTA(L39) or 86Y-DOTA(L39) analogues with lower accumulation in non-target tissues. This low level of background radioactivity provided excellent contrast resulting in high quality PET images, making 64Cu-CB-TE2A(L57)-ReCCMSH(Arg11) a promising melanoma imaging agent.
4.2. Imaging Gene Expression
While numerous cell surface molecules have been successfully targeted for imaging, they represent a small fraction of the proteome of a living cell.505 Since numerous intra- and intercellular regulatory proteins are implicated in cancer initiation and progression, molecular imaging of these biomolecules found within the cytoplasm and nucleus of the cell would provide valuable insight into the phenotype and aggressiveness of cancerous tumors506 and has become an active area of imaging research.
There are several reports in the literature of the use of 86Y, 68Ga and 64Cu radiometals to image mRNA expression.194,507-510 Schlesinger et al. investigated the application of 86Y labeled L-oligonucleotides.194,509 These mirror-image oligonucleotides have an extended biological half-life due to their high resistance to enzyme degradation. Labeled oligonucleotides had a radiochemical purity which fell within a range of 73-98% depending upon the isomer examined. Biodistribution of these systems in normal wistar rats revealed that the radiopharmaceuticals are renally excreted and that they demonstrate appreciable uptake in the adrenal glands, liver and bone. Additionally, Roivainen et al. labeled DOTA (L39)-conjugated antisense oligonucleotides, which were designed to target activated human k-ras oncogene with 68Ga, and these radiopharmaceuticals were evaluated in mice bearing A549 lung carcinoma tumors containing a mutated form of the k-ras oncogene and BxPC3 pancreatic adenocarcinoma cell tumors exhibiting wild type expression of k-ras.511 Radiolabeling did not alter the hybridization properties or protein binding of these systems and small animal PET imaging revealed exceptional visualization of tumors bearing WT k-ras expression, while tumors with the k-ras mutations were barely visible above background. Unfortunately, no quantification of the PET data was provided.
Wang et al. developed a DTPA(L12)-conjugated, 18-mer phosphothioated antisense oligodeoxynucleotide (ODNS) that could be labeled with 111In to image p21waf-1/CIP-1 gene expression at the RNA level.510 The p21waf-1/CIP-1 gene is a prominent early response gene for DNA damage, which encodes a cyclin-dependent kinase inhibitor that regulates progress through the eukaryotic cell cycle. These oligonucleotide imaging agents were evaluated in athymic mice bearing MDA-MB-468 tumor xenografts where the expression of p21waf-1/CIP-1 is induced following the subcutaneous injection of epidermal growth factor (EGF) directly into the tumor. Forty-eight hours after the induced p21waf-1/CIP-1 gene expression, biodistribution studies revealed tumor-to-muscle ratios that were approximately 3- and 2-fold greater than that seen in tumors where EGF induction was not performed or where random ODNS were injected, respectively. Moreover, tumors were easily visualized via gamma camera scintigraphy.
Developing protein nucleic acid based radiopharmaceuticals has also been investigated. Tian and co workers developed 64Cu-DOTA(L39)-PNA-peptide radiopharmaceuticals to image the over-expression of CCND1 mRNA, which is translated into cyclin D1, a key regulator in cell proliferation.512 This radiopharmaceutical was designed with dual specificity, containing a peptide sequence that binds to the insulin-like growth factor receptor (IGFR), which allows the radiopharmaceutical to be internalized into the cell, as well as a nucleic acid sequence that can hybridize to the CCND1 mRNA once in the cytosol. These 64Cu-DOTA(L39)-PNA-peptide radiopharmaceuticals were observed to be stable to transchelation challenge using 100-fold molar excess DTPA (L12), human serum albumin or cysteine at 22°C for 30 minutes, and once injected in vivo, no transchelation to proteins such as superoxide dismutase was observed. Additionally, appreciable tumor uptake was noted that could be blocked with a co-injection of insulin-like growth factor (IGF) and washout was observed to be rapid by 24 h. Moreover tumor-to-muscle ratios were not significantly different between cohorts that received the radiopharmaceutical and the cohorts that received the PNA or peptide mismatch, respectively. Small animal PET/CT imaging of MCF-7, ER+ breast cancer xenografts implanted in female SCID mice revealed significantly faster and higher uptake in these tumors than CCND1 scintographic probes previously described.513 Additional work in this area by this group has also led to the development of 111In-DTPA(L12) conjugated protein nucleic acid (PNAs) antisense molecules that could cross the blood-brain-barrier (BBB) and tumor cell membrane to image either rat glial fibrillary acidic protein (GFAP) or rat caveolin-1 α (CAV).514,515 Together these results demonstrate that it is possible to image endogenous gene expression in brain cancer with sequence specific PNA antisense radiopharmaceuticals conjugated to a drug targeting system.
4.3. Imaging Inflammation and Infection
The imaging of any infection or inflammatory foci which are characterized by enhanced blood flow, enhanced vascular permeability and an influx of white blood cells, has become a valuable tool that physicians can utilize to diagnose disease and monitor therapy.516 There are numerous examples in the literature of non-specific imaging agents that rely on the differences in vascular dilation between healthy and diseased tissue, or targeted radiopharmaceuticals that bind to specific targets present in the inflammation516-519 or infection413-417 process. For example, Lazzeri et al. exploited the high affinity between avidin and biotin for inflammation imaging in patients with skeletal lesions that included orthopedic conditions, osteomyelitis of the trunk, infection/inflammation of prosthetic joint replacements, and patients with suspected osteomeylitis of appendicular bones.520,521 This technique involves an infusion of avidin, which was allowed to localize at the infection site followed 4 h later by an injection of 111In-DTPA(L12)-biotin. Imaging was conducted at 30 minutes and 16-18 h p.i., and to assess its effectiveness it was compared to 99mTc-HMPAO labeled leukocytes scintigraphy. Based upon the results of this study, the 111In-biotin-avidin system was more sensitive at detecting lesions than 99mTc-leukocytes scintigraphy in patients with prosthetic joint replacement, osteomyelitis of appendiuclar bones and osteomyelitis of the trunk, and was determined to be more practical since these procedures reduce patient burden by 50%.
Receptor-specific radiopharmaceuticals that target the cells involved with immune and inflammatory processes have also been designed. For example, van Erd et al.522 and Broekema et al.523 investigated the new imaging agent 111In-DTPA(L12)-DPC11870, where DPC11870 is an antagonist of leukotriene B4 (LTB4) receptor, expressed on the surface of neutrophils (Figure 73). This molecule was evaluated in a model of acute colitis, which was induced in New Zealand white rabbits by infusion of trinitrobenzene sulfonic acid in the descending colon, and was compared to [18F]-FDG and 99mTc-HMPAO granulocytes, which measure metabolic activity and infection response, respectively. Based upon these experiments all three radiotracers revealed the inflamed colon upon scanning, but 111In-DTPA(L12)-DPC11870 was superior to both 99mTc-HMPAO-granulocytes and [18F]-FDG because it exhibited high uptake in the colonic lesions, while generating a low background signal in non-inflamed colon. Additionally, Bhargava et al. was able to label leukocytes with 64Cu using a dual chelator method, which demonstrated an equal efficiency to labeling leukocytes with 111In and was superior to labeling leukocytes with 18F-FDG,524 while Locke and colleagues examined 64Cu-DOTA(L39)-PEG-c(FLFLFK). This radiopharmaceutical targets the Formyl peptide receptor (FPR) located on leukocytes, and acts as an antagonist by selectively binding to neutrophils with a kd of 18 nM, but without inducing neutropenia.525 Small animal imaging studies were performed on mice that were exposed to Klebsiella pneumoniae. Eighteen hours after exposure small animal PET/CT and SUV analysis revealed a 5-fold increase in radiopharmaceutical uptake in animals exposed to the bacteria when compared to the control cohort, and this was corroborated by an examination of the lung myeloperoxidase activity which was determined in both groups of animals.
Figure 73.
The synthetic antagonist DPC11870 targets the leukotriene B4 (LTB4) receptor.
4.4. Imaging Hypoxia and Perfusion
Perfusion and hypoxia imaging have developed into robust research areas since they allow scientists and clinicians to monitor changes in blood flow and image changes in oxygen demand. Both have important implications in the in vivo imaging of myocardial infarction and neurological stroke since changes in perfusion often lead to changes in tissue oxygenation and ischemia.526,527 Imaging hypoxia also has important implications in cancer since tumor hypoxia is believed to increase a tumor’s metastatic potential while reducing its sensitivity to radio- and chemotherapeutic agents.528 Both perfusion and hypoxia imaging have been exhaustively reviewed.63,529-531
Numerous perfusion agents have been developed and evaluated using Ga and Cu radiometals because of their short half lives.532-537 Cutler et al. evaluated the small neutral lipophilic complex 68Ga-tris(2-mercaptobenzyl)amine (L2) as a potential coronary and neurological perfusion agent.538 Radiolabeling of this ligand with 68Ga was observed to be facile and biodistribution studies of this complex in rats demonstrated high uptake in brain and heart after 60 minutes. PET imaging of canines and non-human primates demonstrated that this imaging agent is taken up in normal myocardium and has the potential to be used as a diagnostic PET agent for cerebral blood flow.
Because of the short half lives of 60/61/62Cu, research to develop perfusion agents with these radiometals has also been explored, and the most extensively studied of these perfusion agents is (Cu-PTSM (Cu-L8). Its popularity is attributed to its higher reduction potential when compared to other copper thiosemicarbazone complexes, which have been developed to image tumor hypoxia (vida infra). This physical property facilitates the reduction of Cu(II) to Cu(I) by the normal electron transport chain found in the mitochondria of both normoxic and hypoxic tissues.535,536 This reductive process and its low membrane permeability after reduction explain its utility as a perfusion agent of healthy and diseased tissues. For example, Wallhaus and colleagues demonstrated that flow defects identified by 62Cu-PTSM (62Cu-L8) correlated well with angiography in patients with significant coronary artery disease and the results were comparable to those obtained with 99mTc-Sestamibi and 82Ru.539 Additionally, Flower et al. examined the possibility of using 64Cu-PTSM (64Cu-L8) to monitor blood flow changes in patients who presented with colorectal liver metastases and were prescribed vascular manipulation as a component of loco regional chemo- and radiotherapy. Efficacy rates were as high as 78% in patients receiving these treatments.540 Moreover, Holschenider and colleagues attempted to determine if 64Cu-PTSM (64Cu-L8) could be used to distinguish between active and passive brain function by comparing it to [14C]-iodoantipyrine, a traditional perfusion agent, in Sprague Dawley rats which were being subjected to a motor skills challenge after being injected with each tracer.541
While research had been conducted to develop Ga-based radiopharmaceuticals for hypoxia imaging,534,542,543 the vast majority has been focused on Cu radiopharmaceuticals, which are based upon the hypoxia-selective imaging agent Cu-ATSM (Cu-L9) first reported by Fujibayashi and colleagues.544 Since then, much research has been published on the non-radioactive chemistry of this complex in order to develop new ATSM (L9) derivatives,545-554 and elucidate the relationships between molecular structure and the hypoxia mechanisms active within the living cell.34,64,65,81,555-557 Additionally, ATSM (L9) has been labeled with several different Cu radiometals,558-561 and studied as a PET radiopharmaceutical for hypoxia imaging in lung cancer,562 cervical cancer563-566 and to monitor therapy response after laparoscopic surgery.567 Preclinically, it has been examined as an agent for targeted radiotherapy,62,568-570 examining multidrug resistant tumors,571 and in conjunction with perfusion imaging.572,573 Moreover, 64Cu-ATSM (64Cu-L9) uptake has been correlated with topoisomerase II expression,574 fatty acid synthase expression,575 tissue oxygenation576 and uptake of other PET tracers currently used in the clinic. For example, Dence et al. performed dual tracer experiments that combined autoradiography and small animal PET/CT to correlate the imaging information obtained from 64Cu-ATSM (64Cu-L9) imaging with those from 18F-FMISO, 18F-FDG and 18F-FLT which are clinically-approved tracers for imaging hypoxia, metabolism and cellular proliferation, respectively.577 64Cu-ATSM (64Cu-L9) correlated well with the 18F-FMISO and 18F-FLT, but not strongly with 18F-FDG. This provides a good example of how dual-tracer imaging can yield more detailed information to the clinician about the physiological processes occurring within the imaged tumor.
5.0. Conclusion
Metal-based radiopharmaceutical research has progressed rapidly since the first 99Mo/99mTc generator was produced more than 50 years ago. Since that time great strides have been made in radioisotope production, the coordination chemistry of radiometals, and in correlating the chemical structure of metal based radiopharmaceuticals with their behavior in vivo. For example, radio-copper chelate stability is paramount when developing tumor targeting radiopharmaceuticals so that optimum image quality can be achieved. However, the opposite is true when considering radio-copper radiopharmaceuticals for hypoxia and perfusion imaging. As a result researchers now have the ability to match the physical characteristics of a specific radiometal with the biokinetics of a particular biological targeting molecule leading to the development of diagnostic and therapeutic radiopharmaceuticals which can be tailored to individual disease processes. This impressive achievement has occurred not by chance, but by the dedicated and collaborative efforts of physicists, chemists, biologists, engineers and physicians. Furthermore, as this scientific discipline advances further into the 21st century, this collaboration must continue and is essential if the transition of more of these radiopharmaceuticals from bench to bedside is to occur.
7.0. Acknowledgements
The authors gratefully acknowledge Dr. Riccardo Ferdani, Ph.D. for assistance with the creation of Figure 1, the financial support of NIH grants R01 CA064475 (CJA), CA093375 (EHW) and F32 CA115148 (TJW) and the Gloria G. & Robert E. Lyle Professorship endowment (GRW).
6.0. Glossary
- %IDG
percent injected dose per gram
- aa
amino acid
- BBB
blood brain barrier
- BBN
bombesin
- BFC
bifunctional chelator
- CAV
rat caveolin 1-α
- CN
coordination number
- CT
computed tomography
- DBP
di-(n-butylphosphate)
- Df or DFO
desferrioxamine
- DMF
dimethylformamide
- EDC
1-ethyl-3-(3-dimethyl-aminopropyl)-carbodiimide
- EGF
epidermal growth factor
- Fab
fragment, antigen binding region
- Fc
fragment crystallizable
- FDA
Food and Drug Administration
- FDG
[18F]-fluorodeoxy glucose
- FLT
[18F]-fluoro-L-thymidine
- FMISO
[18F]-fluoromisonidazole
- FPR
formyl peptide receptor
- Fv
fragment variable
- GBq
gigabecquerel
- GFAP
rat glial fibrillary acidic protein
- GRP
gastrin releasing peptide
- GRPR
gastrin releasing peptide receptor
- h
hours
- HDEHP
bis-(2-ethylhexyl)-phosphate
- HEK
human embryonic kidney
- HMPAO
hexamethylpropyleneamine oxime
- HNSCC
head and neck squamous cell carcinoma
- hnTf
human transferrin
- HOPO
hydroxypyridinone
- hplc
high performance liquid chromatography
- IGF
insulin like growth factor
- IGFR
insulin like growth factor receptor
- IgG
immunoglobulin gamma
- kBq
kilobecquerel
- keV
kiloelectron volt
- LTB4
leukotriene B4
- LTB4R
leukotriene B4 receptor
- mAb
monoclonal antibody
- MBq
megabecquerel
- MC-1
melanocortin-1
- MC-1R
melanocortin-1 receptor
- MeV
megaelectron volt
- MRI
magnetic resonance imaging
- NCA
no carrier added
- NET
neuroendocrine tumor
- NIR
near infrared
- N-sucDF
N-Succinyldesferrioxamine B
- NTMF
nuclear track micro filters
- OC
octreotide
- ODNS
oligodeoxynucleotides
- p.i.
post injection
- PEG
polyethylene glycol
- PET
positron emission tomography
- PlGF
placental growth factor
- PNA
protein nucleic acid
- QD
quantum dot
- RIT
radioimmunotherapy
- RP-hplc
reversed phase high performance liquid chromatography
- SA
square antiprismatic
- SCID
severly combined immunodeficiency
- SPECT
single photon emission computed tomography
- SST
somatostatin
- SSTR
somatostatin receptor
- Sulfo-SMCC
sulfosuccinimidyl 4-(n-maleimidomethyl)cyclohexane-1-carboxylate
- TATE
octreotate
- TBP
tributylphosphate
- TFP
10-(3-(4-methyl-piperazin-1-yl)-propyl)-2-trifluoromethyl-10h-phenothiazine
- TOA
trioctyl amine
- TSA
twisted square antiprismatic
- TTA
4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- Y3-OC
tyrosine-3-octreotide
- Y3-TATE
tyrosine-3-octreotate
- α-MSH
alpha melanocyte stimulating hormone
- β−
beta - particle
- β+
positron
- γ
gamma ray
Author Biographies

Carolyn Anderson was born in Superior, WI in 1962, and remained there throughout her school years. In 1985 she graduated Summa Cum Laude with a B.S. in Chemistry from the University of Wisconsin-Superior. In 1984 she received a fellowship to attend the Summer School in Nuclear Chemistry at San Jose State University, and this is where her interest in nuclear and radiochemistry began. She pursued her Ph.D. in Inorganic Chemistry with Prof. Gregory R. Choppin, studying the electrochemistry and spectroscopy of uranium complexes in room temperature molten salts. On completion of her Ph.D. in 1990, she moved to Washington University School of Medicine (WUSM) in St. Louis, MO to carry out postdoctoral research with Prof. Michael J. Welch in the development of radiopharmaceuticals for PET imaging. In 1993 she was promoted to Assistant Professor of Radiology, and she is currently Professor in the departments of Radiology, Biochemistry & Molecular Biophysics and Chemistry. Her research interests include the development of radiometal-labeled tumor receptor-based radiopharmaceuticals for PET imaging and targeted radiotherapy of cancer and cancer metastasis. She greatly enjoys the productive collaboration with Edward Wong and Gary Weisman on the development of novel chelation systems for attaching metal radionuclides to biomolecules for nuclear medicine imaging applications.

Thaddeus J. Wadas was born in Nanticoke, PA in 1974. He received his B.S. degree in Biology from King’s College, Wilkes-Barre, Pa in 1996, and while working in the environmental science industry, completed his second major in Chemistry in 1998. After completing his second major, he pursued graduate studies at the University of Rochester, Rochester, NY where he received his M.S. and Ph.D. degrees in Chemistry under the supervision of Richard Eisenberg, Ph.D. His Ph.D. work focused on the synthesis and characterization of luminescent Pt(II) acetylide complexes for photo-induced charge transfer and light-to-chemical energy conversion. On completion of his Ph.D. he moved to the Washington University School of Medicine in St. Louis, MO to pursue postdoctoral studies with Carolyn Anderson, Ph.D. and develop targeted radiopharmaceuticals for diagnostic imaging and radiotherapy. In 2005 he was the recipient of a National Institutes of Health National Research Service Award (NRSA) Fellowship to study bone metastasis imaging with copper-64-labeled peptides, and in 2009 he was promoted to the position of Instructor at the School of Medicine. His current research interests include the application of combinatorial display methods to radiopharmaceutical development and understanding the respective roles gadolinium based contrast agents and renal insufficiency play in the development of Nephrogenic Systemic Fibrosis.

Edward H. Wong was born in Wuhan, China in 1946 but was raised in Hong Kong where he discovered the joy of mixing chemicals at St. Louis High School. He then majored in chemistry at the University of California at Berkeley and obtained his PhD doing boron hydride synthesis with William N. Lipscomb at Harvard University in 1974. After a post-doctoral stint with M. Frederick Hawthorne at the University of California, Los Angeles, he began his academic career at Fordham University in 1976 before moving to the University of New Hampshire in 1978. He has performed research in main group boron and phosphorus chemistry as well as metal-phosphine coordination chemistry. In recent years, with his colleague Gary Weisman he has focused on the coordination chemistry of cross-bridged tetraamine macrocycles. Together they have also explored applications of these chelators in copper-based radiopharmaceuticals with Carolyn Anderson and her research group.

Gary R. Weisman was born in Mason, Ohio in 1949, receiving his primary and secondary education in the public school system there. He was interested in chemistry from a young age, working with his cousin Thomas J. Richardson in their substantial home laboratories. He earned his B.S. in Chemistry With Distinction at the University of Kentucky in 1971, carrying out research with Robert D. Guthrie. At the University of Wisconsin – Madison, he worked on conformational analysis of hydrazines and their radical cations under the mentorship of Stephen F. Nelsen, earning the Ph.D. in Organic Chemistry in 1976. After a post-doctoral with Donald J. Cram at the University of California, Los Angeles in 1976-1977, he started his independent academic career at the University of New Hampshire, where he has enjoyed both teaching and research. He was Gloria G. and Robert E. Lyle Professor at UNH from 2005-2009 and has been the recipient of Outstanding Teaching awards in 1995 and 2009. He has been a visiting professor at the University of Wisconsin (1986), University of Bristol (1987, 1998), University of Melbourne (2005), and Australian National University (2005). He was a Wilsmore Fellow at the University of Melbourne in 2002. His research interests are in both physical organic and synthetic organic chemistry, with special emphasis on stereochemical aspects. His recent research has centered on the chemistry of polyaza molecules, ligand design and synthesis, and biomedical applications of coordination complexes. He has enjoyed a productive collaboration with Edward H. Wong for almost two decades and more recently with Carolyn J. Anderson.
Contributor Information
Thaddeus J. Wadas, Mallinckrodt Institute of Radiology, Washington University School of Medicine, 510 S. Kingshighway Blvd., Campus Box 8225, St. Louis, MO. 63110 USA.
Edward H. Wong, Department of Chemistry, University of New Hampshire, Durham, NH 03824-3598 USA, Phone: 603-862-1788, Fax: 603-862-4278, ehw@cisunix.unh.edu
Gary R. Weisman, Department of Chemistry, University of New Hampshire, Durham, NH 03824-3598 USA, Phone: 603-862-2304, Fax: 603-862-4278, gary.weisman@unh.edu
Carolyn J. Anderson, Mallinckrodt Institute of Radiology, Washington University School of Medicine, 510 S. Kingshighway Blvd., Campus Box 8225, St. Louis, MO. 63110 USA.
8.0. References
- (1).Society of Nuclear Medicine 2009 http://interactive.snm.org/index.cfm?PageID=5571&RPID=969.
- (2).Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons Inc.; Hoboken, NJ: 2003. [Google Scholar]
- (3).Blower PJ, Lewis JS, Zweit J. Nucl. Med. Biol. 1996;23(8):957. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- (4).Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Curr. Pharm. Des. 2007;13(1):3. doi: 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]
- (5).Smith SV. IDrugs. 2005;8(10):827. [PubMed] [Google Scholar]
- (6).Smith SV. J. Inorg. Biochem. 2004;98(11):1874. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- (7).Shokeen M, Anderson CJ. Acc. Chem. Res. 2009;42(7):832. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zwanziger D, Beck-Sickinger AG. Curr. Pharm. Des. 2008;14(24):2385. doi: 10.2174/138161208785777397. [DOI] [PubMed] [Google Scholar]
- (9).de Jong M, Breeman WAP, Kwekkeboom DJ, Valkema R, Krenning EP. Acc. Chem. Res. 2009;42(7):873. doi: 10.1021/ar800188e. [DOI] [PubMed] [Google Scholar]
- (10).Liu S. Adv. Drug Delivery Rev. 2008;60(12):1347. doi: 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Heeg MJ, Jurisson SS. Acc. Chem. Res. 1999;32(12):1053. [Google Scholar]
- (12).Anderson CJ, Welch MJ. Chem. Rev. 1999;99(9):2219. doi: 10.1021/cr980451q. [DOI] [PubMed] [Google Scholar]
- (13).Anderson CJ, Wadas TJ, Wong EH, Weisman GR. Q. J. Nucl. Med. Mol. Imaging. 2008;52(2):185. [PMC free article] [PubMed] [Google Scholar]
- (14).Rowland DJ, Lewis JS, Welch MJ. J. Cell. Biochem. 2002;(Suppl. 39):110. doi: 10.1002/jcb.10417. [DOI] [PubMed] [Google Scholar]
- (15).McQuade P, Rowland DJ, Lewis JS, Welch MJ. Curr. Med. Chem. 2005;12(7):807. doi: 10.2174/0929867053507397. [DOI] [PubMed] [Google Scholar]
- (16).McQuade P, McCarthy DW, Welch MJ, Valk PE. Metal radionuclides for PET imaging. In: Bailey DL, Townsend DW, Maisey MN, editors. Positron Emission Tomography. Springer-Verlag; London: 2003. p. 251. [Google Scholar]
- (17).Hoffman TJ, Smith CJ. Nucl. Med. Biol. 2009;36(6):579. doi: 10.1016/j.nucmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- (18).Meares CF. Capturing copper for molecular imaging and therapy. In: Chen X, editor. Recent Advances of Bioconjugation Chemistry in Molecular Imaging. Research Signpost; Kerala, India: 2009. p. 227. [Google Scholar]
- (19).Verel I, Visser GWM, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GAMS. J. Nucl. Med. 2003;44(8):1271. [PubMed] [Google Scholar]
- (20).Bass LA, Wang M, Welch MJ, Anderson CJ. Bioconjugate Chem. 2000;11(4):527. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- (21).Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ. J. Med. Chem. 2004;47(6):1465. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- (22).Harrison A, Walker CA, Parker D, Jankowski KJ, Cox JPL, Craig AS, Sansom JM, Beeley NRA, Boyce RA. Nucl. Med. Biol. 1991;18(5):469. doi: 10.1016/0883-2897(91)90107-v. [DOI] [PubMed] [Google Scholar]
- (23).Camera L, Kinuya S, Garmestani K, Wu C, Brechbiel MW, Pai LH, McMurry TJ, Gansow OA, Pastan I, Paik CH. J. Nucl. Med. 1994;35(5):882. [PubMed] [Google Scholar]
- (24).Stimmel JB, Kull FC. Nucl. Med. Biol. 1998;25(2):117. doi: 10.1016/s0969-8051(97)00151-0. [DOI] [PubMed] [Google Scholar]
- (25).Stimmel JB, Stockstill ME, Kull FC., Jr. Bioconjugate Chem. 1995;6(2):219. doi: 10.1021/bc00032a010. [DOI] [PubMed] [Google Scholar]
- (26).Byegard J, Skarnemark G, Skalberg M. J. Radioanal. Nucl. Chem. 1999;241(2):281. [Google Scholar]
- (27).Kukis DL, DeNardo SJ, DeNardo GL, O’Donnell RT, Meares CF. J. Nucl. Med. 1998;39(12):2105. [PubMed] [Google Scholar]
- (28).Jang YH, Blanco M, Dasgupta S, Keire DA, Shively JE, Goddard WA., III J. Am. Chem. Soc. 1999;121(26):6142. [Google Scholar]
- (29).Liu S, Edwards DS. Bioconjugate Chem. 2001;12(1):7. doi: 10.1021/bc000070v. [DOI] [PubMed] [Google Scholar]
- (30).Moreau J, Guillon E, Pierrard J-C, Rimbault J, Port M, Aplincourt M. Chem.--Eur. J. 2004;10(20):5218. doi: 10.1002/chem.200400006. [DOI] [PubMed] [Google Scholar]
- (31).Chong H.-s., Garmestani K, Ma D, Milenic DE, Overstreet T, Brechbiel MW. J. Med. Chem. 2002;45(16):3458. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- (32).Li WP, Ma DS, Higginbotham C, Hoffman T, Ketring AR, Cutler CS, Jurisson SS. Nucl. Med. Biol. 2001;28(2):145. doi: 10.1016/s0969-8051(00)00196-7. [DOI] [PubMed] [Google Scholar]
- (33).Yoo J, Reichert DE, Welch MJ. J. Med. Chem. 2004;47(26):6625. doi: 10.1021/jm0496990. [DOI] [PubMed] [Google Scholar]
- (34).Barnard PJ, Bayly SR, Holland JP, Dilworth JR, Waghorn PA. Q. J. Nucl. Med. Mol. Imaging. 2008;52(3):235. [PubMed] [Google Scholar]
- (35).Jurisson S, Cutler C, Smith SV. Q. J. Nucl. Med. Mol. Imaging. 2008;52(3):222. [PubMed] [Google Scholar]
- (36).McMurry TJ, Pippin CG, Wu C, Deal KA, Brechbiel MW, Mirzadeh S, Gansow OA. J. Med. Chem. 1998;41(18):3546. doi: 10.1021/jm980152t. [DOI] [PubMed] [Google Scholar]
- (37).Broan CJ, Cox J,PL, Craig AS, Kataky R, Parker D, Harrison A, Randall A, Ferguson G. J. Chem. Soc. Perkin Trans. 2. 1991;1:87. [Google Scholar]
- (38).Achour B, Costa J, Delgado R, Garrigues E, Geraldes CFGC, Korber N, Nepveu F, Prata MI. Inorg. Chem. 1998;37(25):6552. doi: 10.1021/ic981268i. [DOI] [PubMed] [Google Scholar]
- (39).Pulukkody KP, Norman TJ, Parker D, Royle L, Broan CJ. J. Chem. Soc., Perkin Trans. 2. 1993;4:605. [Google Scholar]
- (40).Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Science. 1999;284(5415):805. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- (41).Kim B-E, Nevitt T, Thiele DJ. Nat. Chem. Biol. 2008;4(3):176. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- (42).Arnesano F, Banci L, Bertini I, Ciofi-Baffoni S. Eur. J. Inorg. Chem. 2004;8:1583. [Google Scholar]
- (43).Rosenzweig AC. Acc. Chem. Res. 2001;34(2):119. doi: 10.1021/ar000012p. [DOI] [PubMed] [Google Scholar]
- (44).Perk LR, Visser GWM, Vosjan MJWD, Stigter-van Walsum M, Tijink BM, Leemans CR, van Dongen GAMS. J. Nucl. Med. 2005;46(11):1898. [PubMed] [Google Scholar]
- (45).Perk LR, Visser OJ, Stigter-Van Walsum M, Vosjan MJWD, Visser GWM, Zijlstra JM, Huijgens PC, Dongen GAMS. Eu. J. Nucl. Med. Mol. Imaging. 2006;33(11):1337. doi: 10.1007/s00259-006-0160-0. [DOI] [PubMed] [Google Scholar]
- (46).Harris WR, Pecoraro VL. Biochemistry. 1983;22(2):292. doi: 10.1021/bi00271a010. [DOI] [PubMed] [Google Scholar]
- (47).Liu S, Pietryka J, Ellars CE, Edwards DS. Bioconjugate Chem. 2002;13(4):902. doi: 10.1021/bc010134h. [DOI] [PubMed] [Google Scholar]
- (48).Boswell CA, McQuade P, Weisman GR, Wong EH, Anderson CJ. Nucl. Med. Biol. 2005;32(1):29. doi: 10.1016/j.nucmedbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- (49).Jalilian AR, Rowshanfarzad P, Sabet M, Kamalidehlghan M, Mirzaii M, Rajamand AA. Maj. Ulum va Funun-i Hastah-i. 2005;33:1. [Google Scholar]
- (50).Cordero B, Gomez V, Platero-Prats AE, Reves M, Echeverria J, Cremades E, Barragan F, Alvarez S. Dalton Trans. 2008;21:2832. doi: 10.1039/b801115j. [DOI] [PubMed] [Google Scholar]
- (51).Pasquarello A, Petri I, Salmon PS, Parisel O, Car R, Toth E, Powell DH, Fischer HE, Helm L, Merbach AE. Science. 2001;291(5505):856. doi: 10.1126/science.291.5505.856. [DOI] [PubMed] [Google Scholar]
- (52).Mukherjee R. Copper. In: Constable EC, Dilworth J, editors. Comprehensive Coordination Chemistry II: Transition Metal Groups 7 and 8. Vol. 6. Elsevier Limited; San Diego: 2004. p. 747. [Google Scholar]
- (53).Cabbiness DK, Margerum DW. J. Amer. Chem. Soc. 1969;91(23):6540. [Google Scholar]
- (54).Bencini A, Bianchi A, Paoletti P, Paoli P. Coord. Chem. Rev. 1992;120(1):51. [Google Scholar]
- (55).Curtis NF. Nitrogen Ligands. In: Constable EC, Dilworth J, editors. Comprehensive Coordination Chemistry II: Funudamentals. Vol. 1. Elsevier Limited; San Diego: 2004. p. 447. [Google Scholar]
- (56).Liang X, Sadler PJ. Chem. Soc. Rev. 2004;33(4):246. doi: 10.1039/b313659k. [DOI] [PubMed] [Google Scholar]
- (57).Delgado R, Felix V, Lima LMP, Price DW. Dalton Trans. 2007;26:2734. doi: 10.1039/b704360k. [DOI] [PubMed] [Google Scholar]
- (58).Hall MD, Hambley TW. Coord. Chem. Rev. 2002;232(1-2):49. [Google Scholar]
- (59).Fry FH, Jacob C. Curr. Pharm. Des. 2006;12(34):4479. doi: 10.2174/138161206779010512. [DOI] [PubMed] [Google Scholar]
- (60).Hall MD, Failes TW, Yamamoto N, Hambley TW. Dalton Trans. 2007;36:3983. doi: 10.1039/b707121c. [DOI] [PubMed] [Google Scholar]
- (61).Reisner E, Arion VB, Keppler BK, Pombeiro AJL. Inorg. Chim. Acta. 2008;361(6):1569. [Google Scholar]
- (62).Obata A, Yoshimi E, Waki A, Lewis JS, Oyama N, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y. Ann. Nucl. Med. 2001;15(6):499. doi: 10.1007/BF02988502. [DOI] [PubMed] [Google Scholar]
- (63).Vavere AL, Lewis JS. Dalton Trans. 2007;43:4893. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- (64).Holland JP, Barnard PJ, Collison D, Dilworth JR, Edge R, Green JC, McInnes EJL. Chem.--Eur. J. 2008;14(19):5890. doi: 10.1002/chem.200800539. [DOI] [PubMed] [Google Scholar]
- (65).Holland JP, Giansiracusa JH, Bell SG, Wong L-L, Dilworth JR. Phys. Med. Biol. 2009;54(7):2103. doi: 10.1088/0031-9155/54/7/017. [DOI] [PubMed] [Google Scholar]
- (66).Holland JP, Hickin JA, Grenville-Mathers E, Nguyen TN, Peach JM. J. Chem. Res. 2008;12:702. [Google Scholar]
- (67).Heroux KJ, Woodin KS, Tranchemontagne DJ, Widger PCB, Southwick E, Wong EH, Weisman GR, Tomellini SA, Wadas TJ, Anderson CJ, Kassel S, Golen JA, Rheingold AL. Dalton Trans. 2007;21:2150. doi: 10.1039/b702938a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Dhungana S, Taboy CH, Zak O, Larvie M, Crumbliss AL, Aisen P. Biochemistry. 2004;43(1):205. doi: 10.1021/bi0353631. [DOI] [PubMed] [Google Scholar]
- (69).Mies KA, Wirgau JI, Crumbliss AL. BioMetals. 2006;19(2):115. doi: 10.1007/s10534-005-4342-1. [DOI] [PubMed] [Google Scholar]
- (70).Bakaj M, Zimmer M. J. Mol. Struct. 1999;508(1-3):59. [Google Scholar]
- (71).Kaden TA, Gokel GW. Advances in Supramolecular Chemistry. Vol. 3. Elsevier Limited; San Diego: 1993. Functional tetraazamacrocycles: Ligands with many aspects; p. 65. [Google Scholar]
- (72).Bridger GJ, Skerlj RT, Padmanabhan S, Thornton D. J. Org. Chem. 1996;61(4):1519. [Google Scholar]
- (73).Meyer M, Dahaoui-Gindrey V, Lecomte C, Guilard R. Coord. Chem. Rev. 1998;178-180(Pt. 2):1313. [Google Scholar]
- (74).Guilard R, Bessmertnykh AG, Beletskaya IP. Synlett. 1997;10:1190. [Google Scholar]
- (75).Konig B, Pelka M, Klein M, Dix I, Jones PG, Lex J. J. Inclusion Phenom. Macrocyclic Chem. 2000;37(1-4):39. [Google Scholar]
- (76).Kaden TA. Chimia. 2000;54(10):574. [Google Scholar]
- (77).Lewis EA, Allan CC, Boyle RW, Archibald SJ. Tetrahedron Lett. 2004;45(15):3059. [Google Scholar]
- (78).Massue J, Plush SE, Bonnet CS, Moore DA, Gunnlaugsson T. Tetrahedron Lett. 2007;48(45):8052. [Google Scholar]
- (79).Suchy M, Hudson RHE. Eur. J. Org. Chem. 2008;29:4847. [Google Scholar]
- (80).Bharadwaj PK, Potenza JA, Schugar HJ. J. Am. Chem. Soc. 1986;108(6):1351. [Google Scholar]
- (81).Holland JP, Barnard PJ, Collison D, Dilworth JR, Edge R, Green JC, Heslop JM, McInnes EJL, Salzmann CG, Thompson AL. Eur. J. Inorg. Chem. 2008;22:3549. [Google Scholar]
- (82).Bu XH, Zhang ZH, An DL, Chen YT, Shionoya M, Kimura E. Inorg. Chim. Acta. 1996;249(1):125. [Google Scholar]
- (83).Polyakova IN, Poznyak AL, Sergienko VS. Kristallografiya. 2000;45(1):41. [Google Scholar]
- (84).Fomenko VV, Polynova TN, Porai-Koshits MA, Varlamova GL, Pechurova NI. Zh. Strukt. Khim. 1973;14(3):571. [Google Scholar]
- (85).Borzel H, Comba P, Hagen KS, Katsichtis C, Pritzkow H. Chem.--Eur. J. 2000;6(5):914. doi: 10.1002/(sici)1521-3765(20000303)6:5<914::aid-chem914>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- (86).Juran S, Walther M, Stephan H, Bergmann R, Steinbach J, Kraus W, Emmerling F, Comba P. Bioconjugate Chem. 2009;20(2):347. doi: 10.1021/bc800461e. [DOI] [PubMed] [Google Scholar]
- (87).Park G, Dadachova E, Przyborowska A, Lai S.-j., Ma D, Broker G, Rogers RD, Planalp RP, Brechbiel MW. Polyhedron. 2001;20(26-27):3155. [Google Scholar]
- (88).Ma D, Lu F, Overstreet T, Milenic DE, Brechbiel MW. Nucl. Med. Biol. 2002;29(1):91. doi: 10.1016/s0969-8051(01)00287-6. [DOI] [PubMed] [Google Scholar]
- (89).Siegfried L, Kaden TA. Dalton Trans. 2005;6:1136. doi: 10.1039/b417405b. [DOI] [PubMed] [Google Scholar]
- (90).Papini G, Alidori S, Lewis JS, Reichert DE, Pellei M, Gioia Lobbia G, Biddlecombe GB, Anderson CJ, Santini C. Dalton Trans. 2009;1:177. doi: 10.1039/b808831d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Kreher U, Hearn MTW, Moubaraki B, Murray KS, Spiccia L. Polyhedron. 2007;26(13):3205. [Google Scholar]
- (92).Wieghardt K, Bossek U, Chaudhuri P, Herrmann W, Menke BC, Weiss J. Inorg. Chem. 1982;21(12):4308. [Google Scholar]
- (93).Pavlishchuk V, Birkelbach F, Weyhermueller T, Wieghardt K, Chaudhuri P. Inorg. Chem. 2002;41(17):4405. doi: 10.1021/ic011322m. [DOI] [PubMed] [Google Scholar]
- (94).Packard AB, Kronauge JF, Day PJ, Treves ST. Nucl. Med. Biol. 1998;25(6):531. doi: 10.1016/s0969-8051(98)00029-8. [DOI] [PubMed] [Google Scholar]
- (95).Packard AB, Kronauge JF, Barbarics E, Kiani S, Treves ST. Nucl. Med. Biol. 2002;29(3):289. doi: 10.1016/s0969-8051(02)00285-8. [DOI] [PubMed] [Google Scholar]
- (96).Kiani S, Staples RJ, Ted Treves S, Packard AB. Polyhedron. 2009;28(4):775. doi: 10.1016/j.poly.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Kiani S, Staples RJ, Packard AB. Acta Crystallogr., Sect. C. 2002;C58(12):m593. doi: 10.1107/s0108270102019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Walker PS, Bergin PM, Grossel MC, Horton PN. Inorg. Chem. 2004;43(14):4145. doi: 10.1021/ic0497634. [DOI] [PubMed] [Google Scholar]
- (99).Motekaitis RJ, Sun Y, Martell AE, Welch MJ. Can. J. Chem. 1999;77(5-6):614. [Google Scholar]
- (100).Barnard PJ, Holland JP, Bayly SR, Wadas TJ, Anderson CJ, Dilworth JR. Inorg. Chem. 2009;48(15):7117. doi: 10.1021/ic900307f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Riesen A, Zehnder M, Kaden TA. Helv. Chim. Acta. 1986;69(8):2067. [Google Scholar]
- (102).Kumar K, Tweedle MF, Malley MF, Gougoutas JZ. Inorg. Chem. 1995;34(26):6472. [Google Scholar]
- (103).Sun X, Wuest M, Kovacs Z, Sherry AD, Motekaitis R, Wang Z, Martell AE, Welch MJ, Anderson CJ. J. Biol. Inorg. Chem. 2003;8(1-2):217. doi: 10.1007/s00775-002-0408-5. [DOI] [PubMed] [Google Scholar]
- (104).Kalman FK, Baranyai Z, Toth I, Banyai I, Kiraly R, Bruecher E, Aime S, Sun X, Sherry AD, Kovacs Z. Inorg. Chem. 2008;47(9):3851. doi: 10.1021/ic7024704. [DOI] [PubMed] [Google Scholar]
- (105).Aneetha H, Lai Y-H, Lin S-C, Panneerselvam K, Lu T-H, Chung C-S. J. Chem. Soc., Dalton Trans. 1999;16:2885. [Google Scholar]
- (106).Tonei DM, Ware DC, Brothers PJ, Plieger PG, Clark GR. Dalton Trans. 2006;1:152. doi: 10.1039/b512798j. [DOI] [PubMed] [Google Scholar]
- (107).Chapman J, Ferguson G, Gallagher JF, Jennings MC, Parker D. J. Chem. Soc., Dalton Trans. 1992;3:345. [Google Scholar]
- (108).Silversides JD, Allan CC, Archibald SJ. Dalton Trans. 2007;9:971. doi: 10.1039/b615329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Moi MK, Yanuck M, Deshpande SV, Hope H, DeNardo SJ, Meares CF. Inorg. Chem. 1987;26(21):3458. [Google Scholar]
- (110).Sprague JE, Peng Y, Fiamengo AL, Woodin KS, Southwick EA, Weisman GR, Wong EH, Golen JA, Rheingold AL, Anderson CJ. J. Med. Chem. 2007;50(10):2527. doi: 10.1021/jm070204r. [DOI] [PubMed] [Google Scholar]
- (111).Wong EH, Weisman GR, Hill DC, Reed DP, Rogers ME, Condon JP, Fagan MA, Calabrese JC, Lam K-C, Guzei IA, Rheingold AL. J. Am. Chem. Soc. 2000;122(43):10561. [Google Scholar]
- (112).Woodin KS, Heroux KJ, Boswell CA, Wong EH, Weisman GR, Niu W, Tomellini SA, Anderson CJ, Zakharov LN, Rheingold AL. Eu. J. Inorg. Chem. 2005;23:4829. [Google Scholar]
- (113).Lewis EA, Boyle RW, Archibald SJ. Chem. Comm. 2004;19:2212. doi: 10.1039/b406906d. [DOI] [PubMed] [Google Scholar]
- (114).Kotek J, Lubal P, Hermann P, Cisarova I, Lukes I, Godula T, Svobodova I, Taborsky P, Havel J. Chem.-Eur. J. 2003;9(1):233. doi: 10.1002/chem.200390017. [DOI] [PubMed] [Google Scholar]
- (115).Boswell CA, Regino CAS, Baidoo KE, Wong KJ, Milenic DE, Kelley JA, Lai CC, Brechbiel MW. Bioorg. Med. Chem. 2009;17(2):548. doi: 10.1016/j.bmc.2008.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Sargeson AM. Pure Appl. Chem. 1986;58(11):1511. [Google Scholar]
- (117).Bottomley GA, Clark IJ, Creaser II, Engelhardt LM, Geue RJ, Hagen KS, Harrowfield JM, Lawrance GA, Lay PA. Aust. J. Chem. 1994;47(1):143. [Google Scholar]
- (118).Bernhardt PV, Bramley R, Engelhardt LM, Harrowfield JM, Hockless DCR, Korybut-Daszkiewicz BR, Krausz ER, Morgan T, Sargeson AM. Inorg. Chem. 1995;34(14):3589. [Google Scholar]
- (119).Di Bartolo NM, Sargeson AM, Donlevy TM, Smith SV. J. Chem. Soc., Dalton Trans. 2001;15:2303. [Google Scholar]
- (120).Voss SD, Smith SV, DiBartolo N, McIntosh LJ, Cyr EM, Bonab AA, Dearling JLJ, Carter EA, Fischman AJ, Treves ST, Gillies SD, Sargeson AM, Huston JS, Packard AB. Proc. Natl. Acad. Sci. U. S. A. 2007;104(44):17489. doi: 10.1073/pnas.0708436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Smith SV. Q. J. Nucl. Med. Mol. Imaging. 2008;52(2):193. [PubMed] [Google Scholar]
- (122).Cai W, Guzman R, Hsu AR, Wang H, Chen K, Sun G, Gera A, Choi R, Bliss T, He L, Li Z-B, Maag A-LD, Hori N, Zhao H, Moseley M, Steinberg GK, Chen X. Stroke. 2009;40(1):270. doi: 10.1161/STROKEAHA.108.517474. [DOI] [PubMed] [Google Scholar]
- (123).Cai H, Fissekis J, Conti PS. Dalton Trans. 2009;27:5395. doi: 10.1039/b902210d. [DOI] [PubMed] [Google Scholar]
- (124).Ma MT, Karas JA, White JM, Scanlon D, Donnelly PS. Chem. Commun. 2009;22:3237. doi: 10.1039/b903426a. [DOI] [PubMed] [Google Scholar]
- (125).Donnelly PS, Harrowfield JM, Skelton BW, White AH. Inorg. Chem. 2001;40(22):5645. doi: 10.1021/ic010049l. [DOI] [PubMed] [Google Scholar]
- (126).Donnelly PS, Harrowfield JM, Skelton BW, White AH. Inorg. Chem. 2000;39(25):5817. doi: 10.1021/ic000346a. [DOI] [PubMed] [Google Scholar]
- (127).Bandoli G, Dolmella A, Tisato F, Porchia M, Refosco F. Coord. Chem. Rev. 2009;253(1+2):56. [Google Scholar]
- (128).Harris WR, Chen Y, Wein K. Inorg. Chem. 1994;33(22):4991. [Google Scholar]
- (129).Jarjayes O, Mortini F, du Moulinet d’Hardemare A, Philouze C, Serratrice G. Eur. J. Inorg. Chem. 2005;21:4417. [Google Scholar]
- (130).Motekaitis RJ, Martell AE, Koch SA, Hwang J, Quarless DA, Jr., Welch MJ. Inorg. Chem. 1998;37(22):5902. [Google Scholar]
- (131).Francesconi LC, Liu BL, Billings JJ, Carroll PJ, Graczyk G, Kung HF. J. Chem. Soc., Chem. Commun. 1991;2:94. [Google Scholar]
- (132).Zheng YY, Saluja S, Yap GPA, Blumenstein M, Rheingold AL, Francesconi LC. Inorg. Chem. 1996;35(23):6656. doi: 10.1021/ic951535+. [DOI] [PubMed] [Google Scholar]
- (133).Li Y, Martell AE, Hancock RD, Reibenspies JH, Anderson CJ, Welch MJ. Inorg. Chem. 1996;35(2):404. doi: 10.1021/ic941330l. [DOI] [PubMed] [Google Scholar]
- (134).Jung W-S, Chung YK, Shin DM, Kim S-D. Bull. Chem. Soc. Jpn. 2002;75(6):1263. [Google Scholar]
- (135).Ma R, Motekaitis RJ, Martell AE. Inorg. Chim. Acta. 1994;224(1-2):151. [Google Scholar]
- (136).Schuhmacher J, Kaul S, Klivenyi G, Junkermann H, Magener A, Henze M, Doll J, Haberkorn U, Amelung F, Bastert G. Cancer Res. 2001;61(9):3712. [PubMed] [Google Scholar]
- (137).Eder M, Waengler B, Knackmuss S, LeGall F, Little M, Haberkorn U, Mier W, Eisenhut M. Eu. J. Nucl. Med. Mol. Imaging. 2008;35(10):1878. doi: 10.1007/s00259-008-0816-z. [DOI] [PubMed] [Google Scholar]
- (138).Ma R, Murase I, Martell AE. Inorg. Chim. Acta. 1994;223(1-2):109. [Google Scholar]
- (139).Wong E, Caravan P, Liu S, Rettig SJ, Orvig C. Inorg. Chem. 1996;35(3):715. [Google Scholar]
- (140).Blend MJ, Stastny JJ, Swanson SM, Brechbiel MW. Cancer Biother. Radiopharm. 2003;18(3):355. doi: 10.1089/108497803322285107. [DOI] [PubMed] [Google Scholar]
- (141).Wei L, Zhang X, Gallazzi F, Miao Y, Jin X, Brechbiel MW, Xu H, Clifford T, Welch MJ, Lewis JS, Quinn TP. Nucl. Med. Biol. 2009;36(4):345. doi: 10.1016/j.nucmedbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Tsang BW, Mathias CJ, Fanwick PE, Green MA. J. Med. Chem. 1994;37(25):4400. doi: 10.1021/jm00051a018. [DOI] [PubMed] [Google Scholar]
- (143).Sharma V, Beatty A, Wey SP, Dahlheimer J, Pica CM, Crankshaw CL, Bass L, Green MA, Welch MJ, Piwnica-Worms D. Chem. Biol. 2000;7(5):335. doi: 10.1016/s1074-5521(00)00111-3. [DOI] [PubMed] [Google Scholar]
- (144).Hsiao Y-M, Mathias CJ, Wey S-P, Fanwick PE, Green MA. Nucl. Med. Biol. 2009;36(1):39. doi: 10.1016/j.nucmedbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Martell AE, Motekaitis RJ, Clarke ET, Delgado R, Sun Y, Ma R. Supramol. Chem. 1996;6(3-4):353. [Google Scholar]
- (146).Moore DA, Fanwick PE, Welch MJ. Inorg. Chem. 1990;29(4):672. [Google Scholar]
- (147).Clarke ET, Martell AE. Inorg. Chim. Acta. 1991;181(2):273. [Google Scholar]
- (148).Prata MIM, Santos AC, Geraldes CFGC, Lima J. J. P. d. Nucl. Med. Biol. 1999;26(6):707. doi: 10.1016/s0969-8051(99)00041-4. [DOI] [PubMed] [Google Scholar]
- (149).McMurry TJ, Brechbiel MW, Wu C, Gansow OA. Bioconjugate Chem. 1993;4(3):236. doi: 10.1021/bc00021a009. [DOI] [PubMed] [Google Scholar]
- (150).Cox JPL, Craig AS, Helps IM, Jankowski KJ, Parker D, Eaton MAW, Millican AT, Millar K, Beeley NRA, Boyce BA. J. Chem Soc. Perkin Trans 1. 1999:2567. [Google Scholar]
- (151).Studer M, Meares CF. Bioconjugate Chem. 1992;34(4):3691. doi: 10.1021/bc00016a013. [DOI] [PubMed] [Google Scholar]
- (152).Brechbiel MW, McMurry TJ, Gansow OA. Tetrahedron Lett. 1993;34(23):3691. [Google Scholar]
- (153).Andre JP, Macke HR, Zehnder M, Macko L, Acyel KG. Chem. Commun. 1998;12:1301. [Google Scholar]
- (154).Eisenwiener K-P, Prata MIM, Buschmann I, Zhang H-W, Santos AC, Wenger S, Reubi JC, Maecke HR. Bioconjugate Chem. 2002;13(3):530. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- (155).Viola NA, Rarig RS, Ouellette W, Doyle RP. Polyhedron. 2006;25(18):3457. [Google Scholar]
- (156).Heppeler A, Andre JP, Buschmann I, Wang X, Reubi J-C, Hennig M, Kaden TA, Maecke HR. Chem.-Eur. J. 2008;14(10):3026. doi: 10.1002/chem.200701264. [DOI] [PubMed] [Google Scholar]
- (157).Heppeler A, Froidevaux S, Macke HR, Jermann E, Behe M, Powell P, Hennig M. Chem.--Eur. J. 1999;5(7):1974. [Google Scholar]
- (158).Yang C-T, Li Y, Liu S. Inorg. Chem. 2007;46(21):8988. doi: 10.1021/ic7010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Fani M, Andre JP, Maecke HR. Contrast Media Mol. Imaging. 2008;3(2):67. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- (160).Clarke ET, Martell AE. Inorg. Chim. Acta. 1991;190(1):37. [Google Scholar]
- (161).Decristoforo C, Petrik M, von Guggenberg E, Carlsen J, Huisman M, Wester H-J, Virgolini I, Haubner R. J. Lab. Compd. Radiopharm. 2007;50:S23. [Google Scholar]
- (162).Niu W, Wong EH, Weisman GR, Peng Y, Anderson CJ, Zakharov LN, Golen JA, Rheingold AL. Eur. J. Inorg. Chem. 2004;16:3310. [Google Scholar]
- (163).Terova O. Synthesis, Characterization and Inertness Studies of Gallium(III) and Indium(III) Complexes of Dicarboxymethyl Pendant-armed Cross-bridged Cyclam. MS, University of New Hampshire; Durham, NH: 2008. [Google Scholar]
- (164).Ward MC, Roberts KR, Babich JW, Bukhari MA, Coghlan G, Westwood JH, McCready VR, Ott RJ. Int. J. Rad. Appl. Instrum. B. 1986;13(5):505. doi: 10.1016/0883-2897(86)90128-5. [DOI] [PubMed] [Google Scholar]
- (165).Smith-Jones PM, Stolz B, Bruns C, Albert R, Reist HW, Fridrich R, Mäcke HR. J. Nucl. Med. 1994;35(2):317. [PubMed] [Google Scholar]
- (166).Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang Z-F, Waters DJ, Snyder PW, Low PS, Welch MJ, Green MA. Nucl. Med. Biol. 2003;30(7):725. doi: 10.1016/s0969-8051(03)00080-5. [DOI] [PubMed] [Google Scholar]
- (167).Govindan SV, Michel RB, Griffiths GL, Goldenberg DM, Mattes MJ. Nucl. Med. Biol. 2005;32(5):513. doi: 10.1016/j.nucmedbio.2005.04.009. [DOI] [PubMed] [Google Scholar]
- (168).Ye Y, Liu M, Kao JLF, Marshall GR. Biopolymers. 2006;84(5):472. doi: 10.1002/bip.20532. [DOI] [PubMed] [Google Scholar]
- (169).Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP, Banin E. Proc. Natl. Acad. Sci. U. S. A. 2008;105(43):16761. doi: 10.1073/pnas.0808608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Borgias B, Hugi AD, Raymond KN. Inorg. Chem. 1989;28(18):3538. [Google Scholar]
- (171).Borgias BA, Barclay SJ, Raymond KN. J. Coord. Chem. 1986;15(2):109. [Google Scholar]
- (172).Motekaitis RJ, Sun Y, Martell AE. Inorg. Chem. 1991;30(7):1554. [Google Scholar]
- (173).Rose DJ, Zubieta J, Fischman AJ, Hillier S, Babich JW. Inorg. Chem. Commun. 1998;1(5):164. [Google Scholar]
- (174).Ilyukhin AB, Malyarik MA, Petrosyants SP, Davidovich RL, Samsonova IN. Zh. Neorg. Khim. 1995;40(7):1125. [Google Scholar]
- (175).Ilyukhin AB, Malyarik MA, Porai-Koshits MA, Davidovich RL, Logvinova VB. Kristallografiya. 1995;40(4):656. [Google Scholar]
- (176).Maecke HR, Riesen A, Ritter W. J. Nucl. Med. 1989;30(7):1235. [PubMed] [Google Scholar]
- (177).Parker D, Pulukkody K, Smith FC, Batsanov A, Howard JAK. J. Chem. Soc., Dalton Trans. 1994;5:689. [Google Scholar]
- (178).Hsieh W-Y, Liu S. Inorg. Chem. 2004;43(19):6006. doi: 10.1021/ic049973g. [DOI] [PubMed] [Google Scholar]
- (179).Craig AS, Helps IM, Parker D, Adams H, Bailey NA, Williams MG, Smith JMA, Ferguson G. Polyhedron. 1989;8(20):2481. [Google Scholar]
- (180).Matthews RC, Parker D, Ferguson G, Kaitner B, Harrison A, Royle L. Polyhedron. 1991;10(16):1951. [Google Scholar]
- (181).Cole E, Copley RCB, Howard JAK, Parker D, Ferguson G, Gallagher JF, Kaitner B, Harrison A, Royle L. J. Chem. Soc., Dalton Trans. 1994;11:1619. [Google Scholar]
- (182).Bossek U, Hanke D, Wieghardt K, Nuber B. Polyhedron. 1993;12(1):1. [Google Scholar]
- (183).Clarke ET, Martell AE. Inorg. Chim. Acta. 1991;186(1):103. [Google Scholar]
- (184).Liu S, He Z, Hsieh W-Y, Fanwick PE. Inorg. Chem. 2003;42(26):8831. doi: 10.1021/ic0349914. [DOI] [PubMed] [Google Scholar]
- (185).Riesen A, Kaden TA, Ritter W, Maecke HR. J. Chem. Soc., Chem. Commun. 1989;8:460. [Google Scholar]
- (186).Clarke ET, Martell AE. Inorg. Chim. Acta. 1991;190(1):27. [Google Scholar]
- (187).Moerlein SM, Welch MJ. Int. J. Nucl. Med. Biol. 1981;8(4):277. doi: 10.1016/0047-0740(81)90034-6. [DOI] [PubMed] [Google Scholar]
- (188).Mistryukov VE, Sergeev AV, Chuklanova EB, Mikhailov YN, Shchelokov RN. Zh. Neorg. Khim. 1997;42(6):969. [Google Scholar]
- (189).Wang J, Zhao J, Zhang X, Gao J. Rare Met. 2000;19(4):241. [Google Scholar]
- (190).Amin S, Marks C, Toomey LM, Churchill MR, Morrow JR. Inorg. Chim. Acta. 1996;246(1-2):99. [Google Scholar]
- (191).Chang CA, Francesconi LC, Malley MF, Kumar K, Gougoutas JZ, Tweedle MF, Lee DW, Wilson LJ. Inorg. Chem. 1993;32(16):3501. [Google Scholar]
- (192).Vojtisek P, Cigler P, Kotek J, Rudovsky J, Hermann P, Lukes I. Inorg. Chem. 2005;44(16):5591. doi: 10.1021/ic048190s. [DOI] [PubMed] [Google Scholar]
- (193).Corneillie TM, Fisher AJ, Meares CF. J. Am. Chem. Soc. 2003;125(49):15039. doi: 10.1021/ja037236y. [DOI] [PubMed] [Google Scholar]
- (194).Schlesinger J, Koezle I, Bergmann R, Tamburini S, Bolzati C, Tisato F, Noll B, Klussmann S, Vonhoff S, Wuest F, Pietzsch H-J, Steinbach J. Bioconjugate Chem. 2008;19(4):928. doi: 10.1021/bc700453h. [DOI] [PubMed] [Google Scholar]
- (195).Kodama M, Koike T, Mahatma AB, Kimura E. Inorg. Chem. 1991;30(6):1270. [Google Scholar]
- (196).Cox JPL, Jankowski KJ, Kataky R, Parker D, Beeley NRA, Boyce BA, Eaton MAW, Millar K, Millican AT. J. Chem. Soc., Chem. Commun. 1989;12:797. [Google Scholar]
- (197).Spirlet MR, Rebizant J, Loncin MF, Desreux JF. Inorg. Chem. 1984;23(25):4278. [Google Scholar]
- (198).Kang J-G, Na M-K, Yoon S-K, Sohn Y, Kim Y-D, Suh I-H. Inorg. Chim. Acta. 2000;310(1):56. [Google Scholar]
- (199).Maecke HR, Scherer G, Heppeler A, Henig M. Eu. J. Nucl. Med. 2001;28:967. [Google Scholar]
- (200).Onthank DC, Liu S, Silva PJ, Barrett JA, Harris TD, Robinson SP, Edwards DS. Bioconjugate Chem. 2004;15(2):235. doi: 10.1021/bc034108q. [DOI] [PubMed] [Google Scholar]
- (201).Liu S, Edwards DS. Bioconjugate Chem. 2001;12(4):630. doi: 10.1021/bc010013h. [DOI] [PubMed] [Google Scholar]
- (202).Singhal A, Toth LM, Lin JS, Affholter K. J. Am. Chem. Soc. 1996;118(46):11529. [Google Scholar]
- (203).Ekberg C, Kaellvenius G, Albinsson Y, Brown PL. J. Solution Chem. 2004;33(1):47. [Google Scholar]
- (204).Pozhidaev AI, Porai-Koshits MA, Polynova TN. Zh. Strukt. Khim. 1974;15(4):644. [Google Scholar]
- (205).Davidovich RL, Logvinova VB, Teplukhina LV. Koord. Khim. 1992;18(6):580. [Google Scholar]
- (206).Meijs WE, Herscheid JDM, Haisma HJ, Pinedo HM. Appl. Radiat. Isot. 1992;43(12):1443. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- (207).Takahashi A, Yamaguchi H, Igarashi S. Bunseki Kagaku. 1997;46(1):55. [Google Scholar]
- (208).Verel I, Visser GWM, Boellaard R, Boerman OC, van Eerd J, Snow GB, Lammertsma AA, van Dongen GAMS. J. Nucl. Med. 2003;44(10):1663. [PubMed] [Google Scholar]
- (209).Boerjesson PKE, Jauw YWS, Boellaard R, de Bree R, Comans EFI, Roos JC, Castelijns JA, Vosjan MJWD, Kummer JA, Leemans CR, Lammertsma AA, van Dongen GAMS. Clin. Cancer Res. 2006;12(7, Pt. 1):2133. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- (210).Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, Brouwers AH, van Dongen GA, Perk LR, Lub-de-Hooge MN. J. Nucl. Med. 2007;48(8):1313. doi: 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
- (211).Datta A, Raymond KN. Acc. Chem. Res. 2009;42(7):938. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Piel H, Qaim SM, Stocklin G. Radiochim. Acta. 1992;57:1. [Google Scholar]
- (213).Williams HA, Robinson S, Julyan P, Zweit J, Hastings D. Eu. J. Nucl. Med. Mol. Imaging. 2005;32(12):1473. doi: 10.1007/s00259-005-1906-9. [DOI] [PubMed] [Google Scholar]
- (214).Fujibayashi Y, Matsumoto K, Yonekura Y, Konishi J, Yokayama A. J. Nucl. Med. 1989;30(11):1838. [PubMed] [Google Scholar]
- (215).Zweit J, Gordall R, Cox M, Babich JW, Petter GA, Sharma HL, Ott RJ. Eu. J. Nucl. Med. 1992;19(6):418. doi: 10.1007/BF00177368. [DOI] [PubMed] [Google Scholar]
- (216).Haynes NG, Lacy JL, Nayak N, Martin CS, Dai D, Mathias CJ, Green MA. J. Nucl. Med. 2000;41(2):309. [PubMed] [Google Scholar]
- (217).Fukumura T, Okada K, Suzuki H, Nakao R, Mukai K, Szelecsenyi F, Kovacs Z, Suzuki K. Nucl. Med. Biol. 2006;33(6):821. doi: 10.1016/j.nucmedbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- (218).Rowshanfarzad P, Sabet M, Reza Jalilian A, Kamalidehghan M. Appl. Radiat. Isot. 2006;64(12):1563. doi: 10.1016/j.apradiso.2005.11.012. [DOI] [PubMed] [Google Scholar]
- (219).McCarthy DW, Bass LA, Cutler PD, Shefer RE, Klinkowstein RE, Herrero P, Lewis JS, Cutler CS, Anderson CJ, Welch MJ. Nucl. Med. Biol. 1999;26(4):351. doi: 10.1016/s0969-8051(98)00113-9. [DOI] [PubMed] [Google Scholar]
- (220).Szelecsenyi F, Kovacs Z, Suzuki K, Okada K, Fukumura T, Mukai K. Nucl. Instrum. Methods Phys. Res., Sect. B. 2004;222(3-4):364. [Google Scholar]
- (221).Fukumura T, Okada K, Szelecsenyi F, Kovacs Z, Suzuki K. Radiochim. Acta. 2004;92(4-6):209. [Google Scholar]
- (222).Szelecsenyi F, Suzuki K, Kovacs Z, Takei M, Okada K. Nucl. Instrum. Methods Phys. Res., Sect. B. 2002;187(2):153. [Google Scholar]
- (223).Novak-Hofer I, Schubiger PA. Eu. J. Nucl. Med. Mol. Imaging. 2002;29(6):821. doi: 10.1007/s00259-001-0724-y. [DOI] [PubMed] [Google Scholar]
- (224).Schwarzbach R, Zimmermann K, Blauenstein P, Smith A, Schubiger PA. Appl. Radiat. Isot. 1995;46(5):329. doi: 10.1016/0969-8043(95)00010-b. [DOI] [PubMed] [Google Scholar]
- (225).Alekseev IE, Darmograi VV, Marchenkov NS. Radiochem. 2005;47(5):502. [Google Scholar]
- (226).Dasgupta AK, Mausner LF, Srivastava SC. Appl. Radiat. Isot. 1991;42:371. [Google Scholar]
- (227).Kroger LA, DeNardo GL, Gumerlock PH, Xiong CY, Winthrop MD, Shi XB, Mack PC, Leshchinsky T, DeNardo SJ. Cancer Biother. Radiopharm. 2001;16(3):213. doi: 10.1089/10849780152389401. [DOI] [PubMed] [Google Scholar]
- (228).Wun T, Kwon DS, Tuscano JM. BioDrugs. 2001;15(3):151. doi: 10.2165/00063030-200115030-00002. [DOI] [PubMed] [Google Scholar]
- (229).Zimmermann K, Gianollini S, Schubiger PA, Novak-Hofer I. Nucl. Med. Biol. 1999;26(8):943. doi: 10.1016/s0969-8051(99)00067-0. [DOI] [PubMed] [Google Scholar]
- (230).Frier M. Mini-Rev. Med. Chem. 2004;4(1):61. doi: 10.2174/1389557043487510. [DOI] [PubMed] [Google Scholar]
- (231).Zimmermann K, Grunberg J, Honer M, Ametamey S, August Schubiger P, Novak-Hofer I. Nucl. Med. Biol. 2003;30(4):417. doi: 10.1016/s0969-8051(03)00019-2. [DOI] [PubMed] [Google Scholar]
- (232).DeNardo GL, DeNardo SJ, Kukis DL, O’Donnell RT, Shen S, Goldstein DS, Kroger LA, Salako Q, DeNardo DA, Mirick GR, Mausner LF, Srivastava SC, Meares CF. Anticancer Res. 1998;18:2779. [PubMed] [Google Scholar]
- (233).O’Donnell RT, DeNardo GL, Kukis DL, Lamborn KR, Shen S, Yuan A, Goldstein DS, Mirick GR, DeNardo SJ. Clin. Cancer Res. 1999;5(10):3330s. [PubMed] [Google Scholar]
- (234).Mirick GR, O’Donnell RT, DeNardo SJ, Shen S, Meares CF, DeNardo GL. Nucl. Med. Biol. 1999;26(7):841. doi: 10.1016/s0969-8051(99)00049-9. [DOI] [PubMed] [Google Scholar]
- (235).O’Donnell RT, DeNardo GL, Kukis DL, Lamborn KR, Shen S, Yuan A, Goldstein DS, Carr CE, Mirick GR, DeNardo SJ. J. Nucl. Med. 1999;40(12):2014. [PubMed] [Google Scholar]
- (236).Sun X, Anderson CJ. Methods Enzymol. 2004;386:237. doi: 10.1016/S0076-6879(04)86011-7. [DOI] [PubMed] [Google Scholar]
- (237).McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margenau WH, Cutler CS, Anderson CJ, Welch MJ. Nucl. Med. Biol. 1997;24(1):35. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- (238).McCarthy DW, Shefer RF, Klinkowstein RF, Cutler CS, Anderson CJ, Welch MJ. J. Labeled Compd. Radiopharm. 1995;37(9):830. [Google Scholar]
- (239).Kim JY, Park H, Lee JC, Kim KM, Lee KC, Ha HJ, Choi TH, An GI, Cheon GJ. Appl. Radiat. Isot. 2009;67(7-8):1190. doi: 10.1016/j.apradiso.2009.02.060. [DOI] [PubMed] [Google Scholar]
- (240).Le VS, Howse J, Zaw M, Pellegrini P, Katsifis A, Greguric I, Weiner R. Appl. Radiat. Isot. 2009;67(7-8):1324. doi: 10.1016/j.apradiso.2009.02.069. [DOI] [PubMed] [Google Scholar]
- (241).Hermanne A, Tarkanyi F, Takacs S, Kovalev SF, Ignatyuk A. Nucl. Instrum. Methods Phys. Res., Sect. B. 2007;258(2):308. [Google Scholar]
- (242).Hou X, Jacobsen U, Jorgensen JC. Appl. Radiat. Isot. 2002;57(6):773. doi: 10.1016/s0969-8043(02)00170-7. [DOI] [PubMed] [Google Scholar]
- (243).Watanabe S, Iida Y, Suzui N, Katabuchi T, Ishii S, Kawachi N, Hanaoka H, Watanabe S, Matsuhashi S, Endo K, Ishioka NS. J. Radioanal. Nucl. Chem. 2009;280(1):199. [Google Scholar]
- (244).Obata A, Kasamatsu S, McCarthy DW, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y. Nucl. Med. Biol. 2003;30(5):535. doi: 10.1016/s0969-8051(03)00024-6. [DOI] [PubMed] [Google Scholar]
- (245).Avila-Rodriguez MA, Nye JA, Nickles RJ. Appl. Radiat. Isot. 2007;65(10):1115. doi: 10.1016/j.apradiso.2007.05.012. [DOI] [PubMed] [Google Scholar]
- (246).Szajek LP, Meyer W, Plascjak P, Eckleman WC. Radiochim. Acta. 2005;93(4):239. [Google Scholar]
- (247).So L, Pellegrini P, Katsifis A, Howse J, Greguric I. J. Radioanal. Nucl. Chem. 2008;277(2):451. [Google Scholar]
- (248).Spahn I, Coenen HH, Qaim SM. Radiochim. Acta. 2004;92(3):183. [Google Scholar]
- (249).Hilgers K, Stoll T, Skakun Y, Coenen HH, Qaim SM. Appl. Radiat. Isot. 2003;59(5-6):343. doi: 10.1016/s0969-8043(03)00199-4. [DOI] [PubMed] [Google Scholar]
- (250).Abbas K, Kozempel J, Bonardi M, Groppi F, Alfarano A, Holzwarth U, Simonelli F, Hofman H, Horstmann W, Menapace E, Leseticky L, Gibson N. Appl. Radiat. Isot. 2006;64(9):1001. doi: 10.1016/j.apradiso.2005.12.021. [DOI] [PubMed] [Google Scholar]
- (251).Kozempel J, Abbas K, Simonelli F, Zampese M, Holzwarth U, Gibson N, Leseticky L. Radiochim. Acta. 2007;95(2):75. [Google Scholar]
- (252).Hassanein MA, El-Said H, El-Amir MA. J. Radioanal. Nucl. Chem. 2006;269(1):75. [Google Scholar]
- (253).Little FE, Lagunas-Solar MC. Int. J. Appl. Radiat. Isot. 1983;34(3):631. [Google Scholar]
- (254).Nagame Y, Unno M, Nakahara H, Murakami Y. Int. J. Appl. Radiat. Isot. 1978;29(11):615. [Google Scholar]
- (255).Steyn J, Meyer BR. Int. J. Appl. Radiat. Isot. 1973;24(7):369. [Google Scholar]
- (256).Naidoo C, van der Walt TN. Appl. Radiat. Isot. 2001;54(6):915. doi: 10.1016/s0969-8043(00)00360-2. [DOI] [PubMed] [Google Scholar]
- (257).van der Meulen NP, van der Walt TN. Z. Naturforsch., B: Chem. Sci. 2007;62(3):483. [Google Scholar]
- (258).Nayak D, Lahiri S. Appl. Radiat. Isot. 2001;54(2):189. doi: 10.1016/s0969-8043(00)00194-9. [DOI] [PubMed] [Google Scholar]
- (259).Nayak D, Banerjee A, Lahiri S. Appl. Radiat. Isot. 2007;65(8):891. doi: 10.1016/j.apradiso.2007.03.019. [DOI] [PubMed] [Google Scholar]
- (260).Dacosta ACA, Lelite SGF. Biotechnol. Lett. 1991;13:559. [Google Scholar]
- (261).Yamamoto F, Tsukamoto E, Nakada K, Takei T, Zhao S, Asaka M, Tamaki N. Ann. Nucl. Med. 2004;18(6):519. doi: 10.1007/BF02984570. [DOI] [PubMed] [Google Scholar]
- (262).Lambrecht R, Sajjad M. Radiochim. Acta. 1988;43(1):171. [Google Scholar]
- (263).Mirzadeh S, Lambrecht R. J. Radioanal. Nucl. Chem. 1996;202(1-2):7. [Google Scholar]
- (264).Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M, Rosch F. J. Nucl. Med. 2007;48(10):1741. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- (265).Velikyan I, Beyer GJ, Langstrom B. Bioconjugate Chem. 2004;15(3):554. doi: 10.1021/bc030078f. [DOI] [PubMed] [Google Scholar]
- (266).Breeman WA, de Jong M, de Blois E, Bernard BF, Konijnenberg M, Krenning EP. Eu. J. Nucl. Med. Mol. Imaging. 2005;32(4):478. doi: 10.1007/s00259-004-1702-y. [DOI] [PubMed] [Google Scholar]
- (267).Azhdarinia A, Yang DJ, Chao C, Mourtada F. Nucl. Med. Biol. 2007;34(1):121. doi: 10.1016/j.nucmedbio.2006.10.011. [DOI] [PubMed] [Google Scholar]
- (268).Lewis MR, Reichert DE, Laforest R, Margenau WH, Shefer RE, Klinkowstein RE, Hughey BJ, Welch MJ. Nucl. Med. Biol. 2002;29(6):701. doi: 10.1016/s0969-8051(02)00330-x. [DOI] [PubMed] [Google Scholar]
- (269).Lundqvist H, Tolmachev V, Bruskin A, Einarsson L, Malmborg P. Appl. Radiat. Isot. 1995;46(9):859. [Google Scholar]
- (270).Schlyer DJ. Production of radionuclides in accelerators. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons Inc.; Hoboken: 2003. p. 1. [Google Scholar]
- (271).Lubberink M, Tolmachev V, Widstroem C, Bruskin A, Lundqvist H, Westlin J-E. J. Nucl. Med. 2002;43(10):1391. [PubMed] [Google Scholar]
- (272).Tolmachev V, Bernhardt P, Forssell-Aronsson E, Lundqvist H. Nucl. Med. Biol. 2000;27(2):183. doi: 10.1016/s0969-8051(99)00096-7. [DOI] [PubMed] [Google Scholar]
- (273).Nortier FM, Mills SJ, Steyn GF. Appl. Radiat. Isot. 1990;41(12):1201. [Google Scholar]
- (274).Thakare SV, Nair GC, Chakrabarty S, Tomar BS. J. Radioanal. Nucl. Chem. 1999;242(2):537. [Google Scholar]
- (275).Mukhopadhyay B, Lahiri S, Mukhopadhyay K, Ramaswami A. J. Radioanal. Nucl. Chem. 2003;256(2):307. [Google Scholar]
- (276).Shikano K, Katoh M, Shigematsu T, Yonezawa H. J. Radioanal. Nucl. Chem. 1987;119(6):433. [Google Scholar]
- (277).Lewis JS, Laforest R, Lewis MR, Anderson CJ. Cancer Biother. Radiopharm. 2000;15(6):593. doi: 10.1089/cbr.2000.15.593. [DOI] [PubMed] [Google Scholar]
- (278).Casella VR, Grant PM, O’Brien HA., Jr. Radiochim. Acta. 1975;22(1-2):31. [Google Scholar]
- (279).Claessens RAMJ, Janssen AGM, Van den Bosch RLP, De Goeij JJM. Dev. Nucl. Med. 1986;10:46. [Google Scholar]
- (280).Janssen AGM, Claessens RAMJ, Van den Bosch RLP, De Goeij JJM. Appl. Radiat. Isot. 1986;37(4):297. [Google Scholar]
- (281).Abbasi IA, Zaidi JH, Arif M, Waheed S, Subhani MS. Radiochim. Acta. 2006;94(8):381. [Google Scholar]
- (282).Bray LA, Wheelwright EJ, Wester DW, Carson KJ, Elovich RJ, Shade EH, Alexander DL, Culley GE, Atkin SD. Radioact. Radiochem. 1992;3(4):22. [Google Scholar]
- (283).Hsieh BT, Ting G, Hsieh HT, Shen LH. Radioact. Radiochem. 1992;3(4):26. [Google Scholar]
- (284).Happel S, Streng R, Vater P, Ensinger W. Radiat. Meas. 2003;36(1-6):761. [Google Scholar]
- (285).Rane AT, Bhatki KS. Anal. Chem. 1966;38:1598. [Google Scholar]
- (286).Kawashima T. Int. J. Appl. Radiat. Isot. 1969;20(11):806. [Google Scholar]
- (287).Suzuki Y. Int. J. Appl. Radiat. Isot. 1964;15(10):599. doi: 10.1016/0020-708x(64)90004-3. [DOI] [PubMed] [Google Scholar]
- (288).Doering RF, Tucker WD, Stang LGJ. J. Nucl. Med. 1963;4:54. [PubMed] [Google Scholar]
- (289).Chinol M, Hnatowich DJ. J. Nucl. Med. 1987;28(9):1465. [PubMed] [Google Scholar]
- (290).Skraba WJ, Arino H, Kramer HH. Int. J. Appl. Radiat. Isot. 1978;29(2):91. doi: 10.1016/0020-708x(79)90090-5. [DOI] [PubMed] [Google Scholar]
- (291).Chakravarty R, Pandey U, Manolkar RB, Dash A, Venkatesh M, Pillai MRA. Nucl. Med. Biol. 2008;35(2):245. doi: 10.1016/j.nucmedbio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- (292).Pal S, Chattopadhyay S, Das MK, Sudersanan M. Appl. Radiat. Isot. 2006;64(12):1521. doi: 10.1016/j.apradiso.2006.05.003. [DOI] [PubMed] [Google Scholar]
- (293).Roesch F, Qaim SM, Stoecklin G. Appl. Radiat. Isot. 1993;44(4):677. [Google Scholar]
- (294).Sadeghi M, Aboudzadeh M, Zali A, Mirzaii M, Bolourinovin F. Appl. Radiat. Isot. 2009;67(1):7. doi: 10.1016/j.apradiso.2008.08.017. [DOI] [PubMed] [Google Scholar]
- (295).Sadeghi M, Aboudzadeh M, Zali A, Mirzaii M, Bolourinovin F. Appl. Radiat. Isot. 2008;67(1):7. doi: 10.1016/j.apradiso.2008.08.017. [DOI] [PubMed] [Google Scholar]
- (296).Garmestani K, Milenic DE, Plascjak PS, Brechbiel MW. Nucl. Med. Biol. 2002;29(5):599. doi: 10.1016/s0969-8051(02)00322-0. [DOI] [PubMed] [Google Scholar]
- (297).Park LS, Szajek LP, Wong KJ, Pascjak PS, Garmestani K, Googins S, Eckelman WC, Carrasquillo JA, Paik CH. Nucl. Med. Biol. 2004;31(2):297. doi: 10.1016/j.nucmedbio.2003.07.002. [DOI] [PubMed] [Google Scholar]
- (298).Reischl G, Rosch F, Machulla HJ. Radiochim. Acta. 2002;90(4):225. [Google Scholar]
- (299).Yoo J, Tang L, Perkins Todd A, Rowland Douglas J, Laforest R, Lewis Jason S, Welch Michael J. Nucl. Med. Biol. 2005;32(8):891. doi: 10.1016/j.nucmedbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- (300).Lukic D, Tamburella C, Buchegger F, Beyer G-J, Comor Jozef J, Seimbille Y. Appl. Radiat. Isot. 2009;67(4):523. doi: 10.1016/j.apradiso.2008.12.008. [DOI] [PubMed] [Google Scholar]
- (301).Saha GB, Porile NT, Yaffe L. Phys. Rev. 1966;144(3):962. [Google Scholar]
- (302).Link JM, Krohn KA, Eary JF, Kishore R, Lewellen TK, Johnson MW, Badger CC, Richter KY, Nelp WB. J. Labeled Compd. Radiopharm. 1986;23(10-12):1297. [Google Scholar]
- (303).DeJesus OT, Nickles RJ. Appl. Radiat. Isot. 1990;41(8):789. [Google Scholar]
- (304).Zweit J, Downey S, Sharma HL. Appl. Radiat. Isot. 1991;42(2):199. [Google Scholar]
- (305).Hohn A, Zimmermann K, Schaub E, Hirzel W, Schubiger PA, Schibli R. Q. J. Nucl. Med. Mol. Imaging. 2008;52(2):145. [PubMed] [Google Scholar]
- (306).Meijs WE, Herscheid JDM, Haisma HJ, van Leuffen PJ, Mooy R, Pinedo HM. Appl. Radiat. Isot. 1994;45:1143. [Google Scholar]
- (307).Sharma V, Prior JL, Belinsky MG, Kruh GD, Piwnica-Worms D. J. Nucl. Med. 2005;46(2):354. [PubMed] [Google Scholar]
- (308).Sharma V. Bioconjugate Chem. 2004;15(6):1464. doi: 10.1021/bc0498469. [DOI] [PubMed] [Google Scholar]
- (309).Jacob R, Smith T, Prakasha B, Joannides T. Rheumatol. Inter. 2003;23(5):216. doi: 10.1007/s00296-003-0295-2. [DOI] [PubMed] [Google Scholar]
- (310).Turkmen C, Ozturk S, Unal SN, Zulfikar B, Taser O, Sanli Y, Cefle K, Kilicoglu O, Palanduz S. Cancer Biother. Radiopharm. 2007;22(3):393. doi: 10.1089/cbr.2006.328. [DOI] [PubMed] [Google Scholar]
- (311). [accessed August 2009];Cancer Research UK Home Page. http://info.cancerresearchuk.org.
- (312).Gonsalves CF, Brown DB, Carr BI. Exp. Rev. Gastroenterol. Hepatol. 2008;2(4):453. doi: 10.1586/17474124.2.4.453. [DOI] [PubMed] [Google Scholar]
- (313).Nelson K, Vause PE, Jr., Koropova P. Health Phys. 2008;95(5, Suppl.):S156. doi: 10.1097/01.HP.0000318887.65414.15. [DOI] [PubMed] [Google Scholar]
- (314).Khodjibekova M, Szyszko T, Khan S, Nijran K, Tait P, Al-Nahhas A. Rev. Recent Clin. Trials. 2007;2(3):212. doi: 10.2174/157488707781662742. [DOI] [PubMed] [Google Scholar]
- (315).Dijkgraaf I, Boerman OC, Oyen WJG, Corstens FHM, Gotthardt M. Anti-Cancer Agents Med. Chem. 2007;7(5):543. doi: 10.2174/187152007781668733. [DOI] [PubMed] [Google Scholar]
- (316).Vente MAD, Hobbelink MGG, van het Schip AD, Zonnenberg BA, Nijsen JFW. Anti-Cancer Agents Med. Chem. 2007;7(4):441. doi: 10.2174/187152007781058569. [DOI] [PubMed] [Google Scholar]
- (317).Mier W, Haberkorn U, Eisenhut M. Signaling Mol. Targets Cancer Ther. 2007:215. [Google Scholar]
- (318).Gates VL, Atassi B, Lewandowski RJ, Ryu RK, Sato KT, Nemcek AA, Omary R, Salem R. Future Oncol. 2007;3(1):73. doi: 10.2217/14796694.3.1.73. [DOI] [PubMed] [Google Scholar]
- (319).Salem R, Hunter RD. Int. J. Radiat. Oncol., Biol., Phys. 2006;66(2, Suppl.):S83. doi: 10.1016/j.ijrobp.2006.02.061. [DOI] [PubMed] [Google Scholar]
- (320).Welsh JS, Kennedy AS, Thomadsen B. Int. J. Radiat. Oncol., Biol., Phys. 2006;66(2, Suppl.):S62. doi: 10.1016/j.ijrobp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- (321).Pohlman B, Sweetenham J, Macklis RM. Expert Rev. Anticancer Ther. 2006;6(3):445. doi: 10.1586/14737140.6.3.445. [DOI] [PubMed] [Google Scholar]
- (322).Rao AV, Akabani G, Rizzieri DA. Clin. Med. Res. 2005;3(3):157. doi: 10.3121/cmr.3.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Stolz B. Expanding Role of Octreotide I; Presented at the 1st Advances in Oncology, Symposium; Noordwijk, Netherlands. 2002.p. 203. [Google Scholar]
- (324).de Herder WW, Lamberts SWJ. Endocrine. 2003;20(3):285. doi: 10.1385/ENDO:20:3:285. [DOI] [PubMed] [Google Scholar]
- (325).Behr TM, Behe M, Sgouros G. Cancer Biother. Radiopharm. 2002;17(4):445. doi: 10.1089/108497802760363231. [DOI] [PubMed] [Google Scholar]
- (326).Podoloff DA. Curr. Pharm. Des. 2002;8(20):1809. doi: 10.2174/1381612023393882. [DOI] [PubMed] [Google Scholar]
- (327).Virgolini I, Traub T, Novotny C, Leimer M, Fuger B, Li SR, Patri P, Pangerl T, Angelberger P, Raderer M, Burggasser G, Andreae F, Kurtaran A, Dudczak R. Curr. Pharm. Des. 2002;8(20):1781. doi: 10.2174/1381612023393756. [DOI] [PubMed] [Google Scholar]
- (328).Nijsen JFW, Van het Schip AD, Hennink WE, Rook DW, Van Rijk PP, De Klerk JMH. Curr. Med. Chem. 2002;9(1):73. doi: 10.2174/0929867023371454. [DOI] [PubMed] [Google Scholar]
- (329).Vriesendorp HM, Quadri SM. Cancer Biother. Radiopharm. 2000;15(5):431. doi: 10.1089/cbr.2000.15.431. [DOI] [PubMed] [Google Scholar]
- (330).Bal CS, Kumar A. Trop. Gastroenterol. 2008;29(2):62. [PubMed] [Google Scholar]
- (331).Price TJ, Townsend A. Arch. Surg. 2008;143(3):313. doi: 10.1001/archsurg.2007.65. [DOI] [PubMed] [Google Scholar]
- (332).Shimoni A, Nagler A. Leuk Lymphoma. 2007;48(11):2110. doi: 10.1080/10428190701573273. [DOI] [PubMed] [Google Scholar]
- (333).Salem R, Thurston KG. J. Vasc. Interv. Radiol. 2006;17(10):1571. doi: 10.1097/01.RVI.0000236744.34720.73. [DOI] [PubMed] [Google Scholar]
- (334).Salem R, Thurston Kenneth G. J. Vasc. Interv. Radiol. 2006;17(9):1425. doi: 10.1097/01.RVI.0000235779.88652.53. [DOI] [PubMed] [Google Scholar]
- (335).Salem R, Thurston Kenneth G. J. Vasc. Interv. Radiol. 2006;17(8):1251. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- (336).Murthy R, Nunez R, Szklaruk J, Erwin W, Madoff David C, Gupta S, Ahrar K, Wallace Michael J, Cohen A, Coldwell Douglas M, Kennedy Andrew S, Hicks Marshall E. Radiographics. 2005;25(Suppl 1):S41. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- (337).Stipsanelli E, Valsamaki P. Hell. J. Nucl. Med. 2005;8(2):103. [PubMed] [Google Scholar]
- (338).Mulcahy MF. Curr. Treat. Opt. Oncol. 2005;6(5):423. doi: 10.1007/s11864-005-0045-7. [DOI] [PubMed] [Google Scholar]
- (339).Hagenbeek A, Lewington V. Ann. Oncol. 2005;16(5):786. doi: 10.1093/annonc/mdi148. [DOI] [PubMed] [Google Scholar]
- (340).Hernandez MC, Knox SJ. Sem. Oncol. 2003;30(6 Suppl 17):6. doi: 10.1053/j.seminoncol.2003.10.005. [DOI] [PubMed] [Google Scholar]
- (341).Mahmoud-Ahmed AS, Suh JH. Pituitary. 2002;5(3):175. doi: 10.1023/a:1023365200437. [DOI] [PubMed] [Google Scholar]
- (342).Salem R, Thurston Kenneth G, Carr Brian I, Goin James E, Geschwind Jean-Francois H. J. Vasc. Interv. Radiol. 2002;13(9 Pt 2):S223. doi: 10.1016/s1051-0443(07)61790-4. [DOI] [PubMed] [Google Scholar]
- (343).Pharmacother EO, Illidge TM, Bayne MC. Expt. Opin. Pharm. 2001;2(6):953. doi: 10.1517/14656566.2.6.953. [DOI] [PubMed] [Google Scholar]
- (344).Riva P, Franceschi G, Riva N, Casi M, Santimaria M, Adamo M. Eu. J. Nucl. Med. 2000;27(5):601. doi: 10.1007/s002590050549. [DOI] [PubMed] [Google Scholar]
- (345).Sims E, Doughty D, Macaulay E, Royle N, Wraith C, Darlison R, Plowman PN. Clin. Oncol. 1999;11(5):303. doi: 10.1053/clon.1999.9073. [DOI] [PubMed] [Google Scholar]
- (346).Muller C, Schibli R, Krenning Eric P, de Jong M. J. Nucl. Med. 2008;49(4):623. doi: 10.2967/jnumed.107.047704. [DOI] [PubMed] [Google Scholar]
- (347).Mathias CJ, Wang S, Low PS, Waters DJ, Green MA. Nucl. Med. Biol. 1998;26(1):23. doi: 10.1016/s0969-8051(98)00076-6. [DOI] [PubMed] [Google Scholar]
- (348).de Visser M, Janssen PJ, Srinivasan A, Reubi JC, Waser B, Erion JL, Schmidt MA, Krenning EP, de Jong M. Eu. J. Nucl. Med. Mol. Imaging. 2003;30(8):1134. doi: 10.1007/s00259-003-1189-y. [DOI] [PubMed] [Google Scholar]
- (349).Hillairet de Boisferon M, Raguin O, Thiercelin C, Dussaillant M, Rostene W, Barbet J, Pelegrin A, Gruaz-Guyon A. Bioconjugate Chem. 2002;13(3):654. doi: 10.1021/bc015585g. [DOI] [PubMed] [Google Scholar]
- (350).Bussolati G, Chinol M, Chini B, Nacca A, Cassoni P, Paganelli G. Cancer Res. 2001;61(11):4393. [PubMed] [Google Scholar]
- (351).Schoenberger J, Bauer J, Moosbauer J, Eilles C, Grimm D. Curr. Med. Chem. 2008;15(2):187. doi: 10.2174/092986708783330647. [DOI] [PubMed] [Google Scholar]
- (352).Sarda-Mantel L, Hervatin F, Michel J-B, Louedec L, Martet G, Rouzet F, Lebtahi R, Merlet P, Khaw B-A, Le Guludec D. Eu. J. Nucl. Med. Mol. Imaging. 2008;35(1):158. doi: 10.1007/s00259-007-0559-2. [DOI] [PubMed] [Google Scholar]
- (353).Sarda-Mantel L, Michel J-B, Rouzet F, Martet G, Louedec L, Vanderheyden J-L, Hervatin F, Raguin O, Vrigneaud J-M, Khaw BA, Guludec D. Eu. J. Nucl. Med. Mol. Imaging. 2006;33(3):239. doi: 10.1007/s00259-005-1900-2. [DOI] [PubMed] [Google Scholar]
- (354).Ke S, Wen X, Wu Q-P, Wallace S, Charnsangavej C, Stachowiak AM, Stephens CL, Abbruzzese JL, Podoloff DA, Li C. J. Nucl. Med. 2004;45(1):108. [PubMed] [Google Scholar]
- (355).Wen X, Wu Q-P, Ke S, Wallace S, Charnsangavej C, Huang P, Liang D, Chow D, Li C. Cancer Biother. Radiopharm. 2003;18(5):819. doi: 10.1089/108497803770418364. [DOI] [PubMed] [Google Scholar]
- (356).Liu D, Overbey D, Watkinson L, Giblin MF. Bioconjugate Chem. 2009;20(5):888. doi: 10.1021/bc800433y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).Hanaoka H, Mukai T, Habashita S, Asano D, Ogawa K, Kuroda Y, Akizawa H, Iida Y, Endo K, Saga T, Saji H. Nucl. Med. Biol. 2007;34(5):503. doi: 10.1016/j.nucmedbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- (358).Giersing BK, Rae MT, Carballido BM, Williamson RA, Blower PJ. Bioconjugate Chem. 2001;12:964. doi: 10.1021/bc010028f. [DOI] [PubMed] [Google Scholar]
- (359).Perk LR, Stigter-van Walsum M, Visser GWM, Kloet RW, Vosjan MJWD, Leemans CR, Giaccone G, Albano R, Comoglio PM, van Dongen GAMS. Eu. J. Nucl. Med. Mol. Imaging. 2008;35(10):1857. doi: 10.1007/s00259-008-0774-5. [DOI] [PubMed] [Google Scholar]
- (360).Borjesson PKE, Jauw YWS, Boellaard R, de Bree R, Comans EFI, Roos JC, Castelijns JA, Vosjan MJWD, Kummer JA, Leemans CR, Lammertsma AA, van Dongen GAMS. Clin. Cancer Res. 2006;12(7):2133. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- (361).Murata Y, Ishida R, Umehara I, Ishikawa N, Komatsuzaki A, Kurabayashi T, Ishii Y, Ogura I, Ishii J, Okada N, Shibuya H. Nucl. Med. Commun. 1999;20(7):599. doi: 10.1097/00006231-199907000-00002. [DOI] [PubMed] [Google Scholar]
- (362).Kotani J, Kawabe J, Higashiyama S, Kawamura E, Oe A, Hayashi T, Kurooka H, Tsumoto C, Kusuki M, Yamane H, Shiomi S. Ann. Nucl. Med. 2008;22(4):297. doi: 10.1007/s12149-007-0116-x. [DOI] [PubMed] [Google Scholar]
- (363).Van Den Bossche B, Lambert B, De Winter F, Kolindou A, Dierckx RA, Noens L, Van De Wiele C. Nucl. Med. Commun. 2002;23(11):1079. doi: 10.1097/00006231-200211000-00007. [DOI] [PubMed] [Google Scholar]
- (364).Ballani NS, Khan HA, Al-Mohannadi SH, Abu Al-Huda F, Usmani S, Tuli MM, Al-Shemmari SH, Al-Sawagh HF, Al-Enezi FH. Nucl. Med. Commun. 2008;29(6):527. doi: 10.1097/MNM.0b013e3282f81366. [DOI] [PubMed] [Google Scholar]
- (365).Kita T, Watanabe S, Yano F, Hayashi K, Yamamoto M, Iwasaki Y, Kosuda S. Ann. Nucl. Med. 2007;21(9):499. doi: 10.1007/s12149-007-0060-9. [DOI] [PubMed] [Google Scholar]
- (366).Even-Sapir E, Israel O. Eu. J. Nucl. Med. Mol. Imaging. 2003;30(Suppl. 1):S65. doi: 10.1007/s00259-003-1164-7. [DOI] [PubMed] [Google Scholar]
- (367).Nejmeddine F, Raphael M, Martin A, Le Roux G, Moretti J-L, Caillat-Vigneron N. J. Nucl. Med. 1999;40(1):40. [PubMed] [Google Scholar]
- (368).Herman M, Paucek B, Raida L, Myslivecek M, Zapletalova J. Eu. J. Radiol. 2007;64(3):432. doi: 10.1016/j.ejrad.2007.03.003. [DOI] [PubMed] [Google Scholar]
- (369).Sun S-S, Lin C-Y, Chuang F-J, Kao C-H. Clin. Nucl. Med. 2003;28(10):869. doi: 10.1097/01.rlu.0000090949.09692.69. [DOI] [PubMed] [Google Scholar]
- (370).Israel O, Mekel M, Bar-Shalom R, Epelbaum R, Hermony N, Haim N, Dann EJ, Frenkel A, Ben-Arush M, Gaitini D. J. Nucl. Med. 2002;43(10):1295. [PubMed] [Google Scholar]
- (371).Rehm PK. Cancer Biother. Radiopharm. 1999;14(4):251. doi: 10.1089/cbr.1999.14.251. [DOI] [PubMed] [Google Scholar]
- (372).Watanabe R, Iizuka H, Kaira K, Mori T, Takise A, Ito J, Motegi A, Onozato Y, Ishihara H. Radiat. Med. 2006;24(6):456. doi: 10.1007/s11604-006-0043-0. [DOI] [PubMed] [Google Scholar]
- (373).Nakahara T, Togawa T, Nagata M, Kikuchi K, Hatano K, Yui N, Kubo A. Eu. J. Nucl. Med. Mol. Imaging. 2002;29(8):1072. doi: 10.1007/s00259-002-0836-z. [DOI] [PubMed] [Google Scholar]
- (374).Horton MA. Int. J. Biochem. Cell Biol. 1997;29(5):721. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- (375).Albelda SM, Buck CA. FASEB J. 1990;4(11):2868. [PubMed] [Google Scholar]
- (376).Hausner SH, Kukis DL, Gagnon MKJ, Stanecki CE, Ferdani R, Marshall JF, Anderson CJ, Sutcliffe JL. Mol. Imaging. 2009;8(2):111. [PMC free article] [PubMed] [Google Scholar]
- (377).Sutcliffe JL, Liu R, Peng L, DeNardo SJ, Cherry SR, Kukis DL, Hausner SH, Lam KS. J. Nucl. Med. 2007;48(1):69P. [Google Scholar]
- (378).Denardo SJ, Liu R, Albrecht H, Natarajan A, Sutcliffe JL, Anderson C, Peng L, Ferdani R, Cherry SR, Lam KS. J. Nucl. Med. 2009;50(4):625. doi: 10.2967/jnumed.108.056903. [DOI] [PubMed] [Google Scholar]
- (379).Clezardin P. Cell. Mol. Life Sci. 1998;54(6):541. doi: 10.1007/s000180050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Jin H, Varner J. Br. J. Cancer. 2004;90(3):561. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (381).Kimura RH, Cheng Z, Gambhir S, Cochran JR. Cancer Res. 2009;69(6):2435. doi: 10.1158/0008-5472.CAN-08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).McQuade P, Knight LC, Welch MJ. Bioconjugate Chem. 2004;15(5):988. doi: 10.1021/bc049961j. [DOI] [PubMed] [Google Scholar]
- (383).Harris TD, Cheesman E, Harris AR, Sachleben R, Edwards DS, Liu S, Bartis J, Ellars C, Onthank D, Yalamanchili P, Heminway S, Silva P, Robinson S, Lazewatsky J, Rajopadhye M, Barrett J. Bioconjugate Chem. 2007;18(4):1266. doi: 10.1021/bc070002+. [DOI] [PubMed] [Google Scholar]
- (384).Harris TD, Kalogeropoulos S, Nguyen T, Dwyer G, Edwards DS, Liu S, Bartis J, Ellars C, Onthank D, Yalamanchili P, Heminway S, Robinson S, Lazewatsky J, Barrett J. Bioconjugate Chem. 2006;17(5):1294. doi: 10.1021/bc060063s. [DOI] [PubMed] [Google Scholar]
- (385).Harris TD, Kalogeropoulos S, Nguyen T, Liu S, Bartis J, Ellars C, Edwards S, Onthank D, Silva P, Yalamanchili P, Robinson S, Lazewatsky J, Barrett J, Bozarth J. Cancer Biother. Radiopharm. 2003;18(4):627. doi: 10.1089/108497803322287727. [DOI] [PubMed] [Google Scholar]
- (386).Jang B-S, Lim E, Park SH, Shin IS, Danthi SN, Hwang IS, Le N, Yu S, Xie J, Li KCP, Carrasquillo JA, Paik CH. Nucl. Med. Biol. 2007;34(4):363. doi: 10.1016/j.nucmedbio.2007.02.002. [DOI] [PubMed] [Google Scholar]
- (387).Shin IS, Jang B-S, Danthi SN, Xie J, Yu S, Le N, Maeng J-S, Hwang IS, Li KCP, Carrasquillo JA, Paik CH. Bioconjugate Chem. 2007;18(3):821. doi: 10.1021/bc0603485. [DOI] [PubMed] [Google Scholar]
- (388).Yoshimoto M, Ogawa K, Washiyama K, Shikano N, Mori H, Amano R, Kawai K. Int. J. Cancer. 2008;123(3):709. doi: 10.1002/ijc.23575. [DOI] [PubMed] [Google Scholar]
- (389).van Hagen PM, Breeman WAP, Bernard HF, Schaar M, Mooij CM, Srinivasan A, Schmidt MA, Krenning EP, de Jong M. Int. J. Cancer. 2000;90(4):186. [PubMed] [Google Scholar]
- (390).Dumont RA, Hildebrandt I, Su H, Haubner R, Reischl G, Czernin JG, Mischel PS, Weber WA. Cancer Res. 2009;69(7):3173. doi: 10.1158/0008-5472.CAN-08-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (391).Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. J. Nucl. Med. 2005;46(10):1707. [PubMed] [Google Scholar]
- (392).Cao Q, Cai W, Li Z-B, Chen K, He L, Li H-C, Hui M, Chen X. Eu. J. Nucl. Med. Mol. Imaging. 2007;34(11):1832. doi: 10.1007/s00259-007-0451-0. [DOI] [PubMed] [Google Scholar]
- (393).Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, Bremner R, Bading JR, Conti PS. Neoplasia. 2005;7(3):271. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Cao F, Li Z, Lee A, Liu Z, Chen K, Wang H, Cai W, Chen X, Wu JC. Cancer Res. 2009;69(7):2709. doi: 10.1158/0008-5472.CAN-08-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (395).Li Z-B, Cai W, Cao Q, Chen K, Wu Z, He L, Chen X. J. Nucl. Med. 2007;48(7):1162. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- (396).Shi J, Kim Y-S, Zhai S, Liu Z, Chen X, Liu S. Bioconjugate Chem. 2009;20(4):750. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Lee H-Y, Li Z, Chen K, Hsu Andrew R, Xu C, Xie J, Sun S, Chen X. J. Nucl. Med. 2008;49(8):1371. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- (398).Cai W, Chen K, Li Z-B, Gambhir SS, Chen X. J. Nucl. Med. 2007;48(11):1862. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- (399).Wei L, Ye Y, Wadas TJ, Lewis JS, Welch MJ, Achilefu S, Anderson CJ. Nucl. Med. Biol. 2009;36(3):277. doi: 10.1016/j.nucmedbio.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (400).Sprague JE, Kitaura H, Zou W, Ye Y, Achilefu S, Weilbaecher KN, Teitelbaum SL, Anderson CJ. J. Nucl. Med. 2007;48(2):311. [PMC free article] [PubMed] [Google Scholar]
- (401).Jeong Jae M, Hong Mee K, Chang Young S, Lee Y-S, Kim Young J, Cheon Gi J, Lee Dong S, Chung J-K, Lee Myung C. J. Nucl. Med. 2008;49(5):830. doi: 10.2967/jnumed.107.047423. [DOI] [PubMed] [Google Scholar]
- (402).Li Z-B, Chen K, Chen X. Eu. J. Nucl. Med. Mol. Imaging. 2008;35(6):1100. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- (403).Liu Z, Niu G, Shi J, Liu S, Wang F, Liu S, Chen X. Eu. J. Nucl. Med. Mol. Imaging. 2009;36(6):947. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- (404).Reubi JC. Endocr. Rev. 2003;24(4):389. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- (405).Reubi JC, Waser B, Schaer J-C, Laissue JA. Eu. J. Nucl. Med. 2001;28(7):836. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- (406).Rogers BE, Zinn KR, Buchsbaum DJ. Q. J. Nucl. Med. 2000;44(3):208. [PubMed] [Google Scholar]
- (407).Kwekkeboom DJ, Kooij PP, Bakker WH, Maecke HR, Krenning EP. J. Nucl. Med. 1999;40(5):762. [PubMed] [Google Scholar]
- (408).Kwekkeboom DJ, Bakker WH, Kooij PPM, Konijnenberg MW, Srinivasan A, Erion JL, Schmidt MA, Bugaj JE, de Jong M, Krenning EP. Eu. J. Nucl. Med. 2001;28(9):1319. doi: 10.1007/s002590100574. [DOI] [PubMed] [Google Scholar]
- (409).Lebtahi R, Moreau S, Marchand-Adam S, Debray M-P, Brauner M, Soler P, Marchal J, Raguin O, Gruaz-Guyon A, Reubi J-C, Le Guludec D, Crestani B. J. Nucl. Med. 2006;47(8):1281. [PubMed] [Google Scholar]
- (410).Lebtahi R, Le Cloirec J, Houzard C, Daou D, Sobhani I, Sassolas G, Mignon M, Bourguet P, Le Guludec D. J. Nucl. Med. 2002;43(7):889. [PubMed] [Google Scholar]
- (411).Traub T, Petkov V, Ofluoglu S, Pangerl T, Raderer M, Fueger BJ, Schima W, Kurtaran A, Dudczak R, Virgolini I. J. Nucl. Med. 2001;42(9):1309. [PubMed] [Google Scholar]
- (412).Cremonesi M, Ferrari M, Zoboli S, Chinol M, Stabin MG, Orsi F, Maecke HR, Jermann E, Robertson C, Fiorenza M, Tosi G, Paganelli G. Eu. J. Nucl. Med. 1999;26(8):877. doi: 10.1007/s002590050462. [DOI] [PubMed] [Google Scholar]
- (413).Forrer F, Uusijarvi H, Waldherr C, Cremonesi M, Bernhardt P, Mueller-Brand J, Maecke HR. Eu. J. Nucl. Med. Mol. Imaging. 2004;31(9):1257. doi: 10.1007/s00259-004-1553-6. [DOI] [PubMed] [Google Scholar]
- (414).Rodrigues M, Gabriel M, Heute D, Putzer D, Griesmacher A, Virgolini I. Eu. J. Nucl. Med. Mol. Imaging. 2008;35(10):1796. doi: 10.1007/s00259-008-0794-1. [DOI] [PubMed] [Google Scholar]
- (415).Rodrigues M, Traub-Weidinger T, Leimer M, Li S, Andreae F, Angelberger P, Dudczak R, Virgolini I. Eu. J. Nucl. Med. Mol. Imaging. 2005;32(10):1144. doi: 10.1007/s00259-005-1820-1. [DOI] [PubMed] [Google Scholar]
- (416).Becherer A, Szabo M, Karanikas G, Wunderbaldinger P, Angelberger P, Raderer M, Kurtaran A, Dudczak R, Kletter K. J. Nucl. Med. 2004;45(7):1161. [PubMed] [Google Scholar]
- (417).Decristoforo C, Mather SJ, Cholewinski W, Donnemiller E, Riccabona G, Moncayo R. Eu. J. Nucl. Med. 2000;27(9):1318. doi: 10.1007/s002590000289. [DOI] [PubMed] [Google Scholar]
- (418).Meisetschlager G, Poethko T, Stahl A, Wolf I, Scheidhauer K, Schottelius M, Herz M, Wester Hans J, Schwaiger M. J. Nucl. Med. 2006;47(4):566. [PubMed] [Google Scholar]
- (419).Gabriel M, Decristoforo C, Donnemiller E, Ulmer H, Rychlinski CW, Mather SJ, Moncayo R. J. Nucl. Med. 2003;44(5):708. [PubMed] [Google Scholar]
- (420).Anderson CJ, Dehdashti F, Cutler PD, Schwarz SW, Laforest R, Bass LA, Lewis JS, McCarthy DW. J. Nucl. Med. 2001;42(2):213. [PubMed] [Google Scholar]
- (421).Parry JJ, Eiblmaier M, Andrews R, Meyer LA, Higashikubo R, Anderson CJ, Rogers BE. Mol. Imaging. 2007;6(1):56. [PubMed] [Google Scholar]
- (422).Wang M, Caruano AL, Lewis MR, Ballard L, Kelly K, Vanderwaal RP, Anderson CJ. Q. J. Nucl. Med. 2001;45(2):S12. [Google Scholar]
- (423).Wang M, Caruano AL, Lewis MR, Meyer LM, VanderWaal RP, Anderson CJ. Cancer Res. 2003;63(20):6864. [PubMed] [Google Scholar]
- (424).Li WP, Lewis JS, Kim J, Bugaj JE, Johnson MA, Erion JL, Anderson CJ. Bioconjugate Chem. 2002;13(4):721. doi: 10.1021/bc015590k. [DOI] [PubMed] [Google Scholar]
- (425).Edwards WB, Xu B, Akers W, Cheney PP, Liang K, Rogers BE, Anderson C,J, Achilefu S. Bioconjugate Chem. 2008;19(1):192. doi: 10.1021/bc700291m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (426).de Jong M, Breeman WAP, Bernard BF, van Gameren A, de Bruin E, Bakker WH, van der Pluijm ME, Visser TJ, Macke HR, Krenning EP. Eu. J. Nucl. Med. 1999;26(7):693. doi: 10.1007/s002590050439. [DOI] [PubMed] [Google Scholar]
- (427).Lewis JS, Lewis MR, Cutler PD, Srinivasan A, Schmidt MA, Schwarz SW, Morris MM, Miller JP, Anderson CJ. Clin. Cancer Res. 1999;5(10):3608. [PubMed] [Google Scholar]
- (428).Lewis JS, Srinivasan A, Schmidt MA, Anderson CJ. Nucl. Med. Biol. 1999;26(3):267. doi: 10.1016/s0969-8051(98)00105-x. [DOI] [PubMed] [Google Scholar]
- (429).Weisman GR, Rogers ME, Wong EH, Jasinski JP, Paight ES. J. Am. Chem. Soc. 1990;112(23):8604. [Google Scholar]
- (430).Sprague JE, Peng Y, Sun X, Weisman GR, Wong EH, Achilefu S, Anderson CJ. Clin. Cancer Res. 2004;10(24):8674. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- (431).Eiblmaier M, Andrews R, Laforest R, Rogers BE, Anderson CJ. J. Nucl. Med. 2007;48(8):1390. doi: 10.2967/jnumed.107.039990. [DOI] [PubMed] [Google Scholar]
- (432).Milenic DE, Brady ED, Brechbiel MW. Nat. Rev. Drug Discovery. 2004;3(6):488. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- (433).Clifford T, Boswell CA, Biddlecombe GB, Lewis JS, Brechbiel MW. J. Med. Chem. 2006;49(14):4297. doi: 10.1021/jm060317v. [DOI] [PubMed] [Google Scholar]
- (434).Lincke T, Singer J, Kluge R, Sabri O, Paschke R. Thyroid. 2009;19(4):381. doi: 10.1089/thy.2008.0389. [DOI] [PubMed] [Google Scholar]
- (435).Fanti S, Ambrosini V, Tomassetti P, Castellucci P, Montini G, Allegri V, Grassetto G, Rubello D, Nanni C, Franchi R. Biomed. Pharmacother. 2008;62(10):667. doi: 10.1016/j.biopha.2008.01.010. [DOI] [PubMed] [Google Scholar]
- (436).Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Eu. J. Nucl. Med. Mol. Imaging. 2006;33(10):1115. doi: 10.1007/s00259-006-0110-x. [DOI] [PubMed] [Google Scholar]
- (437).Wild D, Schmitt JS, Ginj M, Maecke HR, Bernard BF, Krenning E, de Jong M, Wenger S, Reubi J-C. Eu. J. Nucl. Med. Mol. Imaging. 2003;30(10):1338. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- (438).Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmiti JS, Eisenwiener K, Beglinger C, Macke HR. Int. J. Cancer. 2002;98(6):930. doi: 10.1002/ijc.10295. [DOI] [PubMed] [Google Scholar]
- (439).Hofmann M, Maecke H, Boerner AR, Weckesser E, Schoeffski P, Oei ML, Schumacher J, Henze M, Heppeler A, Meyer GJ, Knapp WH. Eu. J. Nucl. Med. 2001;28(12):1751. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- (440).Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. J. Nucl. Med. 2007;48(4):508. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- (441).Henze M, Schuhmacher J, Hipp P, Kowalski J, Becker DW, Doll J, Macke HR, Hofmann M, Debus J, Haberkorn U. J. Nucl. Med. 2001;42(7):1053. [PubMed] [Google Scholar]
- (442).Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, Dickson J, Caplin M, Ell PJ. Cancer. 2008;112(11):2447. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- (443).Ugur O, Kothari PJ, Finn RD, Zanzonico P, Ruan S, Guenther I, Maecke HR, Larson SM. Nucl. Med. Biol. 2002;29(2):147. doi: 10.1016/s0969-8051(01)00290-6. [DOI] [PubMed] [Google Scholar]
- (444).Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Macke HR, Reubi JC. Proc. Natl. Acad. Sci. U.S.A. 2006;103(44):16436. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (445).Wadas TJ, Eiblmaier M, Zheleznyak A, Sherman CD, Ferdani R, Liang K, Achilefu S, Anderson CJ. J. Nucl. Med. 2008;49(11):1819. doi: 10.2967/jnumed.108.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Science. 1989;244(4905):707. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- (447).Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. J. Clin. Oncol. 2000;18(21):3651. doi: 10.1200/JCO.2000.18.21.3651. [DOI] [PubMed] [Google Scholar]
- (448).Perik PJ, Lub-De Hooge MN, Gietema JA, van der Graaf WTA, de Korte MA, Jonkman S, Kosterink JGW, van Veldhuisen DJ, Sleijfer DT, Jager PL, de Vries EGE. J. Clin. Oncol. 2006;24(15):2276. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- (449).Lub-de Hooge MN, Kosterink JGW, Perik PJ, Nijnuis H, Tran L, Bart J, Suurmeijer AJH, de Jong S, Jager PL, de Vries EGE. Br. J. Pharmacol. 2004;143(1):99. doi: 10.1038/sj.bjp.0705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (450).Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, Sevick-Muraca EM. J. Nucl. Med. 2007;48(9):1501. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- (451).Tang Y, Scollard D, Chen P, Wang J, Holloway C, Reilly RM. Nucl. Med. Commun. 2005;26(5):427. doi: 10.1097/00006231-200505000-00006. [DOI] [PubMed] [Google Scholar]
- (452).Tang Y, Wang J, Scollard DA, Mondal H, Holloway C, Kahn HJ, Reilly RM. Nucl. Med. Biol. 2005;32(1):51. doi: 10.1016/j.nucmedbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- (453).Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A, Williams S, Cole MJ, Ross S, Schwall R. Cancer Res. 2006;67(1):254. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- (454).Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandstroem M, Nilsson FY, Wennborg A, Abrahmsen L, Feldwisch J. Cancer Res. 2007;67(5):2178. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- (455).Wallberg H, Orlova A. Cancer Biother. Radiopharm. 2008;23(4):435. doi: 10.1089/cbr.2008.0464. [DOI] [PubMed] [Google Scholar]
- (456).Breeman WAP, De Jong M, Bernard BF, Kwekkeboom DJ, Srinivasan A, Van Der Pluijm ME, Hofland LJ, Visser TJ, Krenning EP. Int. J. Cancer. 1999;83(5):657. doi: 10.1002/(sici)1097-0215(19991126)83:5<657::aid-ijc15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- (457).Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, Volkert WA. J. Nucl. Med. 2003;44(5):823. [PubMed] [Google Scholar]
- (458).Garrison JC, Rold TL, Sieckman GL, Naz F, Sublett SV, Figueroa SD, Volkert WA, Hoffman TJ. Bioconjugate Chem. 2008;19(9):1803. doi: 10.1021/bc8001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (459).Schuhmacher J, Zhang H, Doll J, Maecke HR, Matys R, Hauser H, Henze M, Haberkorn U, Eisenhut M. J. Nucl. Med. 2005;46(4):691. [PubMed] [Google Scholar]
- (460).Dimitrakopoulou-Strauss A, Hohenberger P, Eisenhut M, Maecke HR, Haberkorn U, Strauss L. J. Nucl. Med. 2006;47(S1):102P. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- (461).Biddlecombe GB, Rogers BE, de Visser M, Parry JJ, de Jong M, Erion JL, Lewis JS. Bioconjugate Chem. 2007;18(3):724. doi: 10.1021/bc060281l. [DOI] [PubMed] [Google Scholar]
- (462).Gasser G, Tjioe L, Graham B, Belousoff MJ, Juran S, Walther M, Kuenstler J-U, Bergmann R, Stephan H, Spiccia L. Bioconjugate Chem. 2008;19(3):719. doi: 10.1021/bc700396e. [DOI] [PubMed] [Google Scholar]
- (463).Rogers BE, Bigott HM, McCarthy DW, Della Manna D, Kim J, Sharp TL, Welch MJ. Bioconjugate Chem. 2003;14(4):756. doi: 10.1021/bc034018l. [DOI] [PubMed] [Google Scholar]
- (464).Parry JJ, Andrews R, Rogers BE. Breast Cancer Res. Treat. 2007;101(2):175. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- (465).Parry JJ, Kelly TS, Andrews R, Rogers BE. Bioconjugate Chem. 2007;18(4):1110. doi: 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- (466).Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, Conti PS. J. Nucl. Med. 2004;45(8):1390. [PubMed] [Google Scholar]
- (467).Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. Proc. Natl. Acad. Sci. U. S. A. 2007;104(30):12462. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (468).Prasanphanich AF, Retzloff L, Lane SR, Nanda PK, Sieckman GL, Rold TL, Ma L, Figueroa SD, Sublett SV, Hoffman TJ, Smith CJ. Nucl. Med. Biol. 2009;36(2):171. doi: 10.1016/j.nucmedbio.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (469).Klein S, Levitzki A. Curr. Opin. Cell Biol. 2009;21(2):185. doi: 10.1016/j.ceb.2008.12.006. [DOI] [PubMed] [Google Scholar]
- (470).Klijn JGM, Berns PMJJ, Schmitz PIM, Foekens JA. Endo. Rev. 1992;13(1):3. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- (471).Kurihara A, Pardridge WM. Cancer Res. 1999;59(24):6159. [PubMed] [Google Scholar]
- (472).Kurihara A, Deguchi Y, Pardridge WM. Bioconjugate Chem. 1999;10(3):502. doi: 10.1021/bc980123x. [DOI] [PubMed] [Google Scholar]
- (473).Reilly RM, Kiarash R, Sandhu J, Lee YW, Cameron RG, Hendler A, Vallis K, Gariepy J. J. Nucl. Med. 2000;41(5):903. [PubMed] [Google Scholar]
- (474).Reilly RM, Kiarash R, Cameron RG, Porlier N, Sandhu J, Hill RP, Vallis K, Hendler A, Gariepy J. J. Nucl. Med. 2000;41(3):429. [PubMed] [Google Scholar]
- (475).Chen P, Cameron R, Wang J, Vallis KA, Reilly RM. J. Nucl. Med. 2003;44(9):1469. [PubMed] [Google Scholar]
- (476).Reilly RM, Chen P, Wang J, Scollard D, Cameron R, Vallis KA. J. Nucl. Med. 2006;47(6):1023. [PubMed] [Google Scholar]
- (477).Hu M, Scollard D, Chan C, Chen P, Vallis K, Reilly RM. Nucl. Med. Biol. 2007;34(8):887. doi: 10.1016/j.nucmedbio.2007.06.010. [DOI] [PubMed] [Google Scholar]
- (478).Reilly RM, Scollard DA, Wang J, Mondal H, Chen P, Henderson LA, Bowen BM, Vallis KA. J. Nucl. Med. 2004;45(4):701. [PubMed] [Google Scholar]
- (479).Velikyan I, Sundberg AL, Lindhe O, Hoeglund AU, Eriksson O, Werner E, Carlsson J, Bergstroem M, Laangstroem B, Tolmachev V. J. Nucl. Med. 2005;46(11):1881. [PubMed] [Google Scholar]
- (480).Niu G, Cai W, Chen K, Chen X. Mol. Imaging Biol. 2008;10(2):99. doi: 10.1007/s11307-007-0123-2. [DOI] [PubMed] [Google Scholar]
- (481).Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Eu. J. Nucl. Med. Mol. Imaging. 2007;34(6):850. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- (482).Li WP, Meyer LA, Capretto DA, Sherman CD, Anderson CJ. Cancer Biother. Radiopharm. 2008;23(2):158. doi: 10.1089/cbr.2007.0444. [DOI] [PubMed] [Google Scholar]
- (483).Wen X, Wu QP, Ke S, Ellis L, Charnsangavej C, Delpassand AS, Wallace S, Li C. J. Nucl. Med. 2001;42(10):1530. [PubMed] [Google Scholar]
- (484).Eiblmaier M, Meyer LA, Watson MA, Fracasso PM, Pike LJ, Anderson CJ. J. Nucl. Med. 2008;49(9):1472. doi: 10.2967/jnumed.108.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Lohela M, Bry M, Tammela T, Alitalo K. Curr. Opin. Cell Biol. 2009;21(2):154. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- (486).Kaplan RN, Rafii S, Lyden D. Cancer Res. 2006;66(23):11089. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. Nature. 2005;438(7069):820. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (488).Chan C, Sandhu J, Guha A, Scollard DA, Wang J, Chen P, Bai K, Lee L, Reilly RM. J. Nucl. Med. 2005;46(10):1745. [PubMed] [Google Scholar]
- (489).Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X. J. Nucl. Med. 2006;47(12):2048. [PubMed] [Google Scholar]
- (490).Wang H, Cai W, Chen K, Li Z-B, Kashefi A, He L, Chen X. Eu. J. Nucl. Med. Mol. Imaging. 2007;34(12):2001. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- (491).Chen K, Li Z-B, Wang H, Cai W, Chen X. Eu. J. Nucl. Med. Mol. Imaging. 2008;35(12):2235. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- (492).Hsu AR, Cai W, Veeravagu A, Mohamedali KA, Chen K, Kim S, Vogel H, Hou LC, Tse V, Rosenblum MG, Chen X. J. Nucl. Med. 2007;48(3):445. [PubMed] [Google Scholar]
- (493).Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Nat. Med. 2007;13(4):504. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- (494).Jemal A, Siegel R, Ward R, Murray T, Xu J, Thun MJ. Cancer J. Clin. 2007;57:43. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- (495).Miao Y, Quinn TP. Front. Biosci. 2007;12(12):4514. doi: 10.2741/2406. [DOI] [PubMed] [Google Scholar]
- (496).Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. J. Nucl. Med. 2001;42(12):1847. [PubMed] [Google Scholar]
- (497).Cheng Z, Chen J, Miao Y, Owen NK, Quinn TP, Jurisson SS. J. Med. Chem. 2002;45(14):3048. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- (498).Chen J, Cheng Z, Miao Y, Jurisson SS, Quinn TP. Cancer. 2002;94(4, Suppl.):1196. doi: 10.1002/cncr.10284. [DOI] [PubMed] [Google Scholar]
- (499).Bapst J-P, Calame M, Tanner H, Eberle AN. Bioconjugate Chem. 2009;20(5):984. doi: 10.1021/bc900007u. [DOI] [PubMed] [Google Scholar]
- (500).McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. J. Med. Chem. 2005;48(8):2985. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- (501).Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. Bioconjugate Chem. 2007;18(3):765. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (502).Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. J. Nucl. Med. 2004;45(1):116. [PubMed] [Google Scholar]
- (503).Wei L, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Vavere AL, Lewis JS. Nucl. Med. Biol. 2007;34(8):945. doi: 10.1016/j.nucmedbio.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (504).Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Lewis JS. J. Nucl. Med. 2007;48(1):64. [PubMed] [Google Scholar]
- (505).Misek DE, Imafuku Y, Hanash SM. Pharmacogenom. J. 2004;5(8):1129. doi: 10.1517/14622416.5.8.1129. [DOI] [PubMed] [Google Scholar]
- (506).Giannios J, Ioannidou-Mouzaka L. Eu. J. Gyn. Oncol. 1997;18(5):387. [PubMed] [Google Scholar]
- (507).Lendvai G, Velikyan I, Estrada S, Eriksson B, Laangstroem B, Bergstroem M. Oligonucleotides. 2008;18(1):33. doi: 10.1089/oli.2007.0104. [DOI] [PubMed] [Google Scholar]
- (508).Lendvai G, Velikyan I, Bergstroem M, Estrada S, Laryea D, Vaelilae M, Salomaeki S, Langstroem B, Roivainen A. Eur. J. Pharm. Sci. 2005;26(1):26. doi: 10.1016/j.ejps.2005.04.017. [DOI] [PubMed] [Google Scholar]
- (509).Schlesinger J, Bergmann R, Klussmann S, Wuest F. Lett. Drug Disc. 2006;3(5):330. [Google Scholar]
- (510).Wang J, Chen P, Mrkobrada M, Hu M, Vallis KA, Reilly RM. Eu. J. Nucl. Med. Mol. Imaging. 2003;30(9):1273. doi: 10.1007/s00259-003-1134-0. [DOI] [PubMed] [Google Scholar]
- (511).Roivainen A, Tolvanen T, Salomaki S, Lendvai G, Velikyan I, Numminen P, Valila M, Sipila H, Bergstrom M, Harkonen P, Lonnberg H, Langstrom B. J. Nucl. Med. 2004;45(2):347. [PubMed] [Google Scholar]
- (512).Tian X, Aruva MR, Zhang K, Shanthly N, Cardi CA, Thakur ML, Wickstrom E. J. Nucl. Med. 2007;48(10):1699. doi: 10.2967/jnumed.107.042499. [DOI] [PubMed] [Google Scholar]
- (513).Tian X, Aruva MR, Qin W, Zhu W, Duffy KT, Sauter ER, Thakur ML, Wickstrom E. J. Nucl. Med. 2004;45(12):2070. [PubMed] [Google Scholar]
- (514).Suzuki T, Zhang Y, Zhang Y.-f., Schlachetzki F, Pardridge WM. Mol. Imaging. 2004;3(4):356. doi: 10.1162/15353500200404145. [DOI] [PubMed] [Google Scholar]
- (515).Suzuki T, Wu D, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. J. Nucl. Med. 2004;45(10):1766. [PubMed] [Google Scholar]
- (516).Boerman OC, Dams ETM, Oyen WJG, Corstens FHM, Storm G. Inflammation Res. 2001;50(2):55. doi: 10.1007/s000110050725. [DOI] [PubMed] [Google Scholar]
- (517).Hughes DK. J. Nucl. Med. Technol. 2003;31(4):196. [PubMed] [Google Scholar]
- (518).Liu S-F, Liu J-W, Lin M-C, Lee C-H, Huang H-H, Lai Y-F. Am. J. Roentgenol. 2007;188(5):W403. doi: 10.2214/AJR.06.0587. [DOI] [PubMed] [Google Scholar]
- (519).Hang LW, Hsu WH, Tsai JJP, Jim YF, Lin CC, Kao A. Rheumatol. Inter. 2004;24(3):153. doi: 10.1007/s00296-003-0346-8. [DOI] [PubMed] [Google Scholar]
- (520).Lazzeri E, Pauwels EKJ, Erba PA, Volterrani D, Manca M, Bodei L, Trippi D, Bottoni A, Cristofani R, Consoli V, Palestro CJ, Mariani G. Eu. J. Nucl. Med. Mol. Imaging. 2004;31(11):1505. doi: 10.1007/s00259-004-1581-2. [DOI] [PubMed] [Google Scholar]
- (521).Lazzeri E, Manca M, Molea N, Marchetti S, Consoli V, Bodei L, Bianchi R, Chinol M, Paganelli G, Mariani G. Eu. J. Nucl. Med. 1999;26(6):606. doi: 10.1007/s002590050428. [DOI] [PubMed] [Google Scholar]
- (522).van Eerd JEM, Oyen WJG, Harris TD, Rennen HJJM, Edwards DS, Liu S, Ellars CE, Corstens FHM, Boerman OC. J. Nucl. Med. 2003;44(7):1087. [PubMed] [Google Scholar]
- (523).Broekema M, Van Eerd JJEM, Oyen WJG, Corstens FHM, Liskamp RMJ, Boerman OC, Harris TD. J. Med. Chem. 2005;48(20):6442. doi: 10.1021/jm050383h. [DOI] [PubMed] [Google Scholar]
- (524).Bhargava KK, Gupta RK, Nichols KJ, Palestro CJ. Nucl. Med. Biol. 2009;36(5):545. doi: 10.1016/j.nucmedbio.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (525).Locke LW, Chordia MD, Zhang Y, Kundu B, Kennedy D, Landseadel J, Xiao L, Fairchild KD, Berr SS, Linden J, Pan D. J. Nucl. Med. 2009;50(5):790. doi: 10.2967/jnumed.108.056127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (526).Heusch G. Br. J. Pharmacol. 2008;153(8):1589. doi: 10.1038/sj.bjp.0707673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (527).Moustafa RR, Baron JC. Br. J. Pharmacol. 2008;153(Suppl. 1):S44. doi: 10.1038/sj.bjp.0707530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (528).Hardee ME, Dewhirst MW, Agarwal N, Sorg BS. Curr. Mol. Med. 2009;9(4):435. doi: 10.2174/156652409788167122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (529).Hwang D-R, Bergmann SR. Radiopharmaceuticals for studying the heart. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons Inc.; Hoboken: 2003. p. 529. [Google Scholar]
- (530).Keng FY. J. Ann. Acad. Med. Singapore. 2004;33(2):175. [PubMed] [Google Scholar]
- (531).Yetkin FZ, Mendelsohn D. Neuroimag. Clin. North Amer. 2002;12(4):537. doi: 10.1016/s1052-5149(02)00029-1. [DOI] [PubMed] [Google Scholar]
- (532).Hoffend J, Mier W, Schuhmacher J, Schmidt K, Dimitrakopoulou-Strauss A, Strauss LG, Eisenhut M, Kinscherf R, Haberkorn U. Nucl. Med. Biol. 2005;32(3):287. doi: 10.1016/j.nucmedbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- (533).Kiratli PO, Tuncel M, Ozkutlu S, Caglar M. Nucl. Med. Commun. 2008;29(10):907. doi: 10.1097/MNM.0b013e328303359f. [DOI] [PubMed] [Google Scholar]
- (534).Kinuya S, Li XF, Yokoyama K, Mori H, Shiba K, Watanabe N, Shuke N, Bunko H, Michigishi T, Tonami N. Nucl. Med. Commun. 2004;25(1):49. doi: 10.1097/00006231-200401000-00007. [DOI] [PubMed] [Google Scholar]
- (535).Takahashi N, Fujibayashi Y, Yonekura Y, Welch MJ, Waki A, Tsuchida T, Sadato N, Sugimoto K, Nakano A, Lee JD, Itoh H. Ann. Nucl. Med. 2001;15(3):293. doi: 10.1007/BF02987849. [DOI] [PubMed] [Google Scholar]
- (536).Fujibayashi Y, Cutler CS, Anderson CJ, McCarthy DW, Jones LA, Sharp T, Yonekura Y, Welch MJ. Nucl. Med. Biol. 1999;26(1):117. doi: 10.1016/s0969-8051(98)00049-3. [DOI] [PubMed] [Google Scholar]
- (537).Plossl K, Chandra R, Qu W, Lieberman Brian P, Kung M-P, Zhou R, Huang B, Kung Hank F. Nucl. Med. Biol. 2008;35(1):83. doi: 10.1016/j.nucmedbio.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (538).Cutler CS, Giron MC, Reichert DE, Snyder AZ, Herrero P, Anderson CJ, Quarless DA, Koch SA, Welch MJ. Nucl. Med. Biol. 1999;26(3):305. doi: 10.1016/s0969-8051(98)00108-5. [DOI] [PubMed] [Google Scholar]
- (539).Wallhaus TR, Lacy J, Stewart R, Bianco J, Green MA, Nayak N, Stone CK. J. Nucl. Cardiol. 2001;8(1):67. doi: 10.1067/mnc.2001.109929. [DOI] [PubMed] [Google Scholar]
- (540).Flower MA, Zweit J, Hall AD, Burke D, Davies MM, Dworkin MJ, Young HE, Mundy J, Ott RJ, McCready VR, Carnochan P, Allen-Mersh TG. Eu. J. Nucl. Med. 2001;28(1):99. doi: 10.1007/s002590000410. [DOI] [PubMed] [Google Scholar]
- (541).Holschneider DP, Yang J, Sadler TR, Galifianakis NB, Bozorgzadeh MH, Bading JR, Conti PS, Maarek JMI. Brain Res. 2008;1234(9):32. doi: 10.1016/j.brainres.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Yang DJ, Ilgan S, Higuchi T, Zareneyrizi F, Oh CS, Liu CW, Kim EE, Podoloff DA. Pharm. Res. 1999;16(5):743. doi: 10.1023/a:1018836911013. [DOI] [PubMed] [Google Scholar]
- (543).Ito M, Yang David J, Mawlawi O, Mendez R, Oh C-S, Azhdarinia A, Greenwell AC, Yu D-F, Kim EE. Acad. Radiol. 2006;13(5):598. doi: 10.1016/j.acra.2006.01.007. [DOI] [PubMed] [Google Scholar]
- (544).Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. J. Nucl. Med. 1997;38(7):1155. [PubMed] [Google Scholar]
- (545).Holland JP, Aigbirhio FI, Betts HM, Bonnitcha PD, Burke P, Christlieb M, Churchill GC, Cowley AR, Dilworth JR, Donnelly PS, Green JC, Peach JM, Vasudevan SR, Warren JE. Inorg. Chem. 2007;46(2):465. doi: 10.1021/ic0615628. [DOI] [PubMed] [Google Scholar]
- (546).Bonnitcha PD, Vavere AL, Lewis JS, Dilworth JR. J. Med. Chem. 2008;51(10):2985. doi: 10.1021/jm800031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (547).Barnard P, Bayly S, Betts H, Bonnitcha P, Christlieb M, Dilworth J, Holland J, Pascu S. Q. J. Nucl. Med. Mol. Imaging. 2008;52(2):174. [PubMed] [Google Scholar]
- (548).Ackerman LJ, West DX, Mathias CJ, Green MA. Nucl. Med. Biol. 1999;26(5):551. doi: 10.1016/s0969-8051(99)00020-7. [DOI] [PubMed] [Google Scholar]
- (549).McQuade P, Martin KE, Castle TC, Went MJ, Blower PJ, Welch MJ, Lewis JS. Nucl. Med. Biol. 2005;32(2):147. doi: 10.1016/j.nucmedbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- (550).Cowley AR, Dilworth JR, Donnelly PS, Heslop JM, Ratcliffe SJ. Dalton Trans. 2007;2:209. doi: 10.1039/b612142j. [DOI] [PubMed] [Google Scholar]
- (551).Cowley AR, Dilworth JR, Donnelly PS, White JM. Inorg. Chem. 2006;45(2):496. doi: 10.1021/ic0514492. [DOI] [PubMed] [Google Scholar]
- (552).Cowley AR, Dilworth JR, Donnelly PS, Gee AD, Heslop JM. Dalton Trans. 2004;16:2404. doi: 10.1039/B406429A. [DOI] [PubMed] [Google Scholar]
- (553).Blower PJ, Castle TC, Cowley AR, Dilworth JR, Donnelly PS, Labisbal E, Sowrey FE, Teat SJ, Went MJ. Dalton Trans. 2003;23:4416. [Google Scholar]
- (554).Castle TC, Maurer RI, Sowrey FE, Went MJ, Reynolds CA, McInnes EJL, Blower PJ. J. Am. Chem. Soc. 2003;125(33):10040. doi: 10.1021/ja035737d. [DOI] [PubMed] [Google Scholar]
- (555).Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng Y, Mullen GED. J. Med. Chem. 2002;45(7):1420. doi: 10.1021/jm0104217. [DOI] [PubMed] [Google Scholar]
- (556).Dearling JLJ, Lewis JS, Mullen GED, Welch MJ, Blower PJ. J. Biol. Inorg. Chem. 2002;7(3):249. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- (557).Burgman P, O’Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Nucl. Med. Biol. 2005;32(6):623. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- (558).Laforest R, Dehdashti F, Lewis JS, Schwarz SW. Eu. J. Nucl. Med. Mol. Imaging. 2005;32(7):764. doi: 10.1007/s00259-004-1756-x. [DOI] [PubMed] [Google Scholar]
- (559).Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. J. Nucl. Med. 1999;40(1):177. [PubMed] [Google Scholar]
- (560).Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. J. Nucl. Med. 2006;47(6):989. [PubMed] [Google Scholar]
- (561).Obata A, Yoshimoto M, Kasamatsu S, Naiki H, Takamatsu S, Kashikura K, Furukawa T, Lewis JS, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y. Nucl. Med. Biol. 2003;30(5):529. doi: 10.1016/s0969-8051(03)00047-7. [DOI] [PubMed] [Google Scholar]
- (562).Dehdashti F, Mintun MA, Lewis JS, Bradley J, Govindan R, Laforest R, Welch MJ, Siegel BA. Eu. J. Nucl. Med. Mol. Imaging. 2003;30(6):844. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- (563).Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Int. J. Radiat. Oncol. Biol. Phys. 2003;55(5):1233. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- (564).Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. J. Nucl. Med. 2008;49(2):201. doi: 10.2967/jnumed.107.048520. [DOI] [PubMed] [Google Scholar]
- (565).Grigsby PW, Malyapa RS, Higashikubo R, Schwarz JK, Welch MJ, Huettner PC, Dehdashti F. Mol. Imaging Biol. 2007;9(5):278. doi: 10.1007/s11307-007-0095-2. [DOI] [PubMed] [Google Scholar]
- (566).Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ, Siegel BA. J. Nucl. Med. 2008;49(7):1177. doi: 10.2967/jnumed.108.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (567).Lewis JS, Connett JM, Garbow JR, Buettner TL, Fujibayashi Y, Fleshman JW, Welch MJ. Cancer Res. 2002;62(2):445. [PubMed] [Google Scholar]
- (568).Aft RL, Lewis JS, Zhang F, Kim J, Welch MJ. Cancer Res. 2003;63(17):5496. [PubMed] [Google Scholar]
- (569).Lewis JS, Laforest R, Buettner TL, Song SK, Fujibayashi Y, Connett JM, Welch MJ. Proc. Natl. Acad. Sci. U.S.A. 2001;98(3):1206. doi: 10.1073/pnas.98.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (570).Obata A, Kasamatsu S, Lewis JS, Furukawa T, Takamatsu S, Toyohara J, Asai T, Welch MJ, Adams SG, Saji H. Nucl. Med. Biol. 2005;32(1):21. doi: 10.1016/j.nucmedbio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- (571).Lewis JS, Dearling JLJ, Sosabowski JK, Zweit J, Carnochan P, Kelland LR, Coley HM, Blower PJ. Eu. J. Nucl. Med. 2000;27(6):638. doi: 10.1007/s002590050557. [DOI] [PubMed] [Google Scholar]
- (572).Black NF, McJames S, Rust TC, Kadrmas DJ. Phys.Med. Biol. 2008;53(1):217. doi: 10.1088/0031-9155/53/1/015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Green MA, Mathias CJ, Willis LR, Handa RK, Lacy JL, Miller MA, Hutchins GD. Nucl. Med. Biol. 2007;34(3):247. doi: 10.1016/j.nucmedbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- (574).Wei L, Easmon J, Nagi RK, Muegge BD, Meyer LA, Lewis JS. J. Nucl. Med. 2006;47(12):2034. [PubMed] [Google Scholar]
- (575).Vavere AL, Lewis JS. Nucl. Med. Biol. 2008;35(3):273. doi: 10.1016/j.nucmedbio.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (576).Lewis JS, Sharp TL, Laforest R, Fujibayashi Y, Welch MJ. J. Nucl. Med. 2001;42(4):655. [PubMed] [Google Scholar]
- (577).Dence CS, Ponde DE, Welch MJ, Lewis JS. Nucl. Med. Biol. 2008;35(6):713. doi: 10.1016/j.nucmedbio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (578).Lederer CM, Shirley VS, editors. Table of Isotopes. 7 ed. John Wiley & Sons; New York: 1978. [Google Scholar]
- (579).Shannon RD. Acta Crystallogr., Sect. A. 1976;A32(5):751. [Google Scholar]
- (580).Baes CF, Jr., Mesmer RE. The Hydrolysis of Cations. Wiley-Interscience; New York: 1976. [Google Scholar]
- (581).Martell AE, Smith RM. Critical Stability Constants, Vol. 3: Other Organic Ligands. Plenum Press; New York: 1977. [Google Scholar]
- (582).Wulfsberg G. Inorganic Chemistry. University Science Books; Sausalito: 2000. [Google Scholar]
- (583).Douglas B, McDaniel D, Alexander J. Concepts and Models of Inorganic Chemistry. Third ed. John Wiley & Sons; New York: 1994. [Google Scholar]
- (584).Hancock RD, McDougall GJ. J. Am. Chem. Soc. 1980;102(21):6551. [Google Scholar]
- (585).Hancock RD, McDougall GJ. Adv. Mol. Relaxation Interact. Processes. 1980;18(2):99. [Google Scholar]
- (586).Martell AE, Hancock RD. Metal Complexes in Aqueous Solutions. Plenum Press; New York: 1996. [Google Scholar]
- (587).Martell AE, Smith RM. Editors Critical Stability Constants, Vol. 1: Amino Acids. Plenum Press; New York: 1974. [Google Scholar]
- (588).Delgado R, De Carmo FM, Quintino S. Talanta. 1997;45(2):451. doi: 10.1016/s0039-9140(97)00157-4. [DOI] [PubMed] [Google Scholar]
- (589).Martell AE, Smith RM. Critical Stability Constants. Plenum Press; New York: 1982. [Google Scholar]
- (590).Bleiholder C, Boerzel H, Comba P, Ferrari R, Heydt M, Kerscher M, Kuwata S, Laurenczy G, Lawrance GA, Lienke A, Martin B, Merz M, Nuber B, Pritzkow H. Inorg. Chem. 2005;44(22):8145. doi: 10.1021/ic0513383. [DOI] [PubMed] [Google Scholar]
- (591).Bevilacqua A, Gelb RI, Hebard WB, Zompa LJ. Inorg. Chem. 1987;26(16):2699. [Google Scholar]
- (592).Garcia E. Synthesis of TACN-derived Chelators and Studies of their Metal Complexes. University of New Hampshire; Durham, NH: 2009. Unpublished work. [Google Scholar]
- (593).Smith RM, Martell AE. Critical Stability Contants. Plenum Press; New York: 1989. [Google Scholar]
- (594).Delgado R, Frausto da Silva JJR. Talanta. 1982;29(10):815. doi: 10.1016/0039-9140(82)80251-8. [DOI] [PubMed] [Google Scholar]
- (595).Chaves S, Delgado R, Dasilva JJRF. Talanta. 1992;39(3):249. doi: 10.1016/0039-9140(92)80028-c. [DOI] [PubMed] [Google Scholar]
- (596).Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ. J. Med. Chem. 2002;45(2):469. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- (597).Delgado R, Sun Y, Motekaitis RJ, Martell AE. Inorg. Chem. 1993;32(15):3320. [Google Scholar]
- (598).Motekaitis RJ, Sun Y, Martell AE, Welch MJ. Inorg. Chem. 1991;30(13):2737. [Google Scholar]
- (599).Evers A, Hancock RD, Martell AE, Motekaitis RJ. Inorg. Chem. 1989;28(11):2189. [Google Scholar]
- (600).Bottari E, Anderegg G. Helv. Chim. Acta. 1967;50(8):2349. [Google Scholar]
- (601).Kumar K, Chang CA, Francesconi LC, Dischino DD, Malley MF, Gougoutas JZ, Tweedle MF. Inorg. Chem. 1994;33(16):3567. [Google Scholar]
- (602).Koudelkova M, Vinsova H, Jedinakova-Krizova V. Czech. J. Phys. 2003;53(Suppl. A, Pt. 2):A769. [Google Scholar]
- (603).Lovqvist A, Humm JL, Sheikh A, Finn RD, Koziorowski J, Ruan S, Pentlow KS, Jungbluth A, Welt S, Lee FT, Brechbiel MW, Larson SM. J. Nucl. Med. 2001;42(8):1281. [PubMed] [Google Scholar]
- (604).Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. J. Nucl. Med. 2007;48(8):1245. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- (605).Asti M, De Pietri G, Fraternali A, Grassi E, Sghedoni R, Fioroni F, Roesch F, Versari A, Salvo D. Nucl. Med. Biol. 2008;35(6):721. doi: 10.1016/j.nucmedbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- (606).Hofmann M, Weitzel T, Krause T. Nucl. Instrum. Methods Phys. Res., Sect. A. 2006;569(2):522. doi: 10.1016/j.nima.2006.08.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (607).van Eerd JEM, Oyen WJG, Harris TD, Rennen HJJM, Edwards DS, Corstens FHM, Boerman OC. J. Nucl. Med. 2005;46(5):786. [PubMed] [Google Scholar]
- (608).Xu H, Baidoo K, Gunn AJ, Boswell CA, Milenic DE, Choyke PL, Brechbiel MW. J. Med. Chem. 2007;50(19):4759. doi: 10.1021/jm070657w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (609).Li WP, Lewis JS, Srinivasan A, Schmidt MA, Anderson CJ. J. Labeled Compd. Radiopharm. 2001;44(suppl. 1):S948. [Google Scholar]
- (610).Lewis JS, Lewis MR, Srinivasan A, Schmidt MA, Wang J, Anderson CJ. J. Med. Chem. 1999;42(8):1341. doi: 10.1021/jm980602h. [DOI] [PubMed] [Google Scholar]
- (611).Wadas TJ, Anderson CJ. Nat. Protoc. 2006;1(6):3062. doi: 10.1038/nprot.2006.431. [DOI] [PubMed] [Google Scholar]
- (612).Liu Z, Yan Y, Liu S, Wang F, Chen X. Bioconjugate Chem. 2009;20(5):1016. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]