Abstract
Hydrogen sulfide (H2S), a volatile sulfur compound, is implicated as a cause of inflammation, especially when it is produced by bacteria colonizing gastrointestinal organs. However, it is unclear if H2S produced by periodontal pathogens affects the inflammatory responses mediated by oral/gingival epithelial cells. Therefore, the aims of this study were 1) to compare the in vitro production of H2S among 14 strains of oral bacteria and 2) to evaluate the effects of H2S on inflammatory response induced in host oral/gingival epithelial cells. P. gingivalis (Pg) produced the most H2S in culture, which, in turn, resulted in the promotion of proinflammatory cytokine IL-8 from both gingival and oral epithelial cells. The up-regulation of IL-8 expression was reproduced by the exogenously applied H2S. Furthermore, the mutant strains of Pg that do not produce major soluble virulent factors, i.e. gingipains, still showed the production of H2S, as well as the promotion of epithelial IL-8 production, which was abrogated by H2S scavenging reagents. These results demonstrated that Pg produces a concentration of H2S capable of up-regulating IL-8 expression induced in gingival and oral epithelial cells, revealing a possible mechanism that may promote the inflammation in periodontal disease.
Keywords: Hydrogen sulfide, Epithelial cells, IL-8, Bacteria, Porphyromonas gingivalis, Gingipain, Pathogenesis, Hydrogen, Innate immune response, Periodontal disease
Introduction
Halitosis is a characteristic symptom of periodontal disease, and is caused by the production of volatile sulphur compounds (VSCs), such as hydrogen sulphide (H2S) and methyl mercaptan, by sulfate-reducing bacteria [1]. Importantly, the major cultivatable periodontal opportunistic pathogens, Porphyromonas gingivalis (Pg), Fusobacterium nucleatum (Fn) and Tannerella forsythia (Tf), are reported to have produced H2S in an in vitro system as measured by gas chromatography [2]. Also, H2S produced by gut bacteria is recognized as a pathogenic factor in inflammatory bowel diseases (IBD) which present features of mucosal inflammatory lesion similar to periodontal disease [3]. In the host biological system, H2S is produced authentically in a manner that causes desulphydration of L-cysteine, cystathionine β-synthetase (CBS) and cystathionine γ-lyase (CSE) [4], indicating its association with the regulation of the cardiovascular and nervous systems, as well as inflammation [5]. Although H2S can be released from either bacteria or the host metabolic system, it is not known whether the H2S released into solution surrounding periodontal bacteria can reach a concentration higher than the one derived from the host metabolic system.
Gingival epithelial cells represent the first line of defense in the gingival crevice. As such, they play a key role in host innate immune response by protecting the host from bacterial challenge with the production of proinflammatory cytokines, such as IL-8, which is a chemotaxis factor for neutrophils [6, 7], and antimicrobial peptides [8], as well as adaptive immune responses [9]. On the other hand, over-expression of these proinflammatory cytokines causes collateral tissue damages. For instance, reactive oxygen species (ROS) produced from the activated neutrophils in response to periodontopathogenic bacteria appears to contribute to tissue destruction in the context of periodontal disease [10]. Therefore, it is thought that over-expression of IL-8 from gingival epithelial cells can promote the local accumulation of neutrophils which results in periodontal tissue damage [11].
Pg is one of the most putatively pathogenic periodontal bacteria, and, in addition to H2S, it produces several other virulence factors. These include extracellular proteases, or gingipains, [12–15], lipopolysaccharide (LPS) [16, 17], and hemagglutinins [18]. However, a recent study discovered that Pg and other putative periodontal pathogens not only colonize the diseased periodontal pocket but also the healthy gingival crevice [19]. Nonetheless, the reason why healthy gingival epithelium remains unaffected in the presence of periodontopathogens such as Pg has not yet been fully investigated.
Based on the lines of evidence described above, we hypothesized that H2S produced from periodontal bacteria contributes to the promotion of inflammatory response by the up-regulation of IL-8 production from gingival epithelial cells. Therefore, the aims of the present study were 1) to monitor the concentration of H2S released from a total of fourteen different oral bacterial strains and 2) to examine the effects of H2S released from Porphyromonas gingivalis (Pg) on the production of proinflammatory cytokine IL-8 from gingival epithelial cells.
Materials and Methods
Bacterial strains and culture
The following oral bacterial strains were used in this study: Porphyromonas gingivalis (Pg) 33277, Pg W83, Tannerella forsythia (Tf) 338, Prevotella intermedia (P. intermedia) 25611, Fusobacterium nucleatum (Fn) 10953, Fusobacterium periodonticum (F. periodonticum) 33693, Peptostreptococcus micros (P. micros) 33270, Streptococcus gordonii (S. gornodii) 10558, Streptococcus sanguis (S. sangius) 10556, Capnocytophaga ochracea (C. ochracea) 25, Capnocytophaga gingivalis (C. gingivalis) 27, Actinobacillus actinomycetemcomitans (Aa) 3826, Aa JP2, Aa Y4, and Pg 33277 mutant strains KDP112 (rgpA−/rgpB−) [20] and KDP137 (rgpA−/kgp−/hagA−) [21]. Especially, Pg 33277 and Pg W83 were grown in brain heart infusion (BHI; BBL) broth medium supplemented with hemine and menadoine (Sigma). T. forsythia was cultured in BHI broth medium containing hemine, menadoine, heat-inactivated fetal bovine serum (FBS), N-acetyl muramic acid, and yeast extract. The remaining bacterial strains were cultured in BHI medium alone or supplemented with yeast extract. All bacteria were cultured in a 37°C chamber under anaerobic conditions (5% H2, 10% CO2 and 85% N2).
Measurement of hydrogen sulfide and hydrogen released from bacteria
Bacteria were cultured in broth medium until they reached late log growth phase, and the concentration of all strains was then adjusted to an optical density (OD 590 nm) of 0.9. Subsequently, the bacterial suspension was centrifuged at 7,000 rpm for 15 min. The supernatants were immediately subjected to measurement for hydrogen sulfide (H2S) or hydrogen (H2) using a needle-type H2S or H2 sensor (Unisense A/S, Denmark) [22, 23]. For each test, the sensor was allowed to stabilize, and three testing points were taken at 5 second intervals. Each bacterial strain grown in broth was examined for the production of H2S and H2 on at least three separate occasions.
Epithelial cell culture
Human gingival epithelial cell line OBA9 [24] and oral (cheek) epithelial cell line OKF6 [25] were used in this study. The cells were cultured in Keratinocyte-Serum Free Medium (K-SFM, Gibco) supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, 50 μg/ml gentamicin and 50 ng/ml amphotericin B (medium A) in humidified atmosphere of 5% CO2 at 37°C.
Treatment of epithelial cells with sodium sulfide (Na2S)
OBA9 or OKF6 cells were seeded at a density of 1 × 104 cells/well in 96-well culture plates coated with type 1 collagen and maintained in medium A until they reached confluence. The cells were treated with or without mitogen phorbol myristate acetate (PMA; 1 μmol/L, Sigma) and immediately exposed to various concentrations of Na2S (VWR), as a H2S donor, in K-SFM alone (medium B) for 24 hr. The culture supernatants were then subjected to enzyme-linked immunosorbent assay (ELISA) for detection of TNF-α, IL-6, and IL-8, as described below.
Transwell assay
Transwell culture plates (24-well format, see Fig 3A) with 0.4-μm pores (Corning) were used to test the effects of soluble small factors released from bacteria on epithelial cells. Epithelial cells in medium A were cultured in the bottom compartment of plates which were pre-treated with type 1 collagen. The cells were treated with PMA (1 μmol/L) in medium B, and bacteria, specifically, Pg W83, Pg KDP112, or Pg KDP137, were cultured in the upper chamber of the Transwell system at various concentrations with or without Morpholine coupled to Rast resin (25mg/ml, Sigma) or 4,6-Dichloro-1,3,5-triazine–resin (25mg/ml, Sigma) in medium B for 24 or 48 hr. The culture supernatants of the bottom well were collected, and the IL-8 concentration was determined by ELISA as described below.
Figure 3. Effect of soluble factors, including H2S, released from PgW83 on IL-8 production in OBA9 and OKF6.
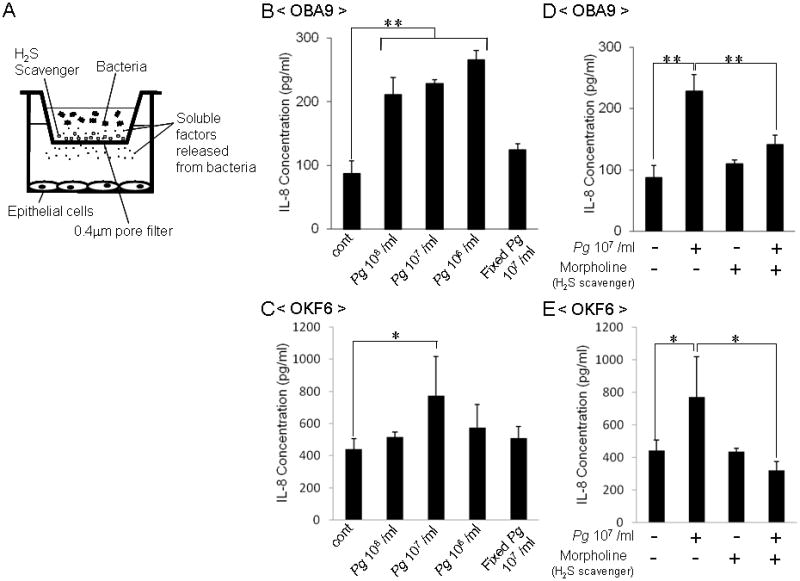
The transwell assay system is illustrated (A). The epithelial cells, OBA9 or OKF6, were cultured in the lower chamber until they reached confluent coverage of surface of tissue culture well. Subsequently, live or fixed PgW83 were applied in the upper chamber with or without H2S scavenger. They were co-cultured for 24 hr in KSF medium containing inflammatory stimulant PMA (1 μmol/L). The concentrations of IL-8 in the culture supernatant of OBA9 (B and D) or OKF6 (C and E) were measured by ELISA. Data indicate the mean ± SD of three cultures. *p < 0.05, **p < 0.01: Values differ significantly (t-test).
Measurement of proinflammatory cytokines
Quantification of the proinflammatory cytokines TNF-α, IL-6 and IL-8 was performed by ELISA kits (PeproTech) following the instructions provided by the manufacturer.
Statistical analysis
Differences between the two groups were analyzed by Student’s t test.
Results
The concentrations of H2S produced from fourteen different oral bacterial strains were monitored using an instrument that accurately measures the concentration of small gas molecules dissolved in solution, including hydrogen (H2) and H2S [22, 23]. Among them, 12 oral bacterial strains are clustered into color-coded complexes as follows: red complex (3 strains), orange complex (4 strains), yellow complex (2 strains) and green complex (3 strains) in descending order according to their degree of virulence in association with clinically diagnosed adult periodontal disease. Two strains of Aa were not classified in any of the color-coded clusters because Aa is more associated with aggressive (juvenile) periodontitis than adult periodontal disease. Since it was recently discovered that hydrogen (H2) produced by intestinal bacteria can provide beneficial effects to the host [22, 23], we also monitored the concentration of H2. The concentrations of H2S detected from two strains of Porphyromonas gingivalis, Pg W83 and Pg 33277, were prominently high (about 600 – 800 μmol/L), whereas 4 strains of orange complex bacteria, F. periodonticum33693, F. nucpoly338, P. intermedia25611, and P. micros33270, also produced a modest concentration of H2S (50 – 300 μmol/L) in descending order (Fig. 1). In contrast, little or no H2S production was detected from the yellow complex, green complex and putative pathogens associated with aggressive (juvenile) periodontitis, including Aa JP2 and Aa Y4 (Fig. 1). H2 production was detected in the 4 strains of bacteria belonging to the orange complex, while the concentrations of such H2 detected from these bacterial strains (30–100 μmol/L) were lower than those of H2S in respective strain bacteria in the orange complex. Exceptionally, T. forsythia338 in the red complex showed a higher production of H2 than H2S (Fig. 1). These results indicated that the red complex bacterium Pg produces a remarkably higher concentration of H2S compared to the other bacteria and that the production of possibly host-beneficial H2 from the bacteria tested was low.
Figure 1. Measurement of H2Sreleased from oral bacteria.
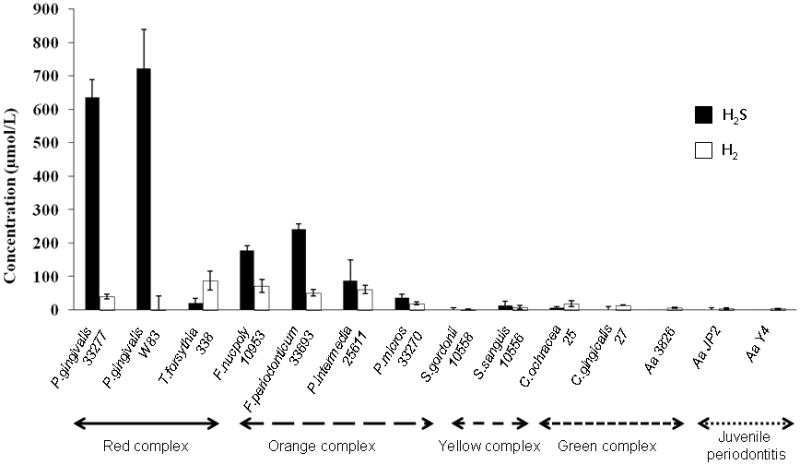
Fourteen bacterial strains that belong to different pathogenesis clustering complexes were cultured in anaerobic conditions until they reached the late log growth phase. The concentration of all strains was adjusted to 0.9 as measured by OD 590 nm. The concentrations of H2S and H2 were measured in the bacterial culture broth using a needle-type H2S or H2 sensor, as described in the Materials & Methods section. Data are shown as the mean ± SD of three different cultures.
To explore whether H2S can affect the host inflammatory response in the context of periodontal disease, the effect of exogenously applied H2S on the production of inflammatory cytokine by the human gingival epithelial cell line OBA9 and oral epithelial cell line OKF6 was examined. The addition of H2S donor, Na2S, to the culture medium did not affect IL-8 production from OBA9 in the absence of PMA (Fig. 2A). However, the addition of Na2S did enhance IL-8 production from OBA9 stimulated with PMA in a dose-dependent manner (Fig. 2A). Similar results were obtained when oral epithelial cell line OKF6 was exposed to Na2S in the presence or absence of PMA (Fig. 2B). Although it was examined whether H2S affects the production of other proinflammatory cytokines, including IL-6 and TNF-α, from OBA9 in the presence or absence of PMA stimulation, no noticeable effects mediated by H2S on these cytokines were observed (data not shown). These results demonstrated that H2S can promote IL-8 production from oral and gingival epithelial cells reacted with PMA, indicating that a predisposed inflammatory condition is required for H2S-mediated promotion of IL-8 production.
Figure 2. Effect of exogenously applied H2S on IL-8 production in OBA9 and OKF6 cells.
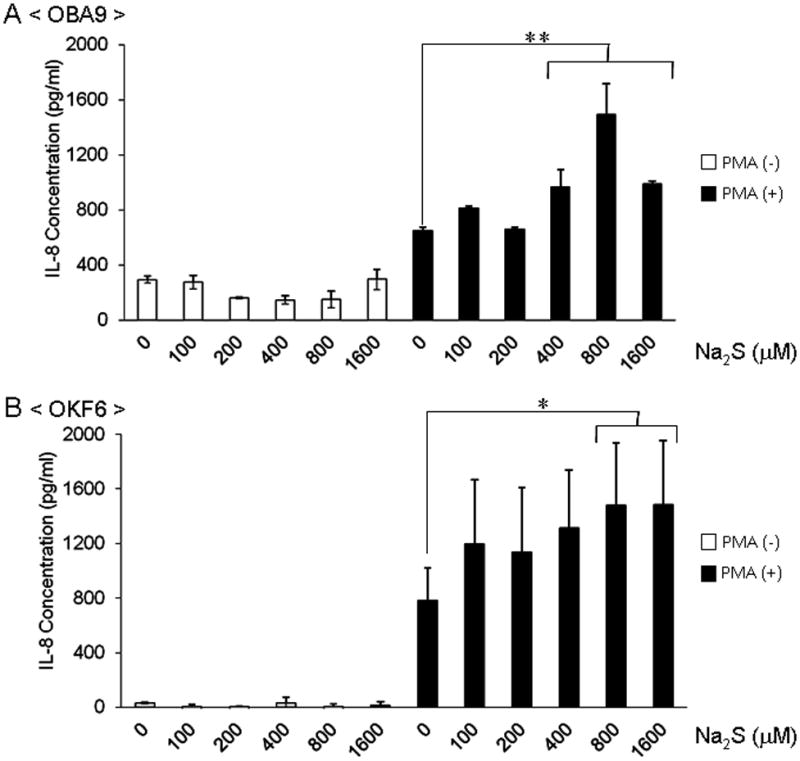
Confluent cultures of gingival epithelial cell line (OBA9) or oral epithelial cell line (OKF6) were exposed to serial dilutions of the H2S donor, Na2S, for 24 hr with or without PMA (1 μmol/L) stimulation. The concentrations of IL-8 in the culture supernatant produced from OBA9 (A) or OKF6 (B) were measured by ELISA. Data indicate the mean ± SD of three cultures. *p < 0.05, **p < 0.01: Values differ significantly (t-test).
In order to examine the effects of H2S derived from oral bacteria on the IL-8 production from epithelial cells, a transwell assay (Fig. 3A) was employed. As a test bacterium, Pg W83 was used because it showed the highest production of H2S (Fig. 1). Pg W83 applied to the transwell (pore size: Ø 0.4 μm; smaller than the size of bacteria) was placed on the OBA9 cells cultured in the bottom compartment of a 24-well tissue culture plate. After incubation for 24 hr, live Pg W83 (106, 107 and 108 bacteria/ml) increased IL-8 production (Fig. 3B). In addition, OKF6 cells co-cultured with Pg W83 in the same transwell assay system showed an increase of IL-8 production at a concentration of 107 bacteria/ml (Fig. 3C). In contrast, co-culture of both OBA9 and OKF6 with killed Pg W83 did not affect their production of IL-8 (Fig. 3B and C). These results suggest that soluble factors released from live bacteria up-regulate the IL-8 production from epithelial cells. The concentration of exogenously applied H2S equaled the concentration of H2S produced by Pg W83, which resulted in the up-regulation of IL-8 (Fig 2). Next, to test if H2S released from Pg W83 is responsible for the promotion of IL-8, OBA9 and OKF6 cells co-cultured with Pg W83 were treated with resin particles coupled to morpholine, which is a H2S scavenger chemical. As expected, morpholine-resin particles abolished the up-regulation of IL-8 production induced by co-culture with Pg W83 in both OBA9 (Fig. 3D) and OKF6 (Fig. 3E). Furthermore, similar results were obtained with 4,6-dichloro-1,3,5-triazine, another H2S scavenger (data not shown).
It is well known that Pg W83 produces arginine-specific cysteine proteinase (Arg-gingipain, RGP) and lysine-specific cysteine proteinase (Lys-gingipain, KGP) extracellularly [26, 27] and that these proteases are recognized as major virulent factors [12–15]. Therefore, it is plausible that either RGP or KGP could also affect IL-8 production in the transwell assay system as performed above. To address this possibility, it was tested if Pg KDP137 (rgpA−/kgp−/hagA− mutant strain of ATCC 33277) affects the IL-8 production from epithelial cells. First, Pg KDP137 produced H2S at a level similar to that of wild-type Pg W83 (Fig. 4A). Co-culture of OBA9 cells with Pg KDP137 in the transwell system for 48 hr increased IL-8 production (Fig. 4C). Furthermore, co-culture of OBA9 cells with Pg KDP112 (rgpA−/rgpB− strain) also enhanced IL-8 production (not shown). Most importantly, the addition of morpholine-resin particles to the co-culture between OBA9 and Pg KDP137 abrogated the increased IL-8 production induced by each of the mutant Pg strains (Fig. 4B), suggesting that H2S, but not gingipains, released from Pg KDP137 is responsible for the increased expression of IL-8 from epithelial cells.
Figure 4. Effects of RGP and KGP deletion mutant strains of Pg on IL-8 production in the culture of gingival epithelial cells.
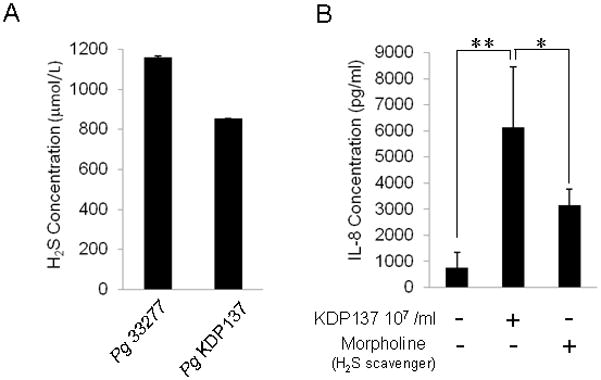
(A) The culture broth of wild-type Pg 33277 or Pg KDP137 (RGP-KGP-HagA triple deficient mutant strain) growing in log growth phase was tested for H2S concentration using a needle-type H2S sensor. Data represent the average ± SD of three cultures. (B) OBA9 cells were cultured in the lower chamber, and Pg KDP137 was applied to the upper chamber of the transwell co-culture system in the presence of PMA (1mmol/L) with or without Morpholine-resin. After co-culture for 48 hr, the concentrations of IL-8 in the culture supernatant were measured by ELISA. Data are the mean ± SD of three cultures. *p < 0.05, **p < 0.01: Values differ significantly (t-test).
Discussion
The present study demonstrated that Pg, a component of the red complex of perio pathogens, releases significantly more hydrogen sulfide than bacteria in the yellow or green complexes, while the levels released by orange complex bacteria fall somewhere in between, suggesting that the pathogenesis mediated by Pg is related to its production of H2S. Most strikingly, adding Na2S, as a source of H2S, up-regulated IL-8 production by OBA9 gingival epithelial cells and by OKF6 oral epithelial cells, both of which were stimulated with PMA. H2S released from live Pg, but not killed Pg, appeared to be responsible for the up-regulation of IL-8 production from PMA-stimulated OBA9 and OKF6 cells because addition of hydrogen sulfide scavenging chemicals suppressed IL-8 production from PMA-stimulated epithelial cells. Up-regulation of IL-8 from PMA-stimulated gingival epithelial cells was still found with mutant strains of Pg that lacked all three classes of gingipains, indicating that the production of these soluble and potent virulence factors did not contribute to IL-8 up-regulation. Consequently, for the first time, this study has demonstrated that the red complex bacterium Pg produces the highest concentration of H2S among the 14 tested strains of periodontal bacteria and that the H2S released from Pg can promote the inflammatory responses induced in gingival and oral epithelial cells, suggesting a novel pathogenic mechanism underlying the development of periodontal disease mediated by periodontal bacteria.
The classical gas chromatograph may not be useful in measuring the concentration of H2S released from bacteria to the surrounding culture medium or host biological fluids, such as saliva, because it is designed to measure atmospheric H2S, not H2S in solution. However, a cutting-edge instrument, which has recently become available, can accurately measure the concentration of small gas molecules dissolved in solution, including hydrogen (H2) and H2S [22, 23]. Therefore, in the present study, H2S produced from a variety of oral bacteria, including both periodontal pathogens and commensal benign bacteria, was first monitored using this instrument. Persson et al. previously reported that red complex bacteria, Pg and Tf, and orange complex bacteria, Fn and Prevotella intermedia (Pi), produce sulfide in the laboratory culture system, as measured using gas chromatography [2]. Since Persson et al. measured the sulfide released to the atmosphere using gas chromatography [2], our results, which measured H2S in solution using a special H2S sensor, as referenced above, (Unisense, Denmark) cannot be compared. Nevertheless, it should be emphasized that both our studies demonstrated that bacteria classified in the red and orange complexes produce a significantly higher concentration of H2S, when compared to the benign, or moderately pathogenic, groups of bacteria in the green and yellow complexes.
The high concentration of H2S produced from Pg (650–1,150 μmol/L) appeared to affect IL-8 production from PMA-stimulated epithelial cells because a concentration of H2S less than 400 μmol/L did not show any significant effect on IL-8 production in PMA-stimulated epithelial cells (Fig. 2). Since physiologically generated H2S is also present at concentrations in the range of 30–100 μM in blood [28], epithelial cells may well tolerate H2S at concentrations lower than 400 μmol/L. Interestingly, even a high concentration of H2S (800 or 1,600 μmol/L) did not induce IL-8 production in epithelial cells in the absence of PMA (Fig. 2), indicating that a predisposed inflammatory condition is required for H2S-mediated promotion of IL-8 production.
The primary biological function mediated by IL-8 is the chemo-attraction of neutrophils, a type of scavenger cell [29]. Neutrophils migrate to the site where IL-8 is released from epithelial cells, and facilitating phagocytosis of bacteria. In general, the production of reactive oxygen species (ROS) released by neutrophils not only kills bacteria but also augments inflammation [30]. Therefore, H2S-mediated up-regulation of IL-8 from epithelial cells should result in enhancement of inflammation at the site, by recruiting excess numbers of neutrophils. The in vivo studies demonstrated that both endogenous H2S and exogenously supplied H2S contribute to increased neutrophil migration to the inflammation induced in the pancreas, lung and liver [31, 32], supporting our premise that H2S released from periodontal bacteria could also promote the chemotaxis of neutrophils to the periodontal pocket.
In conclusion, this study has, for the first time, demonstrated that the red complex periodontal bacterium Pg produces a concentration of H2S capable of up-regulating IL-8 expression induced in gingival and oral epithelial cells, revealing a possible mechanism that may promote the inflammation in periodontal disease.
Acknowledgments
This study was supported by a research grant from Skyview Enterprises and NIH grants DE-018310 and DE-015931. We thank Dr. Koji Nakayama at Kyushu University, Japan, for the strains of Pg KDP112 and Pg KDP137 as well as Lin Martin and Tina Yaskell in the Department of Periodontology at The Forsyth Institute for providing the strains of other periodontal bacteria used in this study. We also acknowledge Olive Tang’s assistance in conducting the laboratory assays. Weilin Chen was supported by The Forsyth Education Outreach Program, and Harrison Mackler was supported by the Medical Fellows Program from the Howard Hughes Medical Institute.
References
- 1.Ratcliff PA, Johnson PW. The relationship between oral malodor, gingivitis, and periodontitis. A review. J Periodontol. 1999;70:485–489. doi: 10.1902/jop.1999.70.5.485. [DOI] [PubMed] [Google Scholar]
- 2.Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5:195–201. doi: 10.1111/j.1399-302x.1990.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 3.Sigusch BW. Periodontitis as manifestation of Crohn’s disease in primary dentition: a case report. J Dent Child (Chic) 2004;71:193–196. [PubMed] [Google Scholar]
- 4.Kasparek MS, Linden DR, Kreis ME, Sarr MG. Gasotransmitters in the gastrointestinal tract. Surgery. 2008;143:455–459. doi: 10.1016/j.surg.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pae HO, Lee YC, Jo EK, Chung HT. Subtle interplay of endogenous bioactive gases (NO, CO and H(2)S) in inflammation. Arch Pharm Res. 2009;32:1155–1162. doi: 10.1007/s12272-009-1806-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang GT, Haake SK, Kim JW, Park NH. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral Microbiol Immunol. 1998;13:301–309. doi: 10.1111/j.1399-302x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 7.Asai Y, Ohyama Y, Gen K, Ogawa T. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect Immun. 2001;69:7387–7395. doi: 10.1128/IAI.69.12.7387-7395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, Seki M, Ouhara K, Sugai M, Taubman MA, Kawai T. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–225. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuyama T, Kawai T, Izumi Y, Taubman MA. Expression of major histocompatibility complex class II and CD80 by gingival epithelial cells induces activation of CD4+ T cells in response to bacterial challenge. Infect Immun. 2005;73:1044–1051. doi: 10.1128/IAI.73.2.1044-1051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canakci CF, Cicek Y, Canakci V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc) 2005;70:619–628. doi: 10.1007/s10541-005-0161-9. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama A, Uehara A, Iki K, Matsushita K, Nakamura R, Ogawa T, Sugawara S, Takada H. Activation of human gingival epithelial cells by cell-surface components of black-pigmented bacteria: augmentation of production of interleukin-8, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor and expression of intercellular adhesion molecule 1. J Med Microbiol. 2002;51:27–33. doi: 10.1099/0022-1317-51-1-27. [DOI] [PubMed] [Google Scholar]
- 12.Hagewald S, Bernimoulin JP, Kottgen E, Kage A. Total IgA and Porphyromonas gingivalis-reactive IgA in the saliva of patients with generalised early-onset periodontitis. Eur J Oral Sci. 2000;108:147–153. doi: 10.1034/j.1600-0722.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- 13.Aduse-Opoku J, Davies NN, Gallagher A, Hashim A, Evans HE, Rangarajan M, Slaney JM, Curtis MA. Generation of lys-gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg-gingipain protease activities. Microbiology. 2000;146( Pt 8):1933–1940. doi: 10.1099/00221287-146-8-1933. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Duncan MJ. Gingipain adhesin domains mediate Porphyromonas gingivalis adherence to epithelial cells. Microb Pathog. 2004;36:205–209. doi: 10.1016/j.micpath.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Sheets SM, Robles-Price AG, McKenzie RM, Casiano CA, Fletcher HM. Gingipain-dependent interactions with the host are important for survival of Porphyromonas gingivalis. Front Biosci. 2008;13:3215–3238. doi: 10.2741/2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bainbridge BW, Darveau RP. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol Scand. 2001;59:131–138. doi: 10.1080/000163501750266710. [DOI] [PubMed] [Google Scholar]
- 17.Hajishengallis G, Martin M, Schifferle RE, Genco RJ. Counteracting interactions between lipopolysaccharide molecules with differential activation of toll-like receptors. Infect Immun. 2002;70:6658–6664. doi: 10.1128/IAI.70.12.6658-6664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride BC, Joe A, Singh U. Cloning of Bacteroides gingivalis surface antigens involved in adherence. Arch Oral Biol. 1990;35(Suppl):59S–68S. doi: 10.1016/0003-9969(90)90132-t. [DOI] [PubMed] [Google Scholar]
- 19.Teles RP, Bogren A, Patel M, Wennstrom JL, Socransky SS, Haffajee AD. A three-year prospective study of adult subjects with gingivitis II: microbiological parameters. J Clin Periodontol. 2007;34:7–17. doi: 10.1111/j.1600-051X.2006.01015.x. [DOI] [PubMed] [Google Scholar]
- 20.Brochu V, Grenier D, Nakayama K, Mayrand D. Acquisition of iron from human transferrin by Porphyromonas gingivalis: a role for Arg- and Lys-gingipain activities. Oral Microbiol Immunol. 2001;16:79–87. doi: 10.1034/j.1399-302x.2001.016002079.x. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Ratnayake DB, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 22.Kajiya M, Sato K, Silva MJ, Ouhara K, Do PM, Shanmugam KT, Kawai T. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun. 2009;386:316–321. doi: 10.1016/j.bbrc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Kajiya M, Silva MJ, Sato K, Ouhara K, Kawai T. Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. Biochem Biophys Res Commun. 2009;386:11–15. doi: 10.1016/j.bbrc.2009.05.117. [DOI] [PubMed] [Google Scholar]
- 24.Kusumoto Y, Hirano H, Saitoh K, Yamada S, Takedachi M, Nozaki T, Ozawa Y, Nakahira Y, Saho T, Ogo H, Shimabukuro Y, Okada H, Murakami S. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2. J Periodontol. 2004;75:370–379. doi: 10.1902/jop.2004.75.3.370. [DOI] [PubMed] [Google Scholar]
- 25.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzpatrick RE, Wijeyewickrema LC, Pike RN. The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 2009;4:471–487. doi: 10.2217/fmb.09.18. [DOI] [PubMed] [Google Scholar]
- 27.Imamura T, Travis J, Potempa J. The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Curr Protein Pept Sci. 2003;4:443–450. doi: 10.2174/1389203033487027. [DOI] [PubMed] [Google Scholar]
- 28.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? Faseb J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1:2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 30.Jacquot J, Tabary O, Le Rouzic P, Clement A. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1703–1715. doi: 10.1016/j.biocel.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. Faseb J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. Faseb J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]


