Abstract
Mutations of the photoreceptor retinol dehydrogenase 12 (RDH12) gene cause the early-onset retinal dystrophy Leber congenital amaurosis (LCA) by mechanisms not completely resolved. Determining the physiological role of RDH12 in photoreceptors is the focus of this study. Previous studies showed that RDH12, and the closely related retinol dehydrogenase RDH11, can enzymatically reduce toxic lipid peroxidation products such as 4-Hydroxynonenal (4-HNE), in vitro. To explore the significance of this activity, we investigated the ability of RDH11 and RDH12 to protect stably transfected HEK-293 cells against the toxicity of 4-HNE. Both enzymes protected against 4-HNE modification of proteins and 4-HNE-induced apoptosis in HEK-293 cells. In the retina, exposure to bright light induces lipid peroxidation, 4-HNE production, and 4-HNE modification of proteins in photoreceptor inner segments, where RDH11 and RDH12 are located. In mouse retina, RDH12 -but not RDH11- protects against adduct formation, suggesting that 4-HNE is a physiological substrate of RDH12. RDH12 -but not RDH11- also protects against light-induced apoptosis of photoreceptors. We conclude that in mouse retina RDH12 reduces 4-HNE to non-toxic alcohol, protecting cellular macromolecules against oxidative modification, and protecting photoreceptors from light-induced apoptosis. This activity is of particular significance to understand the molecular mechanisms of RDH12-induced LCA.
Keywords: Leber congenital amaurosis, lipid peroxidation, 4-hydroxynonenal, retinol dehydrogenase, photoreceptor
Introduction
LCA is a devastating form of vision loss in children. It is the most severe and early-onset form of inherited retinal dystrophy. Causes of autosomal recessive LCA include mutations in the RDH12 gene [1, 2] that encodes a microsomal retinoid dehydrogenase/reductase (RDH) located in photoreceptor inner segments [3–6]. LCA patients with RDH12 mutations experience progressive photoreceptor degeneration affecting both rods and cones. In most cases, visual handicap is diagnosed before one year of age and progresses to legal blindness in early adulthood. The pathogenetic mechanism associated with RDH12 mutations is not completely understood, but correlates with loss of RDH12 enzymatic activity [2, 7, 8].
The substrate specificities of RDH12 and of RDH11, the RDH isoform most closely related to RDH12, have been characterized in vitro [3, 4, 9]. Both enzymes use NADPH as a cofactor to catalyze the reduction of aldehydic substrates, including retinaldehydes such as all-trans retinal, and medium-chain aldehydes such as 4-HNE. However, the extent to which one or both of these groups of molecules serve as physiological substrates of RDH11 and RDH12 in photoreceptor cells has not been established.
Because of their high activities and affinities for retinaldehydes, these enzymes were named retinol dehydrogenases, and a role in the visual cycle that generates the 11-cis retinal chromophore of the rod and cone visual pigments was proposed [3, 4, 8]. The first step of the visual cycle (i.e. reduction of all-trans retinal to all-trans retinol) takes place in photoreceptors outer segments. As both RDH11 and RDH12 are located in photoreceptor inner segments, and their loss-of-function in knockout mice does not limit chromophore synthesis, their contribution to visual cycle activity per se is likely to be indirect [5, 10, 11]. One possibility is that they serve in an auxiliary role in the reduction of all-trans retinal produced in excess of the reductive capacity of the outer segments under certain conditions [12].
The ability of RDH11 and RDH12 to recognize medium chain aldehydes, albeit with lower affinity than for retinaldehydes, suggests that a possible role of these enzymes in photoreceptor cells may be to reduce substrates such as 4-HNE [4, 13, 14]. Medium-chain aldehydes are toxic end-products of lipid peroxidation of membrane polyunsaturated fatty acids. Lipid peroxidation is induced by reactive oxygen species generated in excess during oxidative stress [15]. 4-HNE, one of the most abundant and the most toxic products of lipid peroxidation, is a mediator of the apoptotic response induced by oxidative stress [16–18]. In photoreceptor cells, lipid peroxidation and production of 4-HNE, as well as other toxic lipid peroxidation products, are induced by exposure to light [18]. In addition, although Rdh11 and Rdh12 knockout mice have normal visual function and retinal histology [5, 6, 10, 11, 19], the photoreceptors of Rdh12 knockout mice are more sensitive to light-induced apoptosis than those of wild-type mice [5].
In this study, we tested the hypothesis that RDH11 and RDH12 could protect photoreceptor cells against the toxic effects of 4-HNE. We found that cell lines expressing the wild-type proteins could detoxify 4-HNE and protect the cells from apoptosis, whereas cell lines expressing an inactive RDH12 mutant were not protected. Such detoxification role might be relevant in vivo because we found that light-induced production of 4-HNE co-localized with RDH11 and RDH12 in mouse photoreceptors. Furthermore, RDH12 -but not RDH11- protected against adduct formation and light-induced apoptosis of mouse photoreceptor cells. We conclude that RDH12 is acting as an endogenous detoxifying enzyme for lipid peroxidation products in photoreceptor cells. The loss of RDH12 activity is likely to increase sensitivity of photoreceptor cells to light-induced oxidative injury and thereby contribute to the retinal degeneration phenotype of patients with RDH12 mutations.
Materials and Methods
Materials
Goat polyclonal anti-HNE antibodies, coupled with HRP or biotin, were from Abcam (Cambridge, MA). 4-HNE was from Cayman Chemical (Ann Arbor, MI). Mouse monoclonal anti-Flag coupled with HRP, and all other chemicals were from Sigma (St Louis, MO). Rabbit polyclonal anti-mouse RDH12 antibody was obtained as previously described [13]. Mouse monoclonal anti-human RDH12 antibody was obtained as previously described [6].
Cell Treatment, Quantification of Apoptosis and Adduct Formation
Human embryonic kidney HEK-293 cells were transfected with pTarget, pTarget-RDH11-Flag, and pTarget-RDH12-Flag to generate stable cell lines expressing Flag-tagged versions of mouse RDH11 and RDH12, as described previously [20]. Stable HEK-293 clones expressing human RDH12 variants were generated using previously described expression constructs [8]. Stable cell lines were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (Invitrogen), 100 units/ml penicillin G sodium (Invitrogen), 100 μg/ml streptomycin sulfate (Invitrogen), 0.25 μg/ml amphotericin B (Invitrogen), and 1mg/ml G418 (Sigma). When confluent, cells were incubated with the same medium containing indicated concentrations of 4-HNE for 20 h. Cell apoptosis was quantified the next day using Annexin V-PE Apoptosis Detection Kit from BD Biosciences (San Jose, CA) or CytoTox-ONE Homogenous Membrane Integrity Assay from Promega, (Madison, WI). When using Annexin V-PE Apoptosis Detection Kit, the attached cells were released by tryptic digestion and pooled with floating cells. After 2 washes with phosphate-buffered saline (PBS), cells were resuspended in binding buffer (10 mM Hepes/NaOH (pH 7.4) 140 mM NaCl, 2.5 mM CaCl2) and stained with annexin V-PE according to the manufacturer’s protocol and analyzed using flow cytometry (Epics XL Flow Cytometer from Beckman-Coulter, Fullerton, CA). When using CytoTox-ONE Homogenous Membrane Integrity Assay, 50 μl of medium was removed from each well, and lactate dehydrogenase (LDH) activity was measured according to the manufacturer’s protocol.
To quantify 4-HNE-protein adduct in cells, protein extraction was done using T-PER buffer from Pierce (Rockford, IL) containing protease inhibitors (2 μg/ml aprotinin, 5 μg/ml pepstatin A, 10 μg/ml leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride, final concentrations), according to the manufacturer’s instructions. Protein concentrations were measured using the Coomassie Reagent from Pierce. Dot blot analysis to quantify 4-HNE-protein adduct were carried out as described [18] with some modifications. Briefly, equal aliquots (5 μg) of protein were applied to a 96-well dot blot apparatus (Bio-Rad, Hercules, CA) and then transferred to a nitrocellulose membrane (Bio-Rad) by vacuum filtration. Sample loading was monitored by staining the membrane with Ponceau red (Sigma). Membranes were blotted over night with a 1:1000 dilution of anti-HNE antibody coupled with HRP (abcam). Signals were quantified using SuperSignal West Pico Chemiluminescent Substrate (Pierce) and the digital Kodak Image Station 4000R. Care was taken to ensure that the intensities of detected signals were within the linear range of the camera and that no pixels were saturated.
Animals
Pigmented Rdh11 and Rdh12 knockout mice were previously generated on a mixed genetic background of 129/C57BL/6 [5, 11]. For this study, each line was bred with albino Balb/C mice for 6 generations to generate knockout mice with a Balb/C background. Knockout and wild-type lines were maintained at the Dean A McGee Eye Institute in a 12-h dim light (10 lux)-dark cycle. Two to three-month-old mice were exposed to damaging white fluorescent light (3,000 lux) as described previously for indicated times [13]. After exposure to light, mice were killed immediately or returned to dim cyclic light for indicated times and killed by CO2 inhalation before tissue collection. This method is approved by the Panel of Euthanasia of the American Veterinary Medical Association. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the University of Oklahoma Health Sciences Center (OUHSC) Guidelines for Animals in Research. All protocols were reviewed and approved by the Institutional Animal Care and Use Committees of the OUHSC and the Dean A. McGee Eye Institute.
Preparation of Microsomal Fractions and Assay of 4-HNE-protein Adduct Formation in vitro
Retinas were dissected from two- to three-month-old wild-type and Rdh12 knockout mice and homogenized in sucrose buffer (25 mM sucrose, 10 mM Tris-Cl, pH 7.2, 1 mM EDTA), containing the protease inhibitors described above, using a Polytron PT-1200 CL (Kinematica Inc, Bohemia, NY). Homogenates were centrifuged at 15,000 × g for 10 min at 4°C. The resulting supernatants were then centrifuged at 100,000 × g for 1 h at 4°C, and microsomal pellets were resuspended in storage buffer (50 mM Tris-Cl, pH 7.2, 1 mM EDTA, 20% glycerol, 1 mM dithiothreitol, and the protease inhibitors described above). Protein concentrations were measured and microsomal fractions were stored at −80°C after snap freezing in liquid nitrogen. Equal aliquots (10 μg) of microsomal proteins prepared from retinas of wild-type or Rdh12 knockout mice were incubated in 15 μl of reaction buffer (100 mM Tris-HCl at pH 7.2, 200 mM NaCl, 1 mM dithiothreitol, 1% glycerol) containing indicated concentrations of 4-HNE, with or without NADPH (150 μM), at room temperature for 2 h. Reactions were immediately followed by dot blot analysis as described above with one modification; after loading proteins on the membrane, three washes with PBS, and one wash with PBST were performed to completely eliminate the unbound 4-HNE.
Quantification of Endogenous 4-HNE-Protein Adducts
Two- to three-month-old wild-type and knockout mice were killed after various times of bright light exposure and recovery. Dissected retinas were homogenized in T-PER buffer (Pierce) containing protease inhibitors, according to the manufacturer’s instructions. Protein concentrations were measured and dot blot analyses were carried out as described with 10 μg of protein and using SuperSignal West Femto Chemiluminescent Substrate (Pierce).
Histology, Immunohistochemistry, and Quantification of Photoreceptor Cell Death
Following various times of bright light exposure and recovery, whole eyes were enucleated after orientation of the superior half with a permanent dye. Oriented eyes were embedded in paraffin and sections were cut along the vertical meridian, through the optic nerve head (ONH) for subsequent analysis. For quantitative histology, mice were returned in dim cyclic light for 7 days to allow the retina to clear all dead cells and return to a well-organized morphology. Hematoxylin- and eosin-stained paraffin sections were prepared from each eye and the thickness of the outer nuclear layer (ONL) was measured at 0.24 mm intervals from the optic nerve head (ONH) to the inferior and superior ora serrata. For immunohistochemistry, sections were deparaffinized and incubated in polyclonal affinity-purified anti-RDH12 (1:200) antibody, in blocking buffer (2% Goat serum) overnight at 4°C. Slides were incubated with FITC-coupled anti-rabbit (1:1000) and DAPI diluted in blocking buffer for 30 minutes at room temperature. The tissue was coverslipped using Vectashield mounting medium for immunofluorescence (Vector Laboratories, Burlingame, CA). Detection of 4-HNE-protein adducts was performed using TSA Biotin System (Perkin Elmer, Waltham, MA) excluding the amplification step. After deparaffinization, endogenous peroxidase was quenched with 3% hydrogen peroxide for 10 minutes at room temperature. Slides were incubated overnight at 4°C in 4-HNE antibody coupled with biotin (Abcam), diluted 1:100 in blocking buffer. After washes in PBS, slides were incubated in Streptavidin-HRP diluted 1:100 in blocking buffer. Slides were washed and signal was visualized with HRP chromogenic substrate diaminobenzidine (DAB) (Sigma). Terminal deoxynucleotide transferase dUTP Nick End Labeling (TUNEL) assay for labeling apoptotic cells was performed on paraffin sections using Apo-BrdU-IHC In Situ DNA Fragmentation Assay Kit (BioVision, San Francisco, CA) according to the Staining of Paraffin Embedded Tissue (PET) assay protocol.
Results
RDH11 and RDH12 protect HEK-293 cells against 4-HNE-induced apoptosis
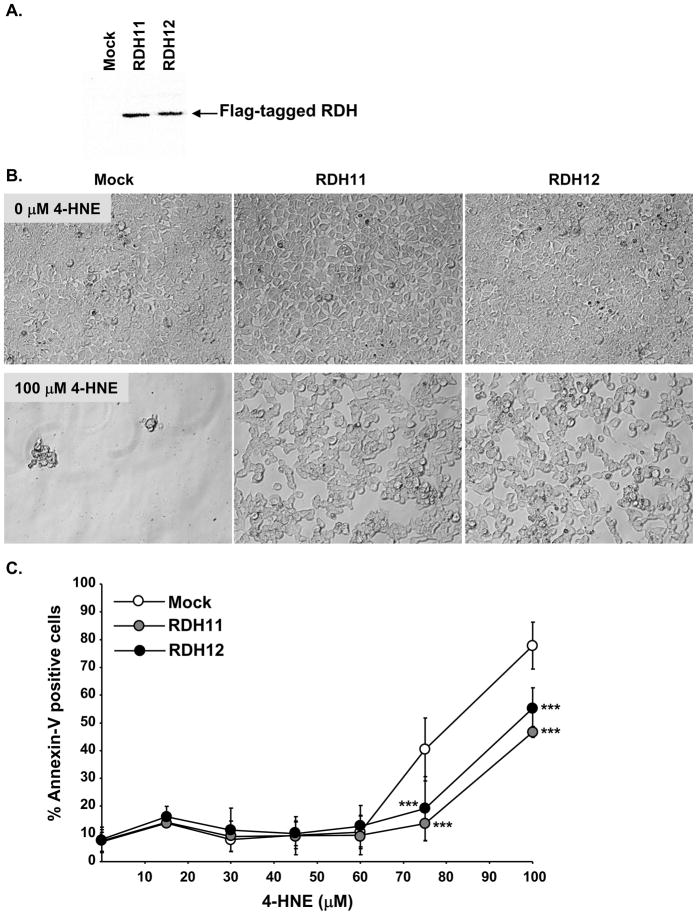
The reducing activities of RDH11 and RDH12 toward 4-HNE were previously shown in vitro using purified recombinant enzyme or the microsomal fraction of transfected cells [4, 9]. We investigated the possibility that enzymatic reduction of 4-HNE by RDH11 and RDH12 could efficiently prevent the toxicity of this compound in cultured cells. Human embryonic kidney HEK-293 cells were used for this study because they have no detectable endogenous RDH11 [9] or RDH12 (Figure 2A). Stable cell lines expressing similar amounts of mouse RDH11, RDH12, or neither of the recombinant enzymes (Figure 1A) were treated with increasing doses of 4-HNE for 20 h. Confluent mock-transfected cells were completely killed by 100 μM 4-HNE, while a significant number of cells expressing either RDH11 or RDH12 remained viable and attached to the dish after treatment with the same dose of 4-HNE (Figure 1B). To quantify this effect, apoptosis was measured using annexin-V staining and flow cytometry. As shown in Figure 1C, 4-HNE did not induce cell death below 60 μM, but a sharp increase in mock-transfected cell death was observed with 4-HNE concentrations above 60 μM, inducing up to 75% annexin-V positive cells at 100 μM of 4-HNE. We observed that cell death was induced by lower concentrations of 4-HNE when the cells were not confluent and if we used cell medium without fetal bovine serum (10%), however our results were more reproducible using the conditions described in Figure 1. At 75 μM 4-HNE, nearly all cells expressing either RDH11 or RDH12 were protected, while about 40% of control cells were annexin-V positive. RDH-induced protection decreased to about 45% when 4-HNE concentration increased to 100 μM. This significant protection demonstrates that RDH11 and RDH12 are able to efficiently detoxify 4-HNE in cells, most likely through their ability to reduce it to a non-toxic alcohol. The data also show that this protection can be overwhelmed with increased oxidation.
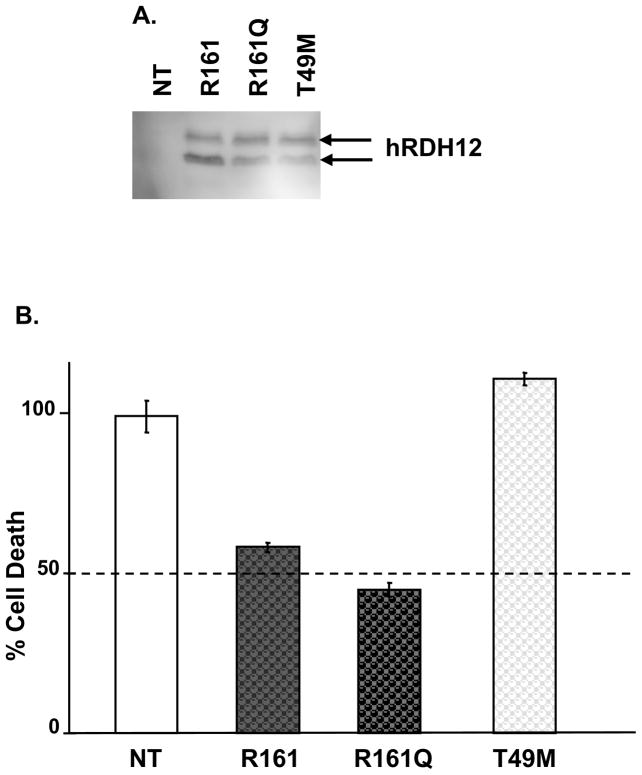
Figure 2.
An inactivating mutation of RDH12 associated with LCA abolishes protection against 4-HNE-induced apoptosis. Cells were transfected with the expression plasmid pcDNA3.1/HIS, expressing human RDH12 variants R161 (wild-type), R161Q (common variant), and T49M (mutant inducing LCA). Stable cell lines were established and maintained by growing the cells in complete DMEM medium containing G418 (1mg/ml). A, Equal aliquots (50 μg) of microsomal fractions were analyzed by Western-blot using anti-hRDH12 antibody (1:1000 dilution) to compare expression levels. B, Cells were treated over night (20 h) with 100 μM of 4-HNE in complete DMEM. LDH activity, released by cells with damaged membranes, was measured in medium to quantify non-viable cells. Percent cell death was calculated for each cell line, according to the manufacturer’s instructions. Percent cell death in each stable cell line was then expressed in comparison to the non-transfected cell death, set at 100%. Graph shows results of 2 independent experiments, each having 3 replicates of the same conditions and error bars denote SD.
Figure 1.
Mouse RDH11 and RDH12 protect HEK-293 cells against 4-HNE-induced apoptosis. Cells were transfected with the expression plasmid pTarget, containing or not Flag-tagged RDH11 or RDH12. Stable cell lines were established and maintained by growing the cells in complete DMEM medium containing G418 (1mg/ml). A, Equal aliquots (50 μg) of cell homogenates were analyzed by Western-blot using anti-Flag antibody (1:1000 dilution) to compare expression levels of RDH11 and RDH12. B, Cells were treated over night (20 h) with indicated concentrations of 4-HNE in complete DMEM containing G418. Pictures of cell dishes subjected to 0 or 100 μM of 4-HNE were taken the next day, after removing the media containing floating cells, and replacing it with PBS. Note complete absence of adhering cells in the mock-transfected clone, in contrast with RDH11- and RDH12-transfected clones in which a significant number of cells are still attached to the dish. C, Quantification of Annexin V positive cells corresponding to apoptotic cells, by flow cytometry. After 20 h treatment with 4-HNE, floating and attached cells are pooled, washed in PBS, and stained with Annexin V-PE before analysis by flow cytometry. Graph shows results of 6 independent experiments. Data points represent the mean and error bars denote SEM. RDH-expressing clones were compared with the control using the Student’s t test for significance. *=p<0.05; **=p<0.001; and ***=p<0.0001.
An inactivating mutation of RDH12 abolishes the protection against 4-HNE-induced apoptosis
We investigated whether the enzymatic activity of RDH12 was necessary for protection against 4-HNE-induced apoptosis using stable HEK-293 cell lines expressing active or inactive variants of this protein. In studies of human RDH12, wild-type R161 and the common variant R161Q have been previously reported to exhibit similar all-trans retinol dehydrogenase activities [7, 8]. On the other hand, the T49M mutant previously associated with LCA [7, 8] showed a dramatic reduction in the ability to produce all-trans retinol from all-trans retinal explained by a defect in cofactor binding [7]. As shown in Figure 2A, all three variants were expressed similarly in stable cell lines. The lower band corresponds to the expected 35 kDa unglycosylated form of RDH12 and is not present in untransfected cells. The upper band corresponds to the glycosylated form(s) of RDH12 and is also specific to RDH12-transfected cells. To quantify the protective effect of these variants, stable cell lines were grown to confluence and treated with 100 μM 4-HNE overnight under conditions described in the previous experiment. The next day, percent cell death was estimated by quantification of lactate dehydrogenase activity released in the medium from the cells with a damaged membrane. In Figure 2B, percent cell death in each stable cell line was compared to non-transfected cell death set at 100%. As expected, both the wild-type and common variant of human RDH12 significantly protected 40 to 50% of cells against 4-HNE-induced apoptosis. On the other hand, the inactive, T49M mutant associated with LCA did not protect the cells. This experiment demonstrates that the enzymatic activity of RDH12 is essential to detoxify 4-HNE in cells.
Protection against 4-HNE-protein adducts formation in HEK-293 cells
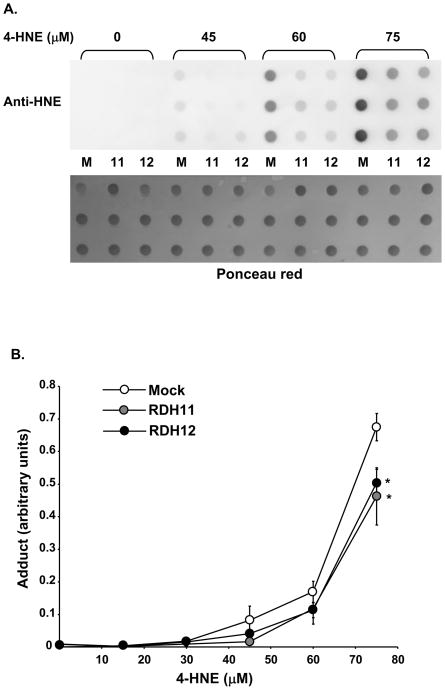
The toxicity of 4-HNE is mediated, in part, by the formation of Michael’s adducts with lysine, histidine, or cysteine residues of proteins, thus inhibiting their physiological functions [21–23]. We quantified 4-HNE-protein adducts in our stable cell lines after incubation with increasing doses of 4-HNE under conditions described in Figure 1, using an antibody specific for 4-HNE-modified proteins. Figure 3A shows a representative example of adduct quantification by dot blot analysis. As shown in Figure 3B, adduct formation was significantly inhibited in cells expressing RDH11 or RDH12. This result demonstrates that both RDH11 and RDH12 protect the cells from apoptosis by inhibiting adduct formation between 4-HNE and proteins.
Figure 3.
Mouse RDH11 and RDH12 protect against 4-HNE-protein adduct formation in HEK-293 cells. Confluent cells were treated overnight (20 h) with indicated concentrations of 4-HNE. A, Whole cell homogenates are prepared and equal aliquots (5 μg) of protein are analyzed by dot blot. M, mock; 11, RDH11; 12, RDH12. Protein loading is first verified by staining the membrane with Ponceau red (lower picture) and the membrane is then incubated with anti-HNE coupled with HRP at 1:1000 dilution. B, Graph shows quantification of adduct formation in 6 independent experiments. Data points represent the mean and error bars denote SEM. RDH-expressing clones were compared with the control using the Student’s t test for significance. *=p<0.05; **=p<0.001; and ***=p<0.0001.
RDH12 protects against 4-HNE-protein adduct formation in microsomal fractions of mouse retina
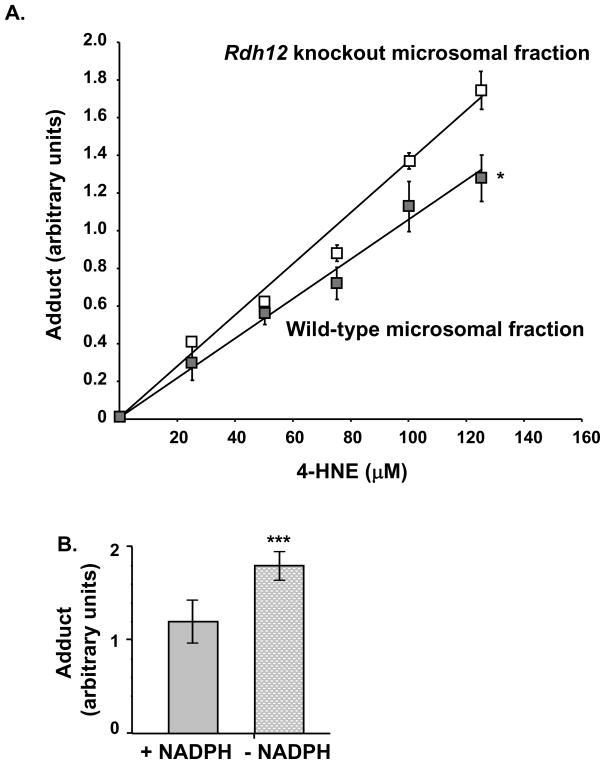
Our studies suggest that RDH12 activity involved in inhibiting 4-HNE adduct formation might be important for protecting photoreceptor cells against toxicity produced by lipid peroxidation. To determine if such protection requires RDH12 function, we prepared microsomes from retinas of wild-type and Rdh12 knockout mice, and incubated them in vitro in reaction buffer containing a range of concentrations of 4-HNE in the presence or absence of NADPH. After 2 h of incubation, reactions were stopped and adducts were quantified by dot blot analysis. As shown in Figure 4A, significantly more adducts were formed with microsomal proteins in the absence than in the presence of RDH12. Figure 4B shows that the protection against adduct formation did not take place in absence of NADPH, the necessary coenzyme of both RDH11 and RDH12. This demonstrates that the protection is mediated by an NADPH-dependent enzymatic activity toward 4-HNE. In the absence of NADPH, the amount of adduct formed is the same as the amount formed in the absence of RDH12 with the membranes from knockout mice. This demonstrates that RDH12 is the only NADPH-dependent enzyme involved in reducing 4-HNE in our preparation of retinal microsomes. The activity of microsomal RDH11 was not significant under these conditions. Our results suggest that in photoreceptor cell membranes in vivo, RDH12 may inhibit adduct formation by reducing 4-HNE to a non-toxic alcohol, thus protecting the cells from cytotoxic effects and apoptosis.
Figure 4.
RDH12 protects against formation of 4-HNE-protein adducts in mouse retinal microsomes. Microsomal fractions were prepared from wild-type and Rdh12 knockout retinas. A, Equal aliquots (10 μg) of microsomal proteins from wild-type or Rdh12 knockout retinas were incubated with indicated concentrations of 4-HNE in reaction buffer containing NADPH, for 2 h at room temperature. Reactions were transferred to the membrane by vacuum filtration, and unbound 4-HNE was washed 3 times with PBS. Protein loading is verified and adduct formation is quantified as described in Figure 3. Graph shows quantification of adduct formation in 3 independent experiments. B, The same experiment is repeated with wild-type retinal microsomes and 125 μM of 4-HNE, with and without NADPH. Graph shows quantification of adduct formation in 3 independent experiments. Data points represent the mean and error bars denote SEM. Results were compared using the Student’s t test for significance. *=p<0.05; **=p<0.001; and ***=p<0.0001.
Exposure to bright light induces production of 4-HNE-protein adduct in mouse photoreceptor inner segments
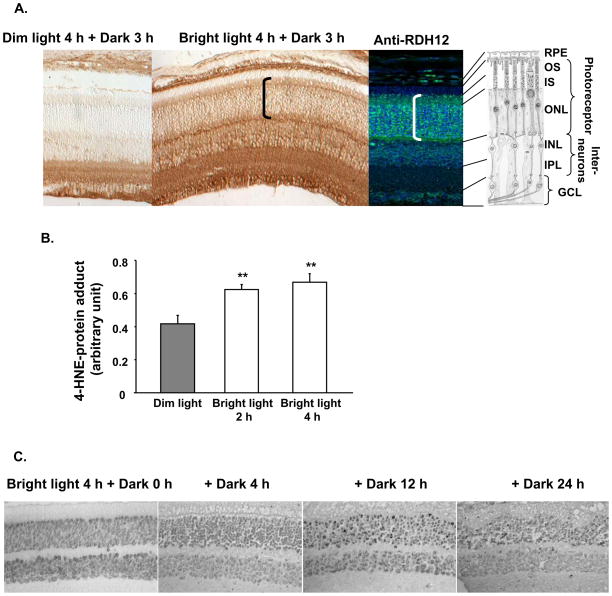
To determine if a role of RDH12 in detoxification of 4-HNE is relevant in vivo, we measured the formation of 4-HNE-protein adducts in Balb/C mouse retina following exposure to bright light. As shown in Figure 5A, light-dependent accumulation of adduct occurs in the retinal pigmented epithelium (RPE), the photoreceptor cells and the interneurons. In photoreceptors, adducts accumulate in the inner segments (IS), cell bodies and synaptic termini (ONL), but not in the outer segments (OS). This pattern is similar to the pattern of RDH12 expression, as shown in Figure 5A and as previously described [5, 6, 13]. RDH11 is also located in mouse photoreceptor inner segments, but not in the outer segments, as previously described [11, 13]. Co-localization of RDH11, RDH12, and 4-HNE in vivo suggests that 4-HNE could be a physiological substrate of these enzymes. In Figure 5B, we quantified the endogenous 4-HNE-protein adducts in retinal homogenates of Balb/C mice raised in dim cyclic light and exposed to bright light for 2 h and 4 h. We found a significant increase of adducts (~60%) in retinas subjected to bright light. This reproduces the previously published 90% increase in Sprague-Dawley albino rats exposed to similar lighting conditions [18]. Finally, in Figure 5C, we show that following exposure to 4 h of bright light, apoptosis of photoreceptors starts 12 h after adducts are formed, as shown by TUNEL-positive photoreceptor nuclei in the outer nuclear layer (ONL). This is supporting the notion that adduct formation is an early event leading to cell death.
Figure 5.
Exposure of Balb/C mice to bright light induces adduct formation in the retina followed by photoreceptor apoptosis. Mice were raised in dim (10 lux) cyclic light until 8 to 12 weeks-old. They were then subjected to bright light (3,000 lux) exposure for indicated times, and transferred to the dark for indicated times. A, Immunohistochemistry of retinal paraffin sections using anti-HNE coupled with biotin at 1:100 dilution (brown signal), and anti-RDH12 at 1:200 dilution (green signal). Pictures were taken at 20 × magnification. RPE, retinal pigmented epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Note the co-localization of adducts and RDH12 in photoreceptors indicated by brackets. B, Immediately after exposure to bright light, mice were killed and retinas were dissected. Whole retinal homogenates were prepared and equal aliquots (10 μg) of retinal homogenates were analyzed by dot blot using anti-HNE coupled with HRP to quantify total 4-HNE-protein adducts. Six mice were used in each group, and the mean and SEM are plotted. C, TUNEL nuclear staining was observed in the ONL at 4 h of exposure to bright light followed by at least 12 h in the dark. Pictures were taken at 40 × magnification.
RDH12 protects against modification of proteins by 4-HNE in mouse retina
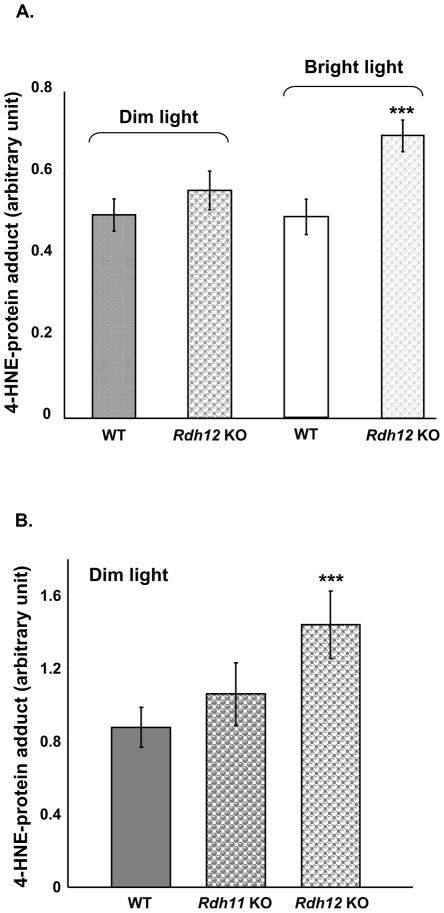
Since light-induced production of 4-HNE colocalizes with RDH11 and RDH12 in photoreceptor inner segments, we investigated whether these enzymes could detoxify endogenous 4-HNE, preventing adduct formation. We first used the original pigmented line of Rdh12 knockout mice [5] to quantify 4-HNE-protein adducts in retinal homogenates of mice raised under dim cyclic light or subjected to bright light, and compared them to the wild-type controls. As shown in Figure 6A, under dim cyclic light, there is no significant difference between the levels of adducts in retinas from wild-type and knockout-mice. After exposure to continuous bright light (3,000 lux) for 48 h, the level of adduct did not increase in the wild-type, but did increase significantly in the knockout by about 40%. Pigmented mice on a mixed genetic background (129/C57BL/6) are more resistant to light-induced damage than Balb/C mice, however 48 h of exposure to 3,000 lux was shown to induce photoreceptor apoptosis in the pigmented Rdh12 knockout, but not in the wild-type [5]. Our results suggest that light-induced apoptosis in pigmented Rdh12 knockout mice might be mediated by formation of toxic 4-HNE-protein adduct.
Figure 6.
RDH12 protects against modification of proteins by 4-HNE in mouse retina. Pigmented wild-type and Rdh12 knockout mice (A) or Balb/C wild-type, Rdh11 knockout and Rdh12 knockout mice (B) were raised in dim cyclic light for 8 to 12 weeks. Pigmented wild-type and Rdh12 knockout mice were then subjected to constant bright light (3,000 lux) for 48 h. Whole retinal homogenates were prepared and equal aliquots (10 μg) of retinal homogenates were analyzed by dot blot using anti-HNE coupled with HRP to quantify total 4-HNE-protein adducts. Six mice were used in each group, and the mean and SEM are plotted. Results were compared using the Student’s t test for significance. *=p<0.05; **=p<0.001; and ***=p<0.0001.
In Figure 6B, we quantified 4-HNE-protein adduct in retinal homogenates of albino Balb/C wild-type, Rdh11 and Rdh12 knockout mice raised under dim cyclic light. Interestingly, the basal level of 4-HNE-modified proteins was significantly higher (60%) in the Rdh12 knockout retina when compared to the wild-type retina. The basal level in Rdh12 knockout mice is similar to the level of 4-HNE-modified proteins in the wild-type retina subjected to acute light stress (Figure 5B). By contrast, in the Rdh11 knockout retina, the basal level of adducts is similar to that of the wild-type. These results suggest that RDH12 might have a specific detoxification role in photoreceptor cells, reducing 4-HNE produced chronically by a basal level of lipid peroxidation taking place in Balb/C retina exposed to dim light. Immediately after exposure to bright light (4h at 3,000 lux), adduct accumulation reached a maximum level that was the same in all three mouse lines (not shown), suggesting that other factors independent of RDH11 or RDH12 are controlling the maximum level of adduct accumulation in Balb/C retina during acute stress.
RDH12 protects photoreceptors against light-induced apoptosis in Balb/C mice
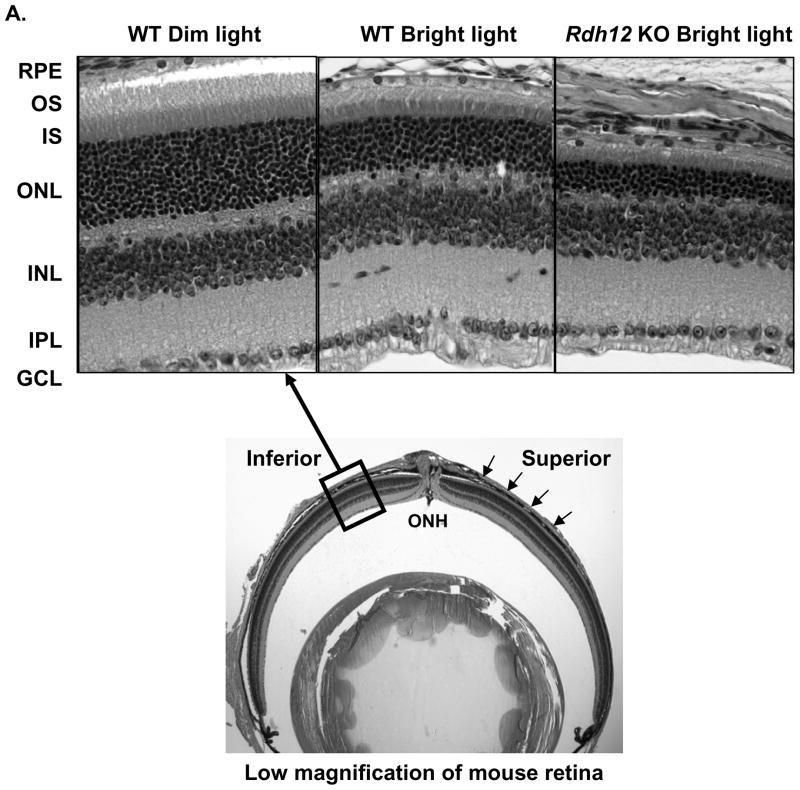
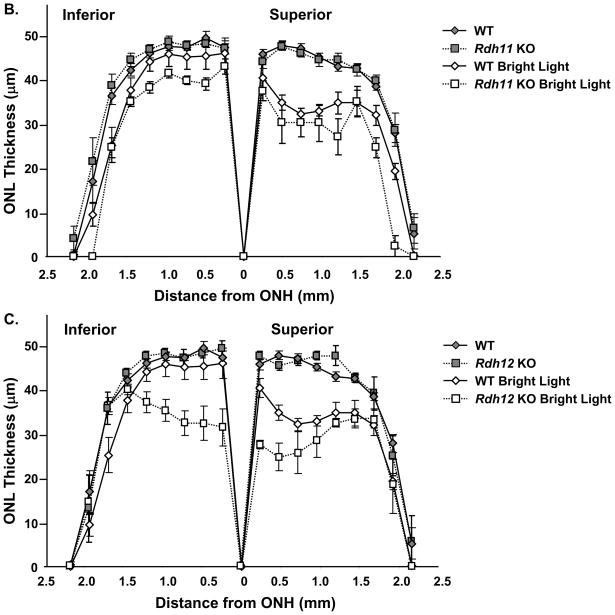
Light microscopic examination of the superior and inferior regions of the retinas from wild-type, Rdh11, and Rdh12 knockout mice under the albino Balb/C background and raised in dim cyclic light showed no difference in retinal structure between the three groups. There were 11–12 rows of photoreceptor nuclei in the outer nuclear layer (ONL), the number usually observed for rodents without retinal degeneration (Figure 7A, left panel). As shown in Figure 7B and C, quantitative analysis of the superior and inferior regions of the ONL layer of the three groups of animals showed no significant differences in the average ONL thickness measured at 0.24 mm intervals from the optic nerve head (ONH) to the inferior and superior ora serrata, indicating that there was no difference in rod photoreceptor viability between the three groups of mice. These results suggest that the Balb/C knockout animals, similarly to the pigmented ones [5, 6, 11, 19], do not exhibit any structural phenotype when they are maintained under dim cyclic light conditions.
Figure 7.
RDH12 protects photoreceptors against light-induced apoptosis in Balb/C mice. Mice were raised in dim cyclic light for 8 to 12 weeks and light damage was induced in wild-type, Rdh11 knockout and Rdh12 knockout by exposure to 3,000 lux of light for 2 h. After light exposure, mice are returned in dim cyclic light for 7 days to allow the retina to clear all dead cells and return to a well-organized morphology. A, Representative sections of wild-type and Rdh12 knockout retinas. Pictures are taken in the inferior retina, 0.5 mm from the optic nerve head (ONH) as indicated by the boxed area on the low magnification of mouse retina. The layer of photoreceptor nuclei (ONL) is thinner in the knockout than in the wild-type after exposure to bright light, indicating more photoreceptor apoptosis in the knockout. The thickness of the outer nuclear layer (ONL) was measured in wild-type and Rdh11 knockout retinas (B) and in Rdh12 knockout retinas (C) at 0.24 mm intervals from the ONH to the inferior and superior ends of retina, as shown by arrows on the low magnification of mouse retina (A, lower panel). Ten mice were used in each group and the mean and SEM are plotted.
The pigmented Rdh12 knockout mouse was previously found to be more sensitive to light-induced photoreceptor apoptosis than the wild-type mouse [5]. To determine whether our albino lines under a Balb/C background exhibit the same hypersensitivity to light damage, mice between 8 and 12 weeks of age were exposed to 3,000 lux illumination for 2 h, a condition predetermined to cause a moderate loss of photoreceptor cells in Balb/C mice [13]. After a 7 days recovery period in dim cyclic light, we measured the extent of photoreceptor cell death. Figure 7A shows representative sections of light-damaged retinas from wild-type and Rdh12 knockout mice. As shown in Figure 7B and C, there was a 25% reduction in rod photoreceptors in the superior region and no significant loss in the inferior region in the wild-type retina after light exposure. The Rdh12 knockout was significantly more affected than the wild-type by exposure to bright light, especially around the ONH (Figure 7C). There was a 40% reduction of rod photoreceptors in the superior and inferior regions. In contrast to the Rdh12 knockout, the Rdh11 knockout retina was only slightly more affected than the wild-type retina by exposure to bright light, across the inferior and superior regions of the retina (Figure 7B). Taken together, these results suggest that RDH12 plays a significant and specific role in detoxifying 4-HNE in photoreceptor cells in the central retina, protecting the cells against light-induced apoptosis.
Discussion
Aldehydic molecules generated during lipid peroxidation have been implicated as causative agents in cytotoxic processes initiated by the exposure of cells or tissues to oxidant stress. 4-HNE is the best recognized and most well studied of the cytotoxic products of lipid peroxidation, and 4-HNE-protein adducts can serve as one of the most useful biomarkers for the occurrence and/or the extent of oxidative stress. The results of our studies lead us to propose that an important physiological function of RDH12 in the photoreceptor cells is to detoxify 4-HNE in order to prevent the chronic accumulation of 4-HNE-protein adducts during exposure to moderate daylight. When this function is perturbed, the threshold of sensitivity to light-induced apoptosis is decreased in photoreceptor cells. We further propose that, in the human retina, cell death resulting from the toxic effects of 4-HNE may be a factor in the development of the early and severe retinal degeneration phenotype observed in LCA patients with RDH12 mutations [24, 25].
More than 30 different sequence variants resulting in RDH12 loss-of-function have been associated with the disease phenotype in LCA patients, suggesting an important function of this enzyme in photoreceptor cells [2, 7, 8]. Data generated from analysis of Rdh12 knockout mice indicate that RDH12 does not limit the efficiency of the visual cycle, but does affect clearance of all-trans retinal, as evidenced by a modest accumulation of the bisretinoid compound A2E in the retina [5, 26]. This modest participation in visual cycle function is not surprising, as RDH12 is located in the inner segments distant from the site of all-trans retinal release from bleached rhodopsin and cone-opsins. Nevertheless, the observed accumulation of A2E in retinas of Rdh12-knockout mice suggests that some degree of retinoid processing occurs in the photoreceptor inner segment. In addition, recent studies suggest that visual cycle efficiency is unlikely to be regulated by RDH activity, in general, as knockout mice deficient in multiple RDH isoforms present in the retina exhibit only minor effects on chromophore synthesis [27].
Previous studies reported that RDH12 can reduce toxic 4-HNE to a non-toxic alcohol in vitro [4] and that 4-HNE is produced in photoreceptor cells [18]. Our studies now show that the reducing activity of RDH12 protects cultured HEK-293 cells from 4-HNE-induced toxicity, as both adduct formation and apoptosis are significantly inhibited by RDH12. This protection requires the enzymatic activity of RDH12, as mutants that are inactive in assays of activity in vitro do not inhibit 4-HNE-induced cell death. Both RDH12 and closely related RDH11 exhibit enzymatic activity toward 4-HNE in vitro [4, 9] and are located in photoreceptor inner segments in mouse [13]. In addition, when expressed at equivalent levels, both isoforms similarly protect transfected cells against 4-HNE toxicity at concentrations ranging from 50 to 100 μM. Such concentrations of 4-HNE are likely to be physiologically relevant, as 4-HNE accumulates in membranes in vivo at concentrations ranging from 10 μM to 5 mM in response to oxidative insults [23].
The possibility that 4-HNE is an important physiological substrate of RDH11 and RDH12 in the photoreceptor cells is supported by our finding that exposure to bright light induced the formation of 4-HNE-protein adducts in mouse photoreceptor inner segments, cell bodies, and synaptic termini, compartments where both isoforms are located. In addition, retinas of both pigmented and albino Rdh12 knockout mice accumulated more 4-HNE adducts, and photoreceptor cells were more sensitive to light-induced apoptosis, than were corresponding wild-type animals on the same genetic background. In contrast, Rdh11 knockout mice did not accumulate significantly higher amounts of 4-HNE adducts nor exhibit hypersensitivity to light-induced apoptosis. This could be explained by the low expression level (about 8-fold lower) of RDH11 relative to RDH12 in mouse retina [13], rather than by a difference in substrate specificity or specific activity, since RDH11 is at least as efficient as RDH12 in protecting against 4-HNE toxicity when present at equivalent levels.
Taken together, our results suggest that an important function of RDH12 in photoreceptor cells is to detoxify 4-HNE and potentially other toxic aldehyde products resulting from lipid peroxidation. The ability of RDH12 to reduce the basal level of 4-HNE protein modification is likely a contributing factor in protecting the photoreceptor cells against light-induced apoptosis. In addition, RDH12 loss-of-function relative to detoxifying 4-HNE could contribute to the pathogenetic mechanism of LCA associated with RDH12 mutations, as absence of endogenous protection could lead to a progressive accumulation of 4-HNE during daily exposure to moderate light. Elevated 4-HNE levels can markedly induce production of intracellular reactive oxygen species [23], leading to an amplification of 4-HNE production. This could result in accumulation of toxic levels of 4-HNE, with toxicity mediated by formation of 4-HNE protein adducts. Adduction of proteins important for phototransduction could result in impaired visual responses. Adduction of proteins important for protecting photoreceptor cells against light-induced damage could result in hypersensitivity to bright light-induced apoptosis, leading to irreversible loss of photoreceptor cells and progressive degeneration of the retina.
Acknowledgments
We thank Dr. Krzysztof Palczewski for the generous gift of the Rdh12 knockout mice. We thank Drs. Robert E. Anderson and John D. Ash for helpful discussions, and Dr. Lixin Zheng for assistance with histology. This work was supported by grants from the National Center for Research Resources (P20RR017703) and the National Eye Institute (R21EY018907 and P30EY012190), the Foundation Fighting Blindness, and by an unrestricted Grant from Research to Prevent Blindness, Inc. to the Departments of Ophthalmology at the University of Oklahoma and the University of Michigan.
List of Abbreviations
- 4-HNE
4-hydroxynonenal
- A2E
diretinoid-pyridinium-ethanolamine
- HEK
human embryonic kidney
- HRP
horseradish peroxidase
- LCA
Leber congenital amaurosis
- NADPH
nicotinamide adenine dinucleotide phosphate reduced form
- ONH
optic nerve head
- ONL
outer nuclear layer
- PBS
phosphate buffered saline
- PBST
phosphate buffered saline with Tween 20
- RDH
retinol dehydrogenase
References
- 1.Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, Rozet JM. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet. 2004;75:639–646. doi: 10.1086/424889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janecke AR, Thompson DA, Utermann G, Becker C, Hèubner CA, Schmid E, McHenry CL, Nair AR, Rèuschendorf F, Heckenlively J, Wissinger B, Nèurnberg P, Gal A. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 3.Haeseleer F, Jang GF, Imanishi Y, Driessen CA, Matsumura M, Nelson PS, Palczewski K. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002;277:45537–45546. doi: 10.1074/jbc.M208882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44:7035–7047. doi: 10.1021/bi050226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA, Palczewski K. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. The Journal of biological chemistry. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurth I, Thompson DA, Rèuther K, Feathers KL, Chrispell JD, Schroth J, McHenry CL, Schweizer M, Skosyrski S, Gal A, Hèubner CA. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Molecular and cellular biology. 2007;27:1370–1379. doi: 10.1128/MCB.01486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun W, Gerth C, Maeda A, Lodowski DT, Van Der Kraak L, Saperstein DA, Hâeon E, Palczewski K. Novel RDH12 mutations associated with Leber congenital amaurosis and cone-rod dystrophy: biochemical and clinical evaluations. Vision research. 2007;47:2055–2066. doi: 10.1016/j.visres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson DA, Janecke AR, Lange J, Feathers KL, Hubner CA, McHenry CL, Stockton DW, Rammesmayer G, Lupski JR, Antinolo G, Ayuso C, Baiget M, Gouras P, Heckenlively JR, den Hollander A, Jacobson SG, Lewis RA, Sieving PA, Wissinger B, Yzer S, Zrenner E, Utermann G, Gal A. Retinal degeneration associated with RDH12 mutations results from decreased 11-cis retinal synthesis due to disruption of the visual cycle. Hum Mol Genet. 2005;14:3865–3875. doi: 10.1093/hmg/ddi411. [DOI] [PubMed] [Google Scholar]
- 9.Kasus-Jacobi A, Ou J, Bashmakov YK, Shelton JM, Richardson JA, Goldstein JL, Brown MS. Characterization of mouse short-chain aldehyde reductase (SCALD), an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2003;278:32380–32389. doi: 10.1074/jbc.M304969200. [DOI] [PubMed] [Google Scholar]
- 10.Kasus-Jacobi A, Birch DG, Anderson RE. Photoreceptor retinol dehydrogenases. An attempt to characterize the function of Rdh11. Advances in experimental medicine and biology. 2006;572:505–511. doi: 10.1007/0-387-32442-9_70. [DOI] [PubMed] [Google Scholar]
- 11.Kasus-Jacobi A, Ou J, Birch DG, Locke KG, Shelton JM, Richardson JA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Edwards AO. Functional characterization of mouse RDH11 as a retinol dehydrogenase involved in dark adaptation in vivo. The Journal of biological chemistry. 2005;280:20413–20420. doi: 10.1074/jbc.M413789200. [DOI] [PubMed] [Google Scholar]
- 12.Chrispell JD, Feathers L, Kurth I, Gal A, Hubner C, Thompson DA. Expression and activity of the retinal dystrophy gene RDH12 in mouse and human retina (abstract) Invest Ophthalmol Vis Sci. 2008 [Google Scholar]
- 13.Kanan Y, Wicker LD, Al-Ubaidi MR, Mandal NA, Kasus-Jacobi A. Retinol dehydrogenases RDH11 and RDH12 in the mouse retina: expression levels during development and regulation by oxidative stress. Investigative ophthalmology & visual science. 2008;49:1071–1078. doi: 10.1167/iovs.07-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SA, Belyaeva OV, Kedishvili NY. Effect of lipid peroxidation products on the activity of human retinol dehydrogenase 12 (RDH12) and retinoid metabolism. Biochim Biophys Acta. 2008;1782:421–425. doi: 10.1016/j.bbadis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Molecular aspects of medicine. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta biochimica Polonica. 2003;50:319–336. [PubMed] [Google Scholar]
- 18.Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Investigative ophthalmology & visual science. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- 19.Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson P. Dalayed dark adaptation in 11-cis-retinol dehydrogenase deficient mice: A role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280:8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanan Y, Jacobi AK, Sawyer K, Mannel DS, Tink JT, Al-Ubaidi MR. An in-vivo assay to identify compounds protective against light induced apoptosis. Advances in experimental medicine and biology. 2008;613:61–67. doi: 10.1007/978-0-387-74904-4_6. [DOI] [PubMed] [Google Scholar]
- 21.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 24.Schuster A, Janecke AR, Wilke R, Schmid E, Thompson DA, Utermann G, Wissinger B, Zrenner E, Gal A. The phenotype of early-onset retinal degeneration in persons with RDH12 mutations. Investigative ophthalmology & visual science. 2007;48:1824–1831. doi: 10.1167/iovs.06-0628. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Schwartz SB, Windsor EA, Roman AJ, Heon E, Stone EM, Thompson DA. RDH12 and RPE65, visual cycle genes causing leber congenital amaurosis, differ in disease expression. Invest Ophthalmol Vis Sci. 2007;48:332–338. doi: 10.1167/iovs.06-0599. [DOI] [PubMed] [Google Scholar]
- 26.Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda A, Shiose S, Okano K, Matosky M, Maeda T, Palczewski K. Retinol dehydrogenase RDH11 protects the mouse retina from light-induced degeneration. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-3749. [DOI] [PubMed] [Google Scholar]