Abstract
CD4 T cells have traditionally been regarded as helpers and regulators of adaptive immune responses; however, a novel role for CD4 T cells as direct mediators of protection against viral infections has emerged. CD4 T cells with cytolytic potential have been described for almost forty years, but their role in host protection against infectious disease is only beginning to be realized. In this review, we describe the current literature identifying these cells in patients with various infections, mouse models of viral infection and our own work investigating the development of cytolytic CD4 cells in vivo and in vitro. CD4 CTL are no longer considered an artefact of cell culture and may play a physiological role in viral infections such as EBV, CMV, HIV and influenza. Therefore, vaccine strategies aimed at targeting CD4 CTL should be developed in conjunction with vaccines incorporating B cell and CD8 CTL epitopes.
Introduction
T lymphocytes are subdivided based on recognition and response to antigen: CD8 T cells recognize peptides of approximately 8-10 amino acids within Major Histocompatibility (MHC) class I proteins while CD4 T cells recognize peptides of approximately 12-15 amino acids in the context of MHC class II molecules. There also exists a functional dichotomy in adaptive immune responsessuch that, CD8 T cells mediate pathogen clearance by active killing of infected host cells, while CD4 T cellssecrete cytokines aiding in the differentiation of B cells to plasma cells and maintaining memory CD8 responses. Indeed, the role of CD4 T cells in the induction and maintenance of adaptive immunity appears to be indirect and includes orchestration of B cell responses, macrophage activation, CD8 memory generation and downregulation of responses after pathogen clearance[1]. CD4 T cells are also further divided into subsets based on cytokines produced and protection against different types of pathogens. For example, Th1 cells secrete IFN-γ and are important against viral and intracellular bacterial infection while Th2 cells secrete IL-4 and IL-5 and provide protection against extracellular parasites [1]. Another subset, termed Th17, has also been described recently that produces IL-17, is important against fungal infections, regulates inflammation during infection and can promote autoimmunity [2]. Finally, T regulatory cells (Treg) are also part of the CD4 T cell lineage and these cells act to maintain peripheral tolerance and downregulateresponses after infection. The development of each subset is controlled by a unique transcription factor and once the developmental program is established, genes that promote the other subsets are silenced.
In addition to the helper functions traditionally assigned to the CD4 T cell subset, a more direct role for CD4 T cells in cell-mediated immunity has recently been appreciated. Although CD4 T cells with cytolytic potential have been described for decades, current data has emerged suggesting that class II restricted CD4 CTL contribute to protective responses against viral and bacterial infections as well as tumor responses. This review will examine the historical data describing CD4 CTL, the models used to study thegeneration and regulation of CD4 CTL, their in vivo relevance and the clinical importance of these cells as vaccine targets or therapeutics.
CD4 T cellswith cytotoxic potential
Class II restricted CD4 effectors with cytolytic potential have been described since the late 1970's[3]. Stimulation of these cells was accomplished with the mixed lymphocyte reaction, an alloreactive response that induces a strong signal in up to 5% of all T cells. Later reports demonstrated that CD4 CTL could be generated in T cell clones that were reactive to influenza[4], poliovirus[5], Epstein Barr virus[6], measles virus [7], and herpes simplex virus[8], suggesting that these cells develop against viral antigens. The appearance of cytolytic CD4 cells during primary infection has also been documented but these cells were identified in mice that lacked the normal complement of CD8 T cells [9; 10]. Taken together, many early reports labeled CD4 CTL as an in vitro artefact and there was speculation whether these cells had any in vivo relevance.
More recently, CD4 cells have been identified in peripheral blood of subjects exposed to CMV [11], EBV[12], and HIV [13] and in mice infected with murinegammaherpesvirus [14]. These circulating CD4 cells appear to be terminally differentiated and are hypothesized to be generated by chronic exposure to antigen[15]. Work with CD4 T cell clones would seem to support this since those cells have been repeatedly stimulated in vitro. However, CD4 CTL have been described in immunocompetent mice infected withLCMV Armstrong strain[16], and influenza (Brown and Swain, submitted), indicating that CD4 CTL can be generated in acute infections. In fact, our work also shows that cytolytic activity in CD4 cells can be generated after just three days in culture with a single primary stimulation [17]. These results indicate that cytolytic CD4 cells arise duringboth chronic and acute infection and may be important against pathogens that evade the classical class I processing pathway.
Two major mechanisms of cell killing have been described for cells of the immune system. One involves binding of a cell surface receptor known as Fas on T cells with Fasligand (FasL) on the target cell. The other mechanism used by CTL is granule exocytotosis whereby perforin and granzyme are secreted by T cells after recognition of peptide antigenin MHC[18]. Both the Fas:FasL and perforin/granzyme pathways culminate in activation of caspases and induction of apoptosis in target cells[19]. The Fas:FasL mediated cytotoxic pathway is believed to be the main pathway of lytic activity by CD4 T cells and is implicated in the downregulation of immune responses. Antigen presenting B cells express high levels of FasL on their surface and are especially sensitive to the Fas mediated pathway of cell death by cytolytic CD4 cells [20]. CD4 CTL have also been shown to lyse A20 mouse B cell lymphomas [21] and human B cell lymphomas transformed by EBV infection [6]. Other reports provide evidence that CD4 T cells can use perforin and granzyme B (grB) [22; 23; 24; 25], and human CD4 CTL can utilize granulysin[26; 27; 28]. Granulysin is not expressed in mice and appears to be important in mycobacterial[26; 27]and fungal infections [29] in humans. Perforin and grB mediated cytotoxicity by CD4 CTL has been shown to be involved in responses against HIV [13], CMV [11; 13], EBV [23], HSV [30], influenza [22], and against B cell lymphocytic leukemia [31]. Therefore, CD4 CTL can use a variety of mechanisms to induce killing of target cells and most of these pathways are not mutually exclusive. Our own data demonstrates that CD4 CTL primarily utilize perforin to lyse target cells, but when IL-2 is limiting, CD4 cells can also use Fas:FasL[17].
A more direct role for CD4 T cell in infectious disease and malignancy
Much of the early work using CD4 T cell clones generated against viral antigens used these cells to demonstrate the ability of CD4 cells to be protective against lethal challenge with virus. Transfer of CD4 CTL prior to inoculation with lethal dose of virus protected mice against influenza infection[4; 22] poliovirus [5] and West Nile virus [32]. In these reports, CD4 cells mediated protection directly, in the absence of CD8 or B cells, or before the primary response in normal mice could develop. Similarly, CD4 T cell clones from patients harboring EBV, can directly lyse EBV infected B cells, or transformed B cellsexpressing EBV proteins [12; 28; 33]. There are also reports of humancytolytic CD4 cells being generated in culture and adoptively transferred to patients with malignancy[34; 35]or in stem cell recipients infected with EBV or CMV[36]. These reports indicate that CD4 CTL have a direct role in protection against lethal virus infection and may be therapeutic against certain malignancies. One hurdle in understanding the nature of protection in these instances is the availability of class II expressing targets in many of these infections. Class II expressing B cells are latently infected with EBVandlysis of these cells can occur any time during viral protein expression or during transformation. In addition, LCMV can infect cells of the immune system, also providing class II expressing targets for CD4 CTL. However, many infections such as CMV and influenza target cells that normally do not express class II molecules, so the role of cytolytic CD4 cells in these infections may be more obscure. It has been demonstrated thatMycobacterium tuberculosis [37], andparainfluenza[38]infection, or treatment with IFN-γ[39]increase the level of class II expression on lung epithelial cells and possibly provide targets for cytolytic CD4 cells. Our results suggest that class II is upregulated on type II epithelial cells after influenza infection and transferred cytolytic CD4 cells are in close proximity to the epithelial layer (Brown and Swain, submitted). Furthermore, we have shown that transferred CD4 CTL can decrease viral titers in the lung four days post lethal infection, before CD8 cells migrate to the lung [22]. Therefore, many infections inducean inflammatory milieu, upregulate class II expression on epithelial andendothelial cells and provide targets for CD4 CTL lysis.
As mentioned previously, CD4 CTL may downregulate immune responses by lysing antigen presenting B cells via Fas:FasL interactions[20]. CD8 CTL also modulate immune responses by eliminating dendritic cells, thus limiting antigen presentation during priming [40; 41]. We do not believe that CD4 CTL modulate APC function in the lymph node during priming after influenza infection since CD4 cells isolated from the draining LN demonstrate low grB expression and lackcytolytic activity. Immunofluorescent studies in influenza infected lungs reveal CD4 CTL in proximity to class II expressing epithelial cells and clustered with class II expressing cells in the lung parenchyma that may be macrophages or dendritic cells (Brown and Swain unpublished observation). While we have yet to definitively identify the class II expressing cell in the lung parenchyma, we speculate thatCD4 CTL may act to downregulate APC populations in the lung as virus is being cleared.
Models of cytolytic CD4 generation and differentiation
While many reports correlate CD4 CTL generation with chronic infection and terminal differentiation state [15; 42], there is a paucity of literature describing the factors that promote and regulate cytolytic activity in developing CD4 effectors. We use a T cell receptor (TCR) transgenic (Tg) model in which all CD4 T cells recognize a peptide from hemagglutinin protein in influenza virus PR8. We have developed an in vivo model in which TCR Tg cells are adoptively transferred to normal, immunocompetent mice, followed by sublethal infection with influenza PR8[43; 44; 45]. Using this model we can investigate the CD4 T cell specific cytolytic response to acute infection with influenza (Brown and Swain, submitted). We have also use these antigen specific CD4 T cells in an in vitro system where naïve T cells are cultured with peptide pulsed antigen presenting cells and various cytokines to determine which factors are required for generation of the cytolytic phenotype [17].
In vivo models
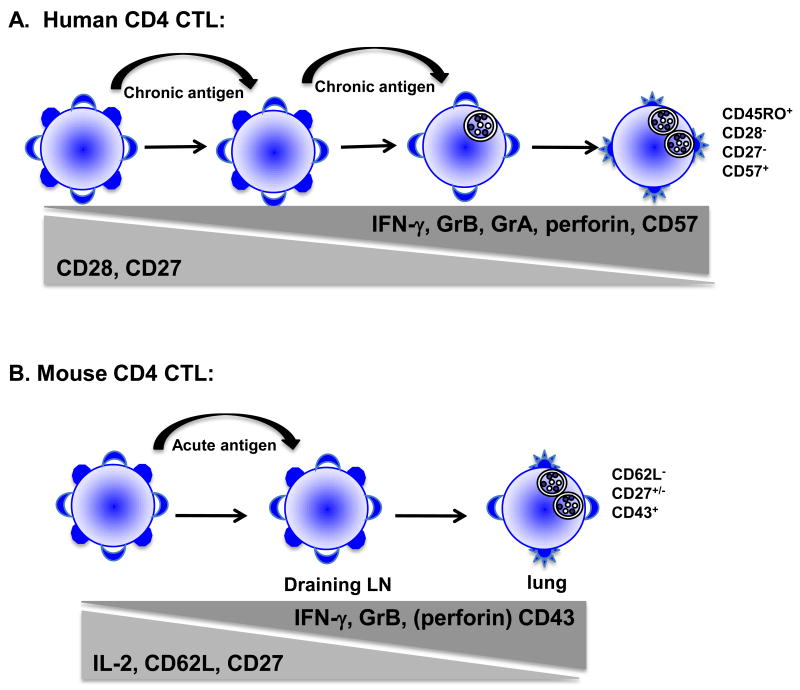
In humans, surface marker analysis of CD4 CTL reveals a circulating cell that is CD45RO+, CCR7-, CD27- and CD28-[13; 46]. Casazza, et al. also correlates CD57+ expression with high levels of granzyme A (grA), grB and perforin[11]. These cells can be isolated from peripheral blood and shown to be cytotoxic directly ex vivo, suggesting that these cells are antigen experienced, terminally differentiated effectors. Recently, in the mouse model of persistent gammaherpes infection, a population of CD27-CD4 cells in the spleen and lung was found to be cytolytic against gHV68 infected targets directly ex vivo and in vivo suggesting that highly differentiated CD4 cells can control this infection by direct cytotoxicity[14]. Our in vivo model confirms these findings as we have demonstrated that CD4 cells isolated from the lung during influenza infection are highly cytolytic against peptide pulsed targets ex vivo. This correlates with acquisition of IFN-γ and loss of IL-2 secretion in the lung as well as loss of CD62L, partial loss of CD27 and increased CD43 expression (Brown and Swain, submitted). Our studies are unique in that we have demonstrated acquisition of cytolytic activity in CD4 cells in vivo in response to an acute infection, rather than a chronic infection. In addition, CD4+grB+ cells are both CD27+ and CD27 in the lungsuggesting that chronic infectionsuch as gammaherpes virus may further differentiate CD4 CTL to CD27- cells while acute infection (influenza) induces a less differentiated cell that is CD27 (Figure 1). It remains to be determined whether both CD27+ and CD27- populations can lyse targets ex vivo, or whether grB expression precedes lytic ability and only the CD27- population is effective killers. Infection of mice with influenza also provides us with a model of in vivo CD4 CTL differentiation since influenza specific CD4 cells from the draining lymph node (dLN) do not lyse peptide pulsed targets while CD4 cells isolated from the lung exhibit high levels of lysis. This correlates with IL-2 secretion in the dLN, followed by switching to IFN-γ secretion in the lung. It remains to be determined whether CD4 CTL receive additional signals in the lung to differentiate to cytolytic cells, or whether they acquire this activity while in transit from the dLN to the site of infection. In addition, it is not known whether the same CD4 cell can act as a “helper” cell in the dLN, then acquire cytolytic and migratory capabilities to become CTL in the lung, or whether these two functionally different cells represent unique subsets. We are actively pursuing these separate hypotheses in our in vivo model of CD4 CTL differentiation.
Figure 1.
A) Model of differentiation of human CD4 CTL. In many chronic infections induce a population of CD4 T cells with cytolytic capacity. These cells are found in peripheral blood and are believed to represent a terminally differentiated effector cell that is CD28-, CD27+, GrB+, perforin+ and in some cases also expresses CD57. B) Differentiation of CD4 CTL in a mouse model of acute infection. In the draining lymph node, CD4 cells produce IL-2, are CD27+ and CD62L+/-. As cells differentiate and migrate to the lung, they lose surface CD62L, acquire CD43 and begin to lose CD27. The ability to secrete IFN-γ and lyse target cells is also acquired as cells migrate to the lung.
In vitro models
Using the in vitro model, we have confirmed what has previously been described for CD4 T cell clones, in that, Th1 polarization correlates with cytotoxicity and protection against influenza [4; 22; 47], poliovirus [5], EBV [6; 33] and West Nile virus [32]. In contrast, Th2 polarized effectors did not demonstrate efficient lysisand IL-4 was shown to inhibit the generation of CD4 CTL when increasing concentrations were added to culture conditions[17]. Furthermore, Th2 effectors were less able to protect mice against lethal influenza infection (Brown and Swain, unpublished observations).
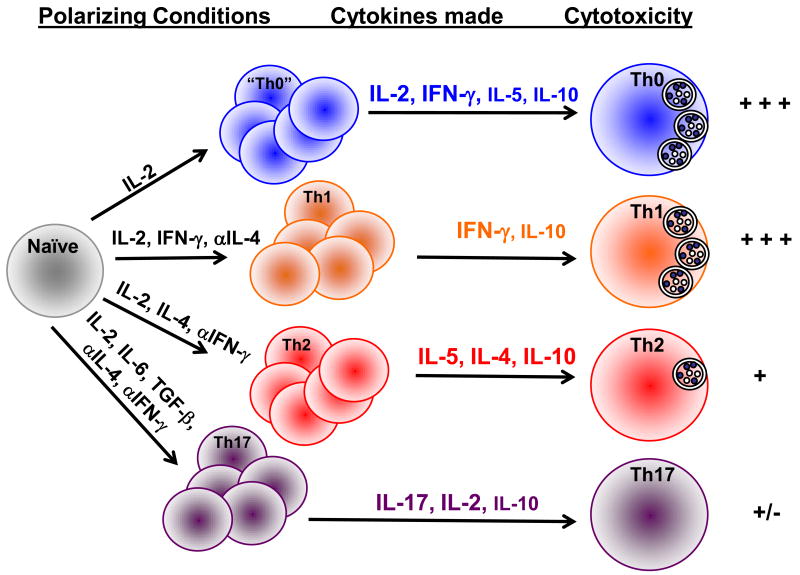
Our in vitro system provides an excellent model with which to define the parameters necessary to drive the differentiation of naïve CD4 T cells to the cytolytic phenotype. Not only do Th1 polarized CD4 effectors exhibit lytic activity, but naïve CD4 cells activated in the presence of antigen and IL-2 alone (Th0) acquire grB expression and perforin mediated cytotoxicity. When compared with Th1, Th2 and Th17 polarized effectors, cells activated with IL-2 only express both Th1 and Th2 type cytokines including IFN-γ and IL-4, and retain the ability to secrete IL-2 [17]. These data suggest that cytolytic cells can be generated without polarizing signals and may represent a less differentiated cell than data obtained with human subjects would indicate (Figure 2). Although Th0 cells can be generated in vitro, demonstrate the highest level of cytotoxicity and correlate with a less differentiated phenotype, CD4 CTL generated in vivo tend to be more differentiated due to Th1 polarizing cytokines. In addition, in vivo generated CD4 CTL are CD62L-, CD43+ and CD27+/-, while a proportion of in vitro generated CD4 effectors are CD62L+ and express moderate levels of CD43 and CD25 [17] and (Brown and Swain, submitted). Furthermore, we have identified a hierarchy in cytolytic activity amongst CD4 T cell subsets with Th0 ≥ Th1 > Th2 > Th17 ([17] and (Moore and Brown, in preparation)). What remains to be determined is whether there is plasticity in these CD4 cell subsets, or whether secondary stimulation with antigen and IL-2 in vitro can induce cytotoxicity in previously polarized subsets.
Figure 2.
Model of CD4 CTL differentiation in vitro. Naïve CD4 cells can be differentiated into various subsets based on the cytokines present early in the culture. Th0 effectors are those incubated in the presence of IL-2 and exhibit high levels of cytotoxicity. Th0 cells also secrete a combination of Th1 and Th2 type cytokines. Th1 effectors also demonstrate high levels of GrB expression and cytotoxicity while Th2 effectors are less able to lyse target cells and Th17 cells demonstrate little, if no cytolytic activity.
Using this in vitro model we have also determined that antigen presenting cells (APC) were absolutely required for the generation of cytolytic CD4 cells and that concentrations of anti-CD3 and anti-CD28 used to activate canonical helper CD4 T cells did NOT induce peptide specific killing[17]. This is in contrast to results obtained from groups using both polyclonal [48]and TCR Tg systems [49]in which CD8 cells activated with anti-CD3/anti-CD28 can lyse target cells effectively. Because our system requires peptide pulsed APC, we attempted to determine whether APC derived cytokines were playing a role in driving CD4 CTL generation. We ruled out IL-12, IL-10, TGF-β, TNF-α and IL-6 using either blocking antibodies or APC deficient in these cytokines in either enhancing or inhibiting CTL activity in CD4 T cells. Our recent work has demonstrated that bone marrow derived dendritic cells (BMDC) are more effective at inducing cytolytic activity when compared to LPS activated B cell blasts or A20 B lymphoma cells. The hierarchy of lytic ability in CD4 T cell subsets remains: Th0 ≥ Th1 > Th2 > Th17, however, Th17 cells show moderate cytotoxicity when generated with BMDC compared to no cytotoxicity when activated with B cell blasts (Moore and Brown, in preparation). Cytokine blocking experiments with BMDC as APC must be repeated in this system and we hypothesize that IL-1 may play a role due to its ability to enhance T cell activation [50; 51]and our observation that BMDC can secrete high amounts of this cytokine compared to B cell blasts.
It remains to be determined whether CD4 CTL represent a terminal differentiation state in Th1 development or whether CD4 CTL are a unique CD4 T cell subset with distinct cellular and molecular requirements for differentiation. How CD4 CTL relate to canonical helper CD4 cells or follicular helper cells are questions we can begin to answer using these in vitro and in vivo models of differentiation. It is clear that CD4 CTL are important in many viral infections whether providing an extra layer of protection in acute infection, or providing primary protection against viruses that evade the immune response by down-regulating class I presentation.
Molecular mechanisms of CD4 CTL differentiation
Many reports have documented the ability of CD4 T cells to lyse class II expressing targets using cell surface expression of FasL binding to Fas expressed on target cells [10; 20; 21]. Our lab and others have shown that CD4 T cells can use grB and perforin to lyse target cells directly ex vivo [22; 23; 24; 25]. CD4 TCR Tg cells that lack perforin, do not lyse peptide expressing targets after sublethalinfluenza infection indicating that the granule exocytosis pathway is the major cytolytic pathway used by CD4 T cells to lyse targets in vivo. This is similar to data identifying cytolytic CD4 cells in peripheral blood that expressgrB and perforinafter infection[11; 13; 42]. Surprisingly, our in vitro model demonstrates that CD4 cells can lyse targets by both FasL- and perforin-mediated mechanisms that aredependent upon peptide dose during effector generation. High levels of peptide, in the 5 μM range, induces 5-10 fold expansion in CD4 cells, high IFN-γ secretion and moderate cytolytic activity that is partially blocked by antibodies to FasL. In contrast, lower amounts of peptide (5-50 nM) induce much less expansion, more Th2 cytokines and high levels of cytolytic activity [17]. This would seem to contradict our earlier finding that Th1 cells have a higher cytolytic activity than Th2 cells, however, it appears that cytokines present early in the priming of naïve CD4 T cells is what dictates whether a naïve CD4 T cell will acquire cytolytic activity. For example, IL-4 present early in the culture will inhibit CD4 CTL generation while IL-12 seems to have no effect. The phenotype of the CD4 CTL, at least in vitro, appears to be a multifunctional cell that can secrete IFN-γ, IL-2, some IL-4 and IL-5 along with cytolytic activity (Figure 2). We have also demonstrated that addition of exogenous IL-2 is required to induce perforin mediated cytotoxicity, especially at low peptide doses, while FasL mediated cytotoxicity can be induced without IL-2 [17].
We are beginning to elucidate the molecular mechanisms involved in IL-2 induced, perforin mediated killing in CD4 T cells. When IL-2 binds to the IL-2 receptor complex, januskinase (JAK) 1 and 3 are activated which in turn phosphorylates the signal transducer and transactivator (STAT) 5. STAT5 can then bind to the perforin gene and upregulate expression [52]. Using CD8 and CD4 human T cell lines, it has been shown that perforin expression is differentially regulated in CD8 vs CD4 T cells. CD8 T cell clones showed increased binding of STAT5 and expressed high levels of perforin in both the resting and activated state while cytolytic CD4 clones demonstrated STAT5 binding and expressed high levels of perforin only during activation [30]. Using a combination of IL-2 deficient, or IL-2Rα (CD25) deficient TCR Tg T cells we can determine the role of IL-2 and STAT5 in promoting perforin mediated cytotoxicity in CD4 T cells. Our preliminary work suggests that IL-2 needs to be present early during T cell priming, and that low (10 ng/ml) amounts of IL-2 are sufficient to upregulategrB and cytotoxicity. The lack of IL-2Rα diminishes cytotoxicity, but grB can still be upregulated in these cells suggesting that signaling through the IL-2 β chain or common γ chain may be sufficient for upregulation of granzyme, but signaling through the high affinity IL-2 receptor complex is necessary for complete cytolytic activity in CD4 T cells. Furthermore, IL-2 induces STAT5 phosphorylation that correlates with cytotoxicity. IL-7, a cytokine that shares the STAT5 signaling pathway as well as the common γ chain receptor also induces cytolytic activity in CD4 T cells, albeit, at lower levels compared to IL-2 (Canfield and Brown, in preparation). Studies are underway to determine whether STAT5is absolutely required for the generation of cytotoxicity in CD4 T cells and the role of other common γ chain cytokines are also being investigated.
Transcription factors have an important role in the development of CD4 T cell subsets, where unique factors simultaneously drive the differentiation of one subset while inhibiting the development of the other subset. It has been shown that the transcription factors T-bet, GATA-3 and RORγt, drive the differentiation of Th1, Th2 and Th17 cells respectively, by inducing cytokine synthesis in a positive feedback mechanism (reviewed in [1]. We hypothesize that CD4 CTL differentiation may be controlled by T-bet since Th1 cells demonstrate effective killing, however, we have also demonstrated that IFN-γ is not necessary for driving peptide specific cytolytic responses in CD4 T cells [17]. We speculate that the Th0 subset, those cells that are the most effective killers, will express T-bet as well as other factors are also be important in the generation of CD8 CTL [53].
Other transcription factors may also play a role in the differentiation of cytolytic CD4 cells, such as T-bet and eomesodermin (Eomes). These factors have been implicated in the differentiation of CD8 CTL since mice deficient in these proteins demonstrate lower cytolytic activity and decreased clearance of LCMV infection [54]. One report demonstrates that Eomes is not expressed in anti-CD3/CD28 activated CD4 cells [55], however, we have shown that this activation pathway does not induce effective antigen specific CD4 cytolytic activity [17]. Our method of inducing CD4 CTL with peptide pulsed APC may in fact induce Eomes, and those experiments are currently in progress. T-bet and Eomes also increase expression of IL-2R β in CD8 CTL, thus increasing responsiveness to IL-2 and IL-15 [56]. In addition, it has been shown that the Runx family of transcription factors is expressed in mature CD8 T cells and Runx-3-/-cells demonstrate reduced cytotoxicity[57]. More recent data demonstrate that Runx3, T-bet and Eomes all cooperate to control the differentiation of cytolytic CD8 cells with T-bet involved in early regulation of IFN-γ, Eomes involved in late regulation of IFN-γ and perforin and Runx3 inducing expression of Eomes as well as perforin, grB and IFN-γ. We hypothesize that more than one transcription factor plays a role in the differentiation of CD4 CTL and studies are underway to determine the molecular profile that correlates with the Th0 subset and highly effective cytotoxicity.
Clinical Implications: vaccine design and immunotherapy
It is clear that CD4 T cells play pivotal roles in immune responses against infection and malignancy as well as pathological conditions such as asthma and autoimmunity. The effects of CD4 T cells during immune responses tend to be indirect, providing cytokine help for antibody production and maintenance of CD8 T cell responses. Cytolytic CD4 cells can exert direct functions against infections and malignancies by lysing class II expressing targets, therefore, vaccines strategies should aim to induce this subset of cells.
Current vaccine strategies for many infections such as influenza rely on generating neutralizing antibodies against the virus. However, outer viral proteins of influenza can mutate rapidly making the current vaccines ineffective and necessary to be reformulated every year. Incorporating both CD4 and CD8 T cell epitopes for many vaccines will help to induce cytolytic cell mediated immunity against many infectious diseases. For example, CD4 T cells are required for control of EBV infection and it has become apparent that much of this control is due to the cytolytic ability of CD4 T cells [14; 33]. Recent vaccine strategies for EBV incorporated CD8 epitopes from LMP2 and CD4 epitopes from EBNA1 and were shown to reactivate both CD8 and CD4 cells in peripheral blood [58]. These strategies can also be applied for the control of EBV transformed malignancies such as Burkitt's lymphoma and certain nasopharyngeal carcinomas that express EBV proteins [14; 33].
New adjuvants must also be developed to target viral and tumor antigens to dendritic cells (DC). Our work suggests that DC are important in inducing CD4 CTL, especially in vivo, and activation of DC with adjuvants that target toll like receptors (TLR) is a possible avenue of development[59]. Synthetic CpGoligonucleotide that targets TLR-9 has been documented to induce Th1 differentiation and provide protective responses against anthrax[60] and vaccinia[61]. Therefore, using adjuvants that enhance cellular immunity will allow the differentiation of cytolytic CD4 cells as well as cytokine secreting cells and increase the efficacy of protective responses against a number of infectious diseases. Indeed, one of the hallmarks of protective vaccination is the generation of a multifunctional CD4 cell that can secrete IL-2, TNF-α and IFN-γ[62]. Perhaps vaccination with Th1 inducing adjuvants can promote differentiation of a multifunctional cell with cytotoxic and cytokine secreting capabilities. Studies using TLR-3 and TLR-7 agonists as vaccines to induce CD4 CTL effectors and memory cells that can act in protection against lethal influenza are ongoing in our laboratory.
In conclusion, cytolytic CD4 T cells are important in many viral infections such as EBV, HIV and CMV and may be generated through repeated antigen stimulation in chronic infections. Mouse models of CD4 CTL generation indicate that these cells are induced by acute infection as well as chronic infections and can primarily utilize the granule exocytosis pathway as a mechanism of cell killing. There remains much to be learned about how these cells are generated, however, IL-2 and dendritic cells appear to be a major players in CD4 CTL differentiation and vaccines designed to activate these pathways should be utilized for infectious diseases and malignancy.
Acknowledgments
The author would like to thank Dr. Susan L. Swain and the Trudeau Institute for guidance, reagents and mice during the early part of this work. In addition, the author thanks Jenna Canfield, Tyler Moore, Alex Vogel and Erik Mellgren for critically reading this manuscript. This work was funded by PHS grants AI-46530, AI-0672, the Trudeau Institute and NCRR COBRE program grant P20 RR15635.
Abbreviations
- APC
antigen presenting cell
- BMDC
bone marrow derived dendritic cell
- CMV
cytomegalovirus
- EBV
Epstein Barr Virus
- grB
granzyme B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Billings P, Burakoff S, Dorf ME, Benacerraf B. Cytotoxic T lymphocytes specific for I region determinants do not require interactions with H-2K or D gene products. J Exp Med. 1977;145:1387–92. doi: 10.1084/jem.145.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infecton. J Exp Med. 1994;180:1273–82. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahon BP, Katrak K, Nomoto A, Macadam AJ, Minor PD, Mills KHG. Poliovirus-specific CD4+ Th1 clones with both cytotxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med. 1995;181:1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikiforow S, Bottomly K, Miller G, Munz C. Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J Virol. 2003;77:12088–104. doi: 10.1128/JVI.77.22.12088-12104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson S, Richert JR, Biddison WE, Satinsky A, Hartzman RJ, McFarland HF. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984;133:754–7. [PubMed] [Google Scholar]
- 8.Yasukawa M, Yakushijin Y, Fujita S. Two distinct mechanisms of cytotoxicity mediated by herpes simplex virus-specific CD4+ human cytotoxic T cell clones. Clin Immunol Immunopathol. 1996;78:70–6. doi: 10.1006/clin.1996.0010. [DOI] [PubMed] [Google Scholar]
- 9.Muller D, Koller BH, Whitton JL, LaPan KE, Brigman KK, Frelinger JA. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992;255:1576–8. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 10.Zajac AJ, Quinn DG, Cohen PL, Frelinger JA. Fas-dependent CD4+ cytotoxic T-cell-mediated pathogenesis during virus infection. Proc Natl Acad Sci U S A. 1996;93:14730–5. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–77. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigh TA, Lin X, Jia H, Hui EP, Chan AT, Rickinson AB, Taylor GS. EBV latent membrane proteins (LMPs) 1 and 2 as immunotherapeutic targets: LMP-specific CD4+ cytotoxic T cell recognition of EBV-transformed B cell lines. J Immunol. 2008;180:1643–54. doi: 10.4049/jimmunol.180.3.1643. [DOI] [PubMed] [Google Scholar]
- 13.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–8. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 14.Stuller KA, Flano E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J Virol. 2009;83:4700–3. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appay V. The physiological role of cytotoxic CD4(+) T-cells: the holy grail? Clin Exp Immunol. 2004;138:10–3. doi: 10.1111/j.1365-2249.2004.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–8. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 17.Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257:69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catalfamo M, Henkart PA. Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin Immunol. 2003;15:522–7. doi: 10.1016/s0952-7915(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 19.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 20.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–12. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 21.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–33. [PubMed] [Google Scholar]
- 22.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–98. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 23.Khanolkar A, Yagita H, Cannon MJ. Preferential utilization of the perforin/granzyme pathway for lysis of Epstein-Barr virus-transformed lymphoblastoid cells by virus-specific CD4+ T cells. Virology. 2001;287:79–88. doi: 10.1006/viro.2001.1020. [DOI] [PubMed] [Google Scholar]
- 24.Williams NS, Engelhard VH. Identification of a population of CD4+ CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–9. [PubMed] [Google Scholar]
- 25.Yanai F, Ishii E, Kojima K, Hasegawa A, Azuma T, Hirose S, Suga N, Mitsudome A, Zaitsu M, Ishida Y, Shirakata Y, Sayama K, Hashimoto K, Yasukawa M. Essential roles of perforin in antigen-specific cytotoxicity mediated by human CD4+ T lymphocytes: analysis using the combination of hereditary perforin-deficient effector cells and Fas-deficient target cells. J Immunol. 2003;170:2205–13. doi: 10.4049/jimmunol.170.4.2205. [DOI] [PubMed] [Google Scholar]
- 26.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167:2734–42. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 27.Klucar P, Barnes PF, Kong Y, Samten B, Tvinnereim A, Spallek R, Nepom GT, Singh M, Shams H. Characterization of effector functions of human peptide-specific CD4+ T-cell clones for an intracellular pathogen. Hum Immunol. 2008;69:475–83. doi: 10.1016/j.humimm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 28.MacArthur GJ, Wilson AD, Birchall MA, Morgan AJ. Primary CD4+ T-cell responses provide both helper and cytotoxic functions during Epstein-Barr virus infection and transformation of fetal cord blood B cells. J Virol. 2007;81:4766–75. doi: 10.1128/JVI.02608-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng CF, Ma LL, Jones GJ, Gill MJ, Krensky AM, Kubes P, Mody CH. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109:2049–57. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 30.Niiya H, Sakai I, Lei J, Azuma T, Uchida N, Yakushijin Y, Hato T, Fujita S, Yasukawa M. Differential regulation of perforin expression in human CD4+ and CD8+ cytotoxic T lymphocytes. Exp Hematol. 2005;33:811–8. doi: 10.1016/j.exphem.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Porakishvili N, Kardava L, Jewell AP, Yong K, Glennie MJ, Akbar A, Lydyard PM. Cytotoxic CD4+ T cells in patients with B cell chronic lymphocytic leukemia kill via a perforin-mediated pathway. Haematologica. 2004;89:435–43. [PubMed] [Google Scholar]
- 32.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181:8568–75. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adhikary D, Behrends U, Moosmann A, Witter K, Bornkamm GW, Mautner J. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J Exp Med. 2006;203:995–1006. doi: 10.1084/jem.20051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landmeier S, Altvater B, Pscherer S, Eing BR, Kuehn J, Rooney CM, Juergens H, Rossig C. Gene-engineered varicella-zoster virus reactive CD4+ cytotoxic T cells exert tumor-specific effector function. Cancer Res. 2007;67:8335–43. doi: 10.1158/0008-5472.CAN-06-4426. [DOI] [PubMed] [Google Scholar]
- 35.Ohminami H, Yasukawa M, Kaneko S, Yakushijin Y, Abe Y, Kasahara Y, Ishida Y, Fujita S. Fas-independent and nonapoptotic cytotoxicity mediated by a human CD4(+) T-cell clone directed against an acute myelogenous leukemia-associated DEK-CAN fusion peptide. Blood. 1999;93:925–35. [PubMed] [Google Scholar]
- 36.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, Decker W, Molldrem JJ, Liu H, Gee AP, Rooney CM, Heslop HE, Dotti G, Brenner MK, Shpall EJ, Bollard CM. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debbabi H, Ghosh S, Kamath AB, Alt J, Demello DE, Dunsmore S, Behar SM. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L274–9. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- 38.Gao J, De BP, Banerjee AK. Human parainfluenza virus type 3 up-regulates major histocompatibility complex class I and II expression on respiratory epithelial cells: involvement of a STAT1- and CIITA-independent pathway. J Virol. 1999;73:1411–8. doi: 10.1128/jvi.73.2.1411-1418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim L, Dominguez M, Yacoub M. Primary human adult lung epithelial cells in vitro: response to interferon-gamma and cytomegalovirus. Immunology. 1993;79:119–24. [PMC free article] [PubMed] [Google Scholar]
- 40.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–52. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Berg PJ, van Leeuwen EM, ten Berge IJ, van Lier R. Cytotoxic human CD4(+) T cells. Curr Opin Immunol. 2008;20:339–43. doi: 10.1016/j.coi.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–70. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 45.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Leeuwen EM, Remmerswaal EB, Vossen MT, Rowshani AT, Wertheim-van Dillen PM, van Lier RA, ten Berge IJ. Emergence of a CD4+CD28- granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol. 2004;173:1834–41. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 47.Lukacher AE, Morrison LA, Braciale VL, Malissen B, Braciale TJ. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985;162:171–87. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 49.Kemp RA, Backstrom BT, Ronchese F. The phenotype of type 1 and type 2 CD8+ T cells activated in vitro is affected by culture conditions and correlates with effector activity. Immunology. 2005;115:315–24. doi: 10.1111/j.1365-2567.2005.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–62. [PubMed] [Google Scholar]
- 51.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–8. [PubMed] [Google Scholar]
- 52.Zhang J, Scordi I, Smyth MJ, Lichtenheld MG. Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. J Exp Med. 1999;190:1297–308. doi: 10.1084/jem.190.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–11. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 54.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–11. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 56.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 57.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–9. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heller KN, Gurer C, Munz C. Virus-specific CD4+ T cells: ready for direct attack. J Exp Med. 2006;203:805–8. doi: 10.1084/jem.20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunological Reviews. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 60.Klinman DM, Xie H, Little SF, Currie D, Ivens BE. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macques. Vaccine. 2004;22:2881–2886. doi: 10.1016/j.vaccine.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Klinman D, Berzofsky JA. Enhancement of CD8+ T cell immunity in the lung by CpG oligodeoxynucleotides increases protective efficacy of a modified vaccinia Ankara vaccine against lethal poxvirus infection even in a CD4-deficient host. J Immunol. 2006;177:6336–43. doi: 10.4049/jimmunol.177.9.6336. [DOI] [PubMed] [Google Scholar]
- 62.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]