Abstract
Calcium-regulated exocytosis is a ubiquitous process in eukaryotes, whereby secretory vesicles fuse with the plasma membrane and release their contents in response to an intracellular calcium surge1. This process regulates diverse cellular functions like plasma membrane repair in plants and animals2,3, discharge of defensive spikes in Paramecium4, and secretion of insulin from pancreatic cells, immune modulators from lymphocytes, and chemical transmitters from neurons5. In animal cells, serine/threonine kinases including PKA, PKC and CaM-kinases have been implicated in calcium-signal transduction leading to regulated secretion1,6,7. Although plants and protozoa also regulate secretion via intracellular calcium, the means by which these signals are relayed have not been elucidated. Here we demonstrate that the Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) is an essential regulator of calcium-dependent exocytosis in this opportunistic human pathogen. Conditional suppression of TgCDPK1 revealed that it controls calcium-dependent secretion of specialized organelles called micronemes, resulting in a block of essential phenotypes including parasite motility, host-cell invasion, and egress. This phenotype was recapitulated using a chemical biology approach, wherein pyrazolopyrimidine-derived compounds specifically inhibited TgCDPK1 and disrupted the parasite life cycle at stages dependent on microneme secretion. Inhibition was specific to TgCDPK1, since expression of a resistant kinase mutant reversed sensitivity to the inhibitor. TgCDPK1 is conserved among apicomplexans and belongs to a family of kinases shared with plants and ciliates8, suggesting that related CDPKs may play a role in calcium-regulated secretion in other organisms. Since this kinase family is absent from mammalian hosts, it represents a validated target that may be exploitable for chemotherapy against T. gondii and related apicomplexans.
The apicomplexan parasite T. gondii has been used as a model for the secretion of numerous proteins from specialized organelles, called micronemes, in response to increased intracellular calcium9. Microneme secretion can be blocked by broad-spectrum serine/threonine kinase inhibitors, and this is not circumvented by addition of calcium ionophores, suggesting kinases mediate the transduction of the calcium signal10. Calcium-dependent protein kinases (CDPKs), have been identified in plants, ciliates, and apicomplexans, but are absent in fungi and animals11. CDPKs can respond to calcium when their calmodulin-like domain binds calcium and releases the kinase domain from an inactive conformation11. Recent structural studies illustrate a novel mechanism of CDPK activation that results from a large-scale intramolecular rearrangement12. Apicomplexans contain a diverse family of CDPKs, some of which have canonical domain structures, while others are more diverse8. In Plasmodium, the causative agent of malaria, gene knockouts of several individual CDPKs have revealed important roles at specific developmental stages8. For example, disruption of CDPK4 in Plasmodium berghei asexual stages leads to differentiation defects in male gametocytes13; a block which currently precludes analysis of its role in other motile stages such as sporozoites. The orthologue of this kinase is called TgCDPK1 in T. gondii and a previous study suggested the ability of KT5926, a pan-specific S/T kinase inhibitor related to staurosporine, to block cell attachment may result from inhibition of this target14. Although KT5926 inhibits TgCDPK1 activity in vitro14, it is unlikely to provide specific inhibition in the parasite, which harbors 11 distinct CDPKs8 that control largely unexplored cellular pathways.
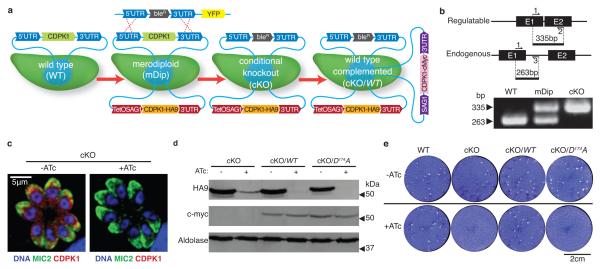
To precisely define the role of TgCDPK1 in the parasite life-cycle, we generated a conditional knockout (cKO) using the tetracycline trans-activator system, previously developed for the study of essential genes in Toxoplasma15. We first engineered a strain expressing a HA9-tagged allele of TgCDPK1 driven by a modified TetOSAG1 promoter, permitting repression of the transgene during growth in anhydrotetracycline (ATc; Fig. 1a). The endogenous TgCDPK1 gene was then replaced by double homologous recombination, generating the cKO as confirmed by PCR analysis (Fig 1a,b). Different alleles, expressed under the SAG1 constitutive promoter, were subsequently introduced into the cKO to test for complementation. Growth of the cKO in ATc resulted in nearly undetectable levels of the HA9-tagged regulatable protein, while the c-Myc-tagged constitutive proteins were stably expressed in the complemented strains (Fig. 1, c and d). As a first assessment of the essentiality of TgCDPK1, we tested the ability of parasites to form plaques on host cell monolayers. Both WT and cKO lines grew normally in the absence of ATc, while the presence of ATc led to a complete block in plaque formation in only the cKO (Fig. 1e). The phenotype was fully rescued when complemented with the wild type allele (cKO/WT; Fig. 1e) but not by a mutant allele where the catalytic aspartate was mutated to an alanine (cKO/D174A; Fig. 1e), indicating that TgCDPK1 function requires an active kinase.
Figure 1. TgCDPK1 is essential for the lytic cycle.
a, Regulatable, HA9-tagged TgCDPK1 was added to wild-type (WT) to create a merodiploid (mDip). Endogenous TgCDPK1 was replaced with phleomycin resistance (bleR) to generate the cKO. Complementation with c-Myc tagged mutant alleles (denoted by cKO/“allele”). b, Multiplexed PCR analysis of TgCDPK1. c, Immunofluorescence analysis of the cKO +/−ATc; endogenous MIC2 (green), HA9-tag (red) and DNA (blue). d, Immunoblot of HA9-tagged regulatable and c-Myc-tagged constitutive TgCDPK1 in cKO and complemented strains +/− Atc. Aldolase, loading control. e, Plaque formation on fibroblast monolayers, +/− ATc for 7 days.
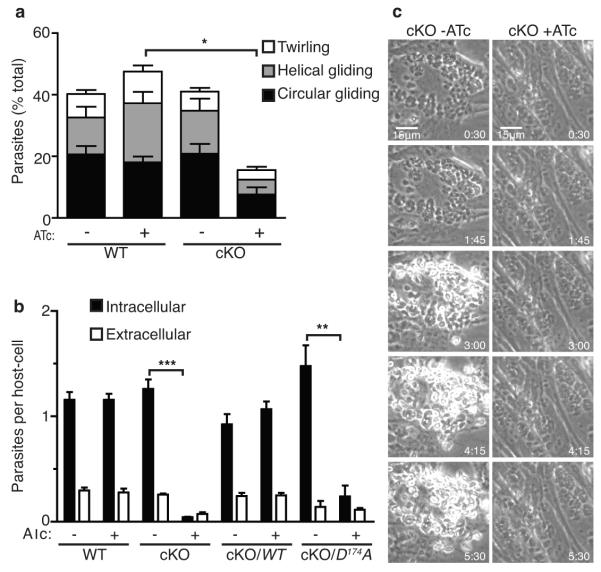
Motility in apicomplexan parasites depends on a unique system whereby adhesins contained in the micronemes are released onto the apical end of the parasite and translocated to the posterior of the cell, thus propelling the parasite forward16. Down regulation of TgCDPK1 by addition of ATc during intracellular growth did not affect parasite replication, yet parasites harvested from such cultures were significantly impaired in all forms of gliding motility (Fig. 2a). Those few cKO parasites that where able to glide, presumably due to leaky suppression, exhibited wild type speeds of motility, indicating that the motor complex itself was unaffected (Supplementary Fig.1). Gliding motility is normally prerequisite for cell invasion16 and consistent with this, the cKO experienced greater than 90% reduction in invasion when grown in the presence of ATc (Fig. 2b). Just as with plaque formation, invasion could be rescued by expression of the constitutive WT allele, but not the kinase-dead allele (Fig. 2b). Interestingly, suppression of TgCDPK1 also resulted in a strong reduction in host cell attachment (Fig. 2b), suggesting it affects an early step in invasion.
Figure 2. TgCDPK1 is required for phenotypes associated with microneme secretion.
a, Types of gliding motility as quantified by video microscopy. Student’s t test; *P < 0.05, mean ± s.e.m., N = 4 experiments. b, Invasion of fibroblasts by WT, cKO and complemented strains. Extracellular and intracellular parasites were differentially stained and enumerated as described in supplementary materials. Student’s t test; ***P<0.0005, **P<0.005, mean ± s.e.m., N = 3 experiments. c, Ionophore-induced egress of the cKO +/− ATc. Time stamp (min:sec) after calcium ionophore addition. See supplementary online videos.
Egress from host cells depends on many of the same cellular pathways required for invasion, and this pathway may naturally be triggered by accumulation of the plant-like hormone abscisic acid17. The cKO parasites grown in the absence of ATc, behaved like WT and rapidly egressed from host cells in response to calcium ionophore treatment, an artificial but potent trigger of egress18 (Fig. 2c, Supplementary Movie 1, Supplementary Table 1). In contrast, virtually all cKO parasites grown in the presence of ATc did not respond to ionophore, remaining immotile within the vacuole (Fig. 2c, Supplementary Movie 2, Supplementary Table 1). Together these experiments indicate that TgCDPK1 is essential for the transduction of the calcium signals regulating gliding motility, invasion, and egress.
All of the above TgCDPK1-dependent phenotypes share a requirement for adhesins stored in micronemes, which undergo calcium-regulated exocytosis, in contrast to other secretory compartments such as dense granules, which are constitutively released9. Interestingly, TgCDPK1 shares a similar expression pattern to known microneme proteins, as detected by microarray analysis of synchronized parasites (95% C.I.; M. Behnke and M. White unpublished data). Together, these data suggested that TgCDPK1 might regulate microneme secretion, releasing, among other proteins, the well-studied adhesin MIC29. Following secretion onto the cell surface, MIC2 is translocated to the cell posterior and shed from the parasite surface by proteolysis, allowing for the detection of secreted MIC2 in the supernatant19. As expected, MIC2 was detected in the supernatant of WT parasites stimulated with ethanol, which is another potent secretagogue that is thought to act through phospholipase C20 (Fig. 3a). In contrast, the amount of MIC2 secreted by the cKO parasites grown in the presence of ATc was nearly undetectable, demonstrating a severe defect in calcium-regulated exocytosis (Fig. 3a). Secretion of MIC2 was restored to wild type levels by the constitutive WT allele, but not by the kinase dead allele (Fig. 3a). Growth of the cKO in ATc did not affect dense granule release (Fig. 3a), demonstrating that TgCDPK1 specifically regulates calcium-dependent exocytosis from micronemes, and not other secretory pathways.
Figure 3. Calcium-dependent microneme secretion requires TgCDPK1.
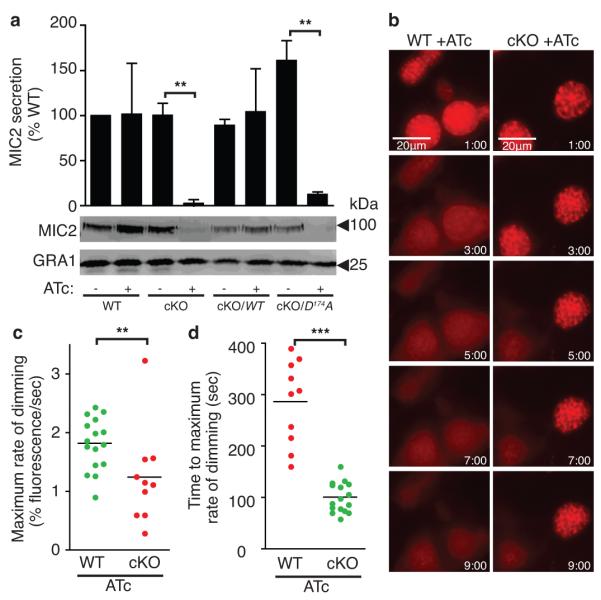
a, Western blot analysis of microneme protein MIC2 secretion following induction by ethanol for 15 min. GRA1 shows constitutive secretion of dense granules. Student’s t test; **P < 0.005, mean ± s.e.m., N = 3 experiments. b, Ionophore-induced permeabilization detected by vacuolar DsRed leakage monitored by fluorescence video-microscopy of strains 90h + ATc . Time stamp (min:sec) after calcium ionophore addition. CytochalasinD added to prevent eggress. c-d, Quantification of maximal rate and timing of florescence loss from rupturing vacuoles. Mann-Whitney test; ***P < 0.0005, **P < 0.005, mean, N = 3 experiments.
Microneme secretion also plays an important role in parasite egress releasing the perforin-like protein TgPLP1 that aids in permeabilization of the parasitophorous vacuole membrane (PVM)21. To assess the role of TgCDPK1 in controlling microneme secretion during egress, we generated WT and cKO lines expressing a constitutively secreted form of DsRed, allowing for the visualization of PVM integrity by live video microscopy. To avoid premature rupture of the vacuole, parasites were treated with cytochalasin D to immobilize them and allow selective monitoring of the kinetics of PVM rupture by leakage of DsRed. As previously reported21, WT parasites rapidly permeabilized the PVM upon calcium-ionophore treatment, releasing DsRed into the host-cell cytoplasm (Fig. 3b, Supplementary Movie 3). All WT vacuoles showed rapid PVM permeabilization (average 1.7 ± 0.5 min, s.d.), whereas approximately 30% of cKO parasites grown in the presence of ATc failed to rupture the PVM (Fig. 3c, Supplementary Movie 4; data not shown). Analysis of those cKO parasite vacuoles that did rupture, showed a significant delay in the timing and rate of DsRed release when compared to WT vacuoles (Fig. 3c, d). Collectively, these results demonstrate a requirement for TgCDPK1 in controlling the release of microneme contents including TgPLP1 during egress. Moreover, the inability of calcium-ionophore to circumvent the requirement for TgCDPK1, places this kinase as the critical transducer downstream of the calcium signal regulating microneme exocytosis.
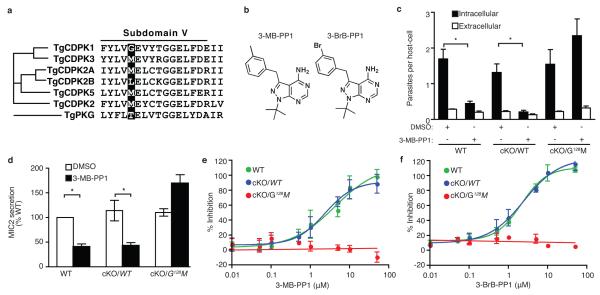
Having established the crucial role of TgCDPK1, we took advantage of the atypical nucleotide-binding pocket of TgCDPK112 to develop a chemical biology approach to further evaluate the essential nature of this kinase. It has been previously reported that the amino acid residue at the “gatekeeper” position within the nucleotide binding pocket radically affects inhibition by pyrazolopyrimidine (PP1) derivatives, which have limited activity against most S/T protein kinases22. Insensitivity is conferred by bulky gatekeeper residues in nearly all kinases of both animal and parasite cells; however, kinases can be rendered fully sensitive by mutation to a small gatekeeper22 (Supplementary Table 2). Fortuitously, TgCDPK1 displays a glycine at this position, which is unique among canonical CDPKs (Fig. 4a) and all other protein kinases in T. gondii (L. Peixoto and D. Roos, unpublished data). This finding predicted that wild type TgCDPK1 would be naturally sensitive to PP1-based inhibitors and, consistent with this a pilot screen of selected derivatives inhibited parasite lytic growth in vitro (Supplementary Fig. 2). Furthermore, purified TgCDPK1 enzyme was extremely sensitive to the compound 3-MP-PP1 (Fig. 4b), while mutation of the glycine gatekeeper to methionine shifted the sensitivity by more than 4 logs (Supplementary Table 2). Complementation of the TgCDPK1 cKO with either wild type or gatekeeper-mutant kinase alleles (cKO/WT and cKO/G128M, respectively) restored plaque formation, demonstrating that the mutation had no major deleterious effect (Supplementary Fig. 3). When treated with PP1 analogs, both WT and cKO/WT parasites were strongly inhibited in host cell attachment and invasion (Fig. 4c), consistent with a recent report that appeared online during the revision of the present work 23. In contrast to this recent report, which failed to provide quantitative analysis of secretion 23, we observed that microneme secretion by extracellular parasites and ionophore-induced egress (Supplementary Fig. 4) were also strongly inhibited by PP1 analogs (Fig. 4d). The reversal of these phenotypes in the G128M mutant confirms that the primary in vivo target of PP1 derivatives is TgCDPK1. Consistent with these effects, 3-MB-PP1 and the related compound 3-BrB-PP1, blocked the ability of the parasite to lyse host cell monolayers (Fig. 4e,f), demonstrating the essential role of TgCDPK1 during in vitro infection. Chemical genetic studies indicate that TgCDPK1 acts independently of the previously characterized cGMP-dependent kinase (PKG), the primary target of trisubstituted pyrole and imidazopyridine kinase inhibitors that also block microneme secretion in T. gondii24,25. Correspondingly, PKG is predicted to be insensitive to PP1 derivatives (Fig. 4a)22. Collectively these findings indicate that both kinases are essential for efficient microneme secretion, possibly reflecting a hierarchical control of this important cellular pathway.
Figure 4. PP1 derivatives specifically inhibit TgCDPK1 and block micronem-mediated functions.
a, Alignment of the kinase sub-domain V highlighting the gatekeeper residue. b, Structures of 3-MB-PP1 and 3-BrB-PP1. c, Effect of 5μM 3-MB-PP1 on host cell invasion. Student’s t test; *P < 0.05, mean ± s.e.m., N = 3 experiments. d, Effect of 5μM 3-MB-PP1 on MIC2 secretion. Student’s t test; *P < 0.05, mean ± s.e.m., N = 3 experiments. e-f, Effect of PP1 derivatives on host lysis by T. gondii +/− 3-MB-PP1 (e) and 3-Br- PP1 (f). Mean ± s.e.m., N = 3 experiments.
Our findings demonstrate that TgCDPK1 acts downstream of the second messenger calcium to regulate exocytosis in T. gondii, thus controlling several essential biological steps in the life cycle. Nearly all mammalian protein kinases normally show very low sensitivity to PP1 derivatives22, making them potential lead compounds for development of specific anti-parasitic drugs. In addition, CDPKs may regulate calcium-dependent exocytosis in related parasites or other organisms like ciliates and plants, representing an evolutionary precedent to calmodulin-dependent kinases that regulate exocytosis in animals.
METHODS SUMMARY
Growth of host cells and parasite strains
T. gondii tachyzoites were maintained by growth in human foreskin fibroblasts, as previously described26. Complemented strains were grown in 3μM pyrimethamine (Sigma) and ATc (Clontech) was added (1.5μg/ml) for 72h unless otherwise indicated. For inhibitor studies, parasites were incubated in the indicated concentration of 3-MB-PP1, 3-BrB-PP1, or DMSO control, for 20 min at RT, prior to use in assays.
Cellular assays
Plaque formation and invasion assays were performed as previously described27,28. Microneme secretion was assayed by monitoring the release of MIC2 into the culture medium following stimulation with 3%FBS and 2% ethanol, 15 min at 37°C, as previously described20. Samples were resolved by SDS-PAGE, blotted and probed with mouse-α-MIC2 (mAb 6D10), and mouse-α-GRA1 (mAb Tg17-43) and quantified by phosphoimager analysis. Egress and PVM permeabilization were monitored by video microscopy following stimulation with 8 μM calcium-ionophore A23187 (Calbiochem). When noted, parasites were pre-treated for 10 min with 2μM Cytochalasin D (Calbiochem) to block motility. The extent and rate of egress, as well as degree of vacuole permeability was quantified using Openlab v. 4.1 (Improvision) as described in the supplementary materials. Host cell lysis was assayed by staining monolayers with crystal violet, 3 days after infection at an MOI of 1.
METHODS
Growth of host cells and parasite strains
T. gondii tachyzoites were maintained by growth in monolayers of human foreskin fibroblasts (HFFs) cultured in Dulbecco’s modified Eagle’s medium containing 10% tetracycline-free fetal bovine serum (HyClone) , 2mM glutamine, 10mM HEPES (pH 7.5), and 20μg/ml gentamicin. Chloramphenicol (20μg/ml; Sigma), phleomycin (5 μg/ml; InvivoGen), ATc (1.5 μg/ml; Clontech), and pyrimethamine (3 μM; Sigma) were added to the media as indicated, and for the maintenance of merodiploid or complemented strains. When noted, parasites were treated with ATc for 72h.
Strain construction
The TgCDPK1 cKO was constructed using tetracycline transactivator system15, as previously described for TgALD126. Briefly, TgCDPK1 (Genbank accession number AF333958) was cloned with a C-terminal HA9-tag into the pTetO7SAG1 vector (obtained from Dominique Soldati), downstream of the inducible promoter, and the CAT selectable marker driven by the SAG1 promoter was introduced at a different site. The TATi-1 strain (obtained from Dominique Soldati), used as the wild type (WT) background in this study, was transfected with the regulatable construct, and stable merodiploids were selected with chloramphenicol29, and cloned by limiting dilution. To generate the knockout construct the Ble selectable marker30 was flanked with 1.5kb upstream of the TgCDPK1 start codon and 1.5kb downstream of the stop codon, followed by a YFP expression cassette26. The knockout construct was linearized and transfected into the merodiploid strain and stable pools were selected through two rounds of phleomycin selection30. Sorting for YFP-negative parasites was used to enrich for successful knockouts and individual clones were isolated by limiting dilution. Knockout of the endogenous TgCDPK1 gene was confirmed in clones by polymerase chain reaction, using primers against consecutive exons and the intervening intron, to distinguish between the endogenous and regulatable genes. Complementing plasmids were constructed by cloning TgCDPK1 with a carboxyl-terminal c-Myc-tag, under the regulation of the SAG1 promoter. The DHFR selectable marker conferring pyramethamine resistance31 was cloned into the complementing vectors. For the inhibitor studies, SAG1 was replaced with the 1.5kb region preceding the TgCDPK1 start codon. Co-transfection with pDHFR-TS31 was used to generate stable clones. Mutations were generated by site directed mutagenesis. Complementing plasmids were transfected into the cKO, stable lines were selected with pyramethamine, and clones were isolated by limiting dilution. To monitor PVM permeabilization, WT and cKO strains were transfected with p30-DsRed21 (obtained from Florence Dzierszinski) and pDHFR-TS for isolation of stable transgenic lines as described above.
Plaque assay
Plaque assays were performed as previously described27. Confluent monolayers of human foreskin fibroblasts in 6-well plates were infected with 200 parasites per well in media with or without 1.5μg/ml ATc (Clontech). 24 h post infection additional media was added reduce the concentration of ATc to 1μg/ml. Monolayers were fixed 7 days post infection and stained with crystal violet. Experiments were repeated three times with triplicate wells / experiment.
Invasion assay
Parasites were harvested in invasion media (Dulbecco’s modified Eagle’s medium containing 20 mM HEPES, pH 7.5, supplemented with 3% FBS). 5 × 106 parasites in a 250μl volume were added to sub-confluent HFF monolayers in 24-well plates and allowed to invade for 20 min. Monolayers were then fixed and stained as previously described28 to distinguish extracellular from total parasites. Three experimental replicates were performed for each strain in each of three separate experiments and parasite numbers per field were normalized to host-cell nuclei. For the inhibitor studies, parasites were incubated in 5μM 3-MB-PP1 or vehicle-only control (DMSO), for 20 min at room temperature, prior to invasion.
Host-lysis assay
Parasites were harvested and incubated in the indicated inhibitor or DMSO concentrations for 20 min at room temperature, prior to incubation with confluent monolayers in 96-well plates at an MOI of 1. For experiments comparing complemented strains, parasites were grown in 1.5μg/ml ATc for 72 h prior to harvesting. Parasites were allowed to invade for 1 hour, monolayers were washed 3-5 times and fresh media containing 1μg/ml ATc was added. The infection was allowed to progress 3 days before fixing with 70% ethanol and staining with crystal violet. Host-cell lysis was determined by measuring absorbance at 570nm in an EL800 microplate reader (BioTek Instruments, Inc.).
Immunofluorescence microscopy
Intracellular parasites were stained as previously described26. MIC2 staining within the micronemes required a 2 min permeabilization with cold 100% ethanol on ice. Staining was performed with rabbit-α-HA9 (Invitrogen) and mouse-α-MIC2 (mAb 6D10), followed by Alexa564-goat-α-rabbit IgG (Invitrogen), Cy5-goat-α-mouse IgG (Jackson) and Sytox green (Invitrogen) for the nuclear stain. Images were collected on a Zeiss LSM 510 confocal microscope.
Video microscopy and quantitation of gliding motility
Gliding and egress were analyzed by video microscopy as previously described32. For gliding, 75 images were taken with exposure times ranging from 50-100 milliseconds with 1 second between exposures. Images were collected and combined into composites using Openlab v. 4.1 (Improvision). ImageJ was used to analyze the images. The ParticleTracker plug-in was used to track cell motility and Cell Counter was used to quantify percent motility.
Ionophore-induced egress and PVM permeabilization
Egress and PVM permeabilization were monitored by video microscopy as described above. Where noted, parasites were pre-incubated for 10 min in media containing 2μM Cytochalasin D (Calbiochem) at 37°C. All dishes were allowed to equilibrate for 5 min on the heated stage, prior to the addition of 8μM calcium ionophore A23187 (Calbiochem). Vacuoles were imaged for up to 10 min after the addition of ionophore. To quantify vacuole permeabilization the fluorescence intensity within a 80μm2 region of each vacuole was measured using Openlab. The values for each vacuole were normalized against the starting (100%) and ending (0%) values for that particular vacuole, and the derivative of the curve was used to find the maximal rate of fluorescence loss and the time when that rate occurred. For the inhibitor studies, parasites were pre-treated with 5μM 3-MB-PP1 or vehicle-only control (DMSO) for 20 min at 37°C, prior to the addition of ionophore.
MIC2 secretion assay
Microneme secretion was assayed as previously described20 by monitoring the release of MIC2 into the culture medium. Secretion was stimulated by treatment with 3%FBS and 2% ethanol, 15 min at 37°C. Parasite lysis was monitored by the release of actin into the medium and remained undetectable in all experiments presented. GRA1 secretion was used as a control for constitutive secretion. Samples were resolved by SDS-PAGE, blotted and probed with mouse-α-MIC2 (mAb 6D10), rabbit-α-TgACT1, and mouse-α-GRA1 (mAb Tg17-43, kindly provided by Marie France Cesbron, Genoble, France). Quantitation was performed by densitometry using a FLA-5000 phosphoimager (Fuji Medical Systems). For the inhibitor studies, parasites were pre-treated with 5μM 3-MB-PP1 or vehicle-only control (DMSO) for 20 min at 37°C, prior to stimulation.
In vitro IC50 determination
Full-length His-tagged TgCDPK1 was cloned into the pET-22b(+) vector (Novagen) and expressed in Escherichia coli BL21. Point mutations were generated using QuickChange site-directed mutagenesis (Stratagene). Proteins were induced with IPTG and purified using nickel-affinity chromatography. Activities of WT and G128M TgCDPK1 were determined using the CycLex CaM Kinase II Assay Kit (MBL International Corporation) according to manufacturer’s instructions. c-Src proteins were expressed and assayed in the presence of various concentrations of the inhibitors as previously described33. IC50 was determined by fitting dose response curve with the GraphPad Prism software.
Statistics
Experiments were repeated three or more times and statistical analyses conducted in Excel using the Students’s test (unpaired, equal variance, two-tailed test) for comparisons with data that fit a normal distribution or the Mann-Whitney test for non-parametric comparisons.
Supplementary Material
Acknowledgements
We thank Gary Ward, Silvia Moreno, and Vern Carruthers for discussions, Florence Dzierszinski for the DsRed plasmid, Dominique Soldati for the Tet-repression system, and Keliang Tang for technical assistance. This work was supported by a predoctoral fellowship from the American Heart Association (S.L.) and a grant from the NIH (L.D.S.).
Footnotes
The authors have no competing interests.
REFERENCES
- 1.Barclay JW, Morgan A, Burgoyne RD. Calcium-dependent regulation of exocytosis. Cell Calcium. 2005;38:343–353. doi: 10.1016/j.ceca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Schapire AL, Valpuesta V, Botella MA. Plasma membrane repair in plants. Trends Plant Sci. 2009;14:645–652. doi: 10.1016/j.tplants.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Vayssie L, Skouri F, Sperling L, Cohen J. Molecular genetics of regulated secretion in paramecium. Biochimie. 2000;82:269–288. doi: 10.1016/s0300-9084(00)00201-7. [DOI] [PubMed] [Google Scholar]
- 5.Chieregatti E, Meldolesi J. Regulated exocytosis: new organelles for non-secretory purposes. Nat. Rev. Mol. Cell Biol. 2005;6:181–187. doi: 10.1038/nrm1572. [DOI] [PubMed] [Google Scholar]
- 6.Choi WS, Chahdi A, Kim YM, Fraundorfer PF, Beaven MA. Regulation of phospholipase D and secretion in mast cells by protein kinase A and other protein kinases. Ann. N. Y. Acad. Sci. 2002;968:198–212. doi: 10.1111/j.1749-6632.2002.tb04336.x. [DOI] [PubMed] [Google Scholar]
- 7.Easom RA. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes. 1999;48:675–684. doi: 10.2337/diabetes.48.4.675. [DOI] [PubMed] [Google Scholar]
- 8.Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–236. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 11.Harper JF, Harmon AC. Plants, symbiosis and parasites: a calcium signalling connection. Nat. Rev. Molec. Cell Biol. 2005;6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 12.Wernimont A, et al. Structural analysis of calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat. Struc. Molec. Biol. 2010 doi: 10.1038/nsmb.1795. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billker O, et al. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 14.Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin- like domain protein kinase. J. Biol. Chem. 2001;276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 15.Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 16.Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 17.Nagamune K, et al. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–211. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo T, Sethi KK, Piekarski G. Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp. Parasitol. 1982;53:179–188. doi: 10.1016/0014-4894(82)90059-5. [DOI] [PubMed] [Google Scholar]
- 19.Carruthers VB, Sherman GD, Sibley LD. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 2000;275:14346–14353. doi: 10.1074/jbc.275.19.14346. [DOI] [PubMed] [Google Scholar]
- 20.Carruthers VB, Moreno SNJ, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] calcium and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 1999;342:379–386. [PMC free article] [PubMed] [Google Scholar]
- 21.Kafsack BF, et al. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bishop AC, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 23.Sugi T, et al. Use of the kinase inhibitor analog 1NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot Cell. 2010 doi: 10.1128/EC.00351-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurnett AM, et al. Purification and molecular characterization of cGMP-dependent protein kinase from Apicomplexan parasites. A novel chemotherapeutic target. J. Biol. Chem. 2002;277:15913–15922. doi: 10.1074/jbc.M108393200. [DOI] [PubMed] [Google Scholar]
- 25.Wiersma HI, et al. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Intl. J. Parasit. 2004;34:369–380. doi: 10.1016/j.ijpara.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Starnes GL, Coincon M, Sygusch J, Sibley LD. Aldolase is essential for energy production and bridging adhesin-actin cytoskeletal interactions during parasite invasion of host cells. Cell Host Microbe. 2009;5:353–364. doi: 10.1016/j.chom.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos DS, Donald RGK, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:28–61. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 28.Huynh MH, et al. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 30.Messina M, Niesman IR, Mercier C, Sibley LD. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene. 1995;165:213–217. doi: 10.1016/0378-1119(95)00548-k. [DOI] [PubMed] [Google Scholar]
- 31.Donald RGK, Roos DS. Stable molecular transformation of Toxoplasma gondii: A selectable dihydrofolate reductase-thymidylate synthase marker based on drug resistance mutations in malaria. Proc. Nat. Acad. Sci. (USA) 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Håkansson S, Morisaki H, Heuser JE, Sibley LD. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol. Biol. Cell. 1999;10:3539–3547. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blair JA, et al. Structure-guided development of affinity probes for tyrosine kinases using chemical genetics. Nat Chem Biol. 2007;3:229–238. doi: 10.1038/nchembio866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.