Abdominal aortic aneurysm (AAA) rupture has been recognised to be a significant cause of mortality for adults aged >60 years in the developed world for some time [1]. AAAs are usually asymptomatic until rupture occurs and screening programs have been shown to reduce mortality in men aged >65 years [2]. Most AAAs detected by ultrasound are <50mm in diameter and there is currently no recognised treatment for these AAAs [3,4]. Studies aimed at understanding the pathogenesis of AAA are important as they may identify targets for novel therapy.
The mechanisms initiating and stimulating progression of AAA are still poorly understood with most knowledge coming from cross-sectional association studies in humans and increasingly from investigations in animal models [4]. Such studies suggest the importance of inflammatory pathways, matrix degradation, thrombosis, haemodynamic forces and a host of associated signalling molecules in AAA pathogenesis [4,5]. Based on the new insights from rodent models a number of novel strategies are being investigated as potential treatments for small AAA [5]. To date there have been very few well designed randomised controlled trials assessing the efficacy of medication in reducing AAA complications in patients [4].
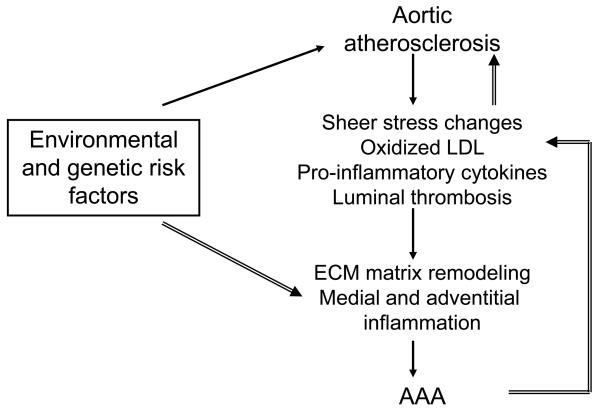
Patients with AAAs frequently have atherosclerosis, with numerous studies showing the association of coronary heart disease and peripheral atherosclerosis with AAA [4,6]. Whether this association between AAA and atherosclerosis is causal or simply due to common risk factors is unknown. One possibility is that an AAA develops as a pathological response to aortic atherosclerosis, a theory first suggested over half a century ago when the term “atherosclerotic aneurysms” was commonly employed but still prevalent today [7,8]. The most compelling argument for a causative role of atherosclerosis in AAA has been that centred on arterial remodeling [9]. A large body of in vitro, animal and histology data suggests that when an arterial luminal stenosis develops compensatory changes occur in the media in response to shear stress alterations [9]. The extracellular matrix remodeling promotes expansion of the artery in an attempt to normalise lumen diameter and shear stresses [9]. Excessive remodeling might explain the severe medial thinning but not perhaps the marked inflammation seen in biopsies of the walls of advanced AAA. Elastin breaks stimulated by medial proteolysis and the diffusion of pro-inflammatory cytokines from inflammatory cells present within atheroma or associated thrombosis could however provide the stimulation for the chronic inflammatory response seen (Figure) [4,5,9]. Based on the premise that atherosclerosis stimulates AAA development all patients with AAA would necessarily have significant atherosclerosis and thus should be considered for indicated medical therapy, as currently advised by AHA guidelines in which AAA is considered an atherosclerotic equivalent [10]. An alternative theory suggests that the development of AAA and atherosclerosis are independent. Shared environmental and genetic risk factors may promote the development of both atherosclerosis and AAA in some patients but the mechanisms involved are distinct. A third perhaps “on the fence” view would be that either aortic atherosclerosis or AAA can develop first and both can subsequently stimulate the development of the other (Figure). Currently evidence to support one of these theories over the other is largely limited to documenting similarities and differences in risk factors and findings within rodent models for atherosclerosis and AAA (Table 1) [4,11-19].
Figure.
According to Theory 1 (filled arrows) environmental and genetic risk factors lead to development of aortic atherosclerosis. Resultant positive remodeling, intimal thrombosis and release of pro-inflammatory cytokines stimulate secondary matrix degradation and adventitial inflammation which promotes AAA development. According to Theory 2 (stripped arrows) environmental and genetic risk factors directly stimulate aortic medial degradation and adventitial inflammation leading to AAA formation which secondarily stimulates intimal atherosclerosis. More likely both pathways act to some extent with the relative proportion varying from patient to patient depending on the risk profile. ECM= Extracellular matrix.
Table.
Some similarities and differences between atherosclerosis and AAA.
| Characteristic | Similarities | Differences |
|---|---|---|
| Clinical risk factors |
Smoking, hypertension, and obesity are common risk factors for AAA and aortic atherosclerosis [4]. |
Diabetes is a negative or neutral risk factors for AAA but important risk factor for atherosclerosis [4]. Male gender and smoking are much more dominant risk factors for AAA than atherosclerosis [4]. |
| Circulating risk factors |
AAA and atherosclerosis have many similar biomarkers, e.g. fibrinogen, CRP and HDL (negative) [11]. |
There are a number of disparate markers for AAA and atherosclerosis, e.g. LDL has no clear association with AAA but is an important risk factor for atherosclerosis [12]. |
| Genetic risk factors |
Family history is an important risk factor for both AAA and atherosclerosis [4]. A locus on chromosome 9p21 is associated with CHD, stroke and AAA [13]. |
Some recognised genetic determinants of atherosclerosis have no consistent association with AAA, e.g. Apolipoprotein E single nucleotide polymorphisms [14]. |
| Histology | Intimal atheroma and thrombosis are usually present in both AAA and atherosclerosis [4]. |
Marked elastin fragmentation and adventitial chronic inflammation is mainly restricted to AAA [4]. |
| Rodent models | Some mice (e.g. Apolipoprotein E deficient) prone to atherosclerosis are also more sensitive to AAA induction [15]. Interventions protective from AAA frequently also reduce atherosclerosis [16]. |
There are examples of differential effects of interventions on AAA and atherosclerosis progression, e.g. TNF and MMP-12 deficiency; and PPAR ligation [17-19]. |
AAA= Abdominal aortic aneurysm; CRP= C-reactive protein; HDL= High density lipoprotein; LDL= Low density lipoprotein; CHD= Coronary hear disease; TNF= Tumour necrosis factor; MMP= Matrix metalloproteinase; PPAR= Peroxisome proliferator-activated receptor.
In the current issue of Arteriosclerosis, Thrombosis and Vascular Biology, Johnsen and colleagues examine data from the well respected Tromso study in an attempt to better elucidate the relationship between atherosclerosis and AAA [20]. The investigators examine the relationship between intimal atherosclerosis and aortic dilatation in a large group of 3282 women and 3164 men aged between 25 and 74 years of age. Atheroma was primarily assessed by estimating the total plaque area of the right common and internal carotid arteries on ultrasound. The luminal diameter of the common femoral artery was also used as a surrogate marker of atherosclerosis severity. The authors report a significant association between carotid artery total plaque area and history of coronary heart disease with AAA prevalence. There was no association between carotid artery total plaque area and aortic diameter within the AAA range, i.e. there was no consistent correlation between atheroma extent and AAA severity. The authors suggest that their findings fit better with atherosclerosis and AAA developing in parallel rather than atherosclerosis directly leading to AAA. The authors are to be commended on tackling this difficult area which has been relatively little studied. They do acknowledge several limitations of their study which make it impossible to make any definitive conclusions on the relationship between atheroma and AAA. The latter particularly includes the cross-sectional nature of the study and the lack of direct atheroma assessment within the aorta. The authors also did not appear to include diabetes in the clinical variables they adjusted for in their analyses. Diabetes is positively associated with atherosclerosis but in contrast has been negatively associated with AAA and therefore is an important risk factor to adjust for [4]. It would indeed be a surprise if the extent of carotid atherosclerosis and AAA size were closely correlated in a cross-sectional study. If atherosclerosis is playing a role in AAA development it is to be expected that its severity within the aorta would be most relevant. While there appears to be a systemic component to atherosclerosis development many regional factors, such as hemodynamic stresses, determinant the distribution of atherosclerosis. Thus it would be expected that carotid and aorta atheroma severity would vary. Thus the findings from the current study are not able to convincingly refute a role for atherosclerosis in AAA.
In our opinion it is likely that multiple mechanisms are responsible for both AAA and atherosclerosis development (including some of those illustrated in the Figure). The relative importance of these different mechanisms is likely to vary from patient to patient and is one of the reasons that standardised therapies for all patients with the same condition is only partially successful. Prospective imaging, and particularly interventional studies, are required to address the value of therapies selectively targeting mechanisms implicated in aortic dilatation and atherosclerosis, respectively, in patients with AAA. Recent human association studies have shown conflicting results on whether drugs that are effective for atherosclerosis, such as statins, inhibit AAA progression [21,22]. Unfortunately randomised controlled trials to assess these types of drugs are unlikely to be feasible. Two current randomised trials however are examining the efficacy of doxycyline and exercise therapy in limited AAA progression. Studies of this type and further carefully designed animal experiments are required to shed further light on the relationship between atherosclerosis and AAA and in particular its therapeutic implications.
Acknowledgments
The authors are support by funding from the NIH, USA (RO1 HL080010), Smart State National and International Research Alliances Program from the Queensland Government and the National Health and Medical Research Council, Australia (project grants 540403, 540404 and 540405). JG and PN hold Practitioner Fellowships from the National Health and Medical Research Council, Australia (431503 and 45805).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 2006. 2009:7–9. MD LCWK1. Accessed at http://www.cdc.gov/nchs/data/dvs/LCWK1_2006.pdf on 22/3/10.
- 2.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203–11. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, Parsons RW, Dickinson JA. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 5.Golledge J, Norman PE. Pathophysiology of abdominal aortic aneurysm relevant to improvements in patients' management. Curr Opin Cardiol. 2009;24:532–8. doi: 10.1097/HCO.0b013e328330c2d3. [DOI] [PubMed] [Google Scholar]
- 6.Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004;14:343–9. doi: 10.1093/eurpub/14.4.343. [DOI] [PubMed] [Google Scholar]
- 7.EISEMAN B, HUGHES RH. Repair of an abdominal aortic vena caval fistula caused by rupture of an atherosclerotic aneurysm. Surgery. 1956;39:498–504. [PubMed] [Google Scholar]
- 8.Kaschina E, Scholz H, Steckelings UM, Sommerfeld M, Kemnitz UR, Artuc M, Schmidt S, Unger T. Transition from atherosclerosis to aortic aneurysm in humans coincides with an increased expression of RAS components. Atherosclerosis. 2009;205:396–403. doi: 10.1016/j.atherosclerosis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–91. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American Association for Vascular Surgery. Society for Vascular Surgery. Society for Cardiovascular Angiography and Interventions. Society for Vascular Medicine and Biology. Society of Interventional Radiology. ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease. American Association of Cardiovascular and Pulmonary Rehabilitation. National Heart, Lung, and Blood Institute. Society for Vascular Nursing. TransAtlantic Inter-Society Consensus. Vascular Disease Foundation ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 11.Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118:2382–92. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golledge J, van Bockxmeer F, Jamrozik K, McCann M, Norman P. Association between serum lipoproteins and abdominal aortic aneurysm. American Journal of Cardiology. doi: 10.1016/j.amjcard.2009.12.076. In press. [DOI] [PubMed] [Google Scholar]
- 13.Helgadottir A, Thorleifsson G, Magnusson KP, Grétarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jääskeläinen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsäter A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemelä M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–24. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 14.Golledge J, Biros E, Cooper M, Warrington N, Palmer LJ, Norman PE. Apolipoprotein E genotype is associated with serum C-reactive protein but not abdominal aortic aneurysm. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2009.09.027. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–7. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 16.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–31. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109:1408–14. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 18.Xanthoulea S, Thelen M, Pöttgens C, Gijbels MJ, Lutgens E, de Winther MP. Absence of p55 TNF receptor reduces atherosclerosis, but has no major effect on angiotensin II induced aneurysms in LDL receptor deficient mice. PLoS One. 2009;4:e6113. doi: 10.1371/journal.pone.0006113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golledge J, Cullen B, Rush C, Moran CS, Secomb E, Wood F, Daugherty A, Campbell JH, Norman PE. Peroxisome proliferator-activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2009.10.027. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnsen SH, Forsdahl SH, Singh K, Jacobsen BK. Atherosclerosis in abdominal aortic aneurysm, a causal event or a process running in parallel? The Tromso study. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.203588. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson CD, Clancy P, Bourke B, Walker PJ, Dear A, Buckenham T, Norman P, Golledge J. Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J. 2010;159:307–13. doi: 10.1016/j.ahj.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlösser FJ, Tangelder MJ, Verhagen HJ, van der Heijden GJ, Muhs BE, van der Graaf Y, Moll FL, SMART study group Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47:1127–33. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]