Abstract
Eosinophils are multifunctional leukocytes involved in various inflammatory processes as well as tissue remodeling and immunoregulation. During inflammation and infection, injured cells and damaged tissues release uric acid and monosodium urate (MSU) crystals as important endogenous danger signals. Uric acid is also implicated in the immunogenic effects of an authentic Th2 adjuvant, aluminum hydroxide. Eosinophils often localize at sites of Th2-type chronic inflammation; therefore, we hypothesized that eosinophils may react to endogenous danger signals. We found that human eosinophils migrate toward both soluble uric acid and MSU crystals in a gradient-dependent manner. Eosinophils incubated with MSU crystals, but not those incubated with uric acid solution, produced elevated levels of IL-6 and IL-8/CXCL8. Other cytokines and chemokines, including IL-1β, IL-10, IL-17, IFN-©, CCL2, CCL3, CCL4, TNF-α, G-CSF, GM-CSF, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and TGF-β, were also produced by eosinophils incubated with MSU crystals. Eosinophils exposed to MSU crystals rapidly (i.e., within 1 minute of exposure) released ATP into the extracellular milieu. Importantly, this autocrine ATP was necessary for eosinophils to produce cytokines in response to MSU crystals, and P2 nucleotide receptors, in particular P2Y2, are likely involved in this positive feedback loop. Finally, at higher concentrations, MSU crystals promoted P2 receptor-dependent release of a granule protein, eosinophil-derived neurotoxin (EDN), and cell death. Thus, human eosinophils may respond to particulate damage-associated endogenous danger signals. These responses by eosinophils to tissue damage may explain the self-perpetuating nature of chronic inflammation in certain human diseases, such as asthma.
INTRODUCTION
Historically, eosinophils have been considered effector cells involved in host protection against helminth infections and in pathological processes in bronchial asthma and allergic diseases (1, 2). Activated eosinophils release toxic granule proteins and pro-inflammatory mediators, which may cause tissue damage and dysfunction (3). Recent evidence suggests that eosinophils may also be involved in tissue remodeling and immunoregulation (4). Eosinophils can synthesize, store and secrete at least 35 inflammatory and immunoregulatory cytokines, chemokines, and growth factors (5), which may play roles in the regulatory functions of eosinophils. In several eosinophil-associated human diseases, for example asthma, eosinophil-derived TGF-β is linked with tissue remodeling (6). In eosinophil-deficient mice, T cell recruitment is impaired and Th2 cytokine production is reduced (7-9). However, the cellular and molecular mechanisms involved for eosinophils to produce these tissue-remodeling and immunoregulatory cytokines are mostly unknown.
Inflammation and immune responses can be considered as reactions to noxious stimuli and conditions, such as infection and injury (10). On one hand, infection with microorganisms, which express pathogen-associated molecular pattern (PAMP)3 molecules, is an example of an exogenous inducer of inflammation. Human eosinophils express TLRs (e.g. TLR7) (11), β2 integrins (12), and protease-activated receptors (13), which may recognize infectious agents. On the other hand, during tissue injury, endogenous molecules released by stressed or damaged tissues, such as ATP, K+ ions, uric acid, high-mobility group box 1 protein (HMGB-1) and S100 calcium-binding protein family members (14-16), may trigger inflammation. Indeed, the immunological actions of a prototypic Th2-type adjuvant, namely aluminum hydroxide (alum), are likely mediated by induction of endogenous uric acid (17). These damage-associated molecular pattern (DAMP) molecules or danger signals may play important roles in asthma and allergic disorders (18). However, whether and how eosinophils respond to DAMPs or danger signals is not clear.
Herein, we describe the eosinophils’ attraction to a prototypic danger signal, namely monosodium urate (MSU) crystals (19). Eosinophils cultured with MSU crystals produce large quantities and various kinds of cytokines and chemokines. Importantly, the autocrine release of ATP likely provides a pivotal positive feedback signal in eosinophils exposed to MSU crystals. Thus, human eosinophils may respond to certain DAMPs released by injured cells or damaged tissues. This previously unrecognized capacity of eosinophils to respond to danger signals may explain the self-perpetuating nature of chronic inflammation in certain human diseases, such as asthma.
MATERIALS AND METHODS
Reagents
Recombinant human IL-33 and human eotaxin were from R&D Systems (Minneapolis, MN). Recombinant human IL-5 was a generous gift from the Schering-Plough Research Institute (Kenilworth, NJ). Anti-CD16-conjugated immunomagnetic beads and anti-FITC-conjugated immunomagnetic beads were from Miltenyi Biotec (Auburn, CA). FITC-conjugated anti-human CD14 mAb was from BD Bioscience (San Jose, CA). Percoll was from GE Healthcare Biosciences AB (Uppsala, Sweden). Alpha-calf serum (α-CS) and FCS were from HyClone (Thermo Fisher Scientific, Waltham MA); α-CS was heat-inactivated at 56 °C for 30 minutes before use. RPMI 1640 medium was from Gibco® (Invitrogen/ Life Technologies, Carlsbad, CA). EGTA, uric acid, MSU crystals, exo-ATPase inhibitor (ARL 67156), apyrase, ATP, ATP periodate oxidized sodium salt (oATP), adenosine 5′-(3-thiotriphosphate) tetralithium salt (ATPγS), UTP, and Nonidet P-40 were obtained from Sigma-Aldrich (St. Louis, MO). Uric acid was dissolved in the incubation medium at 1 mg/ml and diluted to desired concentrations. No crystal formation of uric acid was observed under an inverted microscope at the concentrations used in this study (i.e. 0.1 mg/ml or less). The MSU crystals were suspended in the incubation buffer (e.g. RPMI 1640 medium) at 10 mg/ml and diluted serially. Suramin sodium salt was from EMD Chemicals (Gibbstown, NJ). KN-62 was from TOCRIS Bioscience (Ellisville, MO). Rabbit polyclonal anti-P2Y2 antibody was from Thermo Fisher Scientific. Control rabbit IgG was from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell isolation
Eosinophils were isolated from the peripheral blood of 65 healthy volunteers, having no history of asthma or allergic diseases or having mild hay fever, by negative selection with anti-CD16 microbeads, as previously described with minor modifications (20). Briefly, granulocytes were incubated with an equal volume of anti-CD16-conjugated magnetic beads, FITC-conjugated anti-human CD14 mAb, and anti-FITC-conjugated magnetic beads on ice for 30 min. This protocol consistently yielded ≥99% eosinophil purity (mean 99.4%). The responses of eosinophils from normal individuals and from patients with mild hay fever were not quantitatively different; therefore, the data were pooled. The Mayo Clinic Rochester Institutional Review Board approved the protocol to obtain blood from volunteers; all provided informed consent.
Eosinophil migration assay
Eosinophil migration through a membrane was examined using a 24-well Transwell insert system (Thermo Fisher) (21). These inserts with porous bottoms (pore, 3μm) serve as the upper chambers, and ordinary tissue culture plate wells serve as the lower chambers. Eosinophils were suspended in RPMI 1640 medium supplemented with 10% FCS at 1×106 cells/ml. One hundred microliters of the eosinophil suspension was added to the upper chamber, and 500 μl of serial dilutions of uric acid solution or MSU crystal suspension was added to the lower chamber. Eotaxin at 100 ng/ml was used as a positive control. Alternatively, 100 μl of eosinophil suspension with 0.1 mg/ml uric acid solution or MSU crystal suspension was added to the upper chamber, and 500 μl of 0.1 mg/ml uric acid solution or MSU crystal suspension was added to the lower chamber in a checkerboard fashion. After 2 h incubation at 37 °C and 5% CO2, the cells that migrated to the lower chambers were collected and counted by light microscopy. Results show the ratio (percent) of migrated cells to the initial total number of cells.
Cytokine production
Purified eosinophils (1 × 106 cells/ml) were resuspended in RPMI 1640 with 10% FCS. Cells were incubated with medium alone or serial dilutions of MSU crystal suspensions or uric acid solution in 96-well tissue culture plates for 24 h at 37 °C and 5% CO2 (22). After culture, supernatants were collected, and concentrations of IL-6 and IL-8 were measured by ELISA kits according to the manufacturer’s directions (R&D Systems). Sensitivities for IL-6 and IL-8 were 4 pg/ml. The supernatants were also analyzed by a Bio-Plex human 27 cytokine assay kit and the Bio-Plex suspension array system (Bio-Rad Laboratories). All assays were conducted in duplicate. In some experiments, ATP (20-500 μM), ATP©S (20-500 μM) or UTP (100 μM) was used to stimulate eosinophils instead of MSU crystals, and the exo-ATPase inhibitor, ARL 67156 (12.5~200 μM), was used to block the effects of endogenous ATPase.
To examine the role of endogenous ATP in the eosinophils’ response to MSU crystals, pharmacological agents, including apyrase (5-20 U/ml), oATP (10-300 μM), or suramin (50-500 μM), were added to eosinophils cultured with MSU crystals. To examine the role of the P2Y2 nucleotide receptor in the eosinophils’ cytokine responses to MSU crystals, eosinophils were preincubated with serial dilutions of rabbit polyclonal anti-P2Y2 Ab or control rabbit IgG for 15 minutes before addition of MSU crystal suspensions. To examine the involvement of the P2X7 nucleotide receptor, eosinophils were preincubated with an antagonist for the P2X7 receptor, KN-62 (50 nM), for 15 minutes before addition of MSU crystal suspensions, ATP, or UTP.
Eosinophil degranulation assay
Eosinophil degranulation was quantitated by the eosinophil-derived neurotoxin (EDN) released into cell-free supernatants (23). Briefly, freshly isolated eosinophils were suspended in HBSS with 25mM HEPES and 0.01% gelatin (for 3 h culture), or RPMI 1640 supplemented with 10 mM HEPES and 10% FCS (for 24 h culture) at 2.5 × 105 cells. Cells were added to the wells of 96-well tissue culture plates and incubated with serial dilutions of MSU crystal suspensions (1~10 mg/ml) with or without 1 ng/ml IL-33 or 1 ng/ml IL-5 for 3 h or 24 h at 37°C and 5% CO2. After incubation, cell-free supernatants were collected and stored at −20 °C before EDN was measured by ELISA. In some experiments, to examine the role of extracellular calcium in eosinophil degranulation, 1 mM EGTA was added to the culture. To examine the role of P2 receptors, eosinophils were preincubated with serial dilutions of oATP for 15 minutes before the addition of 3 mg/ml MSU crystal suspensions.
The EDN ELISA was performed as described earlier (24) using anti-human EDN mAbs (clones 167-6C5 and 167-2G4) made at Mayo Clinic Rochester. The lowest point of the standard curve was 0.09 ng/ml. All assays were conducted in duplicate.
Cell viability assay
Viability of cultured eosinophils was assessed by double staining with FITC-conjugated annexin V and propidium iodide (PI) and flow cytometry analysis. Briefly, eosinophils (5.0×105 cells) were suspended in HBSS buffer or RPMI 1640 medium, as described above, and incubated with medium alone or serial dilutions of MSU crystal suspensions or uric acid solutions in 5 ml polystyrene round-bottom tubes (BD Biosciences, Bedford, MA) for 3 h or 24 h at 37 °C and 5% CO2. After washing, cells were suspended in binding buffer and stained with FITC-conjugated annexin V and PI, according to the procedure recommended by the manufacturer (Annexin V-FITC Kit, Miltenyi Biotech, Auburn, CA). At least 10,000 cells were analyzed using flow cytometry (FACScan; BD Biosciences) and Becton Dickinson Lysis II software. The percentages of apoptotic cells (annexin V-positive and PI-negative) and dead cells (annexin V-positive and PI-positive) were determined. Alternatively, immediately after a 3 h-degranulation assay with MSU and oATP as described above, eosinophils were stained with PI and fluorescein diacetate (Sigma-Aldrich) and at least 200 cells were analyzed using a hemacytometer and an epifluorescent microscope.
Expression of P2Y2 nucleotide receptor
Eosinophil surface expression of P2Y2 nucleotide receptor was examined by flow cytometry. Purified eosinophils (1 × 106 cells) were resuspended in PAB buffer (PBS with 3% BSA and 0.1% sodium azide) and incubated with rabbit anti-human P2Y2 Ab or control rabbit IgG for 60 min on ice. After washing with PAB buffer, cells were incubated with FITC-conjugated F(ab’)2 of goat anti-rabbit IgG for 30 min on ice. Cells were washed twice with PAB, fixed with 1% paraformaldehyde for 20 min and were analyzed using flow cytometry (FACScan) and Becton Dickinson Lysis II software.
ATP release
To examine ATP release by eosinophils, purified eosinophils were suspended at 1×106/ml in HBSS with 25 mM HEPES and 0.01% gelatin and incubated with medium alone or 0.1 mg/ml MSU crystal suspension in polypropylene tubes for up to 20 min at 37 °C. After incubation, the reaction was stopped by centrifuging the tubes at 4 °C and collecting the cell-free supernatants. To quantitate ATP release, the ATP concentrations in the supernatants were measured with an ATP Determination Kit (BioAssay Systems,Hayward, CA) and a luminometer.
Statistical analysis
Results were expressed as means +/− SEMs. Statistical analysis was performed by using one-way ANOVA (Tukey-Kramer multiple comparisons test) or differences between two sample groups were analyzed with the paired Student’s t-test. P values <0.05 were considered statistically significant.
RESULTS
Soluble uric acid and MSU crystals induce eosinophil chemotaxis
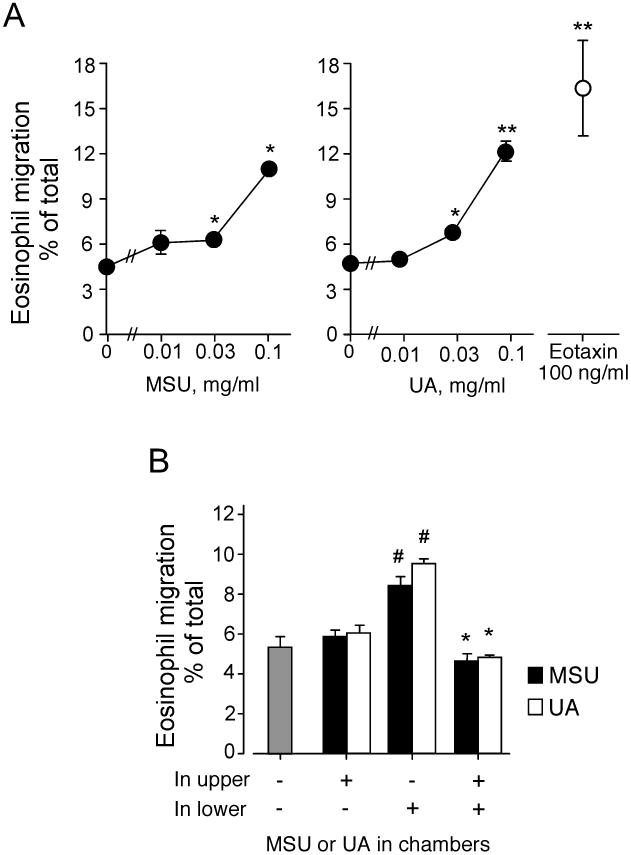
Sites of chronic inflammation or tissue fibrosis frequently contain eosinophils (4, 25, 26). In addition, eosinophils can accumulate even in the absence of acquired immune responses (26, 27) or before antigen sensitization (28). After dying cells release uric acid, MSU crystals have been identified as a danger signal (19). Therefore, we examined whether MSU crystals or soluble uric acid could induce eosinophil migration. Both MSU crystal suspensions and uric acid solutions ≥ 0.03 mg/ml induced eosinophil migration (p<0.05, n=5) that appeared to increase up to 0.1 mg/ml MSU or uric acid (Figure 1A). The eosinophil migration induced by MSU crystals or uric acid was roughly 70% compared to an authentic eosinophil chemotactic factor, eotaxin at 100 ng/ml. Uric acid concentrations >0.1 mg/ml were not examined because the solute crystallized.
FIGURE 1.
MSU crystals and soluble uric acid induce eosinophil chemotaxis. (A) Human eosinophil suspensions were added to the upper chamber, and MSU crystal suspensions or uric acid solutions were added to the lower chamber. Eotaxin, 100 ng/ml, in the lower chamber was used as a positive control. After 2 h at 37 °C, the cells that migrated to the lower chambers were collected and counted with light microscopy. The data show the ratio (percent) of migrated cells to the total number of input cells. Results show the mean±SEM from five different eosinophil preparations. * and **; significant differences compared with medium alone in the lower chamber (p<0.05 and p<0.01, respectively). (B) Eosinophil suspensions with or without 0.1 mg/ml MSU crystals or uric acid were added to the upper chamber, and 0.1 mg/ml MSU crystals or uric acid was added to the lower chamber in a checkerboard fashion. After 2 h at 37 °C, the cells in the lower chambers were collected and counted as in (A); the data are summarized as in (A). Results show the mean±SEM from five different eosinophil preparations. #; significant differences compared with medium alone in both upper and lower chambers (p<0.05). *; significant differences compared with MSU crystals or uric acid only in the lower chamber (p<0.05).
We used a checkerboard experiment to characterize eosinophil migration. Eosinophils with or without the MSU crystal suspension or uric acid solution (each at 0.1 mg/ml) were placed in the upper chamber, and the MSU crystal suspension or uric acid solution (0.1 mg/ml) was placed in the lower chamber. MSU crystals or uric acid in the lower chamber, but not in the upper chamber, induced eosinophil migration compared to medium alone (p<0.05, n=5) (Figure 1B). MSU crystals or uric acid in the upper chamber abolished eosinophil migration to the lower chamber. Therefore, eosinophil migration likely requires a gradient of MSU crystals or uric acid.
MSU crystals, but not uric acid, induce robust cytokine production
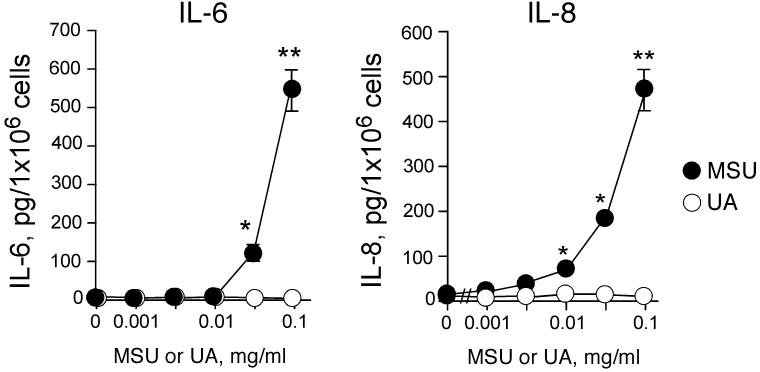
To examine whether MSU crystals or uric acid induce eosinophil cytokine production, eosinophils were incubated with MSU crystals or uric acid, and the levels of IL-6 and IL-8 in the cell-free supernatants were measured by ELISA. MSU crystals induced robust production of both IL-6 and IL-8 (Figure 2). Cytokine production was observed with MSU crystals at 0.03 mg/ml (for IL-6) or 0.01 mg/ml (for IL-8), and increased up to 0.1 mg/ml MSU crystals. In contrast, uric acid (≤0.1 mg/ml) did not induce IL-6 or IL-8 production.
FIGURE 2.
MSU crystals, but not soluble uric acid, induce IL-6 and IL-8 production by human eosinophils. Purified eosinophils were incubated with medium alone or serial dilutions of MSU crystal suspensions or uric acid (UA) solution for 24 h at 37 °C. Concentrations of IL-6 and IL-8 in the cell-free supernatants were measured by ELISA. Results show the mean±SEM from five different eosinophil preparations. * and **; significant differences compared with medium alone (p<0.05 and p<0.01, respectively).
We used a Bio-Plex assay to examine a panel of cytokines and chemokines produced by eosinophils cultured with MSU crystal suspensions for 24 h. As shown in Table I, several cytokines were produced, including pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), a Th1 cytokine (IFN-γ), a Th17 cytokine (IL-17), a regulatory cytokine (IL-10), growth factors (G-CSF, GM-CSF), tissue remodeling factors (epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), TGF-β, and chemokines (CXCL8, CCL2, CCL3, CCL4). Similar to the IL-6 and IL-8 results (Figure 2), the production of these cytokines and chemokines increased up to 0.1 mg/ml MSU crystal suspension. In contrast, Th2 cytokines, such as IL-4, IL-5, and IL-13, and other cytokines and chemokines, including IL-2, IL-7, IL-9, IL-12p70, IL-15, CCL5, CCL10, CCL11, nerve growth factor (NGF), and platelet-derived growth factor (PDGF)-bb, were undetectable. Altogether, human eosinophils exposed in vitro to MSU crystals produce several cytokines, growth factors, and chemokines.
Table I.
Production of cytokines and chemokines by eosinophils stimulated with MSU crystalsa
| Monosodium urate (MSU) crystals, mg/ml | |||
|---|---|---|---|
| 0 | 0.03 | 0.1 | |
| Concentrations of cytokines detected, pg/ml | |||
| IL-1β | 0±0 | 27±4 * | 57±6 ** |
| IL-1Rα | 1±1 | 20±4 * | 48±4 ** |
| IL-6 | 0±0 | 204±30 ** | 717±101 ** |
| IL-8/CXCL8 | 29±2 | 785±129 ** | 3840±1220 ** |
| IL-10 | 0±0 | 1±0 | 9±1 ** |
| IL-17 | 0±0 | 1±0 | 14±4 * |
| FGF | 4±1 | 8±2 | 23±4 * |
| G-CSF | 0±0 | 5±1 * | 39±6 ** |
| GM-CSF | 1±0 | 3±1 | 16±5 * |
| IFN-γ | 0±0 | 5±1 * | 29±5 ** |
| MCP-1/CCL2 | 5±1 | 20±2 * | 47±6 ** |
| MIP-1α/CCL3 | 0±0 | 28±5 * | 115±18 ** |
| MIP-1β/CCL4 | 26±2 | 585±99 * | 2050±516 ** |
| TNF-α | 2±1 | 35±6 * | 118±18 ** |
| VEGF | 3±0 | 6±1 * | 17±2 * |
| TGF-β | 10±3 | 61±13 * | 88±7 ** |
Undetectable in any samples: IL-2, IL-4, IL-5, IL-7, IL-9, IL-12 p70, IL-13, IL-15, RANTES/CCL5, IP-10/CXCL10, PDGF-bb, Eotaxin/CCL11, NGF
Eosinophils were incubated with medium alone or indicated concentrations of MSU crystal suspensions (0.03 or 0.1 mg/ml) for 24 h at 37° C. Concentrations of chemokines and cytokines in cell-free supernatants were determined by Bio-Plex. Results show the mean±SEM from five different eosinophil preparations.
significant differences compared with medium alone (p<0.05 and p<0.01, respectively)
significant differences compared with medium alone (p<0.05 and p<0.01, respectively)
MSU crystals induce modest EDN release from eosinophils
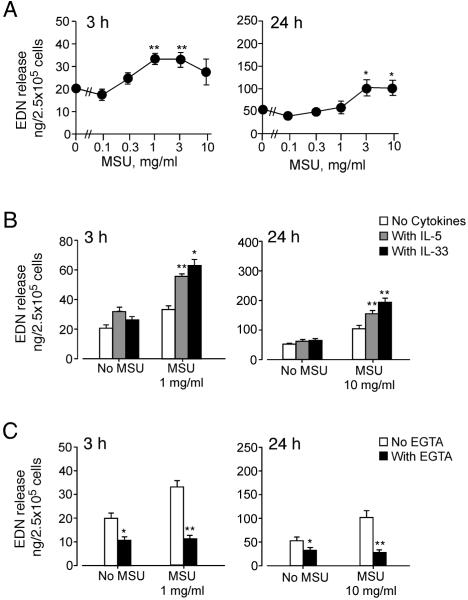
We then examined whether eosinophils stimulated with MSU crystals release granule proteins. Eosinophils were stimulated with MSU crystal suspensions for 3 h or 24 h, and EDN released into supernatants was measured by ELISA. MSU crystals induced modest EDN release with ≥ 1 mg/ml by 3 h or with ≥ 3 mg/ml or higher by 24 h (p<0.01 and <0.05, respectively) (Figure 3A). The EDN release required about a 10-fold more concentrated suspension of MSU crystals (Figure 3) compared to the concentrations needed to induce chemotaxis or cytokine production (Figures 1 and 2). Furthermore, at the optimal MSU crystal concentrations (~3 mg/ml), the quantity of released EDN was about 3% of total EDN; this was considerably less than the percent of total EDN typically induced by authentic secretagogues for human eosinophils [e.g. 1 μM platelet-activating factor (PAF) induces 40% of total EDN] (29). Soluble uric acid at 0.1 mg/ml or lower showed no effects on EDN release (data not shown).
FIGURE 3.
MSU crystals induce modest degranulation of eosinophils, which is regulated by cytokines and extracellular calcium. (A) Eosinophils were incubated with MSU crystal suspensions for 3 h or 24 h at 37 °C. EDN released into supernatants was measured by ELISA. Results show the mean±SEM from eight (for 3 h) or four (for 24 h) different eosinophil preparations. * and **; significant differences compared with medium alone (p<0.05 and p<0.01, respectively). (B) Eosinophils were incubated with or without 1 mg/ml (for 3 h) or 10 mg/ml (for 24 h) MSU crystal suspensions at 37 °C with or without 1 ng/ml IL-33 or 1 ng/ml IL-5. EDN released into supernatants was measured by ELISA. Results show the mean±SEM from eight (for 3 h) or four (for 24 h) different eosinophil preparations. * and **; significant differences compared with the samples with MSU but no cytokines (p<0.05 and p<0.01, respectively). (C) Eosinophils were incubated with or without 1 mg/ml (for 3 h) or 10 mg/ml (for 24 h) MSU crystal suspensions at 37 °C with or without 1 mM EGTA. EDN released into supernatants was measured by ELISA. Results show the mean±SEM from eight (for 3 h) or four (for 24 h) different eosinophil preparations. * and **; significant differences compared with the samples without EGTA (p<0.05 and p<0.01, respectively).
Because the EDN release induced by MSU crystals was modest, we investigated whether the MSU crystals mediated an unknown toxic or cytolytic effect. Thus, we examined the effects of IL-5 and IL-33, which also activate the effector functions of human eosinophils (24). Suboptimal concentrations of IL-5 alone (1 ng/ml) or IL-33 alone (1 ng/ml) induced modest EDN release (Figure 3B). IL-5 and IL-33 enhanced EDN release induced by 1 mg/ml or 10 mg/ml MSU crystals at 3 h and 24 h, respectively (p<0.05 or p<0.01). Furthermore, increased intracellular calcium concentration ([Ca2+]i) is a key triggering step in the coupling of stimulus to eosinophil degranulation (30). When we used 1 mM EGTA to chelate extracellular calcium, eosinophils did not degranulate in response to MSU crystals (Figure 3C). These findings suggest that eosinophil degranulation induced by MSU crystals is controlled under physiological conditions (e.g. cytokines, extracellular calcium).
Exposure to MSU crystals modulates eosinophil viability
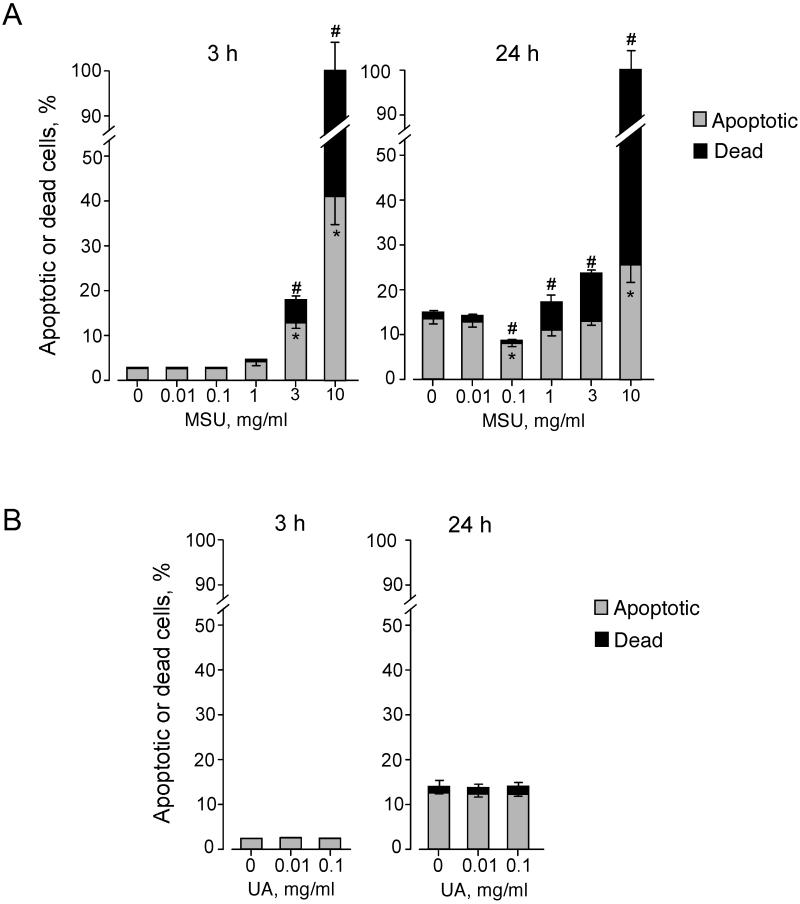
Eosinophil cytokine production declined when cells were incubated with > 1 mg/ml MSU crystal suspensions (Figure 2). Therefore, we examined the effects of different concentrations of MSU crystals on eosinophil viability. Incubation of eosinophils for 3 h with medium alone or MSU crystals at 0.01 mg/ml or 0.1 mg/ml did not affect eosinophil viability (Figure 4A); ≤ 2.9% and 0.2% of the cells were apoptotic and dead, respectively. In contrast, MSU crystals at 3 mg/ml or 10 mg/ml increased the proportions of both apoptotic and dead cells (p<0.01, n=5). When eosinophils were incubated with medium alone for 24 h, the proportion of apoptotic cells increased to about 13% of total cells, consistent with increased apoptosis of eosinophils when they are cultured without any growth factors for a prolonged period (31). This increase in eosinophil apoptosis at 24 h was partially inhibited by 0.1 mg/ml MSU crystals (p<0.01, n=5). In contrast, MSU crystals at ≥ 1 mg/ml increased the proportion of dead cells at 24 h. Soluble uric acid at concentrations ≤0.1 mg/ml showed no effects on eosinophil viability (Figure 4B). Thus, MSU crystals at low concentrations (e.g. 0.1 mg/ml) likely inhibit eosinophil apoptosis and promote cytokine production, but at higher concentrations they induce cell death.
FIGURE 4.
MSU crystals modulate eosinophil viability. (A) Eosinophils were incubated with medium alone or serial dilutions of MSU crystal suspensions for 3 h or 24 h at 37 °C. (B) Eosinophils were incubated with medium alone or serial dilutions of soluble uric acid (UA) for 3 h or 24 h at 37 °C. Cell apoptosis and death were examined by double staining with FITC-conjugated annexin V and PI and flow cytometry analysis. The percentages of apoptotic cells (annexin V-positive and PI-negative) and dead cells (annexin V-positive and PI-positive) were determined. The proportion of cells with annexin V-negative and PI-positive was less than 0.5%. Results show the mean±SEM from five (A) or three (B) different eosinophil preparations. Downward error bars and upward error bars indicate SEM for apoptotic cells and dead cells, respectively. * and #; significant differences compared with percent apoptotic cells and dead cells in medium alone, respectively (p<0.01).
Endogenous ATP is involved in the eosinophils’ responses to MSU crystals
What could be the molecular mechanisms involved in the eosinophils’ responses to MSU crystals? No cellular receptors for MSU crystals have been identified (32). Interestingly, endogenous ATP is critically involved in cytokine production by monocytes stimulated with TLR ligands (33) and in the directed migration of neutrophils induced by FMLP (34). Therefore, we hypothesized that autocrine ATP is involved in the eosinophils’ responses to MSU crystals.
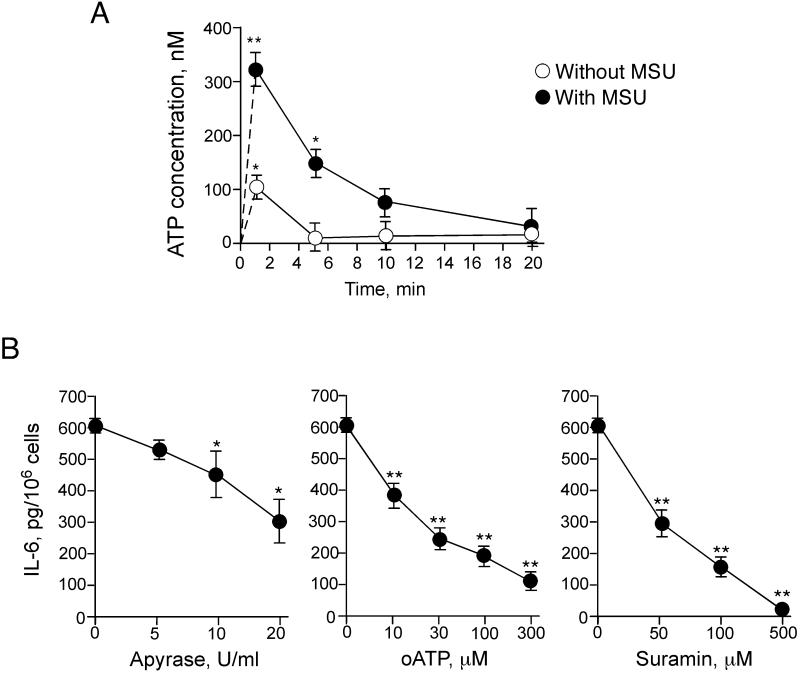
To test the hypothesis, we first measured the concentrations of ATP in the culture supernatants of eosinophils. ATP was detectable within 1 minute after placing the eosinophils in a test tube with medium alone (Figure 5A); ATP levels returned to baseline levels within 5 minutes, likely due to the effects of ecto-ATPase activity. Importantly, when MSU crystals were added to eosinophils, the peak extracellular ATP level was about 3 times higher than that with eosinophils with medium alone, and these detectable levels of ATP were more persistent. Thus, eosinophils exposed to MSU crystals quickly release ATP.
FIGURE 5.
Autocrine release of ATP by MSU-stimulated eosinophils is involved in IL-6 production. (A) Eosinophils were incubated with medium alone or 0.1 mg/ml MSU crystal suspension in polypropylene tubes for up to 20 min at 37 °C. The reaction was stopped by centrifugation at 4 °C. The concentrations of ATP in the supernatants were measured by using an ATP Determination Kit. Results show the mean±SEM from three different eosinophil preparations. * and **; significant differences compared with the samples without MSU (p<0.05 and p<0.01, respectively). (B) Eosinophils were incubated with 0.1 mg/ml MSU crystal suspensions with or without serial dilutions of apyrase (5-20 U/ml), oATP (10-300 μM), or suramin (50-500 μM) for 24 h at 37 °C. Concentrations of IL-6 in supernatants were measured by ELISA. Results show the mean±SEM from three to six different eosinophil preparations. * and **; significant differences compared with the samples without inhibitors (p<0.05 and p<0.01, respectively).
We next examined whether this released ATP is involved in cytokine production by eosinophils stimulated with MSU crystals. To this end, we neutralized the increases in ATP by exogenously administering an ATP-hydrolyzing enzyme, apyrase (33, 35). Apyrase partially (~50%), but significantly inhibited IL-6 production by eosinophils stimulated with MSU crystals (p<0.05, Figure 5B). We next examined the effects of broad-range inhibitors of P2 receptors, as receptors for ATP. Oxidized ATP (oATP) is a derivative of ATP, and it covalently modifies nucleotide-binding proteins (33, 35). oATP potently inhibited IL-6 production by eosinophils stimulated with MSU crystals with an IC50 of about 20 μM (Figure 5B). Suramin is a broad-spectrum antagonist for P2 receptors (36, 37), and it strongly inhibited IL-6 production by eosinophils (IC50 50 μM) and abolished the response at 500 μM (Figure 5B). Thus, autocrine ATP interacting with P2 receptors is likely involved in the MSU crystal-induced production of IL-6 by eosinophils.
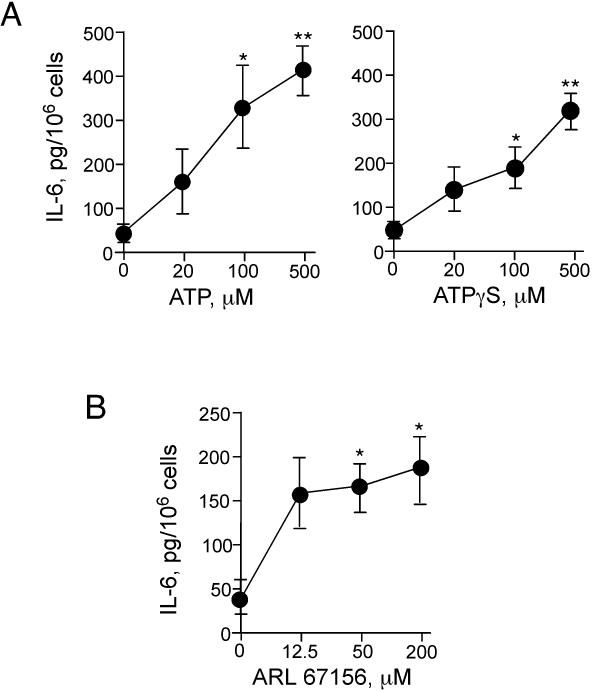
We also examined whether the increased extracellular ATP levels around the eosinophils in media only (without MSU crystals) is sufficient to induce cytokine production. Addition of exogenous ATP or ATPγS alone to eosinophils induced robust IL-6 production (Figure 6A). Furthermore, inhibition of the eosinophils’ ATPase activity by an exto-ATPase inhibitor, ARL 67156, enhanced IL-6 production (without MSU crystals) (p<0.05, n=5) (Figure 6B). Thus, the ability of eosinophils to produce IL-6 appears to be tightly regulated by the extracellular levels of ATP and the activity of a catalytic enzyme(s).
FIGURE 6.
Exogenous ATP and inhibition of exo-ATPase activity trigger IL-6 production by eosinophils. (A) Eosinophils were incubated with ATP or ATP□S for 24 h at 37 °C. Concentrations of IL-6 in cell-free supernatants were measured by ELISA. Results show the mean±SEM from five different eosinophil preparations. * and **; significant differences compared with medium alone (p<0.05 and p<0.01). (B) Eosinophils were incubated with an exo-ATPase inhibitor, ARL 67156, for 24 h at 37 °C. Concentrations of IL-6 in cell-free supernatants were measured by ELISA. Results show the mean±SEM from five different eosinophil preparations. *; significant differences compared with medium alone (p<0.05).
P2Y2 receptor is likely involved in the eosinophils’ cytokine responses to ATP and MSU crystals
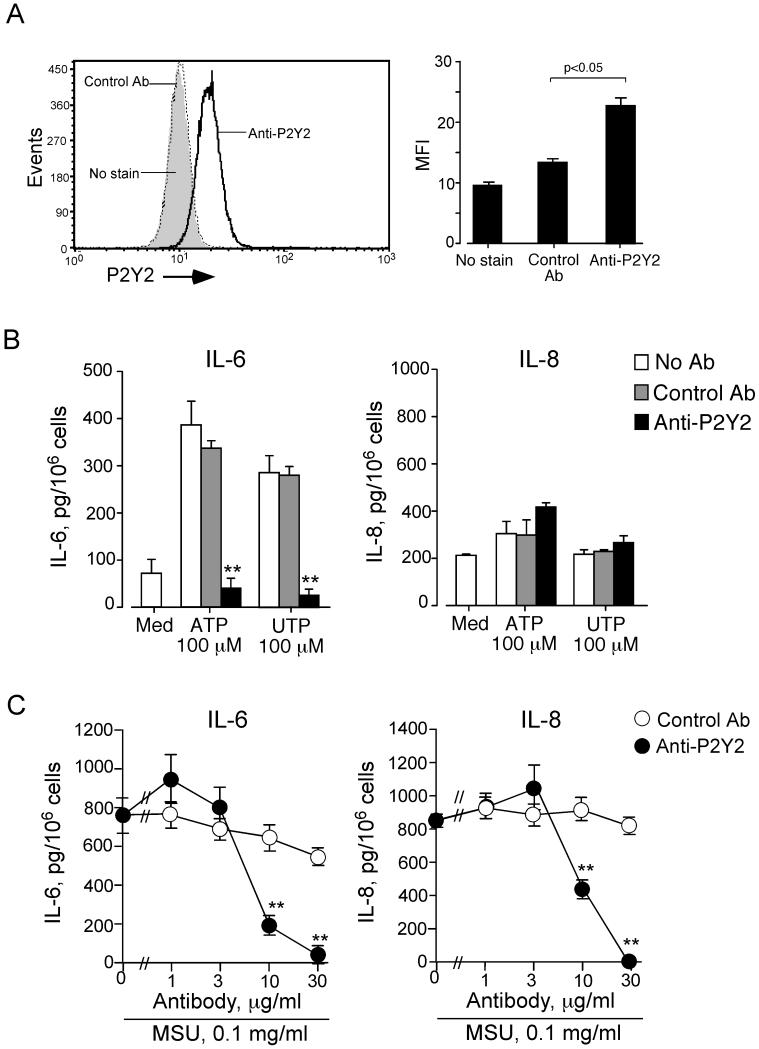
Because oATP and suramin may block other receptors besides the P2 receptors (e.g., the sphingosine-1-phosphate receptor-3) (36), we used an Ab and pharmacological agents to dissect the role for specific P2 receptors in the eosinophils’ responses to ATP and MSU crystals. Several nucleotide receptors, both P2X and P2Y subfamilies, have been detected in human eosinophils by mRNA levels (38). The P2Y2 receptor has been implicated in neutrophil chemotaxis mediated by endogenous ATP (34). Therefore, we suspected a role for the P2Y2 receptor in the eosinophils’ response to ATP. Flow cytometry consistently detected the surface expression of P2Y2 protein in freshly isolated human eosinophils from six different donors (Figure 7A).
FIGURE 7.
Human eosinophils express the P2Y2 receptor, and anti-P2Y2 Ab blocks the eosinophils’ responses to exogenous ATP, UTP and MSU crystals. (A) Eosinophils were incubated with rabbit anti-human P2Y2 Ab or control Ab. Cells were then incubated with FITC-conjugated goat anti-rabbit IgG, fixed with 1% paraformaldehyde, and analyzed by flow cytometry. Left panel shows a representative histogram. Right panel shows a summary with eosinophils from six different donors. Results show the mean±SEM of mean fluorescence intensity (MFI). *; significant differences compared with control Ab (p<0.05). (B) Eosinophils were preincubated with or without rabbit polyclonal anti-P2Y2 Ab or control Ab (20 μg/ml) for 15 minutes and cultured with medium alone, 100 μM ATP or 100 μM UTP for 24 h at 37 °C. Concentrations of IL-6 and IL-8 in the supernatants were measured by ELISA. Results show the mean±SEM from five different eosinophil preparations. **; significant differences compared with control Ab (p<0.01). (C) Eosinophils were preincubated with or without anti-P2Y2 Ab or control Ab (1-30 μg/ml) for 15 minutes and cultured with 0.1 mg/ml MSU crystal suspension for 24 h at 37 °C. Concentrations of IL-6 and IL-8 in the supernatants were measured by ELISA. Results show the mean±SEM from six different eosinophil preparations. **; significant differences compared with control Ab (p<0.01).
To examine the role of the P2Y2 receptor in the eosinophils’ response to ATP, we incubated eosinophils with anti-P2Y2 Ab or control Ab and stimulated the cells with 100 μM ATP. Anti-P2Y2 Ab nearly abolished the ATP-induced IL-6 production, but the control Ab did not (Figure 7B), suggesting that P2Y2 is involved in the eosinophils’ IL-6 production in response to exogenous ATP. Furthermore, UTP, an agonist for P2Y2 and P2Y4 receptors (39), potently induced IL-6 production, which was also abolished by anti-P2Y2 Ab (Figure 7B). Therefore, perturbation of eosinophil P2Y2 by exogenous ATP or UTP is likely sufficient to induce IL-6 production. In contrast, neither ATP nor UTP showed significant effects on IL-8 production (Figure 7B).
To examine the role of the P2Y2 receptor in the eosinophils’ IL-6 and IL-8 production in response to MSU crystals, we incubated eosinophils with MSU crystals in the presence of anti-P2Y2 Ab or control Ab. Anti-P2Y2 Ab inhibited the eosinophils’ production of both IL-6 and IL-8; control Ab showed minimal effects (Figure 7C). An antagonist for the P2X7 receptor, KN-62 (50 nM), showed no effects on IL-6 or IL-8 production induced by MSU crystals (data not shown). Altogether, by recognizing autocrine ATP, P2Y2 likely plays a pivotal role in the eosinophils’ cytokine response to MSU crystals.
ATP-mediated autocrine feedback loops are likely involved in MSU-induced eosinophil degranulation
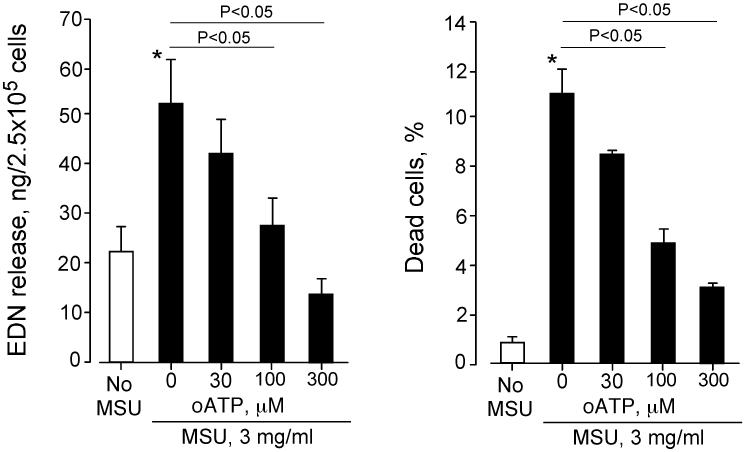
Because eosinophil viability decreased when cells were incubated with higher concentrations of MSU crystals (e.g. ≥ 1 mg/ml, Figure 4), we asked whether the EDN released at these concentrations of MSU (Figure 3) was a result of passive cell death or of ATP-mediated cell activation. To address this, we examined the effects a broad-range inhibitor of the P2 receptors, namely oATP, on EDN release and cell viability. Eosinophils incubated with 3 mg/ml MSU crystals showed significant EDN release and increased cell death, compared to those incubated without MSU (p<0.05, Figure 8). This MSU-induced EDN release was significantly inhibited by oATP in a concentration-dependent manner, suggesting that P2 receptors are involved in EDN release. Importantly, cell death was also inhibited by treating the cells with oATP, suggesting that loss of cell viability is also mediated by P2 receptors. These findings suggest that, at high concentrations, MSU crystals promote P2 receptor-dependent release of the eosinophil granule protein, EDN, and cell death.
FIGURE 8.
Blockade of P2 receptors with oATP inhibits EDN release and decreases cell death induced by MSU crystals. Eosinophils were preincubated with serial dilutions of oATP for 15 min and incubated with or without 3 mg/ml MSU crystal suspensions for 3 h at 37 °C. (A) EDN released into supernatants was measured by ELISA. (B) Eosinophil viability was examined by staining cells with PI and fluorescein diacetate and by analyzing at least 200 cells with an epifluorescent microscope. The percentages of dead cells (PI-positive and flurorescein diacetate-negative) were determined. Results show the mean±SEM from five different eosinophil preparations. *; significant difference compared with no MSU (p<0.05).
DISCUSSION
When we exposed human eosinophils to MSU crystals, these cells produced abundant quantities of IL-6 and several other cytokines and chemokines, including Th1 cytokines, pro-inflammatory cytokines, and chemokines, but no Th2 cytokines. Autocrine production of ATP likely plays a pivotal role in this process by providing a positive feedback, as demonstrated by the inhibitory effects of exogenous ATPase, broad-spectrum P2 antagonists and anti-P2Y2 Ab. Addition of exogenous ATP or blockade of endogenous ATPase activity also induced IL-6 production by eosinophils.
MSU crystals have been identified as a danger signal formed after release of uric acid from dying cells or damaged tissues (19, 40). The physiological significance of these eosinophil responses to MSU crystals or damaged tissues remains open for speculation. Eosinophils may participate in immunological surveys to recognize tissue damage or injury. For example, in mice injected with B16 melanoma cells, infiltration by eosinophils was observed in the necrotic regions of tumors even without acquired immune responses (26, 27). In naive mice injected intraperitoneally with alum, splenic eosinophils were recruited, and these eosinophils primed the early antigen-specific IgM antibody response (28). More recently, eosinophils have been shown to recognize a prototypic damage-associated molecular pattern molecule, HMGB-1, which is released by necrotic cells (41). Eosinophils release various cytokines (5, 42), and eosinophil-derived factor(s) may be involved in the recruitment of Th2-type CD4+ T cells in mouse models of allergic airway inflammation (8, 9). Furthermore, eosinophils have an antigen-presenting capability (43). Therefore, reacting to a danger signal, such as MSU crystals and HMGB-1, may be a part of the eosinophils’ innate immune responses, leading to the regulation of adaptive immunity in a Th2-type environment. Furthermore, the chronic and waxing-and-waning nature of eosinophilic airway diseases, such as asthma and chronic rhinosinusitis, may be explained by a self-perpetuating mechanism involving tissue injury, release of damage-associated molecules, and eosinophil infiltration and inflammatory mediator release.
Extracellular ATP has gained attention as a mediator of intercellular communication and an autocrine mediator (44, 45). An in vivo mouse model of asthma implicates extracellular ATP in the induction and maintenance of Th2-type airway inflammation (46). The intracellular concentration of ATP ranges between 5-10 mM, and cell death or even ‘cellular stress’ will release large amounts of ATP into the pericellular space (47, 48). While information is limited regarding triggers for ATP release by inflammatory cells (33), we observed rapid ATP release (within 1 minute) from eosinophils exposed to MSU crystals. A non-lytic mechanism likely mediates this fast response, because when eosinophils are exposed to MSU crystals and cultured for 24 h, they synthesize new proteins, namely IL-6 and IL-8 (Figure 2). Furthermore, eosinophil viability was not compromised by the concentration of MSU crystals (i.e. 0.1 mg/ml) that induced robust ATP release (Figures 4 and 5). A recent study suggests that MSU crystals induce the aggregation of cellular membranes, particularly the cholesterol components, without involving specific cellular receptors (32). Membrane deformation caused by mechanical stress induces the release of cellular ATP from mammalian cells (49, 50). Eosinophils exposed to MSU crystals may release ATP as a result of ‘cellular stress’ caused by alterations in cell membrane structure. The extracellular concentration of ATP is tightly regulated by ubiquitous ecto-ATPase activity (47). Because addition of an ecto-ATPase inhibitor, ARL 67156, alone was sufficient to induce IL-6 production (Figure 6) without any additional stimuli, eosinophils are themselves tightly regulated. Thus, a fine balance between ‘stress’-induced ATP release and ATP catalysis likely plays a pivotal role in regulating the eosinophils’ cytokine production. Whether a ‘stress’ status of eosinophils or reduced activity of ecto-ATPase activity or both may explain the increased effector functions of eosinophils in diseased organs (51) remains unknown.
Because suramin and oATP broadly block P2 receptors, the exact P2 receptors involved in mediating the effects of autocrine ATP in eosinophils remain to be elucidated. Expression of a P2 receptor, P2Y2, was clearly observed on the cell surface of freshly isolated eosinophils (Figure 7A). Both exogenous ATP and UTP induced robust IL-6 production (Figure 7B), which was abolished by anti-P2Y2 Ab. In contrast, previous findings showed that exogenous ATP, but not UTP, induced IL-8 production by eosinophils, which was partially inhibited by a P2X7 antagonist, KN-62 (52). Therefore, the agonists and intracellular signaling mechanisms required for IL-6 and IL-8 production by eosinophils are probably different. Interestingly, both IL-6 and IL-8 production induced by MSU crystals were inhibited by anti-P2Y2 Ab. Thus, activation of P2Y2 by endogenous ATP released by MSU-exposed eosinophils is likely sufficient to provoke robust IL-6 production. On the other hand, endogenous ATP may be necessary but insufficient to induce robust IL-8 production. Altogether, our observations suggest a pivotal role for P2Y2 in the eosinophils’ responses to endogenous and exogenous ATP and subsequent production of certain cytokines. These results need to be verified when more selective P2 receptor agonist/antagonist and/or gene-deficient animals become widely available. To date, human eosinophils express mRNA for P2X1, P2X4, P2X7, P2Y1, P2Y2, P2Y4, P2Y11, and P2Y14 (38, 53-55). For technical reasons, the siRNA knockdown of specific mRNA has been unsuccessful in human eosinophils (data not shown).
Eosinophils release EDN in response to relatively high concentrations (i.e. 1 mg/ml or higher) of MSU crystals (Figure 3). The magnitude of EDN release in response to MSU crystals was small and about 10-fold less than that induced by authentic agonists (e.g. PAF) for human eosinophils. Nonetheless, MSU-induced EDN release is likely mediated by autocrine ATP, similarly to cytokine production, because blockade of P2 receptors by oATP significantly inhibited EDN release (Figure 8). Increased cell viability when eosinophils are pretreated with oATP also suggests that eosinophil cell death induced by MSU crystals is likely the result of ATP-mediated cell activation and degranulation, but unlikely due to direct cytotoxicity by MSU crystals. Eosinophil degranulation activated by physiologic or immunological stimuli, such as immobilized sIgA or ligation of FcγRII, induces cell death (56, 57). Thus, eosinophil exposure to MSU crystals likely induces migration, robust cytokine production, and modest degranulation. Indeed, dissociation between cytokine production and EDN release was observed when eosinophils were incubated with soluble IgA immune complex or stimulated with ligation of FcγRII by soluble antibody (56, 57).
Overall, preformed MSU crystals were highly stimulatory for cytokine production by eosinophils (Figure 2), whereas soluble uric acid was not. Thus, ATP release and cytokine production probably requires physical interaction between particulate MSU crystals and eosinophils. Interestingly, soluble uric acid induced eosinophil chemotaxis in a gradient-dependent manner (Figure 1). Uric acid is relatively insoluble in sodium-rich biological fluids (up to 70 μg/ml); uric acid remains soluble in certain buffers up to 1,600 μg/ml (19, 58, 59). Because the cytosol contains high concentrations of uric acid (i.e., 4,000 μg/ml) (19), the local environmental around dying cells should become supersaturated with uric acid when cells lyse, favoring the formation of MSU crystals. Our data suggest that a chemical phase transition of uric acid could be key in triggering distinct cellular functions (e.g. chemotaxis vs. cytokine production) of human eosinophils. Perhaps, soluble uric acid in tissues relatively remote from dying cells may be chemotactic, but MSU crystals in close proximity to the dying cells may cause cytokine production.
In conclusion, human eosinophils respond to an endogenous danger signal, MSU crystals, resulting in robust production of a wide variety of cytokines and chemokines. Finding the precise P2X and P2Y receptor(s) involved in the eosinophils’ recognition of autocrine ATP in mediating these responses will be challenging, but could lead to new therapeutic approaches that suppress eosinophilic inflammation and disease pathology.
ACKNOWLEDGEMENTS
The authors thank Ms. Cheryl Adolphson for editorial assistance and Ms. LuRaye Eischens for secretarial assistance. The authors also thank Mr. James Checkel for technical assistance.
Footnotes
Supported in part by Grant AI34486 from the National Institutes of Health and by the Mayo Foundation
- ATPγS
- adenosine 5′-(3-thiotriphosphate) tetralithium salt
- alum
- aluminum hydroxide
- oATP
- ATP periodate oxidized sodium salt
- DAMP
- damage-associated molecular pattern
- EDN
- eosinophil-derived neurotoxin
- EGF
- epidermal growth factor
- ARL 67156
- exo-ATPase inhibitor
- FGF
- fibroblast growth factor
- HMGB-1
- high-mobility group box 1 protein
- [Ca2+]i
- intracellular calcium concentration
- MSU
- monosodium urate
- PAMP
- pathogen-associated molecular pattern
- PAF
- platelet-activating factor
- PI
- propidium iodide
- VEGF
- vascular endothelial growth factor
REFERENCES
- 1.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv. Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J. Allergy Clin. Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Lacy P, Moqbel R. Eosinophil cytokines. Chem. Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 6.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–482. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J. Exp. Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 11.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 12.Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J. Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J. Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- 14.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 15.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 17.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin. Exp. Allergy. 2009;39:12–19. doi: 10.1111/j.1365-2222.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 20.Ide M, Weiler D, Kita H, Gleich GJ. Ammonium chloride exposure inhibits cytokine-mediated eosinophil survival. J. Immunol. Methods. 1994;168:187–196. doi: 10.1016/0022-1759(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 21.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. Am. J. Respir. Cell Mol. Biol. 1997;16:455–463. doi: 10.1165/ajrcmb.16.4.9115757. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Horie S, Gleich GJ, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J. Allergy Clin. Immunol. 1996;98:371–381. doi: 10.1016/s0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- 24.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J. Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 26.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 28.Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J. Leukoc. Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J. Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 30.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J. Allergy Clin. Immunol. 2003;111:923–932. quiz 933. [PubMed] [Google Scholar]
- 31.Stern M, Meagher L, Savill J, Haslett C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J. Immunol. 1992;148:3543–3549. [PubMed] [Google Scholar]
- 32.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 35.Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, Wagner JA. Augmentation of cutaneous immune responses by ATP gamma S: purinergic agonists define a novel class of immunologic adjuvants. J. Immunol. 2005;174:7725–7731. doi: 10.4049/jimmunol.174.12.7725. [DOI] [PubMed] [Google Scholar]
- 36.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 38.Mohanty JG, Raible DG, McDermott LJ, Pelleg A, Schulman ES. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: activation of purinergic P2Y receptors. J. Allergy Clin. Immunol. 2001;107:849–855. doi: 10.1067/mai.2001.114658. [DOI] [PubMed] [Google Scholar]
- 39.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 2009;183:5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- 42.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J. Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 45.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 46.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr., Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 47.Bours MJ, Swennen EL, Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 49.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem. Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 50.Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br. J. Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busse WW, Nagata M, Sedgwick JB. Characteristics of airway eosinophils. Eur. Respir. J. Suppl. 1996;22:132s–135s. [PubMed] [Google Scholar]
- 52.Idzko M, Panther E, Bremer HC, Sorichter S, Luttmann W, Virchow CJ, Jr., Di Virgilio F, Herouy Y, Norgauer J, Ferrari D. Stimulation of P2 purinergic receptors induces the release of eosinophil cationic protein and interleukin-8 from human eosinophils. Br. J. Pharmacol. 2003;138:1244–1250. doi: 10.1038/sj.bjp.0705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di Virgilio F, Luttmann W. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486:217–224. doi: 10.1016/s0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- 54.Ferrari D, la Sala A, Panther E, Norgauer J, Di Virgilio F, Idzko M. Activation of human eosinophils via P2 receptors: novel findings and future perspectives. J. Leukoc. Biol. 2006;79:7–15. doi: 10.1189/jlb.0505286. [DOI] [PubMed] [Google Scholar]
- 55.Idzko M, Dichmann S, Panther E, Ferrari D, Herouy Y, Virchow C, Jr., Luttmann W, Di Virgilio F, Norgauer J. Functional characterization of P2Y and P2X receptors in human eosinophils. J. Cell. Physiol. 2001;188:329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- 56.Bartemes KR, Cooper KM, Drain KL, Kita H. Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions. J. Allergy Clin. Immunol. 2005;116:827–835. doi: 10.1016/j.jaci.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Kim JT, Schimming AW, Kita H. Ligation of Fc gamma RII (CD32) pivotally regulates survival of human eosinophils. J. Immunol. 1999;162:4253–4259. [PubMed] [Google Scholar]
- 58.Iwata H, Nishio S, Yokoyama M, Matsumoto A, Takeuchi M. Solubility of uric acid and supersaturation of monosodium urate: why is uric acid so highly soluble in urine? J. Urol. 1989;142:1095–1098. doi: 10.1016/s0022-5347(17)39003-1. [DOI] [PubMed] [Google Scholar]
- 59.Kippen I, Klinenberg JR, Weinberger A, Wilcox WR. Factors affecting urate solubility in vitro. Ann. Rheum. Dis. 1974;33:313–317. doi: 10.1136/ard.33.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]