Abstract
Although risk assessments are typically conducted on a chemical-by-chemical basis, the 1996 Food Quality Protection Act required the US Environmental Protection Agency to consider cumulative risk of chemicals that act via a common mechanism of toxicity. To this end, we are conducting studies with mixtures of chemicals to elucidate mechanisms of joint action at the systemic level with the end goal of providing a framework for assessing the cumulative effects of reproductive toxicants.
Previous mixture studies conducted with antiandrogenic chemicals are reviewed briefly and two new studies are described in detail. In all binary mixture studies, rats were dosed during pregnancy with chemicals, singly or in pairs at dosage levels equivalent to approximately one half of the ED50 for hypospadias or epididymal agenesis. The binary mixtures included: androgen receptor (AR) antagonists (vinclozolin plus procymidone), phthalate esters (DBP plus BBP and DEHP plus DBP), a phthalate ester plus an AR antagonist (DBP plus procymidone), a mixed mechanism androgen signaling disruptor (linuron) plus BBP, and two chemicals which disrupt epididymal differentiation through entirely different toxicity pathways: DBP (AR pathway) plus 2,3,7,8 TCDD (AhR pathway). We also conducted multi-component mixture studies combining several “antiandrogens” together. In the first study, seven chemicals (four pesticides and three phthalates) that elicit antiandrogenic effects at two different sites in the androgen signaling pathway (i.e. AR antagonist or inhibition of androgen synthesis) were combined. In the second study, three additional phthalates were added to make a ten chemical mixture.
In both the binary mixture studies and the multi-component mixture studies, chemicals that targeted male reproductive tract development displayed cumulative effects that exceeded predictions based upon a response addition model and most often were in accordance with predictions based upon dose addition models. In summary, our results indicate that compounds that act by disparate mechanisms of toxicity to disrupt the dynamic interactions among the interconnected signaling pathways in differentiating tissues produce cumulative dose-additive effects, regardless of the mechanism or mode of action of the individual mixture component.
Keywords: Reproductive system; Male reproduction; Antiandrogens and 2,3,7,8 TCDD
Introduction
Although risk assessments have been historically conducted on a chemical-by-chemical basis, regulatory agencies are beginning to consider cumulative risk of chemicals. However, a consistent framework for cumulative risk assessment has yet to be developed. It is well known that humans (Calafat et al. 2008; Eskenazi et al. 1999; Landrigan et al. 1999; Silva 2004; Wolff et al. 2008; Wolff et al. 2007), fish (Ankley et al. 2007; Jobling et al. 1998; Jobling and Tyler 2006; Jobling et al. 2006) and wildlife (Hall and Thomas 2007) are continuously exposed to multiple contaminants. The chemicals found in some aquatic systems include pesticides (Hela et al. 2005; Jaspers et al. 2006) and industrial chemicals (Hall and Thomas 2007), as well as pharmaceuticals and hormones (Durhan et al. 2006; Kolpin et al. 2002).
The effects of mixtures of chemicals like the ubiquitous phthalates are of concern since humans are exposed to multiple phthalates at one time (Silva 2004; Wolff et al. 2007). In order to address this issue, in 2006 the U.S. Environmental Protection Agency (EPA) requested that the National Academy of Sciences (NAS) establish a panel to provide the EPA with recommendations on whether to perform a cumulative risk assessment of the phthalates. This review will present the major conclusions of the NAS panel (Box from p 116 of the NAS report) and discuss the data from our laboratory on the reproductive effects of mixtures.
Our working hypothesis is that chemicals that disrupt a common system or tissue during development contribute to cumulative toxicity and should therefore, be included in cumulative risk assessments. The results of our studies support this hypothesis and are in agreement with the conclusions of the NAS Report (2008) (Table 1). This represents a shift from the current cumulative risk assessment process which only includes chemicals which share a narrowly defined mechanism of action.
Table 1.
Main points from National Academy of Science report: “Phthalates and Cumulative Risk Assessment: The Task Ahead”
|
As a result of growing concerns about mixtures, the field of “mixtures toxicology” is emerging as an area of increasing scientific and regulatory focus. For example, in 1996 the EPA began considering the cumulative risk of chemicals that act via a common mechanism of toxicity as mandated in the Food Quality Protection Act (FQPA). The EPA’s Offices of Water and Research and Development and the EPA Superfund, Solid Waste and Air Programs also have ongoing programs in the area of mixtures toxicology. In this regard, the research from our laboratory, described herein, is intended to contribute to the development of a guidance framework for assessing cumulative risk to reproduction and development from exposures during pregnancy.
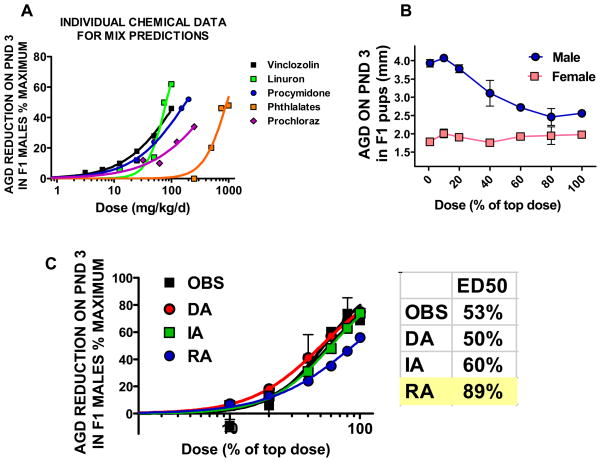
Our research has included mixtures of pesticides, phthalates and 2,3,7,8 TCDD. These chemicals disrupt sexual differentiation by acting as androgen receptor (AR) antagonists, inhibitors of fetal testosterone synthesis or as an aryl hydrocarbon receptor (AhR) agonist (Figures 1 and 2).
Figure 1.
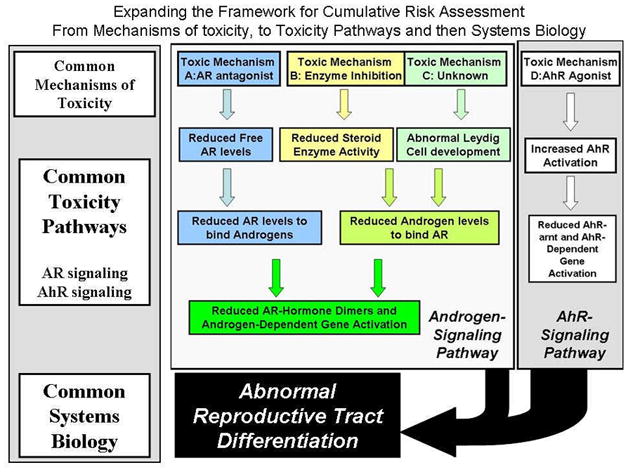
A diagram showing how different mechanisms of toxicity and disruption of diverse toxicity pathways (Androgen- versus Ah receptor signaling pathways) converge as an integrated network of pathways to disrupt the development of the reproductive tract during a critical developmental period.
Figure 2.
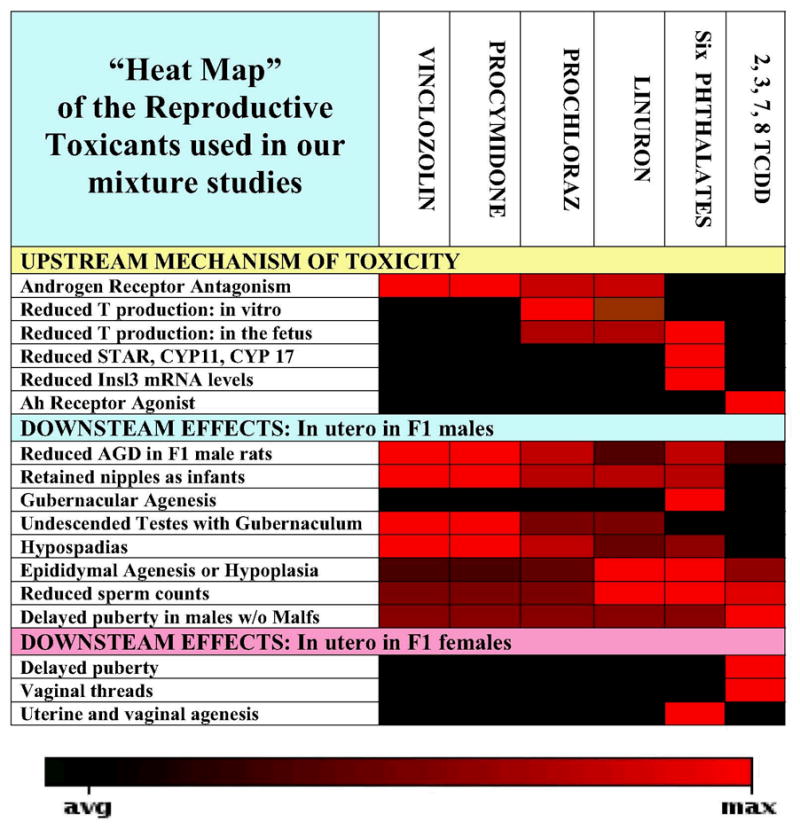
“Heat map” displaying the intensity of effects of each chemical in vitro, in short-term in vivo screens and on F1 male and female offspring after exposure in utero during sexual differentiation. The red shading indicates stronger effects whereas the black areas indicate the absence of effects. Reviewing the columns associated with each chemical describes the 1) known mechanisms of toxicity as determined from in vitro and short-term in vivo screening studies and 2) the overall phenotype in the male offspring after exposure during fetal life. Comparing the different chemicals by rows (the endpoints) allows one to compare the relative potencies displayed by the toxicants to one another. For each endpoint, we predict that all the chemicals with shading (light red to dark red) will interact jointly when combined in a mixture, but those with black squares will have no effect when included in the mixture.
We initially conducted a series of binary mixture studies exposing pregnant dams during fetal sexual differentiation with pairs of chemicals (Gray et al. 2001b; Hotchkiss et al. 2004a; Howdeshell et al. 2007; Howdeshell et al. 2008b; Rider et al. 2009). In the binary studies, rats were dosed with chemicals singly or in pairs at dosage levels equivalent to about one half of the ED50 for hypospadias or epididymal agenesis (2 × 2 factorial designs). We predicted that by itself each chemical in a pair would cause a minimal rate of malformations, whereas when two chemicals were mixed together they would produce predictable, dose-additive effects on common target tissues. We found that the binary combinations produced cumulative, dose-additive effects on the androgen-dependent tissues that were a common component of each chemical’s phenotype. Several of the studies have been reviewed in detail previously (Gray 2002; Gray et al. 2001a; Howdeshell et al. 2008b; Rider et al. 2008; Rider et al. 2009) and will only be briefly reviewed, whereas the phthalate plus dioxin binary study is new and will be presented in more detail.
In addition to the binary studies, we also have conducted three mixture studies with larger numbers of chemicals. One study combined five phthalates together in the mixture to determine if they produced dose-additive effects on fetal testosterone production (Howdeshell et al. 2008b), fetal testis gene expression and postnatal development of the male and female offspring. These phthalates all shared a common mechanism of toxicity. The remaining two multi-component mixture studies combined chemicals that elicit antiandrogenic effects at two different sites in the androgen signaling pathway (i.e. AR antagonist or inhibition of androgen synthesis). One study combined seven chemicals together and the second combined 10 chemicals in the mixture.
The paper that follows will 1) briefly describe the mechanisms and modes of toxicity in vitro and in vivo of the individual chemicals that we selected to study in our research program since these have been reviewed in detail previously (Gray et al. 2001b; Howdeshell et al. 2008b; Rider et al. 2008; Rider et al. 2009), 2) describe the mathematical modeling procedures that we use to describe the effects of the individual chemicals and derive predictions for how the mixtures will behave, 3) present a summary of the results of our mixture studies on chemicals that act via common mechanisms of toxicity (Section A), chemicals that act via disparate mechanisms of toxicity but disrupt the same signaling pathway (Section B), and chemicals that disrupt common developing tissues via alteration of diverse signaling pathways (Section C), and 4) describe a proposed framework for cumulative risk assessment based upon disruption of a common developing system. The review will include new data from two studies, one a multi-component mixture study of ten chemicals that disrupt androgen signaling by diverse mechanisms of toxicity and another study combining chemicals that disrupt different signaling pathways, the aryl hydrocarbon receptor (AhR) agonist 2,3,7,8 TCDD and dibutyl phthalate that reduces fetal testosterone levels.
Mechanisms and modes of action of the individual chemicals used in mixture studies
Over the last several decades our laboratory has studied the postnatal effects of in utero exposure to a variety of environmental chemicals with diverse endocrine and non-endocrine mechanisms of reproductive toxicity. We are now using the dose response information from these studies to design mixture studies to determine how chemicals from different classes with similar and diverse mechanisms and modes of action interact in the mixture. The objective of our research is to define a framework for conducting cumulative risk assessments of reproductive toxicants.
The chemicals selected for our current mixture studies are shown in Figure 2. In this figure, the red indicates stronger effects whereas the black areas indicate the absence of effects. Reviewing the columns associated with each chemical describes the 1) known mechanisms of toxicity as determined from in vitro and short-term in vivo screening studies and 2) the overall phenotype in the male offspring after exposure during fetal life. Comparing the different chemicals by rows (the endpoints) allows one to compare the relative potencies displayed by the toxicants to one another. For each endpoint, we predict that all the chemicals with shading (dark red to light red) will interact jointly when combined in a mixture, but those with black squares will have no effect when included in the mixture. For example, of the chemicals in this chart, only the phthalates reduce insulin-like peptide hormone-3 (insl3) and cause gubernacular agenesis. Therefore, combining a phthalate with any of the other chemicals in the chart will not enhance the phthalate-induced reduction in insl3 mRNA levels in the fetal testis or increase the incidence of gubernacular agenesis.
Dicarboximide fungicides: Vinclozolin and Procymidone
Of the dicarboximide fungicides, vinclozolin (Kelce et al. 1994), iprodione (Blystone et al, in prep) and procymidone (Hosokawa et al. 1993; Nellemann et al. 2003; Ostby et al. 1999; Vinggaard et al. 1999) act as AR antagonists in vitro and/or in vivo. These pesticides, or their metabolites, competitively inhibit the binding of androgens to AR which leads to an inhibition of androgen-dependent gene expression in vitro and in vivo (Kelce and Wilson 1997).
Linuron (herbicide)
While some toxicants disrupt sexual differentiation predominantly via one mechanism of toxicity (i.e. AR antagonists or inhibitors of testosterone synthesis), some pesticides including linuron and prochloraz act via dual mechanisms of toxicity. These pesticides display AR antagonist activity and inhibit testosterone synthesis with varying potencies.
The herbicide linuron is an AR antagonist in vitro (Lambright et al. 2000; McIntyre et al. 2002a; McIntyre et al. 2002b; McIntyre et al. 2000; Turner et al. 2003). In contrast to some AR antagonists, neither short term (Lambright et al. 2000; O’Connor et al. 2002) nor long-term (Gray et al. 1999) administration with linuron induces elevated serum LH levels.
Prochloraz (fungicide)
Prochloraz is a fungicide that also disrupts reproductive development and function by several modes of action (Noriega et al. 2005; Vinggaard et al. 2005; Vinggaard et al. 2006). Prochloraz inhibits the steroidogenic enzymes 17, 20 lyase and aromatase and it also is an AR antagonist (Blystone et al. 2007). In a study in which rat dams were dosed from GD 14–18, Wilson et al. (Wilson et al. 2004) found that prochloraz reduced fetal testis testosterone levels and increased progesterone production tenfold on GD 18 without affecting Leydig cell insl3 mRNA levels.
Phthalates
In utero, some phthalates alter male and female reproductive tract differentiation via unknown mechanisms of action. The mode of action in the male involves altered Leydig cell migration and differentiation and abnormal gonocytes development (Hallmark et al. 2007; Mahood et al. 2005; Mahood et al. 2006; Parks et al. 2000). The Leydig cell alterations result in reductions in fetal testis testosterone production, and mRNA levels for key proteins in the steroidogenic pathway including StAR and CYP11, as well as insl-3, which is critical for gubernacular development and testis descent (Hughes and Acerini 2008; Kumagai et al. 2002).
2,3,7,8 Tetrachlorodibenzo Dioxin (TCDD)
TCDD binds with high affinity to the cytosolic AhR. This basic-helix-loop-helix receptor acts as a transcription factor in a manner similar to the steroid hormone receptors. Although the normal role of this transcription factor in development is unknown, gestational TCDD treatment induces malformations of the external genitalia and subfertility in female rat (Gray and Ostby 1995) (Flaws et al. 1997; Gray et al. 1997a) and hamster (Wolf et al. 1999) offspring and alters reproductive development of the male rat (Gray et al. 1995; Gray et al. 1997b) (Mably et al. 1992) (Simanainen et al. 2004) and hamster (Gray et al. 1995).
Several studies have shown that TCDD administration does not reduce adult (Gray et al. 1995; Theobald et al. 2000) or fetal androgen levels in the male rat or mouse (Ko et al. 2004) (Haavisto et al. 2001; Haavisto et al. 2006), even though several of the effects in the male offspring include suppressed development of some androgen-dependent tissues. In mice, it is known that TCDD directly inhibits the androgen-dependent processes by which the urogenital sinus of fetal mice forms prostatic epithelial buds (Lin et al. 2004) without affecting androgen levels (Akingbemi et al. 2004). Additional mechanistic studies proposed that TCDD acted directly on AhR, ARNT, and AhR-induced transcripts in the periprostatic mesenchyme (Vezina et al. 2008; Vezina et al. 2009). This tissue intimately contacts urogenital sinus epithelium where buds are specified and they proposed that activation of AhR signaling disrupted dorsoventral patterning of the urogenital sinus, reprogramming the areas where prostatic buds are specified, and prostate lobes are formed.
Administration of TCDD on the 15th day of gestation (GD 15) at doses lower than or equal to 1 μg/kg both demasculinizes and feminizes male rat reproductive morphology and behavior. In our first series of studies, Long Evans Hooded and Holtzman rats were dosed by gavage with 1 μg TCDD/kg on GD 8 (a period of major organogenesis), or Syrian hamsters, a species relatively insensitive to the lethal effects of TCDD, were dosed on GD 11 (a period equivalent to GD 15 in the rat), with TCDD at 2 μg/kg. In Long Evans and Holtzman F1 male rats exposed on GD 15 or hamsters exposed on GD 11, puberty (preputial separation) was delayed by about 3 days, ejaculated sperm counts were reduced by at least 58% and epididymal sperm storage was reduced by 30%. Testicular sperm production was less affected. The sex accessory glands were also reduced in size in Long Evans offspring treated on GD 15 in spite of the fact that serum testosterone levels, testosterone production by the testis in vitro, and AR levels were not reduced. Some reproductive measures, like anogenital distance and male sex behavior, were altered by TCDD-treatment in rat but not hamster offspring (Gray et al. 1995).
In the F1 female offspring, in utero TCDD exposure (1 μg/kg on gestational day (GD) 15) induces cleft phallus, vaginal thread formation and reduces ovarian weight (Flaws et al. 1997; Gray and Ostby 1995; Gray et al. 1997b). TCDD treatment on GD 15 at 0, 0.05, 0.20 or 0.80 μg/kg, delays vaginal opening and induces malformations of the external genitalia in the female progeny (cleft phallus and persistent vaginal thread formation (Flaws et al. 1997; Gray and Ostby 1995; Gray et al. 1997b).
Mixture Modeling
Over the past several decades, research in several laboratories including our own has described the effects of individual chemicals on the reproductive development of male rats. Individual chemical data can be used in mathematical models to make predictions about the potential effects of mixtures on male reproductive tract development. Predicted mixture responses can then be compared to the observed effects of the mixtures to determine the type of joint action (dose addition, response addition, synergy, antagonism) exhibited by the mixture.
For our mixture studies, most of the individual data were compiled from studies conducted in our laboratory over the past twenty years (see individual chemical data in Rider et al. 2008). Occasionally, data from the literature were used to increase the robustness of the individual chemical dose-response analyses (i.e. add data points to a partial dose-response curve). While these historical data included studies conducted by different researchers with several rat strains and slightly different dosing schedules, all studies included exposure to the chemical during the critical in utero window of male rat reproductive tract differentiation. The use of historical data as input for mixture modeling introduces a certain degree of variability, but is necessary given that developmental studies with an in utero exposure and a postnatal evaluation of the offspring are resource intensive precluding the inclusion of all the individual dose response groups within the mixture study. In addition, we found surprisingly good agreement between overlapping studies within our lab and between labs. We attribute this agreement to the relatively standardized procedures for measuring organ weights and identifying reproductive tract malformations among labs from which data were used. The greatest limitation in the modeling of mixtures of chemicals in utero is that complete dose response curves cannot be generated for each individual chemical for many endpoints because the systemic toxicity of some the chemicals restrains using high dosage levels.
Assumptions are a necessary part of risk assessment as data sets are often incomplete. Therefore, the utility of a given mixture toxicity model depends in part on whether it can consistently produce accurate predictions even when input data contain logical assumptions. We present our mixture work here as a body of evidence supporting the presumption that the dose addition model consistently provides a relatively good fit to observed data despite the use of imperfect input data.
Using GraphPad Prism 5.0 software, we transformed the data to fit a 0 to 100% scale. For continuous endpoints (AGD and organ weights), we converted the data to percent change from the control value and Prism input included the mean, standard error and sample size for each group. For malformation data, we presented the data as percent incidence. We then graphed the data on a log-linear scale and fit the data with a sigmoidal (variable slope) equation in Graphpad Prism (see Equation 1):
| Equation 1 |
where Y is the response, X is the chemical dose, Top and Bottom refer to the minimum and maximum effect calculated from the data and ED50 is the exposure dose eliciting a 50 % response. The parameters (Hill slope and ED50) generated from the logistic fit to the individual chemical data were used in models to make predictions of the mixture responses.
Predicted responses from models versus observed responses
We modeled the observed mixture responses (means, standard errors and sample sizes) using Prism software and generated predictions of the mixture effects with three types of models (described in more detail in (Rider et al. 2008).
dose addition
response addition, and
integrated addition
The dose addition model is commonly applied to mixtures of chemicals that have the same mechanism of action (Altenburger et al. 2000; Silva et al. 2002), whereas the response addition model has been proposed as an alternative for use with mixtures containing chemicals with different mechanisms of action (Backhaus et al. 2000). The integrated addition model represents a melding of the dose and response addition models in an attempt to deal with mixtures containing chemicals with similar and dissimilar mechanisms of toxicity (Altenburger et al. 2005; Rider et al. 2008; Rider and LeBlanc 2005).
Application of the dose addition (DA) model requires putting chemicals in equivalent terms by accounting for the individual chemical potencies. This is done by dividing the concentration of the individual chemical in the mixture by the ED50 of that chemical (see equation 2). Once this is accomplished, these adjusted doses can be added to arrive at a total mixture dose. The predicted response is then calculated by determining the response level that corresponds with the total mixture dose. In summary, the slope and ED50 derived from individual chemical dose-response analyses are input into the dose addition equation to calculate the overall response of the mixture associated with any given mixture dose.
The dose addition equation that we used to calculate predicted responses of mixtures is:
| Equation 2 |
where R is the response to the mixture, Di is the concentration of chemical i in the mixture, ED50i is the concentration of chemical i that causes a 50% response, and ρ′ is the average Hill slope (i.e. slope associated with a logistic fit of the individual chemical dose-response curve) associated with the chemicals.
The response addition (RA) model or independent joint action model has been suggested as the more appropriate model for mixtures of chemicals with different mechanisms of action (Greco et al. 1992). The equation for response addition is based on probability theory and is expressed as:
| Equation 3 |
Because this equation is specific to situations in which response level increases with increasing dose, endpoints were converted to fit this criterion (e.g. AGD, which decreases in males with increasing antiandrogen dose, was expressed as change from control).
The integrated addition (IA) model provides an intermediate model that incorporates both dose and response addition (Altenburger et al. 2005; Rider and LeBlanc 2005; Teuschler et al. 2004). Chemicals with the same mechanism of action are grouped and the total dose associated with each group is calculated using dose addition. The groups are then combined using response addition.
The integrated addition model is expressed mathematically as:
| Equation 4 |
Identifying a Framework for Cumulative Risk Assessment
The following discussion will present a summary of the data that supports the conclusions of the NAS report and our hypothesis that the framework for cumulative risk assessments should be broadly based upon disruption of common systems or target tissues rather than narrowly based upon specific mechanisms of toxicity.
Mixtures Section A. Chemicals that act via common mechanisms of toxicity
androgen receptor (AR) antagonists (vinclozolin plus procymidone)
phthalate esters (DBP plus BBP)
phthalate esters (DEHP plus DBP)
Mixtures Section B. Chemicals that disrupt a common pathway via disparate mechanisms of toxicity (i.e. disruption of the androgen signaling pathway)
a phthalate ester plus an AR antagonist (DBP plus procymidone)
a pesticide plus a phthalate (linuron plus BBP)
a multi-component mixture of seven chemicals (vinclozolin, procymidone, prochloraz, linuron, DBP, BBP, and DEHP)
a multi-component mixture study of ten chemicals (DiHP, DPP and DiBP and the chemicals used in the seven chemical study (above).
Mixtures Section C. Chemicals that disrupt a common tissue via different signaling pathways and diverse mechanisms of toxicity (i.e. disruption of the androgen and AhR signaling pathways in the fetal male rat reproductive tract)
2,3,7,8 tetrachlorodibenzo dioxin plus a phthalate (TCDD plus DBP).
Several different frameworks have been proposed for including chemicals in a cumulative risk assessment ranging from one limited to chemicals that clearly display a common cellular and molecular mechanism of action, to a much broader framework based upon disruption of a common target tissue (Figures 1 and 2). The broader framework was recommended by a recent NAS panel report (Table 1). The frameworks presented here include grouping chemicals in a cumulative risk assessment based upon 1) common mechanisms of toxicity, 2) disruption of common signaling pathways (a toxicity pathway approach) or 3) a broader approach, based upon disruption of common reproductive target tissues in the fetus (a systems approach including multiple signaling pathways) (Figure 1).
If cumulative risk assessments were conducted on the 11 chemicals (six phthalates, four pesticides and TCDD) discussed herein using the framework based upon common mechanisms of toxicity, then they would be clustered into at least 4 different assessments (i.e. 1. AR antagonists, 2. inhibitors of fetal testosterone production, 3. dual mechanism antiandrogens, and 4. AhR-mediated toxicant). The assumption would be that chemicals with different mechanisms of toxicity interact independently and that the mixture outcome could be predicted by response addition modeling, whereas dose addition models would over-predict the effects. In reviewing the data one finds that dose addition models consistently yield more accurate predictions than those from either integrated addition or response addition models.
Grouping the chemicals for cumulative risk assessments based upon disruption of a common signaling pathway or toxicity pathway rather than common mechanisms of toxicity assumes that chemicals that disrupt androgen signaling will act jointly and the effects can be predicted by dose addition modeling but these chemicals would act independently from those that disrupt the same tissue via the AhR pathway. This would result in the 11 chemicals in the current study being grouped into two independent risk assessments (i.e. 1. androgen signaling disruptors and 2. AhR signaling disruptor).
Grouping the chemicals for cumulative risk assessments based upon disruption of a common system or target tissue via multiple pathways and mechanisms of toxicity assumes that chemicals that disrupt androgen signaling and AhR signaling will act jointly and the effects of all 11 chemicals can be predicted by a single dose addition model. This is the approach recommended in the NAS report (Table 1), and the approach supported by our studies. Under this framework, all 11 chemicals in our studies would be included in a single cumulative risk assessment (i.e. chemicals that target the developing reproductive tract).
Mixtures Section A. Disruption of reproductive differentiation by common mechanisms of toxicity
A.1 Mixtures of AR antagonists, vinclozolin and procymidone
In the late 1990s and in 2005 the EPA began an examination of whether some or all members of the dicarboximide class of fungicides, which includes vinclozolin, iprodione and procymidone, shared a common mechanism of toxicity. At that time, the scientific information on the mechanisms of toxicity of the imide group of dicarboximide fungicides was incomplete and the EPA did not, therefore, recommend a cumulative risk assessment of vinclozolin, procymidone and iprodione (http://www.epa.gov/opp00001/reregistration/vinclozolin/) (http://www.epa.gov/oppsrrd1/REDs/procymidone_tred.pdf). Since then several studies from our laboratory and other laboratories have been completed that address these uncertainties. These studies demonstrate that vinclozolin and procymidone share a common mechanism of toxicity and interact in a cumulative manner (Gray et al. 2001b; Nellemann et al. 2003). In our AR antagonist binary study (Gray et al. 2001b), in utero exposure to vinclozolin alone resulted in 10% incidence of hypospadias and 0% incidence of vaginal pouch development in male rats, while procymidone exposure resulted in 0% incidence of either malformation. The combination exposure, however, resulted in 96% incidence of hypospadias and 54% incidence of vaginal pouch in treated animals.
A.2 Alteration of fetal Leydig cell differentiation and hormone production by phthalates
The effects of mixtures of phthalates are a concern since humans are exposed to multiple phthalates at one time (Silva 2004; Wolff et al. 2007). In order to address this issue, in 2006 the EPA requested that the NAS establish a panel to provide the EPA with recommendations on how to address the cumulative effects of the phthalates. The data from several of the phthalate mixture studies presented herein were given to the NAS panel for their reanalysis and evaluation (http://www8.nationalacademies.org/cp/projectview.aspx?key=48860).
We conducted three studies with mixtures of phthalate esters. The first study combined two phthalate esters with a common active metabolite (DBP and BBP). The second study combined two phthalate esters with different active metabolites (DEHP and DBP) (Howdeshell et al. 2007). In the third phthalate mixture study, we combined five phthalates and examined the effects of the mixture on fetal testosterone synthesis and gene expression levels and postnatal effects in the male and female offspring (Howdeshell et al. 2008c) (Howdeshell et al in prep). The results from all three of these studies were consistent with predictions based on a dose addition model for inducing abnormal development in utero in male rats.
Exposure to the individual chemicals resulted in no malformations or low incidences of malformations whereas the combination exposures typically resulted in 50% or greater incidences of malformations (Howdeshell et al. 2007).
In the third mixture study, we assessed the cumulative effects on fetal testosterone production, gene expression levels and postnatal development of the male and female offspring following in utero exposure to a mixture of five phthalates: DBP, di-iso-butyl phthalate (DiBP), BBP, diethyl hexyl phthalate (DEHP), and di-n-pentyl (DPP) (Howdeshell et al. 2008c). First, we characterized the dose response effects of six individual phthalates [BBP, DBP, DEHP, diethyl phthalate (DEP), DiBP, and DPP] on gestation day (GD) 18 testicular testosterone production following exposure of Sprague Dawley rats on GD8-18. BBP, DBP, DEHP, and DiBP were equipotent (ED50 of 440 ± 16 mg/kg/d), DPP was about 3-fold more potent (ED50=130 mg/kg/d) and DEP had no effect on fetal testosterone production.
We hypothesized that co-administration of the five antiandrogenic phthalates would reduce testosterone production and insulin-like-3, StAR and Cyp11a mRNA levels and induce male reproductive tract malformations in a dose-additive fashion since they act via a common mode of toxicity. In the five phthalate mixture study, dams were dosed at 100, 80, 60, 40, 20, 10, 5 or 0% of the mixture. The top dose contained 1300 mg of total phthalates/kg/d including BBP, DBP, DEHP, DiBP (300 mg/kg/d per chemical) and DPP (100 mg DPP/kg/d). This mixture ratio was selected such that each phthalate would contribute equally to the reduction in testosterone. As hypothesized, fetal testosterone production (Howdeshell et al. 2008c) and gene expression levels were reduced and male reproductive tract malformations were increased (Howdeshell et al in prep) in a dose-additive manner.
From these mixture studies, we conclude that above pairs of chemicals target the androgen signaling pathway via the same mechanism of action and produced dose additive effects when presented as mixtures. These results indicate that these chemicals would be good candidates for cumulative risk assessment and supports the use of the dose addition model for determining the effects of these mixtures.
Mixtures Section B. Disruption of the androgen signaling pathway by disparate mechanisms of toxicity: AR antagonism and inhibition of fetal testosterone synthesis
Environmental chemicals can disrupt the androgen signaling pathway in the male rat fetus during sexual differentiation and alter this process via several diverse endocrine mechanisms of toxicity (Figure 2). EDCs known to interfere with the androgen signaling pathway include dicarboximide fungicides (e.g., vinclozolin (Kelce et al. 1994), organochlorine based insecticides (e.g., p,p=DDT and p,p=DDE (Kelce et al. 1995), conazole fungicides (e.g., prochloraz (Noriega et al. 2005; Vinggaard et al. 1999), plasticizers (e.g., phthalates), polybrominated diphenyl ethers (PBDEs; (Gray et al. 2004; Stoker et al. 2005) and urea-based herbicides (e.g., linuron) (Lambright et al. 2000; McIntyre et al. 2000)..
Mixture Studies with Pesticides and Phthalates with diverse modes of toxicity
We conducted two binary mixture studies to determine how procymidone and linuron interacted in mixtures with phthalates. In the first study, we combined BBP (reduces fetal testosterone) with linuron, an antiandrogen with multiple mechanisms of action (AR antagonist and reduces fetal testosterone levels) (Hotchkiss et al. 2004b) (Wilson et al. 2004). The second study combined DBP with the AR antagonist procymidone. In these studies, pregnant rats were dosed on GD14-18 with either the individual compounds or the binary mixture at a dose level equivalent to approximately one half of the ED50 value for malformations. Following this, we conducted a study with a mixture of seven chemicals that included vinclozolin, procymidone, linuron, prochloraz, and three phthalates DBP, DEHP and BBP (Rider et al. 2008) and we will present new data herein on a multi-component mixture study with a ten chemical mixture (the seven chemicals listed above and three additional phthalates)..
B.1 Benzyl butyl phthalate plus linuron
In the BBP and linuron study, in utero exposure to BBP alone elicited a 0% incidence of hypospadias and vaginal pouch formation and 12% incidence of epididymal agenesis in male rats. In utero exposure to linuron alone resulted in 0% incidence of hypospadias and vaginal pouch development and 63% incidence of epididymal agenesis. However, exposure to the combination resulted in cumulative effects with males displaying 56%, 40%, and 97% incidence of hypospadias, vaginal pouch, and epididymal agenesis, respectively ((Hotchkiss et al. 2004b).
B.2 Di-n-butyl phthalate plus procymidone
In the procymidone plus DBP study, procymidone or DBP alone induced low incidences of hypospadias (1.5% and 0%, respectively) and vaginal pouch (0%) whereas the males treated with the combination of procymidone and DBP displayed 49% and 27% incidences of hypospadias and vaginal pouch, respectively indicating that the interaction was at least dose additive.
B.3 Seven chemical mixture study, including vinclozolin, procymidone, prochloraz, linuron and three phthalates or six phthalates
To further test our hypothesis, we designed two multi-component mixture studies, one with seven antiandrogenic chemicals and the other with ten chemicals (see below). The chemicals in these multi-component mixtures alter the androgen signaling pathway via diverse mechanisms of action including AR antagonists (vinclozolin and procymidone), mixed mechanism chemicals that bind to the AR and decrease testosterone production (linuron and prochloraz) and testosterone synthesis inhibiting phthalates (BBP, DBP, and DEHP in the seven chemical study (Rider et al. 2008) and BBP, DBP, DiBP, DEHP, DPP and DiHP in the new ten chemical study (see below). According to the current mixtures paradigm, these mixtures should conform to a model of integrated addition. However, we found that models of integrated addition or response addition frequently underestimated the effects of these mixtures.
B.4. Ten chemical mixture study (original data)
The main objective of this study was to expand upon our previous seven chemical mixture study by including a greater number of chemicals in order to more clearly define the differences in predictions generated from the dose addition model versus the integrated and response addition models. Increasing the number of chemicals allowed for the use of lower concentrations of individual chemicals in the mixture further testing our working hypothesis that chemicals present below their no observed adverse effect levels can contribute to mixture toxicity as long as they have a common target.
The dosage levels of each chemical at each dilution of the mixture is shown in Table 2. The ED50 values of the individual chemicals used in the models to predict the effects of the mixture are shown in Table 3. When compared to the previous seven chemical study, this study included three more phthalates (DiBP, DiHP and DPP), slightly larger numbers of litters per dose group and a larger number of dilutions of the top dose in order to provide greater distinction among the model predictions in the higher dosage ranges where the dose-, integrated- and response-addition models separate.
Table 2.
Chemical doses in the ten chemical mixture study.
| Mixture groups (% of top dose) | |||||||
|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | 20 | 10 | 0 | |
| Chemical | mg/kg/d | mg/kg/d | mg/kg/d | mg/kg/d | mg/kg/d | mg/kg/d | mg/kg/d |
| DBP | 150 | 120 | 90 | 60 | 30 | 15 | 0 |
| DIBP | 150 | 120 | 90 | 60 | 30 | 15 | 0 |
| BBP | 150 | 120 | 90 | 60 | 30 | 15 | 0 |
| DEHP | 150 | 120 | 90 | 60 | 30 | 15 | 0 |
| DIHP | 150 | 120 | 90 | 60 | 30 | 15 | 0 |
| DPP | 50 | 40 | 30 | 20 | 10 | 5 | 0 |
| VINCLOZOLIN | 30 | 24 | 18 | 12 | 6 | 3 | 0 |
| PROCYMIDIONE | 30 | 24 | 18 | 12 | 6 | 3 | 0 |
| LINURON | 40 | 32 | 24 | 16 | 8 | 4 | 0 |
| PROCHLORAZ | 60 | 48 | 36 | 24 | 12 | 6 | 0 |
| Total Dose (mg/kg/d) | 960 | 768 | 576 | 384 | 192 | 96 | 0 |
| Necropsy Litter # (pup #) | 2 (6) | 2 (8) | 4 (22) | 3 (17) | 3 (14) | 4 (26) | 5 (26) |
| AGD Litter # (pup #) | 3 (21) | 2 (8) | 4 (30) | 3 (17) | 3 (15) | 4 (28) | 6 (35) |
Table 3.
Dose-response parameters (ED50 and Hill slope) for the ten chemicals included in the new multi-component mixture study. Parameters were derived from a logistic fit to individual chemical dose-response data.
| Vinclozolin | Linuron | Procymidone | Phthalates DBP, DiBP, BBP, DEHP, DiHP* |
Prochloraz | More Potent Phthalate (DPP) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ED50 (mg/kg) |
Hill slope |
ED50 (mg/kg) |
Hill slope |
ED50 (mg/kg) |
Hill slope |
ED50 (mg/kg) |
Hill slope |
ED50 (mg/kg) |
Hill slope |
ED50 (mg/kg) |
Hill slope |
|
| AGD | 123.2 | 0.96 | 81.1 | 2.68 | 179.8 | 0.95 | 935.0 | 2.11 | 562.7 | 0.82 | 311.7 | 2.11 |
| PND 13 nips | 35.3 | 1.80 | 5394.0 | 0.38 | 90.0 | 1.28 | 775.3 | 25.6 | 336.3 | 1.06 | 258.4 | 25.6 |
| Hypospadias | 100.0 | 7.64 | 104.7 | 42.1 | 191.2 | 16.0 | 1000.0 | 16.0 | 137.9 | 11.8 | 333.3 | 16.0 |
| Ectopic testes | 177.8 | 3.63 | 228.4 | 10.93 | 500.0 | 11.4 | 538.7 | 12 | 500.0 | 11.4 | 180.0 | 12 |
| Epididymal agenesis | 4000 | 5.16 | 81 | 3.66 | 7640 | 5.16 | 581.6 | 5.55 | 295.6 | 5.34 | 193.87 | 5.55 |
| Epididymal weight | 4000.0 | 3.41 | 7600.0 | 4.71 | 154.4 | 3.41 | 601.7 | 6.70 | 590.2 | 1.33 | 200.6 | 6.70 |
| Seminal vesicle weight | 103.9 | 1.03 | 116.2 | 4.43 | 253.7 | 7.30 | 708.4 | 14.87 | 333.6 | 2.64 | 236.1 | 14.87 |
| Ventral prostate weight | 176.9 | 0.74 | 146.6 | 1.60 | 276.1 | 1.17 | 987.4 | 12.64 | 189.9 | 2.68 | 329.1 | 12.64 |
| Levator Ani Bulbocavernosus Muscles weight | 141.0 | 3.20 | 205.0 | 2.16 | 258.0 | 3.20 | 962.0 | 3.00 | 321.0 | 2.98 | 321.0 | 3.00 |
Phthalate (DBP, DiBP, BBP, DEHP, and DiHP) parameters were based on individual chemical dose-response data for DBP (for which the most complete dose-response data were available) because previous studies with incomplete dose-response data from the other phthalates in this group supported the assumption that these phthalates were approximately equipotent.
Materials and Methods
Animals
Thirty timed pregnant Sprague-Dawley rats were purchased from Charles River Breeding Laboratory (Raleigh, NC) on gestation day (GD) 2. Animals were housed individually in clear polycarbonate cages (20 cm × 25 cm × 47 cm) with a bedding of heat-treated laboratory-grade pine shavings (Northeastern Products, Warrensburg, NY). Animals were fed Purina Rat Chow 5008 (pregnant and lactating females) or Purina Rat Chow 5001 (weanling and adult rats) and provided with access to filtered municipal drinking water (Durham, NC) ad libitum. The current study was conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee.
Treatment Administration
Our goal in dose selection of the seven chemical study (four pesticides and three phthalates) was to use doses of each chemical that would contribute to the induction of malformations approximately equally in the mixture toxicity. The mixture contained each chemical at 1/7th of its respective ED100 for inducing reproductive tract malformations. In the current study with ten chemicals, we kept the mixture ratio for the four pesticides and total phthalate dose constant with the seven chemical study.
Since this study has six phthalates (see below), rather than three, the contribution of each of the six phthalates in the mixture was half of the contribution of the three phthalates in the seven chemical study. Thirty pregnant rats (n= 4 (mixture groups) to 6 dams (control group); table 2) were dosed with the vehicle (corn oil at 2.5 ml/kg; Sigma) or a dilution of the high dose of the mixture by oral gavage on GD14-18.
It is noteworthy that the goal of these studies is to fit the full dose response curve for as many endpoints as possible in order to determine which model best predicts the observed effects of the mixture. The goal of these studies is not to establish no-observed-adverse-effect levels, which would require using larger numbers of litters in the low dose range and a longer intrauterine exposure period.
Chemical Information (reported purity)
Benzyl n-butyl phthalate (BBP) – 98%, Aldrich – CAS 85-68-7; lot # 08523JQ; cat # 3,850-1.
Di (n-butyl) phthalate (DBP) – 98%, Sigma – CAS 84-74-2; lot # 109F0386; cat # D-2270.
Diethylhexyl phthalate or Phthalic Acid bis(2-Ethylhexyl Ester) (DEHP) – Aldrich – CAS 117-81-7, cat # D201154, lot # 09428JC, purity = 99%.
Diisobutyl Phthalate (DiBP) – Aldrich – CAS 84-69-5, cat # 152641, lot # 10314LC, purity = 99%.
Diisoheptyl Phthalate (DiHP) – Aldrich – CAS 71888-89-6, cat # 376671, lot # 06905EC, purity = technical grade.
Dipentyl Phthalate (DPP) - Fluka – CAS 131-18-0, ca t#80154, lot #11151652, purity = 99 %.
3-(3, 4-dichlorophenyl)-1-methoxy-1-methylurea (Linuron) – 99%, Crescent Chemical Company - CAS 330-55-2; cat # W-146400; lot # 70226.
N-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl)-1H-imidazole-1-carboxamide (Prochloraz) –99.5%, Riedel-de-Haen – CAS 067747-09-5; cat # 45631; lot # 9060X.
N-(3, 5-dichlorophenyl)-1, 2-imethylcyclopropane-1, 2-dicarboxamide (Procymidone) – 99%, Chem Services – CAS 32809-16-8; cat # PS-2126; lot # 231-100A.
N-3, 5-dichlorophenyl-5-methyl-5-vinyl-1, 3-oxazolidine-2, 4-dione (Vinclozolin) – 99.4%,, Riedel-de-Haen – CAS 50471-44-8; cat # 35625; lot # 9126X.
Vehicle – Corn Oil – Sigma – CAS 8001-30-7; cat # C-8267; lot # 126K0117.
Male offspring assessment
On postnatal day (PND) 3, all pups were sexed, weighed, and anogenital distances (AGD) were measured with a dissecting microscope with an ocular micrometer (Hotchkiss et al. 2004b) (Figure 3). At PND 14, each male rat was examined in a blinded manner for areolae or nipples. At about 200 days of age, all male rats were anesthetized with CO2, decapitated, and examined for reproductive tract alterations. The ventral surface of each male rat was shaved and examined for the presence of nipples. Rats were then assessed for abnormalities including cleft phallus, hypospadias, epididymal agenesis, testicular atrophy, undescended testes, fluid filled testes, ventral prostate or seminal vesicle agenesis, and malformations of the gubernacular cords (elongated or absent). Weights were taken on body, glans penis, ventral prostate, seminal vesicles, testes, epididymis, and levator ani bulbocavernous muscle (LABC) (Figure 4a).
Figure 3.
Effects of the individual chemicals on AGD at 3 days of age (Fig 3A). Phthalates refers to the group of phthalates (DBP, DiBP, DiHP, BBP, and DEHP) which have similar potency and are represented by the dose-response data from DBP and DEHP. DPP is not shown on this graph, but was three times as potent as the other phthalates and was assumed to have a dose-response curve with a similar shape as that of the other phthalates. Observed (OBS) effects of the mixture on AGD at 3 days of age (Fig 3B, male and female) and observed and predicted effects in our new multi-component mixture study with ten chemicals (four pesticides and six phthalates) on AGD in male rats at 3 days of age (Fig 3C). Since AGD is linear in the low dose range, all models (dose (DA), integrated (IA) and response addition (RA) provided similarly accurate predictions for the observed reductions in AGD. The ED50s as % of the top dose (100%) are shown for the observed and predicted effects of the mixture on F1 male rat AGD at three days of age. Predicted ED50 values with shading fall outside of the 95% confidence limits surrounding the ED50 calculated from the observed data. RA provides less accurate predictions than either DA or IA models. Values on the figures are means and standard errors of observed responses and predicted responses.
Figure 4.
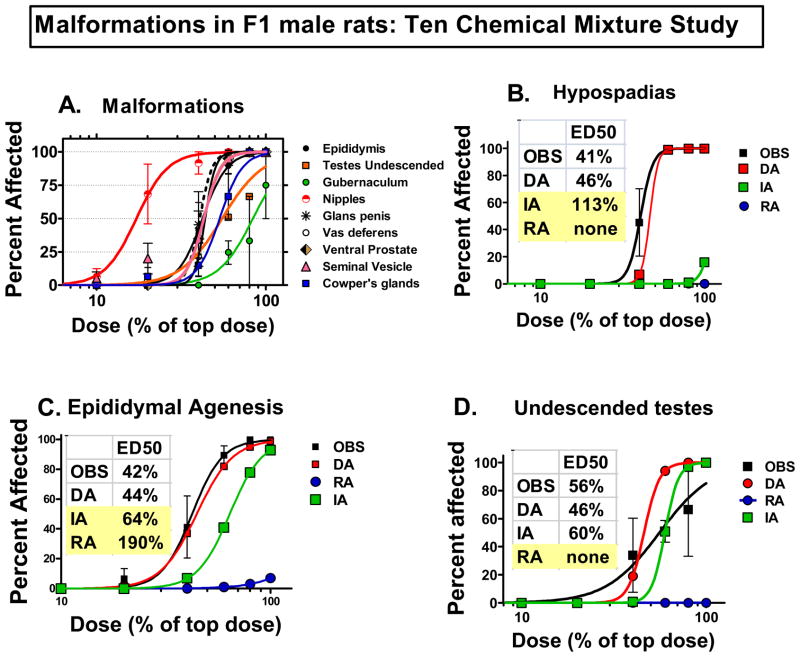
Summary of the observed (Fig 4A) and predicted effects in our new multi-component mixture study with ten chemicals (four pesticides and six phthalates) on reproductive tract malformations detected in adult F1 males. Individual chemical data that went into models can be found in Rider et al. 2008. Dose addition (DA) models more accurately predicted the effects of the mixture of ten chemicals on the incidences of hypospadias (Fig 4B) and epididymal agenesis (Fig 4C). The integrated addition (IA) model under-predicts effects and the response addition (RA) model predicts no malformations at even the highest dose. The ED50s as % of the top dose (100%) are shown for the observed and predicted effects of the mixture on F1 male rat malformations (hypospadias, epididymal agenesis and undescended testes (Fig 4D)). Predicted ED50 values with shading fall outside of the 95% confidence limits surrounding the ED50 calculated from the observed data. Only the DA model accurately predicted the observed incidences of all three of these malformations. Values on the figures are means and standard errors of observed responses and predicted responses.
AGD, retained nipple and organ weight data were analyzed using litter mean values on SAS 9.1 with PROC GLM (p<0.05 was the critical value for statistical significance). The incidence of reproductive tract malformations was similarly analyzed using the litter mean incidence of each malformation. Prism output from the observed mixture responses included the ED50 (with standard errors and 95% confidence limits (CL)), and the goodness of fit (R Square) of the observed data to the best-fit model. For statistical purposes, we compared the ED50s of the observed responses with the dose, response and integrated addition predictions of the ED50s. Predicted ED50s differed significantly from the observed ED50 if the predicted value was outside of the 95% CL of the ED50 of the observed response.
Results
In the ten chemical study, no maternal toxicity (maternal weight loss or weight gain) or treatment-related pup mortality was observed (data not shown). The body weight of the male offspring was significantly reduced (by Dunnett’s post hoc test) in the 100% dose group. A significant incidence of female-like retained nipples was observed in the 20% to 100% dose groups, whereas most other tissues were significantly affected at 40% of the top dose and above. The gubernaculum was the least sensitive tissue, being affected only at 60% of the top dose and above.
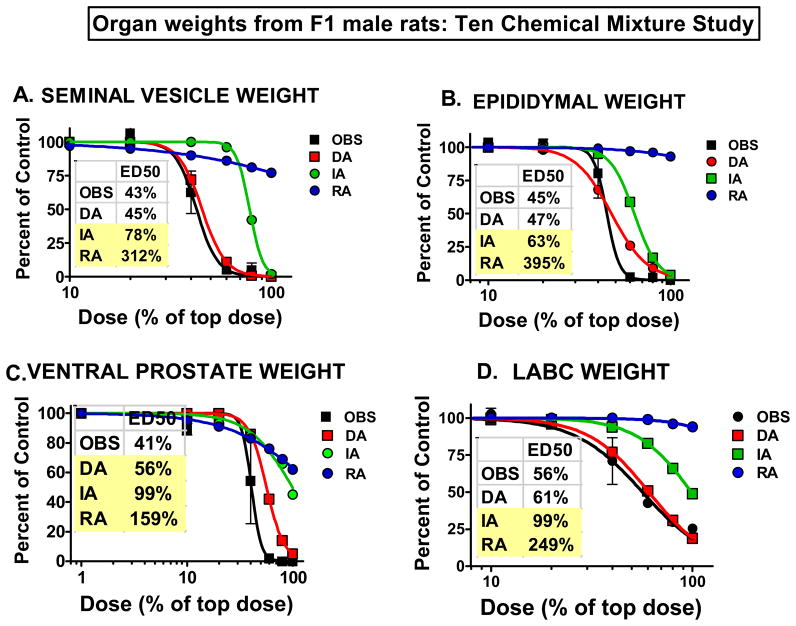
When the androgen-dependent endpoints were analyzed, dose addition models, provided estimates of mixture responses that closely approximated the observed responses (Figures 3, 4, 5). For example, hypospadias was observed in all of the high dose animals as predicted by dose addition (Figure 4b) whereas integrated and response addition models only predicted that about 20% or 0%, respectively, would be affected. Epididymal agenesis also was accurately predicted by dose addition, but not integrated or response addition models (Figure 4c). For undescended testes, both dose addition and integrated addition models were of similar accuracy but response addition was not useful (Figure 4d). In addition, for the androgen dependent seminal vesicle, epididymal, ventral prostate and LABC weights (Figure 5a,b,c,d) dose addition provided the most accurate predictions of the effects of the ten chemical mixture. Clearly, integrated and response addition models grossly under predict many of the effects of such mixtures indicating that a framework based narrowly upon common mechanisms of toxicity underestimates the risk associated with exposure to multi-component mixtures such as those used here.
Figure 5.
Summary of the observed and predicted effects in our new multi-component mixture study with ten chemicals (four pesticides and six phthalates) on reproductive tract organ weights measured in adult F1 males. Individual chemical data that went into models can be found in Rider et al. 2008. The ED50s as % of the top dose (100%) are shown for the observed and predicted effects of the mixture on F1 male rat seminal vesicle (Fig 5A), epididymal (Fig 5B), ventral prostate (Fig 5C) and LABC (Fig 5D) weights. Predicted ED50 values with shading fall outside of the 95% confidence limits surrounding the ED50 calculated from the observed data. The reductions in seminal vesicle, ventral prostate, epididymal and LABC weights were all more accurately predicted by dose addition (DA) than by either integrated addition (IA) or response addition (RA) models. The DA model accurately predicted all the reductions except for the reduction in ventral prostate weight, where it slightly under-predicted the magnitude of the effect. However, it was still clearly the “best” model as compared to IA or RA predictions. Values on the figures are means and standard errors of observed responses and predicted responses.
Since all the chemicals used in the mixtures discussed in this section disrupt the androgen signaling pathway, albeit via different mechanisms of toxicity, it is clear that a cumulative risk assessment framework based upon mechanisms of toxicity would not adequately predict the effects of these two multi-component mixtures of chemicals with different mechanisms of toxicity.
Mixtures Section C. Chemicals that disrupt a common tissue via different signaling pathways and diverse mechanisms of toxicity. Disruption of the Androgen and AhR Signaling Pathways in the fetal male rat reproductive tract. (Original data)
C.1 2,3,7,8 tetrachlorodibenzo dioxin (TCDD ) plus a phthalate (DBP)
The purpose of this study was to determine whether the joint action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and DBP on the development of the male and female rat reproductive tract resulted in cumulative effects that exceeded response addition predictions. The TCDD and DBP mixture study further contributes to our understanding of the mechanisms of joint action of chemicals that disrupt androgen dependent reproductive development. This study will help to further define the boundaries of chemicals that should be included in a cumulative risk assessment of “antiandrogens” because they are dose additive.
The doses of TCDD and DBP selected for this study are based upon previous dose response studies with the individual chemicals. Wilker et al. (Wilker et al. 1996) found that a one time in utero dose of 2 μg/kg resulted in epididymal malformations in 27% of male rats. Our previous studies with DBP showed that a dose of 500mg/kg/day during GD 14-18 resulted in epididymal agenesis in about 25% of male rats (Hotchkiss et al. 2004b; Howdeshell et al. 2007). For the mixture study, we wanted to select doses of the two chemicals that were approximately equipotent and that would induce significant malformations in reproductive organs if the chemicals did display dose additivity. Therefore, the high dose of our mixture consisted of TCDD at 2 μg/kg administered once on GD14 and DBP administered at 500mg/kg/day on GD14-18.
We expected that TCDD and DBP would act jointly and produce cumulative disruptions of “common” reproductive tissues, including epididymal and testicular differentiation, resulting in reduced epididymal and testis weights, as well as reduced sperm counts. We also expected that since the constellation of effects for DBP and TCDD differ, there would be some effects that would only be disrupted by one of the two chemicals and would result in effect levels congruent with response additive predictions for those endpoints (i.e. cleft phallus and vaginal threads in females and delayed puberty in males would result from TCDD but not DBP treatment; retained nipples in males would result from DBP but not TCDD treatment).
Chemical information
Di(n-butyl) phthalate (DBP) – CAS # 84-74-2, lot #, purity, density, ordering information: Sigma catalog #; 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) – CAS # 1746-01-6, gift from Dr L. Birnbaum; Vehicle = laboratory grade corn oil – CAS # 8001-30-7.
Methods
Thirty adult (about 90 days of age) timed-pregnant Sprague-Dawley rats were purchased from Charles River (Raleigh, NC) and shipped to the laboratory on GD 3 (day after mating is counted as GD1) and housed, as above. The study included 5 treatment (Table 4) groups with 4 pregnant dams per group. On gestational day 14, dams were weighed and assigned to one of the 5 treatment groups to ensure that the treatment groups were equally weighted.
Table 4.
Doses used in the DBP and 2,3,7,8-TCDD preliminary mixture study.
| Treatment (% of top mixture dose) | |||||
|---|---|---|---|---|---|
| Vehicle control (0) | TCDD alone | DBP alone | Mix1 (100) | Mix2 (65) | |
| TCDD (μg/kg) | 0 | 2 | 0 | 2 | 1.3 |
| DBP (mg/kg) | 0 | 0 | 500 | 500 | 320 |
| Litter # (pup #) | 4 (22) | 3 (5) | 4 (21) | 4 (21) | 3 (21) |
Dams in all groups were dosed once with either TCDD or vehicle on GD14 and with either DBP or vehicle on GD14-18. The dose groups included a control, DBP (500 mg/kg/d) alone, TCDD (2 μg/kg/d) alone), the 100% mixture group (DBP (500 mg/kg/d) and TCDD (2 μg/kg/d)), or a 65% mixture group (DBP (320 mg/kg/d) and TCDD (1.3 μg/kg/d)).
Endpoints measured during development included:
Anogenital distance (AGD; PND3) – AGD measured under a dissecting microscope with a 15x ocular micrometer. AGD represents the distance between the base of the genital papilla and the rostral end of the anal opening;
Nipple retention (PND13–15 and at necropsy) – measurement of the position and numbering of areolae/nipples;
Timing of puberty observed as preputial separation and vaginal opening (starting at PND35 and 32, respectively and monitored until completion);
-
Mature F1 males were necropsied beginning at PND120 after they were anesthetized with CO2 and euthanized by decapitation and examined for
Nipple retention,
External malformations – hypospadias or abnormal glans penis,
Internal malformations – epididymal agenesis, gubernacular malformations (agenesis or elongation), testicular malformations (fluid filled testes, undescended testes, etc.), ventral prostate or seminal vesicle agenesis, and
Androgen dependent organ weight changes – glans penis, ventral prostate, seminal vesicle, testes, epididymides, levator ani/bulbocavernosus muscle.
Epididymal sperm numbers, using the methods described in (Howdeshell et al. 2008a).
The observed effects of the mixture of 2 μg/kg/d TCDD plus 500 mg/kg/d DBP were compared with the values predicted by response addition using a t-test to determine if the mixture effects were greater than the response addition predicted values. For some of the endpoints the response addition predictions were equivalent to levels induced by either DBP (malformations of the vas deferens, epididymis, testis, and epididymal weight for example) or TCDD (testis weight and epididymal sperm numbers) alone. In these instances, the high dose mixture group was compared by t-test to either the DBP or TCDD alone group, as appropriate. Since malformations of the liver and external genitalia were seen only in the high dose mixture group, the mixture group was compared with the control, DBP alone and TCDD groups using Fisher Exact test to determine if the mixture effect was significantly greater than the incidence predicted by response addition.
Results of the TCDD plus DBP mixture study
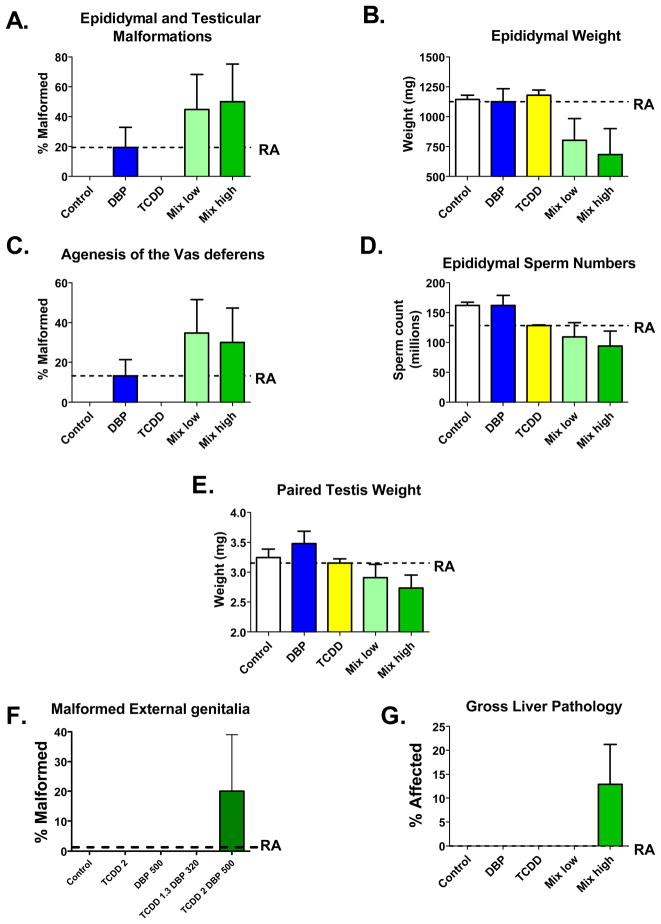
In the TCDD plus DBP study, the incidences of malformed organs in the 100% and 65% mixture groups exceeded response addition for the epididymal, testicular, vas deferens, hypospadias and liver malformations and for testis and epididymal weights (Figure 7). However, of these effects, only the reduction in epididymal weight was statistically significant at the p<0.05 levels whereas the other contrasts resulted in p values between 0.10 and 0.20, likely due to the relatively small number of litters used in this preliminary study.
The increases in gross liver pathology and malformations of the external genitalia and vas deferens were unanticipated since TCDD is not known to induce these malformations and the gross lesions in the liver have not been seen previously with either chemical alone. For a few endpoints (ventral prostate weight, seminal vesicle weight, and levator ani bulbocavernosus weight) both chemicals appeared to contribute to the effects, but the mixture response did not exceed response addition predictions (data not shown). In this study, there were no consistent effects on maternal toxicity or effects on F1 growth or mortality (data not shown), but these endpoints need to be reevaluated in a larger study.
In summary, the endpoints targeted by both DBP and TCDD (decreased epididymal weight and epididymal agenesis) produced responses that exceeded those expected if the chemicals had acted independently through response addition. Some endpoints that we had hypothesized to be targeted by one chemical (i.e. malformations of the vas deferens and external genitalia) also demonstrated mixture responses that exceeded those predicted by response addition. Other endpoints that were targeted by only one chemical (nipple retention and delay in preputial separation) had mixture responses that were consistent with response addition predictions. The mixture of DBP and TCDD caused liver malformations that were not elicited by either of the chemicals alone.
If confirmed, these observations would considerably expand the framework for cumulative risk assessment, and for this reason, we plan to build upon this study by examining a more complete DBP plus TCDD mixture dosing regime in order to further elucidate the target-dependent differences in mixture responses observed in this study.
Conclusions
Individual chemical dose-response data can be incorporated into models of mixture toxicity in order to test hypotheses regarding which type of model (dose addition or response addition) most accurately reflects observed responses to mixture exposures. Despite our relatively robust collection of individual reproductive toxicant dose-response data, gaps existed and necessitated the incorporation of practical assumptions. For example, we had incomplete dose-response data for many of the phthalates (BBP, DiHP, and DiBP). The data points that we did have overlapped with our complete dose-response data for DBP and DEHP, therefore, we made the assumption that these five phthalates were equipotent and we used the parameters from the DBP and DEHP dose-response curves for all of those phthalates. In addition, limited numbers of dosage levels and sample sizes constrained the precision of dose-response analyses. Although it is desirable to have more dose groups and larger numbers of litter per dose group, even small transgenerational studies use considerable resources. For example, a small study with only 4 groups and 5 litters per dose group generates about 200 male and female offspring that are evaluated over a 6 month period after birth. Lastly, the dose addition model we employed in this work used the average of the Hill slopes from the mixture components, which adds another source of inaccuracy. However, we put forth that the intentionally large differences in predictions resulting from response addition versus dose addition models overshadow the relatively minor inaccuracies in individual data and model assumptions (see example in Figure 4B). The goal of this work was not to perform an exhaustive cumulative risk assessment on these chemicals, but to elucidate the mechanisms of joint chemical action and thereby, inform procedures by which chemicals are selected for inclusion in cumulative risk assessments.
The weight of evidence from the mixtures studies described here clearly indicates that compounds that target the same tissue, regardless of their specific mechanism of action, display cumulative, dose-additive effects when present in combination. This conclusion is supported by the work of others. Gray et al. (2001) and Nellemann et al. (2003) found that mixtures of vinclozolin and procymidone resulted in dose additive decrease in androgen-sensitive organ weights, androgen levels, and AR-dependent gene expression in castrated, testosterone-treated male rats. Birkhoj et al. (2004) assessed the in vitro and in vivo effects of a combination of five pesticides with dissimilar mechanisms of action and found androgen-sensitive endpoint responses greater than would be expected using response addition modeling. In a recent study conducted by Drake et al. (2009), dexamethasone appeared to exacerbate the reproductive anomalies induced by in utero exposure of male rats to dibutyl phthalate. This further demonstrates that assaults to male reproductive development can come from a host of sources (chemical and physical stress) to act in concert.
Taken together, these findings suggest a modification of the approach for cumulative risk assessments from one based upon “common mechanism of toxicity” to one that includes the cumulative assessment of chemicals that disrupt development of common tissues. This would result in a target organ and timing based approach rather than one based on a narrowly defined mechanism of toxicity. We propose that the primary focus should be on the biological system or the target tissue rather than the mechanism of toxicity or even a single signaling pathway. Normal development of the fetal reproductive tract tissues is almost certainly dependent upon the interconnecting and dynamic, inductive signaling pathways that converge on the earliest genes that initiate sexual differentiation in the rat fetus, as seen in the liver and pancreas (Wandzioch and Zaret 2009). These inductive networks are dynamic and change within hours. The different signaling pathways function in parallel to induce normal expression of different early genes, and disrupting or delaying any signal in the network will result in permanent alterations of the normal sexual phenotype. For this reason, we propose that a cumulative risk assessment should include all chemicals which target that system during the same critical developmental period.
Figure 6.
A summary of the binary mixture study with DBP and 2,3,7,8 TCDD, a mixture of chemicals that disrupts diverse receptor signaling pathways (AR and AhR) is presented here. The incidences of malformed organs in the 100% (TCDD at 2 μg/kg on GD 14 and DBP at 500 mg/kg/d GD 14-18) and 65% mixture group exceeded response addition (RA) for the epididymal and testicular (Fig 6A), vas deferens (Fig 6C), external genitalia (Fig 6F) and liver (Fig 6G) malformations and epididymal (Fig 6B) and testis weights (Fig 6E) and epididymal sperm numbers (Fig 6D). The increases in gross liver pathology and malformations of the external genitalia were unanticipated since TCDD is not known to induce hypospadias and the gross lesions in the liver have not been seen previously with either chemical alone. Values on the figures are means and standard errors of observed responses. The line identified as RA is the response level predicted by the RA model for the effect of the high dose mixture of TCDD and DBP. The effect of the high dose mixture was significantly greater (p<0.03 by t-test of litter mean values) than the level predicted by the response addition model.
Acknowledgments
This work was supported in part by NTP, NIEHS/EPA Interagency Cooperative Research Agreement HHS Y1-ES-8014-01; EPA RW75922855-01-0. C. Rider was funded by the NCSU/EPA Cooperative Training Program CT833235-01-0 and NIH Pathway to Independence grant 1K99ES016806.
Footnotes
Publisher's Disclaimer: DISCLAIMER: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, ORD, U. S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Altenburger R, Backhaus T, Boedeker W, Faust M, Scholze M, Grimme LH. Predictability of the toxicity of multiple chemical mixtures to Vibrio fischeri: Mixtures composed of similarly acting chemicals. Environmental Toxicology and Chemistry. 2000;19:2341–2347. [Google Scholar]
- Altenburger R, Schmitt H, Schuurmann G. Algal toxicity of nitrobenzenes: Combined effect analysis as a pharmacological probe for similar modes of interaction. Environmental Toxicology and Chemistry. 2005;24:324–333. doi: 10.1897/04-032r.1. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Brooks BW, Huggett DB, Sumpter JP. Repeating History: pharmaceuticals in the environment. Environmental science & technology. 2007;41:8211–8217. doi: 10.1021/es072658j. [DOI] [PubMed] [Google Scholar]
- Backhaus T, Scholze M, Grimme LH. The single substance and mixture toxicity of quinolones to the bioluminescent bacterium Vibrio fischeri. Aquatic toxicology (Amsterdam, Netherlands) 2000;49:49–61. doi: 10.1016/s0166-445x(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Blystone CR, Lambright CS, Howdeshell KL, Furr J, Sternberg RM, Butterworth BC, Durhan EJ, Makynen EA, Ankley GT, Wilson VS, LeBlanc GA, Gray LE. Sensitivity of Fetal Rat Testicular Steroidogenesis to Maternal Prochloraz Exposure and the Underlying Mechanism of Inhibition. Toxicological Sciences. 2007;97:65–74. doi: 10.1093/toxsci/kfm055. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, Gray LE, Ankley GT. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environmental health perspectives. 2006;114(Suppl 1):65–8. doi: 10.1289/ehp.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environmental health perspectives. 1999;107(Suppl 3):409–19. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws JA, Sommer RJ, Silbergeld EK, Peterson RE, Hirshfield AN. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces genital dysmorphogenesis in the female rat. Toxicology and applied pharmacology. 1997;147:351–62. doi: 10.1006/taap.1997.8295. [DOI] [PubMed] [Google Scholar]
- Gray LE. Emerging Issues related to endocrine disrupting chemicals and environmenal androgens and antiandrogens. In: Hutzinger O, editor. The Handbook of environmental chemistry. Vol. 3. Springer-Verlag; Heidelberg: 2002. pp. 209–248. [Google Scholar]
- Gray LE, Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicology and applied pharmacology. 1995;131:108–18. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Wolf C, Lambright C, Wilson V, Noriega N. Toxicant-induced hypospadias in the male rat. Advances in experimental medicine and biology. 2004;545:217–41. doi: 10.1007/978-1-4419-8995-6_14. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicology and applied pharmacology. 1995;133:285–94. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- Gray LE, Lambright C, Parks L, Tyl RW, Orlando EF, Guillette LJ, Wolf CJ, Seeley JC, Chang T-Y, Wilson V, Hotchkiss A, Ostby JS, editors. Antiandrogenic Effects of Environmental Endocrine Disruptors. Springer-Verlag; New York: 2001a. [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Human reproduction update. 2001b;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS, Kelce WR. A dose-response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans Hooded rat offspring. Toxicology and applied pharmacology. 1997a;146:11–20. doi: 10.1006/taap.1997.8223. [DOI] [PubMed] [Google Scholar]
- Gray LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicology and industrial health. 1999;15:94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- Gray LE, Wolf C, Mann P, Ostby JS. In utero exposure to low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive development of female Long Evans hooded rat offspring. Toxicology and applied pharmacology. 1997b;146:237–44. doi: 10.1006/taap.1997.8222. [DOI] [PubMed] [Google Scholar]
- Greco W, Unkelbach HD, Poch G, Suhnel J, Kundi M, Bodeker W. Consensus on concepts and terminology for combined-action assessment: The Saariselka agreement. Archives of Complex Environmental Studies. 1992;4:65–60. [Google Scholar]
- Haavisto T, Nurmela K, Pohjanvirta R, Huuskonen H, El-Gehani F, Paranko J. Prenatal testosterone and luteinizing hormone levels in male rats exposed during pregnancy to 2,3,7,8-tetrachlorodibenzo-p-dioxin and diethylstilbestrol. Mol Cell Endocrinol. 2001;178:169–79. doi: 10.1016/s0303-7207(01)00425-7. [DOI] [PubMed] [Google Scholar]
- Haavisto TE, Myllymaki SA, Adamsson NA, Brokken LJ, Viluksela M, Toppari J, Paranko J. The effects of maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on testicular steroidogenesis in infantile male rats. International journal of andrology. 2006;29:313–22. doi: 10.1111/j.1365-2605.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Thomas GO. Polychlorinated biphenyls, DDT, polybrominated diphenyl ethers, and organic pesticides in United Kingdom harbor seals (Phoca vitulina)--mixed exposures and thyroid homeostasis. Environmental toxicology and chemistry/SETAC. 2007;26:851–61. doi: 10.1897/06-310r.1. [DOI] [PubMed] [Google Scholar]
- Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe RM. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environmental health perspectives. 2007;115:390–6. doi: 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hela DG, Lambropoulou DA, Konstantinou IK, Albanis TA. Environmental monitoring and ecological risk assessment for pesticide contamination and effects in Lake Pamvotis, northwestern Greece. Environmental toxicology and chemistry/SETAC. 2005;24:1548–56. doi: 10.1897/04-455r.1. [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Murakami M, Ineyama M, Yamada T, Koyama Y, Okuno Y, Yoshitake A, Yamada H, Miyamoto J. Effects of procymidone on reproductive organs and serum gonadotropins in male rats. J Toxicol Sci. 1993;18:111–24. doi: 10.2131/jts.18.111. [DOI] [PubMed] [Google Scholar]
- Hotchkiss A, Parks-Saldutti L, Ostby J, Lambright C, Furr J, Vandenbergh J, Gray JLE. A mixture of the “antiandrogens’ linuron and betyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biology of reproduction. 2004a;71:1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, Gray LE. A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biology of reproduction. 2004b;71:1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE., Jr Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci. 2007;99:190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci. 2008a;102:371–82. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008b;108:168–76. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008c;105:153–65. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Acerini CL. Factors controlling testis descent. European journal of endocrinology/European Federation of Endocrine Societies 159 Suppl. 2008;1:S75–82. doi: 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- Jaspers VL, Covaci A, Voorspoels S, Dauwe T, Eens M, Schepens P. Brominated flame retardants and organochlorine pollutants in aquatic and terrestrial predatory birds of Belgium: levels, patterns, tissue distribution and condition factors. Environ Pollut. 2006;139:340–52. doi: 10.1016/j.envpol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environmental science & technology. 1998;32:2498–2506. [Google Scholar]
- Jobling S, Tyler CR. Introduction: The ecological relevance of chemically induced endocrine disruption in wildlife. Environmental health perspectives. 2006;114(Suppl 1):7–8. doi: 10.1289/ehp.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, Tyler CR, van Aerle R, Santos E, Brighty G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environmental health perspectives. 2006;114(Suppl 1):32–9. doi: 10.1289/ehp.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE. Environmental Hormone Disruptors: Evidence that Vinclozolin Developmental Toxicity is Mediated by Antiandrogenic Metabolites. Toxicology and applied pharmacology. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–5. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Wilson EM. Environmental antiandrogens: Developmental effects, molecular mechanisms, and clinical implications. Journal of Molecular Medicine-Jmm. 1997;75:198–207. doi: 10.1007/s001090050104. [DOI] [PubMed] [Google Scholar]
- Ko K, Moore RW, Peterson RE. Aryl hydrocarbon receptors in urogenital sinus mesenchyme mediate the inhibition of prostatic epithelial bud formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology and applied pharmacology. 2004;196:149–55. doi: 10.1016/j.taap.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environmental science & technology. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277:31283–6. doi: 10.1074/jbc.C200398200. [DOI] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, Gray LE. Cellular and molecular mechanisms of action of linuron: An antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicological Sciences. 2000;56:389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, Wolff MS. Pesticides and inner-city children: exposures, risks, and prevention. Environmental health perspectives. 1999;107(Suppl 3):431–7. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Rasmussen NT, Moore RW, Albrecht RM, Peterson RE. 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits prostatic epithelial bud formation by acting directly on the urogenital sinus. The Journal of urology. 2004;172:365–8. doi: 10.1097/01.ju.0000124989.02257.38. [DOI] [PubMed] [Google Scholar]
- Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 3. Effects on spermatogenesis and reproductive capability. Toxicology and applied pharmacology. 1992;114:118–26. doi: 10.1016/0041-008x(92)90103-y. [DOI] [PubMed] [Google Scholar]
- Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–23. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- Mahood IK, McKinnell C, Walker M, Hallmark N, Scott H, Fisher JS, Rivas A, Hartung S, Ivell R, Mason JI, Sharpe RM. Cellular origins of testicular dysgenesis in rats exposed in utero to di(n-butyl) phthalate. International journal of andrology. 2006;29:148–54. doi: 10.1111/j.1365-2605.2005.00574.x. discussion 181–5. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PM. Male rats exposed to linuron in utero exhibit permanent changes in anogenital distance, nipple retention, and epididymal malformations that result in subsequent testicular atrophy. Toxicol Sci. 2002a;65:62–70. doi: 10.1093/toxsci/65.1.62. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Sar M, Wallace DG, Foster PM. Effects of in utero linuron exposure on rat Wolffian duct development. Reproductive toxicology (Elmsford, NY) 2002b;16:131–9. doi: 10.1016/s0890-6238(02)00010-2. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW, Foster PM. Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat. Toxicology and applied pharmacology. 2000;167:87–99. doi: 10.1006/taap.2000.8998. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicological Sciences. 2003;71:251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Noriega NC, Ostby J, Lambright C, Wilson VS, Gray LE. Late gestational exposure to the fungicide prochloraz delays the onset of parturition and causes reproductive malformations in male but not female rat offspring. Biology of reproduction. 2005;72:1324–1335. doi: 10.1095/biolreprod.104.031385. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Frame SR, Ladics GS. Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol Sci. 2002;69:92–108. doi: 10.1093/toxsci/69.1.92. [DOI] [PubMed] [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE. The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicology and industrial health. 1999;15:80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS, Gray LE., Jr A mixture of seven antiandrogens induces reproductive malformations in rats. International journal of andrology. 2008;31:249–62. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Rider CV, LeBlanc GA. An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicological Sciences. 2005;87:520–528. doi: 10.1093/toxsci/kfi247. [DOI] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, Gray LE., Jr Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicologic pathology. 2009;37:100–13. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- Silva Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000 (vol 112, pg 331, 2004) Environmental health perspectives. 2004;112:A270–A270. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing” - Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environmental science & technology. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Simanainen U, Haavisto T, Tuomisto JT, Paranko J, Toppari J, Tuomisto J, Peterson RE, Viluksela M. Pattern of male reproductive system effects after in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive rat lines. Toxicol Sci. 2004;80:101–8. doi: 10.1093/toxsci/kfh142. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicology and applied pharmacology. 2005;207:78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Teuschler LK, Rice GE, Wilkes CR, Lipscomb JC, Power FW. Feasibility study of cumulative risk assessment methods for drinking water disinfection byproduct mixtures. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2004;67:755–777. doi: 10.1080/15287390490428224. [DOI] [PubMed] [Google Scholar]
- Theobald HM, Roman BL, Lin TM, Ohtani S, Chen SW, Peterson RE. 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits luminal cell differentiation and androgen responsiveness of the ventral prostate without inhibiting prostatic 5alpha-dihydrotestosterone formation or testicular androgen production in rat offspring. Toxicol Sci. 2000;58:324–38. doi: 10.1093/toxsci/58.2.324. [DOI] [PubMed] [Google Scholar]
- Turner KJ, McIntyre BS, Phillips SL, Barlow NJ, Bowman CJ, Foster PM. Altered gene expression during rat Wolffian duct development in response to in utero exposure to the antiandrogen linuron. Toxicol Sci. 2003;74:114–28. doi: 10.1093/toxsci/kfg096. [DOI] [PubMed] [Google Scholar]
- Vezina CM, Allgeier SH, Moore RW, Lin TM, Bemis JC, Hardin HA, Gasiewicz TA, Peterson RE. Dioxin causes ventral prostate agenesis by disrupting dorsoventral patterning in developing mouse prostate. Toxicol Sci. 2008;106:488–96. doi: 10.1093/toxsci/kfn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina CM, Lin TM, Peterson RE. AHR signaling in prostate growth, morphogenesis, and disease. Biochemical pharmacology. 2009;77:566–76. doi: 10.1016/j.bcp.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinggaard AM, Christiansen S, Laier P, Poulsen ME, Breinholt V, Jarfelt K, Jacobsen H, Dalgaard M, Nellemann C, Hass U. Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol Sci. 2005;85:886–97. doi: 10.1093/toxsci/kfi150. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Hass U, Dalgaard M, Andersen HR, Bonefeld-Jorgensen E, Christiansen S, Laier P, Poulsen ME. Prochloraz: an imidazole fungicide with multiple mechanisms of action. International journal of andrology. 2006;29:186–191. doi: 10.1111/j.1365-2605.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Joergensen EC, Larsen JC. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicology and applied pharmacology. 1999;155:150–60. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science (New York, NY) 2009;324:1707–10. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker C, Johnson L, Safe S. Effects of developmental exposure to indole-3-carbinol or 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive potential of male rat offspring. Toxicology and applied pharmacology. 1996;141:68–75. doi: 10.1006/taap.1996.0261. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, Gray LE. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicology letters. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol Sci. 1999;51:259–64. doi: 10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environmental health perspectives. 2008;116:1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environmental health perspectives. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]