Abstract
Sleep disturbance among methadone-maintained patients is highly prevalent. A full understanding of sleep disturbance requires polysomnographic (PSG) measures along with subjective sleep quality measures. The goal of this study was to describe our experiences in performing at-home unattended PSG in this population. Participants had a Pittsburgh Sleep Quality Index (PSQI) score of six or higher, indicating clinically significant insomnia, and sixty five percent of eligible persons agreed to enroll. Among 88 participants (53% female, 82% Caucasian, mean methadone dose 105 mg/day), each undergoing 2 nights of home PSG, we initiated 165 out of a maximum of 176 recordings. Overall, 81.7% of participants provided at least one night of “acceptable” PSG data of at least four hours duration. Urine toxicologies on PSG nights demonstrated that benzodiazepine use was common. We conclude that unattended polysomnography is feasible in a population of persons receiving methadone maintenance treatment. PSG signal quality and overall study success rates were similar to those in non-substance using populations.
Keywords: sleep, methadone, opiate dependence, home PSG
INTRODUCTION
The disease burden of the opioid-dependent drug user is substantial, related to transmission of infectious diseases, chronic medical conditions, and frequent hospitalizations.1 Over one million opioid-dependent patients enter methadone maintenance treatment each year in the United States, with over 225,000 persons enrolled in treatment programs (MMTP) at any given time.
Sleep disturbance among methadone-maintained patients is highly prevalent, and, as with alcohol-dependent patients,2,3 sleep difficulties may contribute to relapse risk. At least three-quarters of people in methadone maintenance surpass the threshold for clinically meaningful sleep disturbance.4,5 Data in these studies were based solely on self-report, however, and persons with difficulty sleeping may misperceive their sleep quality and quantity in comparison with polysomnographic measurement.6 Thus, a more complete understanding of sleep disturbance in patients in MMTP requires polysomnographic measures along with subjective sleep quality measures.
Unattended home polysomnography using portable digital recorders is an emerging methodology in sleep medicine and sleep research. Termed “Level II” devices, home sleep monitors record electroencephalography (EEG), electrooculography (EOG), electromyography (EMG), and respiratory parameters, the same measures that are recorded in laboratory polysomnography. Comparable results are obtained from home and in-lab recordings in populations without substance use disorders.7-9 Reasons for employing home polysomnography include lower costs,8,10 the ability to study a greater number of individuals, and evidence that sleep recorded in the home is free from “first-night” effects of an in-lab recording, and therefore more naturalistic and representative of usual sleep.11
Opioid-dependent persons, even those in treatment, may demonstrate less stable lifestyles than the general population--higher rates of psychiatric distress, family problems, and relationship difficulties--potentially limiting the feasibility of performing at-home polysomnography.12 On the other hand, these same issues may also pose logistical difficulties in obtaining in-lab polysomnography in methadone maintenance patients in terms of cancellations, drop-outs/no-shows, and transportation issues. No studies to date have evaluated the viability of home polysomnography in individuals in MMTP.
Our group has an ongoing clinical trial investigating whether bedtime treatment with trazodone improves sleep quality compared to placebo among persons enrolled in MMTP who report sleep problems. This trial includes at-home polysomnography to provide an objective measure of sleep (the main dependent measure). The goal of this paper is to describe our experiences in performing at-home unattended polysomnography in a methadone-maintained population with insomnia who are enrolling in a trazodone treatment trial.
METHODS
Participants were recruited between January 2006 and May 2008 from eight Methadone Maintenance Treatment clinics in the Providence, RI metropolitan area. Study research assistants (RA’s) posted flyers to recruit individuals with trouble sleeping, and clinic staff made clients aware of the study. Interested MMTP clients were screened by study staff in person at their respective clinics during clinic dosing hours, usually between 6AM and noon.
Eligibility criteria included a Pittsburgh Sleep Quality Index (PSQI) score of six or higher13 indicating clinically significant insomnia, ability to speak, read, and understand English, and plans to continue methadone maintenance for at least six months. Individuals with current or past symptoms suggestive of schizophrenia, bipolar disorder, any psychotic disorder or gross cognitive dysfunction per an RA’s assessment (see below) were excluded. Other exclusion criteria included: current use (last 30 days) of trazodone; inability or refusal to terminate the use of proerectile agents; inability or refusal to use birth control throughout the study period in female participants; the presence of contraindications to the study medication, including pregnancy/lactation, ischemic heart disease, known obstructive sleep apnea, poorly controlled diabetes mellitus; probation/parole requirements that might interfere with protocol participation; inability to identify contact persons; and unstable housing such as a shelter or halfway house.
Eligible individuals provided informed consent. The study was approved by the Institutional Review Boards of Butler Hospital and Rhode Island Hospital. Participants agreed to four assessments over six months (baseline, 1, 3, and 6 months) with two 2-night home sleep studies performed at the baseline and one month. Participants were also asked to complete daily sleep diaries for two weeks prior to the baseline assessment. Participants logged bed and wake times, rated subjective sleep quality, and recorded study medication and other drug use. Participants received monetary compensation at each of their study assessments, but no compensation was provided for study eligibility screening.
Enrollment Interview
At baseline, participants were interviewed to assess demographics, drug and/or alcohol use and dependence, addiction severity, drug cravings, and commitment to abstinence. Questionnaires also included assessments of sleep quality, fatigue, psychological symptoms, health-related quality of life, medical conditions and medications, and service utilization. The baseline interview included a checklist of symptoms of potential medication side effects, to be compared to an identical list completed at follow-ups after participants were randomized to trazodone or placebo.
Polysomnography
Compumedics (Charlotte, NC, USA) Safiro and Siesta units were used for polysomnographic (PSG) recordings following standard home PSG study protocols.14 Sleep was measured using electroencephalography recorded from C3 and C4 referenced to the contralateral mastoids, electrooculography from ROC and LOC, and electromyography from submental surface electrodes. Respiration was monitored with nasal/oral thermocouples or thermistors, nasal pressure transducers, pulse oximetry, and surface intercostal and abdominal piezo crystal respiration belts. EKG was monitored with surface electrodes on the chest and side.
Participants were scheduled for two consecutive nights of baseline unattended polysomnography. Two RA’s were present at each PSG set-up to help ensure the safety and comfort of participants and staff. The RA’s went through a formal training, and were required to perform three acceptable sleep studies independently prior to collecting study data. The quality performance of individual technicians was monitored using feedback of PSG results from the Registered Polysomnographic Technologist (RPSGT) who scored the studies. The RPSGT maintained >90% concordance with a second trained sleep scorer.
Before set up, participants performed a breathalyzer and provided a urine sample for toxicological analysis of cocaine, opiates other than methadone, tetrahydrocannabinol (THC), and benzodiazepines (6-Panel KO Autosplit Drug Test Cup, Drug Detection Devices, Ltd., Alpharetta, GA, USA). Evidence of drug/alcohol use was not a basis for discontinuing the evening’s study, although RA’s were instructed not to continue if a participant exhibited symptoms of intoxication.
When PSG hook-up was complete, the portable sleep monitor was placed in a waist pack that the participant wore throughout the night. Technicians tested electrode impedances and viewed the recordings on laptop computers before leaving the participant’s home. The following morning, two research assistants met the participant at his/her home, or asked the participant to bring the equipment to the MMT clinic. When equipment was returned, RA’s administered a brief survey about bed and wake times, sleep quality and medication/illicit drug use.
Because sleep studies were conducted using unattended home polysomnography, technical difficulties, including artifacts and sensor loss, were not detectable in “real time.” In accordance with the criteria set in the Sleep Heart Health Study(SHHS),15 our a priori criteria for an acceptable polysomnogram stipulated that the record must contain at least 4 hours of sleep period time (SPT), including at least one scorable EEG, oximetry, and a respiratory signal during the total recording time. Studies that did not meet these criteria were deemed “unacceptable” per SHHS protocol.
In order to quantify data lost to artifact and sensor loss, we randomly selected 20 polysomnograms (10 each from night 1 and night 2). Each study was examined in 30 second increments or “epochs” for presence of artifact or missing data in the following leads: C3 EEG, C4 EEG, right EOG, left EOG, chin EMG, abdominal effort, thoracic effort, oximetry, nasal pressure transducer, and thermocouple. A signal was deemed uninterpretable if it was missing or obscured by artifact for ≥ half the epoch.
RESULTS
From January 30, 2006 through May 31, 2008, 332 individuals in MMT were screened for the study. Of these, 136 were eligible and 88 agreed to participate. The most common reasons for ineligibility were unstable housing (n=89), plans to leave methadone treatment within six months (n=46), and a PSQI score less than 6 (n=32). Participant demographic information is shown in Table 1. The cohort was 46.6% male with a mean methadone dose of 105 mg per day. Eighty-two percent of participants lived with another person and 44.3% had children in the household.
Table 1.
Demographic data for 88 participants
| Male | 46.6% |
| Caucasian | 81.8% |
| Average age | 37.6 years |
| Duration of MMT (weeks) | Mean: 71.8 Median: 38.3 |
| Average MMT dose | 104.9 mg/day |
| % Living alone | 18.2% |
| % With children in the home | 44.3% |
| % With children <5y.o. in the home | 19.3% |
Polysomnography
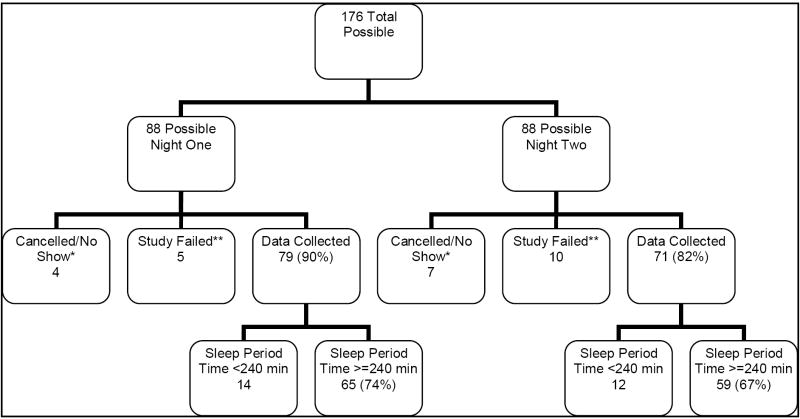
At the baseline PSG assessment, based upon a study protocol of 2 nights of PSG in 88 participants, a maximum of 176 recordings were possible (Figure 1). Research staff initiated PSG recordings on 165 nights: 84 on night 1 and 81 on night 2. Only one participant explicitly refused to continue after his first night of polysomnography, and one refused polysomnography before any PSG. Two participants had unstable housing situations after enrollment, and continued in the study without any polysomnography. Other reasons PSG recordings were not initiated include a death in a participant’s family and participants who were not at home (“no-shows”).
Figure 1. Outcomes for 176 Total Possible Baseline PSG’s.
*Studies were cancelled by research staff if a participant lost stable housing, or at the request of the participants. A participant was considered a “no-show” for a recording if he/she was not at home by 30 minutes past an appointment.
**A study was considered failed if the recording was initiated but sensor loss (equipment failure or participant non-compliance) resulted in no useable data.
Among the 165 complete PSG nights, 15 nights (5 night one, 10 night two) provided no usable data, due to sensor loss or participant non-compliance. Because research staff left the home after PSG equipment was in place, participants could have accidentally (excessive movement) or willfully removed key electrodes and caused study failure. Some patients reported that sweating made electrode adherence difficult. Twenty-six recordings had less than 4 hours of SPT. Overall, 81.7% of participants provided at least one night of acceptable PSG data.
Of the 20 randomly selected recordings we examined, 3 had no lead loss or artifact in any signal for the entire recording, and fourteen had no data loss in EEG, EOG or EMG signals for the entire recording, thus allowing for complete sleep staging in 17 of 20. Respiratory data were more susceptible to artifact and sensor loss with 11 of 20 records missing at least one epoch of data. Nevertheless, all recordings were sufficient to evaluate fully for sleep-disordered breathing.
Substance Use
Urine samples were obtained before 71 of the 84 night one recordings and 73 of the 81 night two recordings (Table 2). Twenty-three of 71 participants tested positive for benzodiazepines on night one. Of those who tested negative for benzodiazepines or did not receive toxicology on night one, 4 of 51 tested positive on night two. Other positive urinalyses were for tetrahydrocannabinol (24/71 on night one and 5/51 new positives on night two), cocaine (17/71 on night one and 3/57 new positives on night two), and opiates other than methadone (12/71 on night one and 1/62 new positives on night two). We found no association between positive urine toxicology and either study failure or unacceptable PSG based on at least 4 hours of sleep period time.
Table 2.
Urinalysis results.
| Night 1 | Night 2 (New positives*) | |
|---|---|---|
| Benzodiazepines | 23/71 (32.4%) | 4/51 (7.8%) |
| Tetrahydrocannabinol | 24/71 (33.8%) | 5/51 (9.8%) |
| Cocaine | 17/71 (23.9%) | 3/57 (5.3%) |
| Opiates | 12/71 (16.9%) | 1/62 (1.6%) |
| BAL >0 | 2/81 (2.5%) | 1/75 (1.3%) |
| More than one substance | 23/71 (32.4%) | 5/51 (9.8%) |
Night 2 results are for participants who tested negative for a particular substance on Night 1 or who did not receive urinalysis on Night 1.
Participants were asked to report any medication or substance use in the four hours before bedtime or during the night. Substances reported were marijuana (n=5 both on night 1 and night 2), Tylenol PM (n=2 night 1 and night 2), benzodiazepines (n=6 night 1 and n=2 night 2), melatonin (n=1 night 1 and night 2), trazodone (n=1 night 1), heroin (n=1 night 1), cocaine (n=1 night 1 and night 2), and alcohol (n=1 night 2).
Participant Burden
Sleep quality and discomfort caused by the equipment were assessed by self-report the morning after each sleep study. More than three-quarters of participants (77.4% night 1, 77.8% night 2) reported sleeping better than or the same as usual. Participant discomfort due to specific aspects of the PSG is described in Table 3.
Table 3.
Self-reported discomfort due to specific aspects of PSG recording.
| Night 1 | Night 2 | |
|---|---|---|
| Leads on head | 29.8% | 21.0% |
| Probe on finger | 31.0% | 16.1% |
| Belts around chest/stomach | 20.2% | 17.3% |
| Nasal cannulas | 15.5% | 19.8% |
DISCUSSION
Our data indicate that at-home unattended polysomnography is feasible in a population of opiate dependent persons receiving methadone maintenance treatment. There was high interest in study participation with sixty-five percent of eligible persons agreeing to enroll. Once enrolled, moreover, 93% of scheduled baseline PSG’s were initiated. In addition, the quality of the data in terms of hours of adequate sleep recording was high. The Sleep Heart Health Study, which examined unattended PSG in a large group of individuals from the general population, obtained at least four hours of PSG data in 90.6% of recordings.16 In our trial, four hours of data was successfully obtained on at least one baseline night from 81.7% of participants. Unlike SHHS however, we included only persons with insomnia, and thus four hours of contiguous recording may be too strict a threshold for an “acceptable” study in this population.
Sensor loss in our study was not higher than in studies researching other populations. In the first large-scale study examining signal loss during unattended PSG, Kapur, et al., documented rates of sensor loss comparable to those we observed.16 Sensor loss rarely rendered the sleep staging uninterpretable and sleep-disordered breathing was fully evaluable.
Nonetheless, researchers working with drug dependent persons should be prepared for unique design considerations. First, due to the impoverishment of a high proportion of participants, sleep technicians may be asked to enter apartments and houses that are poorly lit and unclean. Evening study technicians often had to be very flexible and accommodating. Participants were not always home, or awake, when they said they would be. Evening staff had participant phone numbers and were instructed to wait for 30 minutes before canceling the study. Fortunately, study staff was usually able to locate participants or wake them, and few studies were cancelled. On occasion, staff arrived at the scheduled location to find a message instructing them to go to a different location for the study. As in all home PSG studies, children and pets were occasionally a distraction to both the participant and the technicians.
Overnight PSG’s can be uncomfortable, requiring participants to have paste and tape applied to their heads and faces, and to wear two nasal cannulas. While we had low levels of self-reported discomfort during PSG nights, we do not know the extent to which discomfort contributed to lead loss in our study or whether opioid-dependent patients experience more discomfort or react differently during a PSG recording than those without opiate dependence.
On several occasions, we encountered difficulties retrieving the sleep monitoring equipment on the morning following a study. If a participant was not home or at the MMT clinic at the appointed time, RA’s telephoned the participant and his/her contacts and could almost always locate the equipment. On one occasion, the evening study technicians were able to supply information regarding a participant’s workplace, and an RA retrieved the equipment there.
The use of illicit drugs on PSG nights was an expected finding. We collected both self-report and toxicological data to quantify in future studies if drug use has effects on polysomnographic data (e.g. sleep staging) or on the efficacy of our medication intervention. Toxicological data prior to the first recording indicated that nearly one-third of participants had used benzodiazepines in the past week. We do not know if this was prescribed or non-prescribed benzodiazepine use. Study staff asked participants not to use any sleep aids on PSG nights; only six persons reported using benzodiazepines on nights one or two, and the number of newly positive toxicologies was similar. The limitations of toxicologic analysis makes it difficult to determine actual drug use during PSG nights. In addition, participants were unwilling/unable to provide urine samples for toxicology on 24 baseline nights.
In this population we anticipated high rates of study failure due to difficult home circumstances and chaotic lifestyles, particularly in cases of ongoing illicit substance use. In addition, we were studying a population with subjective sleep complaints. Yet the present data demonstrate the feasibility of performing unattended home polysomnography in methadone-maintained persons with insomnia. Signal quality and overall PSG study success rates were high. We have demonstrated that home PSG will have research and clinical use in the evaluation of sleep disorders among methadone-maintained persons when properly trained staff are aware of the population-specific issues likely to arise.
Acknowledgments
This study was funded by The National Institute on Drug Abuse, Grant DA20479-2; Clinical Trial NCT 00253890
Footnotes
Dr. Stein is a recipient of NIDA Mid-Career Award DA00512
Reference List
- 1.Stein MD. Medical consequences of substance abuse. Psychiatric Clinics of North America. 1999 June;22(2):351–70. doi: 10.1016/s0193-953x(05)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Feige B, Scaal S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007 January;31(1):19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 4.Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend. 2006 April 28;82(2):103–10. doi: 10.1016/j.drugalcdep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, Anderson BJ. Sleep disturbances among methadone maintained patients. J Subst Abuse Treat. 2004 April;26(3):175–80. doi: 10.1016/S0740-5472(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976 December;133(12):1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 7.Ferber R, Millman R, Coppola M, Fleetham J, Murray CF, Iber C, McCall V, Nino-Murcia G, Pressman M, Sanders M. Portable recording in the assessment of obstructive sleep apnea. ASDA standards of practice Sleep. 1994 June;17(4):378–92. doi: 10.1093/sleep/17.4.378. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher EC, Stich J, Yang KL. Unattended home diagnosis and treatment of obstructive sleep apnea without polysomnography. Arch Fam Med. 2000 February;9(2):168–74. doi: 10.1001/archfami.9.2.168. [DOI] [PubMed] [Google Scholar]
- 9.Fry JM, DiPhillipo MA, Curran K, Goldberg R, Baran AS. Full polysomnography in the home. Sleep. 1998 September 15;21(6):635–42. doi: 10.1093/sleep/21.6.635. [DOI] [PubMed] [Google Scholar]
- 10.Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. European Respiratory Journal. 2003;21:253–259. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 11.Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988 June;11(3):273–6. doi: 10.1093/sleep/11.3.273. [DOI] [PubMed] [Google Scholar]
- 12.Hayaki J, Stein MD, Lassor JA, Herman DS, Anderson BJ. Adversity among drug users: relationship to impulsivity. Drug Alcohol Depend. 2005 April 4;78(1):65–71. doi: 10.1016/j.drugalcdep.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997 December;20(12):1077–85. [PubMed] [Google Scholar]
- 15.Iber C, Redline S, Kaplan Gilpin AM, Quan SF, Zhang L, Gottlieb DJ, Rapoport D, Resnick HE, Sanders M, Smith P. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004 May 1;27(3):536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 16.Kapur VK, Rapoport DM, Sanders MH, Enright P, Hill J, Iber C, Romaniuk J. Rates of sensor loss in unattended home polysomnography: the influence of age, gender, obesity, and sleep-disordered breathing. Sleep. 2000 August 1;23(5):682–8. [PubMed] [Google Scholar]