Abstract
Background. Monitoring changes in glomerular filtration rate (GFR) is the recommended method for assessing the progression of kidney disease. The aim of this study was to assess the decline of graft function defined by the annualized change in GFR and the factors which affect it.
Methods. Four thousand four hundred and eighty-eight patients, transplanted during the years 1990, 1994, 1998 and 2002 in 34 centres in Spain with allograft survival of at least 1 year, were included in the study. GFR was estimated using the four-variable equation of the Modification of Diet in Renal Diseases (MDRD) study. Linear mixed effects model was applied to determine the relation between the covariates and the annualized change in GFR after transplantation.
Results. The average GFR at 12 months was 51.4 ± 18.9 mL/min/1.73 m2; most patients were in stage 3 of chronic kidney disease classification. The average patient slope, calculated in a linear model with varying-intercept and varying-slope without covariates, was −1.12 ± 0.05 mL/min/year (slope ± standard error). Some variables were related to both the 12-month GFR (intercept) and the slope: recipient gender, hepatitis C virus (HCV) status, estimated GFR (eGFR) at 3 months and proteinuria at 12 months. Some variables were only related to the slope of eGFR: time on dialysis, primary renal disease and immunosuppression. Others affected only the 12-month GFR: donor age, delayed graft function, acute rejection and systolic blood pressure at 12 months. Higher graft function at 3 months had a negative impact on the GFR slope. Cyclosporine-based immunosuppression had a less favourable effect on the rates of change in allograft function.
Conclusions. There was a slow decline in GFR. Poor graft function was not associated with an increased rate of decline of allograft function. Immunosuppression with cyclosporine displayed the worst declining GFR rate.
Keywords: glomerular filtration rate, immunosuppression, kidney transplantation
Introduction
The new immunosuppressive drugs have decreased the incidence of rejection and have improved short-term graft survival. However, there has been little or no improvement in late allograft failure [1,2]. The lack of long-term improvement in graft outcomes has been attributed to the increased mortality of recipients with functioning grafts, mostly by cardiovascular diseases, and to graft losses by chronic allograft nephropathy, 50% of losses being due to patient death and the remainder to loss of function [3]. On the other hand, it has been reported that graft function measured by serum creatinine early after transplantation was an important predictive factor of graft survival [4]. Consequently, improving and maintaining early graft function could reduce the rate of late graft failure. However, serum creatinine is not an accurate index of graft function. The Kidney Disease Outcome Quality Initiative (K/DOQI) [5] guidelines have recommended measuring graft function in primary renal diseases by estimated creatinine clearance (eCrCl) or glomerular filtration rate (eGFR) including variables such as age, sex and race. Monitoring changes in GFR has been established as the recommended method for assessing the progression of kidney disease (K/DOQI).
As in most native kidney diseases, GFR declines progressively over time in renal transplant recipients. Several studies from the USA and Canada have shown that the decline of renal function can be calculated post-transplantation from the slope of eCrCl or eGFR beyond 6 or 12 months after transplantation [6–12]. But, in most of these studies, the decline of graft function was calculated by linear least squares regression analysis or by the increment between two measurements. However, there are other statistical methods to estimate the decline of graft function. For example, the measurement of progression of renal disease in the Modification of Diet in Renal Disease Study (MDRD) was estimated using linear mixed effects models [13].
The purpose of the present study was to evaluate, in a large Spanish population of renal transplant recipients, the rate of decline of graft function after transplantation and the factors associated with this change, making special emphasis on baseline graft function and immunosuppression using the linear mixed effects model.
Materials and methods
Patients
From a total of 4842 adult renal transplant recipients from 34 centres receiving a renal allograft in Spain during the years 1990, 1994, 1998 and 2002, 4488 were included in the study. The inclusion criteria were to be recipients of a single organ, with the graft functioning 12 months after transplantation and >2 years of follow-up. Patients were followed up until graft loss, death or December 2005, whichever occurred before. The mean follow-up was 74.0 ± 43.9 months. Altogether, 26 667 reviews were performed, corresponding to a mean of six reviews per patient.
Methods
Data concerned recipients (age, gender, primary renal disease, time on dialysis, type of dialysis, height and weight, serology to hepatitis C and B virus and last panel-reactive antibodies), donors (age, gender, type of donor, cause of donor death and serology to hepatitis C and B virus), grafts [human leucocyte antigen (HLA) mismatches, warm and cold ischaemia times, re-anastomosis time, immediate graft function, rejection episodes and immunosuppression]. Clinical and biochemical variables (haemoglobin, serum creatinine, proteinuria, blood glucose, serum lipids) were collected at 3 months, 12 months and yearly until the end of the follow-up.
Delayed graft function was defined by haemodialysis requirement during the first week. Acute rejection was defined at each centre based on clinical and/or histological data. Immunosuppressive treatment was recorded at each visit and classified into three groups: (i) cyclosporine-based, (ii) tacrolimus-based and (iii) calcineurin inhibitor (CNI)-free therapy. The CrCl was estimated by the Cockcroft–Gault equation: CrCl (mL/min) = [(140 − age) × weight] / [72 × SCr (mg/dL)] × (0.85 if female). The estimated GFR (eGFR) was estimated using the abbreviated MDRD equation: eGFR (mL/min/1.73 m2) = exp(5.228 − 1.154) × ln(SCr) − 0.203 × ln(age) − (0.299 if female) + (0.192 if black). The study was approved by the ethical committee of the Hospital de Bellvitge. Medical record review was performed according to Spanish law with reference to clinical data confidentiality protection. A blinded code was assigned to each participating hospital in order to take into consideration centre effect.
Statistics
Patient characteristics were described as mean and standard deviation for continuous variables and frequency for categorical variables. All available creatinine measurements recorded were included in the serial GFR estimates. Progression of chronic kidney disease (CKD) was analysed by the mixed effects model:
where i = 1, 2,..., 4488. GFRi is the GFR for the ith patient, β coefficients correspond to the random effects, which allow the intercept and the slope to be different for each patient, a and b are the vector of fixed effects, X is a vector of the covariates, and ei is a vector of residuals [14,15]. In order to take into account the correlation between successive patient measures, we considered a model with first-order autoregressive structure for the residuals. The coefficient of interaction terms, b Xi year, measures the influence of these covariates in the change of the slope according to the estimated model. The time origin (year = 0) was fixed at 12 months.
The covariates included in the model were: (i) Recipient: age, gender, primary renal disease, time on dialysis, hepatitis C virus (HCV) serology; (ii) Donor and graft: age, gender, type of donor, number of transplants, cold ischaemia time, delayed graft function, acute rejection, year of transplantation and graft function at 3 months; (iii) Characteristics at 12 months: blood pressure, body mass index (BMI), proteinuria, immunosuppression and graft function estimated as chronic kidney disease stages. For calculations, time on dialysis and proteinuria were converted to natural logarithms. Univariate and multivariate analysis were performed. We explored a multivariate model, which included all clinical relevant variables, and selected a final model retaining as many variables as possible using de AKAIKE Information Criterion (AIC). We estimated the slope in the multivariate analysis by two models: in the first, only the available eGFR were used; in the second, we imputed a GFR of 10 mL/min/1.73 m2 to patients who returned to dialysis at the date of graft failure.
Results
Characteristics of the patients
The patient characteristics are contained in Table 1; some data are missing, and the number of patients did not reach 4488. There was a progressive increase of recipient age from 42.5 ± 12.2 years in 1990 to 48.3 ± 13.3 years in 2002 (P = 0.000). Donor age also increased from 32.7 ± 14.6 in 1990 to 46.4 ± 16.4 in 2002 (P = 0.000). Time on dialysis decreased from 3.8 ± 3.4 years in 1990 to 3.1 ± 3.8 years in 2002 (P = 0.000) as well as cold ischaemia time from 20.8 ± 6.6 h in 1990 to 18.1 ± 6.0 in 2002 (P = 0.000). Most patients were on haemodialysis, and they received a kidney from a deceased donor. Immunosuppression at 12 months was cyclosporine-based in 70.4% and tacrolimus-based in 23.2%, and only 6.4% of patients were in CNI-free immunosuppression. During the follow-up, 842 recipients lost their grafts. The causes of graft loss were: death with graft function (36.0%), biopsy-confirmed chronic allograft nephropathy (22.2%), chronic allograft nephropathy without histological confirmation (27.4%), recurrent glomerulonephritis (3.3%), de novo glomerulonephritis (2.9%) and others (8.2%).
Table 1.
Characteristics of the patients and CKD progression (univariate analysis)
| Variable | Mean ± SD or % | Coefficient ± SE | t |
|---|---|---|---|
| Age at transplant (years) | 45.9 ± 13.1 | 0.020 ± 0.004 | 5.24 |
| Gender (male/female) | 2819/1669 | −0.358 ± 0.100 | −3.52 |
| Time on dialysis (years) | 3.3 ± 3.7 | −0.278 ± 0.048 | −5.78 |
| Type of dialysis (vs haemodialysis) | |||
| Haemodialysis | 3842 (85.6%) | 0.229 ± 0.179 | 1.28 |
| Peritoneal dialysis | 418 (9.6%) | −0.088 ± 0.298 | −0.30 |
| Both | 117 (2.7%) | ||
| HCV status (negative/positive) | 3578/576 | −0.766 ± 0.136 | −5.63 |
| Primary renal disease (vs diabetes) | |||
| Diabetes | 237 (5.3%) | ||
| Chronic glomerulonephritis | 1007 (22.5%) | 0.784 ± 0.248 | 3.01 |
| Interstitial nephritis | 570 (12.7%) | 0.604 ± 0.302 | 2.00 |
| Nephroangiosclerosis | 315 (7.0%) | 0.680 ± 0.269 | 2.52 |
| Polycystic disease | 596 (13.3%) | 1.017 ± 0.270 | 2.78 |
| Unknown/other | 1763 (39.3%) | 0.738 ± 0.249 | 2.96 |
| Donor age (years) | 41.8 ± 16.9 | 0.002 ± 0.003 | 0.66 |
| Donor gender (male/female) | 2906/1582 | 0.059 ± 0.103 | 0.57 |
| Cold ischaemia time (hours) | 19.2 ± 7.1 | 0.009 ± 0.006 | 1.41 |
| Type of donor (deceased/living) | 4425/63 | −0.864 ± 0.438 | −1.97 |
| HLA mismatches (n) | 3.2 ± 2.5 | −0.009 ± 0.041 | −0.22 |
| Number of transplant (first/re-transplant) | 3950/538 | −0.703 ± 0.154 | −4.57 |
| Delayed graft function (no/yes) | 2952/1275 | 0.077 ± 0.112 | 0.69 |
| Acute rejection (no/yes) | 3204/1211 | −0.159 ± 0.107 | −1.50 |
| Immunosuppression at 12 months (vs CsA) | |||
| Cyclosporine | 3163 (70.5%) | ||
| Tacrolimus | 1044 (23.3%) | 0.382 ± 0.178 | 2.15 |
| No calcineurin inhibitors | 133 (2.9%) | 1.135 ± 0.332 | 3.42 |
| eGFR at 3 months (mL/min/1.73 m2) | 51.2 ± 20.0 | −0.026 ± 0.002 | −10.00 |
| BMI at 12 months (kg/m2) | 26.4 ± 4.3 | 0.016 ± 0.016 | 1.02 |
| Proteinuria at 12 months (g/24 h) | 0.3 ± 0.8 | −0.064 ± 0.125 | −5.15 |
| Systolic blood pressure at 12 months (mmHg) | 139 ± 18 | −0.002 ± 0.003 | −0.68 |
| Diastolic blood pressure at 12 months (mmHg) | 81 ± 11 | −0.001 ± 0.001 | 0.12 |
| Total cholesterol at 12 months (mg/dL) | 219 ± 46 | 0.001 ± 0.001 | 1.21 |
| Triglycerides at 12 months (mg/dL) | 148 ± 71 | 0.250 ± 0.125 | 2.01 |
| CKD stage at 12 months (vs stage 5) | |||
| Stage 5 | 22 (0.49%) | ||
| Stage 4 | 459 (10.27%) | −0.674 | −0.76 |
| Stage 3 | 2656 (59.44%) | −1.057 | −1.22 |
| Stage 2 | 1210 (27.08%) | −1.732 | −1.99 |
| Stage 1 | 121 (2.71%) | −4.804 | −5.26 |
The coefficient represents the interaction of the covariate and the time. The differences are statistically significant when t >2.
HCV, hepatitis C virus; CsA, cyclosporine; BMI, body mass index; CKD, chronic kidney disease.
Graft function
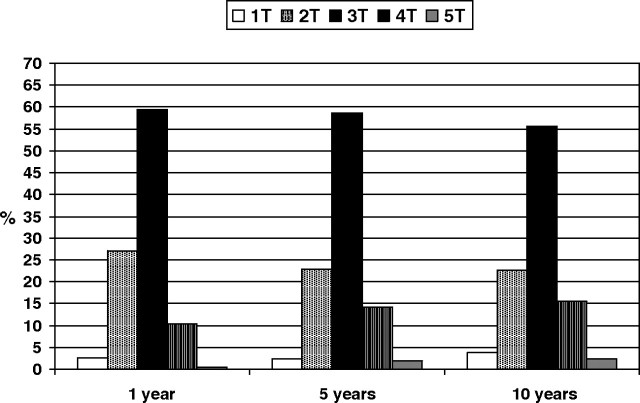
At 12 months, the mean serum creatinine of the 4488 patients was 1.6 ± 1.5 mg/dL, median (range 0.5–6.7 mg/dL). The mean creatinine clearance (n = 4079) determined by the Cockroft–Gault equation was 60.7 ± 21.8 mL/min, median 58.5 mL/min (range 11–162 mL/min), and the mean eGFR calculated by the MDRD study equation was 51.7 ± 18.8 mL/min/1.73 m2, median 49.02 mL/min/1.73 m2 (range 7.9–139 mL/min/1.73 m2). Proteinuria at 12 months measured as gram per 24 h was available in 4288 recipients. The distribution of CKD stages at 12 months for stages 1–5 was 2.7%, 27.1%, 59.4%, 10.3% and 0.5% according to eGFR. A very similar CKD stage distribution was observed at 5 and 10 years (Figure 1).
Fig. 1.
Distribution of chronic kidney disease stages at 1, 5 and 10 years.
CKD progression
The eGFR rate declined from 12 months to last visit, with a mean of −1.26 ± 6.24 mL/min/year (mean ± standard deviation) and median of −0.752 mL/min (range from −68.7 to 39.1 mL/min/year) when we determined the slope by applying the least squares regression for each patient. If we used the linear mixed effects models, varying intercept and slope for each patient, the decline was −1.12 ± 0.05 mL/min/1.73 m2 per year (coefficient ± standard error) (Figure 2).
Fig. 2.
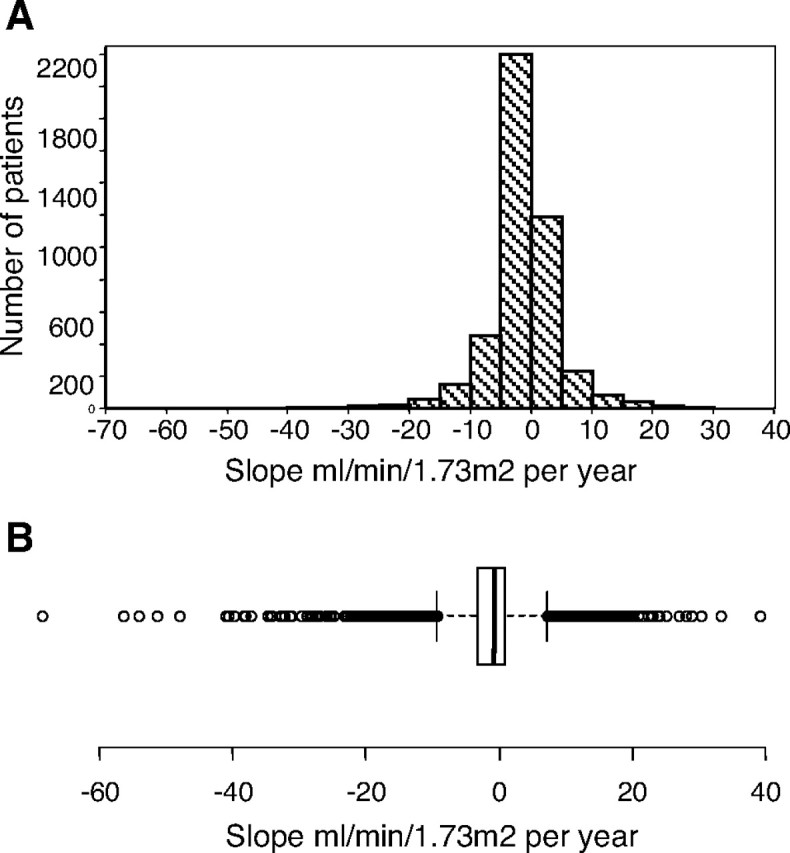
(A) Distribution of GFR slope. (B) The figure illustrates the median of the slopes, the interquartile range and the maximum and minimum values.
In the mixed effects model, we have used all the baseline characteristics described in Table 1 to analyse the evolution of eGFR along the time of follow-up. In the univariate analysis, the variables correlated with GFR at 12 months (intercept) were: recipient age and sex, donor age and sex, HLA mismatches, delayed graft function, acute rejection, GFR at 3 months, immunosuppression, BMI, proteinuria, blood pressure and serum lipids at 12 months. Table 1 shows the coefficient and the standard error of covariates associated with the GFR slope. The coefficients represent the interaction (covariate × time). For continuous variables, the coefficient is the mean of the change in GFR decline for each unit change in the predictor variable while maintaining the other covariates fixed in the model. For example, mean GFR decline was 0.020 ± 0.004 mL/min/1.73 m2 per year faster for each year of age. For binary variables such as recipient and donor sex, type of donor, delayed graft function and acute rejection at 12 months, the coefficient is the mean difference in GFR decline between the two groups. For example, mean GFR decline was 0.358 ± 0.100 mL/min/year faster in female than in male recipients. For variables with more than two categories, such as primary renal disease and treatment group, having k possibilities or categories without a definite order, we constructed a series of k-1 binary dummy variables, coding one as reference. The coefficient for the other categories is the mean difference in GFR decline between this category and the reference. Diabetes mellitus was the reference in primary renal disease variable and cyclosporine in the immunosuppressive treatment. For example, mean GFR decline was 1.135 ± 0.332 mL/min/year slower in CNI-free-treated recipients than in cyclosporine-treated recipients.
In the univariate analysis, among the baseline characteristics of the patients, age at transplant, sex, time on dialysis, HCV status and renal disease were associated with GFR slope. The number of transplants and re-transplantation was also associated with GFR slope. There was no association with donor age, donor sex, type of donor, cold ischaemia time, delayed graft function and acute rejection. Concerning patient characteristics at 12 months, proteinuria and immunosuppression were associated with GFR slope, and there was no association with body mass index, systolic and diastolic blood pressure, serum cholesterol levels, triglycerides levels and graft function expressed as CKD stages.
The variables significantly associated with GFR intercept (value at 12 months after transplant) and slope in the multivariate analysis are expressed in Table 2; the number of patients included in the final analysis was 3502. Several variables: donor gender, HCV status, donor age, delayed graft function, acute rejection, eGFR at 3 months, proteinuria at 12 months and systolic blood pressure at 12 months had an impact on the 12-month eGFR. Some variables affected both the 12-month eGFR and the subsequent slope: donor gender, HCV status, eGFR at 3 months and proteinuria at 12 months. Other variables affected only the slope of eGFR: time on dialysis, primary renal disease and immunosuppression. There was a great variability in inter- and intra-patient slope; the square root of the estimated variance inter-patients was 1.5, and the square root of the residual variance (intra-patients) was 9.4. As expected, the estimated stationary autocorrelation was moderately large (0.44), indicating a fair amount of autocorrelation amongst the model residuals.
Table 2.
Association between selected variables and GFR at 12 months (intercept) and GFR slope (multivariate analysis)
| Variable | Coefficient ± ES | t | P |
|---|---|---|---|
| Variables affecting intercept | |||
| Recipient gender (male/female) | −1.523 ± 0.394 | −3.868 | 0.000 |
| HCV status (negative/positive) | −1.789 ± 0.567 | −3.153 | 0.002 |
| Donor age (years) | −0.222 ± 0.012 | −17.927 | 0.000 |
| Delayed graft function | −0.804 ± 0.412 | −1.954 | 0.050 |
| Acute rejection (no/yes) | −2.179 ± 0.421 | −5.179 | 0.000 |
| eGFR at 3 months (mL/min/1.73 m2) | 0.604 ± 0.011 | 52.264 | 0.000 |
| Proteinuria at 12 months (g/24 h) | −0.438 ± 0.067 | −6.516 | 0.000 |
| Systolic blood pressure at 12 months (mmHg) | −0.024 ± 0.010 | −2.389 | 0.017 |
| Variables affecting slope | |||
| Recipient gender by time | −0.505 ± 0.103 | −4.916 | 0.000 |
| Time on dialysis (years) by time | −0.201 ± 0.052 | −3.871 | 0.000 |
| HCV status (vs negative) by time | −0.407 ± 0.136 | −3.001 | 0.003 |
| Primary renal disease (vs diabetes) by time | |||
| Chronic glomerulonephritis | 1.051 ± 0.274 | 3.829 | 0.001 |
| Interstitial nephritis | 0.750 ± 0.324 | 2.314 | 0.000 |
| Nephroangiosclerosis | 1.028 ± 0.294 | 3.491 | 0.000 |
| Polycystic disease | 1.301 ± 0.293 | 4.442 | 0.000 |
| Unknown/other | 0.993 ± 0.276 | 3.596 | 0.000 |
| eGFR at 3 months (mL/min/1.73 m2) by time | −0.023 ± 0.003 | −8.945 | 0.000 |
| Proteinuria at 12 months (mg/day) by time | −0.098 ± 0.018 | −5.545 | 0.000 |
| Immunosuppression 12 months (vs cyclosporine) by time | |||
| Tacrolimus | 0.415 ± 0.193 | 2.147 | 0.032 |
| No calcineurin inhibitors | 0.874 ± 0.341 | 2.562 | 0.010 |
HCV, hepatitis C virus.
When we analysed a model in which we imputed a GFR of 10 mL/min on the date of graft failure to the patients who returned to dialysis, there were no significative changes in the results. In order to explore a non-linear change of GFR, we tested a model with a quadratic term for the time, finding it not relevant.
Discussion
The distribution of CKD stages was similar to other studies performed in Spain [16,17] and in other countries [18,19], and most of the patients were in the 3T stage. Furthermore, this distribution of CKD stages did not change with the length of follow-up, and no significant differences were observed at 1, 5 and 10 years after transplantation. We have observed a very slow decline rate of graft function (−1.12 ± mL/min per 1.73 m2 per year), lower than that previously reported from single-centre studies [6,10,11, 20] or from registries [7,8]. These differences could be due to the characteristics of the population studied, but age at transplant, graft function at starting the study and length of follow-up were not different to those of our patients. Another possible explanation could be a better control of other variables such as blood pressure, serum lipid levels or proteinuria, and finally, the differences could also be explained by the method used to calculate the slope, mixed effect analysis in our study and single regression in most previous studies. A common finding in our and other studies when analysing the decline in graft function over time was the great inter-patient variability in the slope of GFR [9]. Patients with slower progression of graft function deterioration and patients with improvements suggest that the grafts still retain a certain capacity of hypertrophy as is typical of solitary native kidneys in response to uninephrectomy.
The multivariate analysis demonstrated that some variables were independently associated with the 12-month eGFR such as recipient gender, HCV status, donor age, delayed graft function, acute rejection, eGFR at 3 months, proteinuria and systolic blood pressure at 12 months. Donor age and acute rejection were the factors with the strongest association with the 12-month graft function. Several of these variables were associated with 6-month Cockcroft–Gault estimate of CrCl in other studies [6,7,20]. In contrast, our data did not confirm any association of HLA mismatches, panel-reactive antibodies type of donor and 12-month graft function [6,7,20]. The influence of HLA matching and panel-reactive antibodies in the evolution of graft function could support the importance of immunological factors in allograft function; a more potent immunosuppression could explain our differences with the other studies. Concerning the type of donor, the number of living donors was too small to establish robust comparisons with deceased donors.
When we analysed the variables associated with the slope of eGFR, some variables (donor age, delayed graft function and rejection) associated with graft function at 12 months did not affect the slope of eGFR, but the time on dialysis, diabetes as primary renal disease and immunosuppression did. Graft function at 3 months, proteinuria, recipient gender and time on dialysis were the variables which showed the strongest association with the slope of eGFR. The influence of initial graft function in the progression of graft failure has been investigated in several previous works. Gill et al. [7] found, in 40 963 renal transplant recipients, a small but significantly more rapid decline in GFR in patients with a higher baseline GFR, and their findings are supported by other single-centre studies [6]. Djamali et al. have investigated the evolution of graft function according to CKD stages [11], and they observed a more rapid decline in graft function in early stages (1T and 2T) than in late stages (3T and 4T). We found a similar tendency in our study in the univariate and multivariate analysis. These data demonstrated that there is no increased rate of function loss at lower levels of GFR and that grafts with GFR <30 mL/min/1.73 m2 may be stabilized for long periods of time. As has been stated [6], this is an important finding mainly in the current era in which the percentage of older and suboptimal donors in our country is very high and is still increasing, and supports the utilization of grafts from old donors despite worse eGFR when compared with younger donors.
Proteinuria, usually at low levels, is quite common after kidney transplantation. It has been identified as a risk factor of poor graft survival [21,22]. After transplantation, proteinuria may be due to various allograft pathologies and/or may be a side effect of immunosuppression. However, no previous studies have evaluated its influence in the decline of graft function. Our study showed that proteinuria at 12 months was one of the most important factors negatively associated with graft function decline. These findings strengthen the recommendations that antiproteinuric measures should be applied liberally to transplant recipients with proteinuria. As in other studies [6,7], female recipients experienced a more rapid rate of decline, the reason for which could be a higher sensitization. Recipient HCV serology before transplant had a negative influence in the decline of graft function. There are no data in previous studies about the effects of HCV infection on GFR decline. The influence of HCV infection on graft survival is controversial [23]. The impact of HCV status in the slope could be explained by the increased incidence of proteinuria and chronic allograft nephropathy in this population [24]. As in the general population, diabetes mellitus, as a primary renal disease, was associated with a higher decline in graft function than all other diseases, but two previous studies did not find this association in renal transplant recipients [11,12].
CNI, cyclosporine and tacrolimus, are the most commonly used immunosuppressive agents; both share the same immunosuppressive mechanisms, and both are nephrotoxic. There are a few studies about the influence of immunosuppressive regimens in the decline of graft function. In a registry study of 40 963 first kidney transplant recipients, Gill et al. 2004 [8] observed a slower decline in GFR in tacrolimus-treated patients and in patients who did not receive CNI when compared with patients who received cyclosporine microemulsion, and patients receiving mycophenolate mofetil also had a slower decline in GFR than those who received azathioprine. Flechner et al. [25] have emphasized the differences in GFR slopes between sirolimus-based and cyclosporine-based immunosuppression in a randomized, prospective trial. Cyclosporine recipients had a negative slope, while sirolimus recipients had a positive slope. On examining the effects of immunosuppression on graft function, we also found that cyclosporine-treated recipients had a more rapid decline than tacrolimus-treated and CNI-free immunosuppression recipients. This could support the belief that tacrolimus displays lower toxicity than cyclosporine. However, control trials have shown no differences on patient and graft survival [26] nor in the incidence of morphologic characteristics consistent with chronic allograft nephropathy [27] in recipients treated with tacrolimus when compared with those on treatment with cyclosporine. Patients on tacrolimus had a shorter follow-up than patients on cyclosporine and a different number of GFR estimations that could influence the results.
Our study has some limitations as it is a retrospective study, with significant differences in the length of follow-up and consequently in the number of GFR measurements. The assessment of graft function was performed by the abbreviated MDRD equation which is one of the methods of estimation of GFR recommended by K/DOQI guidelines. However, this method of estimation of GFR was considered to have a low precision and accuracy when compared with iothalamate GFR, and its slope underestimated the rate of functional loss [10]. But more precise methods of estimation of graft function are not always available, and are more expensive and time consuming.
Acknowledgments
The authors thank Luis Miguel Molinero for performing the statistical analysis and Mary Harper for her assistance in preparing the English version of this article.
Conflict of interest statement. None declared.
References
- 1.Meier-Kriesche H-U, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplanit. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Marcén R, Fernández-Rodriguez A, Rodriguez-Mendiola N, et al. Evolution of rejection rates and kidney graft survival: a historical analysis. Transplant Piroc. 2009;41:2357–2359. doi: 10.1016/j.transproceed.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Excerpts from the United States Renal Data System 2008 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2009;53:S1–S372. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311–318. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 5.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease. Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 6.Gourishankar S, Hunsicker LG, Jhangri GS, et al. The stability of the glomerular filtration rate after transplantation is improving. J Am Soc Nephrol. 2003;14:2387–2394. doi: 10.1097/01.asn.0000085019.95339.f0. [DOI] [PubMed] [Google Scholar]
- 7.Gill JS, Tonelli M, Mix CH, et al. The change in allograft function among long-term kidney transplant recipients. J Am Soc Nephrol. 2003;14:1636–1642. doi: 10.1097/01.asn.0000070621.06264.86. [DOI] [PubMed] [Google Scholar]
- 8.Gill JS, Tonelli M, Mix CH, et al. The effect of maintenance immunosuppression medication on the change in kidney allograft function. Kidney Int. 2004;65:692–699. doi: 10.1111/j.1523-1755.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Gaston RS, Gourishankar S, et al. Long-term deterioration of kidney allograft function. Am J Transplant. 2005;5:1405–1414. doi: 10.1111/j.1600-6143.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 10.Gera M, Slezak JM, Rule AD, et al. Assessment of changes in kidney allograft function using creatinine-based estimates of glomerular filtration rate. Am J Transplant. 2007;7:880–887. doi: 10.1111/j.1600-6143.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 11.Djamali A, Kendziorski C, Brazy PC, et al. Disease progression and outcomes in chronic kidney disease and renal transplantation. Kidney Int. 2003;64:1800–1807. doi: 10.1046/j.1523-1755.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 12.Kukla A, Adulla M, Pascual J, et al. CKD stage-to-stage progression in native and transplant kidney disease. Nephrol Dial Transplant. 2008;23:693–700. doi: 10.1093/ndt/gfm590. [DOI] [PubMed] [Google Scholar]
- 13.The Modification on Diet in Renal Disease Study Group (prepared by Hunsicker LG, Adler S, Caggiula et al) Predictors of the progression of renal disease in the Modification on Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 14.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. Springer. 2000 [Google Scholar]
- 15.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; 2008. [Google Scholar]
- 16.Marcén R, Pascual J, Tenorio M, et al. Chronic kidney disease in renal transplant recipients. Transplant Proc. 2005;37:3718–3720. doi: 10.1016/j.transproceed.2005.09.101. [DOI] [PubMed] [Google Scholar]
- 17.Marcén R, del Castillo D, Capdevila L, et al. Achieving chronic kidney disease treatment targets in renal transplant recipients: results from a cross-sectional study in Spain. Transplantation. 2009;87:1340–1346. doi: 10.1097/TP.0b013e3181a23837. [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan V, Karpinski J, Nair RC, et al. The burden of chronic kidney disease in renal transplant recipients. Am J Transplant. 2003;4:262–269. doi: 10.1046/j.1600-6143.2003.00315.x. [DOI] [PubMed] [Google Scholar]
- 19.Ansell D, Udayarej UP, Steenkamp R, et al. Chronic renal failure in kidney transplant recipients. Do they receive optimum care? Data from the UK Renal Registry. Am J Transplant. 2007;7:1167–1176. doi: 10.1111/j.1600-6143.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 20.Hunsicker G, Bennett LA. Acute rejection reduces creatinine clearance (Ccr) at 6 months following renal transplantation but does not affect subsequent slope of Ccr (Abstract) Transplantation. 1999;67:S83. [Google Scholar]
- 21.Halimi J-M, Laouad I, Buchler M, et al. Early low-grade proteinuria; causes, short-term evolution and long-term consequences in renal transplantation. Am J Transplant. 2005;5:2281–2288. doi: 10.1111/j.1600-6143.2005.01020.x. [DOI] [PubMed] [Google Scholar]
- 22.Amer H, Fidler ME, Myslak M, et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant. 2007;7:2748–2756. doi: 10.1111/j.1600-6143.2007.02006.x. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez-Gil B, Morales JM. Transplantation in the patients with hepatitis C. Transplant Int. 2009;22:1117–1131. doi: 10.1111/j.1432-2277.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud IM, Elhabashi AF, Elsawy E, et al. The impact of hepatitis C virus viremia on renal graft and patient survival: a 9-year prospective study. Am J Kidney Dis. 2004;43:131–139. doi: 10.1053/j.ajkd.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Flechner SM, Kurian SM, Solez K, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant. 2004;4:1776–1785. doi: 10.1111/j.1600-6143.2004.00627.x. [DOI] [PubMed] [Google Scholar]
- 26.Vincenti F, Jensik SC, Filo RS, et al. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation. 2002;73:775–782. doi: 10.1097/00007890-200203150-00021. [DOI] [PubMed] [Google Scholar]
- 27.Solez K, Vincenti F, File R. Histopathologic findings from 2-ýear protocol biopsies from US multicenter kidney transplant trial comparing tacrolimus versus cyclosporine. Transplantation. 1998;66:1736–1740. doi: 10.1097/00007890-199812270-00029. [DOI] [PubMed] [Google Scholar]