Abstract
Alzheimer's disease (AD) is characterized by cognitive impairment, progressive neurodegeneration and formation of amyloid-β (Aβ)-containing plaques and neurofibrillary tangles composed of hyperphosphorylated tau. The neurodegenerative process in AD is initially characterized by synaptic damage accompanied by neuronal loss. In addition, recent evidence suggests that alterations in adult neurogenesis in the hippocampus might play a role. Synaptic loss is one of the strongest correlates to the cognitive impairment in patients with AD. Several lines of investigation support the notion that the synaptic pathology and defective neurogenesis in AD are related to progressive accumulation of Aβ oligomers rather than fibrils. Abnormal accumulation of Aβ resulting in the formation of toxic oligomers is the result of an imbalance between the levels of Aβ production, aggregation and clearance. Aβ oligomers might lead to synaptic damage by forming pore-like structures with channel activity; alterations in glutamate receptors; circuitry hyper-excitability; mitochondrial dysfunction; lysosomal failure and alterations in signaling pathways related to synaptic plasticity, neuronal cell and neurogenesis. A number of signaling proteins, including fyn kinase; glycogen synthase kinase-3β (GSK3β) and cyclin-dependent kinase-5 (CDK5), are involved in the neurodegenerative progression of AD. Therapies for AD might require the development of anti-aggregation compounds, pro-clearance pathways and blockers of hyperactive signaling pathways.
INTRODUCTION
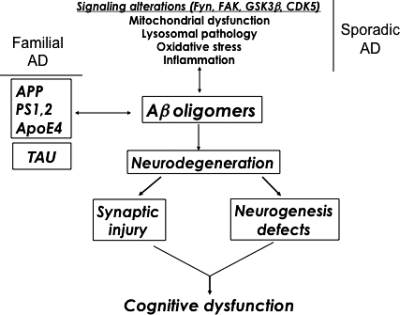
It is estimated that over 5 million people live with Alzheimer's disease (AD) in the USA, and it is predicted that by the year 2025 there will be an average 50% increase in patients with AD (1). AD is a leading cause of dementia in the aging population (2). Patients with AD experience symptoms including cognitive alterations, memory loss and behavioral changes (3,4). The dementia in AD is associated with neurodegeneration that is characterized initially by synaptic injury (5–7) followed by neuronal loss (8). This is accompanied by astrogliosis (9), microglial cell proliferation (10,11) and the presence of neurofibrillary tangles composed of dystrophic neurites and hyperphosphorylated tau (5,12–16). More recent studies have uncovered evidence, suggesting that another component to the neurodegenerative process in AD might include the possibility of interference with the process of adult neurogenesis in the hippocampus (17,18; Fig. 1). In transgenic (tg) animal models of AD, previous studies have shown significant alterations in the process of adult neurogenesis in the hippocampus (19–23).
Figure 1.
Mechanisms of neurodegeneration in AD. Defective cellular processes can lead to the accumulation of Aβ dimers, trimers and oligomers, which in turn contribute to neurogenesis defects and synaptic damage.
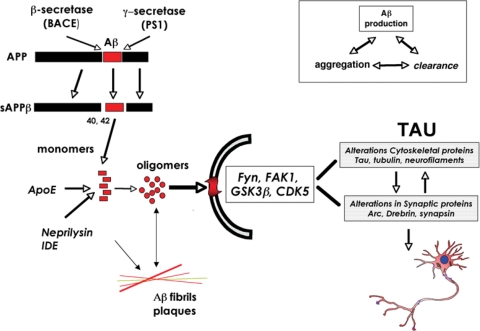
Of the various neuropathological features of AD, cognitive impairment in patients with AD is closely associated with synaptic loss in the neocortex and limbic system (7,24,25). Several lines of investigation support the notion that the pathogenesis of AD is related to progressive accumulation of amyloid-β (Aβ) protein, which is derived from the proteolysis of Aβ precursor protein [APP (26–28)]. Abnormal accumulation of Aβ is the result of an imbalance between the levels of Aβ production, aggregation and clearance (Fig. 2). Aβ clearance is mediated by proteolytic enzymes such as neprilysin (29), chaperone molecules such as apoE (30), lysosomal [e.g. autophagy (31)] and non-lysosomal pathways [e.g. proteasome (32)]. While in familial forms of AD, mutations result in an increased Aβ production or aggregation, in sporadic AD, failure of the clearance mechanisms might play a central role (Fig. 2). Progressive accumulation of Aβ results in the formation of Aβ oligomers (33) and fibrils which are the principal components of the plaque. Most evidence supports the notion that the Aβ oligomers rather than the fibrils are responsible for the synapto-toxic effects of Aβ [(34,35) Fig. 2].
Figure 2.
APP metabolism, Aβ oligomerization and signaling involvement in the mechanisms of synaptic damage in AD. Proteolytic cleavage of APP by β- and γ-secretase results in the generation of the Aβ1–42 monomer, which under pathological conditions can assemble into potentially toxic oligomers. Enzymes such as Neprilysin and insulin-degrading enzyme (IDE) can degrade the Aβ monomer, whereas oligomers can be sequestered into fibrillar aggregates in plaques. Oligomers may be the toxic Aβ species that contribute to de-regulation of signaling pathways (Fyn, FAK, GSK3β, CDK5) and result in alterations to cytoskeletal and synaptic proteins and subsequent synaptic and neuronal damage. Aβ accumulation is mediated by factors including rates of peptide production, aggregation and clearance.
Sporadic forms of AD generally afflict patients later in life, with onset of sporadic AD occurring usually between the ages of 60 and 70 (36). Although patients with sporadic disease constitute the majority of the affected population, ∼10–15% of patients have a genetically-linked familial form of AD (FAD). These patients often have earlier onset of the disease, and it is associated with mutations in several genes, including APP, tau and presenilin-1 [PS1 (37–41), Fig. 1]. Animal models of AD have been developed based on these familial mutations, and a number of models that express high levels of mutant APP recapitulate several of the neuropathological, neurodegenerative and behavioral characteristics of the spectrum of disease in human patients (42–44).
Most efforts toward developing tg models have been focused on overexpression of mutant APP in combination with mutant PS1. A summary of the FAD mutations of APP reproduced in tg mouse models is presented in ref. (16). Previously developed tg animal models have shown that it is possible to reproduce certain aspects of AD pathology over a shorter period of time (42–44).
In one such model, lines of tg mice express hAPP751 cDNA containing the London (Lon, V717I) and Swedish (Swe, K670N/M671L) mutations under the regulatory control of the mThy1 gene [mThy1-hAPP751 (16,45)]. While expression of mutant hAPP under the PDGF-β promoter results in the production of diffuse (and some mature) plaques (46,47), tg expression of mutant hAPP under the mThy1 (48) and PrP (49,50) promoters favors the formation of mature plaques in the hippocampus and neocortex.
This suggests that the differential patterns of Aβ deposition might be dependent on the specific neuronal populations selected by the promoter, levels of expression and topographical distribution of the transgene and levels of Aβ1–40 and Aβ1–42. Extensive investigation of these animal models has led to better understanding of the neuropathological alterations and some of the pathways involved in AD pathogenesis; however, the molecular mechanisms are still not entirely clear, and other deficits may play a role in the cognitive alterations in AD.
Loss of synapses (7,51,52) and axonal pathology (53) are probably key neuropathological features leading to dementia in these neurodegenerative disorders; however, other factors may contribute. In addition to the alterations in synaptic plasticity and neuronal integrity in mature neuronal circuitries, the neurodegenerative process in AD has recently been shown to be accompanied by alterations in neurogenesis (17,18,22,23,54–56). This suggests that the pathogenesis of AD may represent a two-pronged attack on the brain, contributing to degeneration of mature neurons, and disruption of the neurogenic niches in the adult brain [(16) Fig. 1].
Defective neurogenesis and AD
In addition to the alterations in synaptic plasticity and neuronal integrity in mature neurononal circuitries, the neurodegenerative process in AD have recently been shown to be accompanied by alterations in neurogenesis (17,18,22,23,54–56). Although there are some controversies over whether neurogenesis is increased (54) or decreased (17,18) in the pathogenesis of AD, more recent studies suggest that apparent increases in markers of neurogenesis in the brains of AD patients may be related to glial and vasculature-associated changes (17).
Animal models of APP overexpression present a more complex picture; however, in support of the more recent studies in human AD patients, a number of animal models of FAD display significantly reduced neurogenesis compared with non-tg controls [(21,23,56,57), for a more comprehensive review of neurogenic alterations in FAD-linked mouse models, see ref. (58)]. Taken together, these studies suggest that the pathogenesis of AD may be characterized by not only a loss of mature neurons but also by alterations in neural progenitor cells (NPCs) in neurogenic niches such as the dentate gyrus (DG) of the hippocampus. However, the molecular mechanisms involved in defective neurogenesis in AD and in animal models of FAD remain to be fully elucidated.
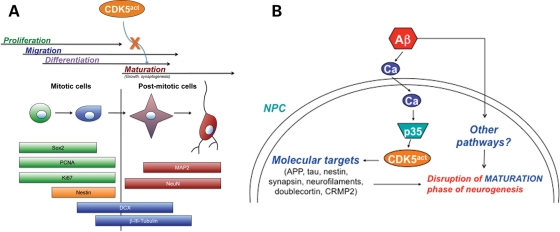
Neurogenesis in the mature healthy central nervous system occurs throughout adult life (59) in the olfactory bulb, the subventricular zone (SVZ) and the DG of the hippocampus (60). Neurogenesis is a complex process characterized by several progressive steps, including NPC proliferation, migration, differentiation (cell fate commitment) and maturation, including growth and synaptogenesis (Fig. 3). Moreover, during any one of these stages, survival and apoptosis may play a role in the net outcome of neurogenesis and numbers of surviving neural progeny in the adult hippocampus. Furthermore, each of these phases may be regulated by distinct molecular mechanisms, and could be susceptible to changes induced by pathological conditions in disease states. For studies of neurogenesis in both the SVZ and DG, characterization of different markers is used to distinguish between stages of the neurogenic process (Fig. 3); however, there is much overlap in expression of the different markers and phases themselves. Markers of cell division (Sox2, PCNA, Ki67, or BrdU in BrdU-treated cells or animals) or NPC-specific markers (nestin) are often used to identify cells in the progenitor cell (proliferative) phase of neurogenesis [(59,61) Fig. 3]. For later stages in the process, markers such as doublecortin (DCX) or β-III Tubulin are utilized to detect progeny in the early neuroblast phase (newly born neurons, often migratory) or immature new neurons, respectively [(62) Fig. 3]. For cells that are committed to a neuronal fate, eventually these progeny will be immunopositive with markers such as NeuN, MAP2 or synaptic markers (Fig. 3). Neurogenesis in the DG is an active process in the mature brain and plays a key role in synaptic plasticity, memory and learning (63). Environmental enrichment has been shown to stimulate neurogenesis and improve the performance in memory tasks in mice (64–66). Mechanisms of neurogenesis in the fetal brain have been extensively studied, and pathways such as the wnt (67) and Notch (68,69) signaling cascades play an important role in this process. However, less is known about the factors regulating neurogenesis in the adult nervous system and their role in neurodegenerative disorders.
Figure 3.
Aberrant activation of signaling molecules such as CDK5 (CDK5act) might impair adult neurogenesis during the cell maturation stage. (A) Schematic model of the stages that comprise the neurogenic process of NPC development in the adult brain, and representative markers that can be utilized to identify cells in various phases of development. (B) Aberrant signaling through CDK5 and other pathways might disrupt the maturation stage of adult neurogenesis in the pathogenesis of AD.
Neurodegeneration in AD: the role of Aβ oligomers
During aging and in the progression of AD, synaptic plasticity and neuronal integrity are disturbed (5–8,55). Although the precise mechanisms leading to neurodegeneration in AD are not completely clear, most studies have focused on the role of APP and its products in AD pathogenesis (26,33,70). Recent studies suggest that alterations in the processing of APP, resulting in the accumulation of Aβ and APP C-terminal products, might play a key role in the pathogenesis of AD [(71,72) Fig. 2]. In this context, previous studies have shown that Aβ1–42, a proteolytic product of APP metabolism (Fig. 2), accumulates in the neuronal endoplasmic reticulum (73) and extracellularly (12,74,75). Several products are derived from APP through alternative proteolytic cleavage pathways, and enormous progress has recently been made in identifying the enzymes involved [(33,76–79) Fig. 2].
The primary pathogenic event triggering synaptic loss and selective neuronal cell death in these disorders is the subject of debate (51,80); however, recent studies suggest that nerve damage might result from the conversion of normally non-toxic monomers to toxic oligomers [(34,81–83) Fig. 2], whereas larger polymers and fibers that often constitute the plaques might not be as toxic (84,85). Various lines of evidence suggest that the direct abnormal accumulation of Aβ oligomers in the nerve terminals might lead to the synaptic damage and ultimately to neurodegeneration in AD (33). A number of recent studies have begun to investigate the possibility that Aβ oligomers might interfere with synaptic function by altering synaptic proteins such as post-synaptic density-95 [PSD95 (86–89)], scaffold proteins such as Shank (90) and glutamate receptors (91).
In summary, the potential role of neurotoxic Aβ oligomers has emerged as a topic of considerable interest in recent years (34,35,83,92).
Molecular pathways of neurodegeneration in AD
The mechanisms through which of Aβ monomers, oligomers and other APP metabolites might lead to synaptic damage and neurodegeneration is not completely clear. A number of possibilities are under investigation, including the formation of pore-like structures with channel activity (93–96); alterations in glutamate receptors and excitotoxicity (97–101); circuitry hyper-excitability (102); mitochondrial dysfunction (103,104); lysosomal failure (105) and alterations in signaling pathways related to synaptic plasticity, neuronal cell death and neurogenesis.
Previous studies have shown that a number of signaling proteins, including fyn kinase (106–109), glycogen synthase kinase-3β [GSK3β (110–113)] and cyclin-dependent kinase-5 [CDK5 (114–116)], are involved in the neurodegenerative progression of AD (Fig. 1). Other signaling pathways that have been investigated include members of the MAPK family such as ERK (117–121) and JNK (122–124) as well as other pathways such as p21-activated kinase (125).
Abnormal activation of signaling pathways might lead to synaptic failure and altered neurogenesis by promoting abnormal Tau phosphorylation and aggregation (126–128), cytoskeletal abnormalities (129), activating caspase pro-apototic pathways (130–132) and activating calcium and calpain dependent proteolysis [(133,134) Fig. 2].
Contribution of the CDK5 pathway to neurodegeneration in AD, and potential role for this pathway in the mechanisms of defective neurogenesis
In AD, the neurodegenerative process has been linked with hyperactivation of CDK5 and its activators p35 and p25 (115,116,135). Furthermore, levels of CDK5 are increased in the brains of AD patients (136). CDK5 is the predominant CDK found in the brain, is highly expressed in neurons and plays an important role in synaptic plasticity and neuronal development (137). CDK5 is a Ser-Thr protein kinase with postmitotic activity that phosphorylates KSP motifs on cytoskeletal (MAP1b, tau, NF, nestin, DCX, CRMP2), synaptic proteins (PSD95, synapsin, cadherin) and transcription factors [MEF2 (138–140)]. While in dividing neurons CDKs are activated by cyclins, in the nervous system CDK5 is activated by forming a complex with p35 or p39 (139,141). The primary activator of CDK5 is p35 (142), which under high calcium conditions is cleaved by calpain into p25 (134). While CDK5 activation via complex formation with p35 is associated with physiological activation of CDK5, the truncated p25 form hyperactivates CDK5 and leads to abnormal phosphorylation of substrates such as tau (143). Through these effects, CDK5 and p35/p25 may play a critical role in neuroplasticity in the pathogenesis of AD.
Although the hyperactivation of CDK5/p35/p25 has been associated with the pathogenesis of neurodegenerative diseases such as AD (Figs 1 and 3), its physiological function has been implicated in critical functions such as neuroblast migration (144–146) and synaptic plasticity (147,148). Furthermore, the Cdk5/p35 complex localizes to the leading edge of axonal growth cones (149) where it regulates neurite outgrowth in mature cortical neurons (150). More recently, CDK5 has been shown to be essential for adult neurogenesis (151,152). In this context, it is possible that the neurogenesis deficits in AD might be related to alterations in CDK5 activity in NPCs.
Recent evidence in support of this possibility suggests that the neurodegenerative process in patients with AD might not only target mature neurons, but also interfere with the process of neurogenesis (22,23,56). Studies demonstrating that in mice deficient in this kinase and its activator (p35) neuronal development and migration is arrested (137,144,153) support the notion that CDK5 plays an important role in neurogenesis in the developing brain. In the adult nervous system the role of the p35-CDK5 signaling pathway in neurogenesis is less well understood. The mechanisms through which AD-related molecular changes interfere with neurogenesis in the adult brain might involve signaling alterations (e.g. CDK5/p35/p25) analogous to those involved in the neurodegenerative process (Fig. 3).
In this context, in models of AD, Aβ has been shown to impair neurogenesis via calpain activation and p35 deregulation (154); however, the downstream effectors involved and the consequences of CDK5 and p35/p25 manipulation remain to be revealed. CDK5 may mediate alterations in neurogenesis in AD via aberrant phosphorylation of CDK5 substrates, which include cytoskeletal (neurofilaments, nestin) (155), synaptic proteins [e.g. synapsin (156)], among others (Fig. 3). Previous studies have shown that the Aβ/CDK5 neurotoxic pathway may involve the destabilization of microtubules (157) since CDK5 can associate with microtubules indirectly (158) and its substrates include microtubule-associated proteins (MAPs). Since CDK5 plays a role both in synaptic function and neuronal integrity, then abnormal activation of this molecule by Aβ might impair the functioning of mature neurons and also contribute to alterations in neurogenesis by impairing cell maturation (Fig. 3). Elucidating the signaling pathways and downstream molecular targets involved in the deregulation of neurogenesis is important to fully understand the mechanisms of neuroplasticity in AD (Fig. 3).
Therapeutical strategies for AD
The focus in past years for AD therapies has been to improve memory by activating cholinergic neurotransmission (159) and, more recently, anti-oxidants (160) and blockers of calcium channels (161) have been utilized. In recent years, the focus has been on reducing Aβ or Tau deposition. The alternative approach is to protect selective neuronal populations and promote synaptic formation and neurogenesis.
Several possibilities are currently being tested to reduce Aβ accumulation, including (i) anti-aggregation molecules that block oligomers and fibrils, (ii) regulators of APP proteolysis by blocking the β- or γ-secretase pathways or increasing α-secretase activity, (iii) regulation of APP processing by modulating cholesterol and lipid metabolism, (iv) reducing APP production (e.g. siRNA), (v) increasing Aβ clearance with antibodies, ApoE and other chaperones (e.g. HSP70), (vi) increasing clearance via lysosomal and proteasomal pathways, (vii) increasing Aβ clearance by increasing degradation (e.g. NEP and IDE delivery) and (viii) blocking signaling pathways and receptors activated by neurotoxic Aβ oligomers (e.g. Fyn kinase, GSK3β and CDK5 inhibitors and glutamate receptor blockers).
Neuroprotective strategies include the use of neurotrophic factors (e.g. brain-derived neurotrophic factor, nerve growth factor), neuroprotective peptides [e.g. cerebrolysin (162)], anti-oxidants [e.g. curcumin, vitamin E (163)] and calcium channel blockers [e.g. memantine (164)]. Tau is also an important target, and in this context recent studies have shown that in a Tau-deficient background APP transgenic mice are protected from the toxic effects of Aβ (165). Tau has been targeted by reducing Tau synthesis or decreasing Tau phosphorylation with compounds such as lithium (166,167). In addition to the traditional delivery methods and strategies with oral small molecules, new approaches are currently been tested, including gene therapy, vaccination, changes in lifestyle that enhance neurogenesis, intra-thecal drug delivery and use of compounds bound to lipids.
Conflict of Interest statement. None declared.
FUNDING
This work was supported by National Institutes of Health grants AG5131, AG11385, AG10435, NS44233 and AG18440.
REFERENCES
- 1.Hebert L.E., Scherr P.A., Bienias J.L., Bennett D.A., Evans D.A. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2004;62:1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- 2.Ashford J.W. APOE genotype effects on Alzheimer's disease onset and epidemiology. J. Mol. Neurosci. 2004;23:157–165. doi: 10.1385/JMN:23:3:157. doi:10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- 3.Katzman R. Alzheimer's disease. N. Engl. J. Med. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- 4.Budson A.E., Price B.H. Memory dysfunction. N. Engl. J. Med. 2005;352:692–699. doi: 10.1056/NEJMra041071. doi:10.1056/NEJMra041071. [DOI] [PubMed] [Google Scholar]
- 5.Terry R., Hansen L., Masliah E. Structural basis of the cognitive alterations in Alzheimer disease. In: Terry R., Katzman R., editors. Alzheimer Disease. New York: Raven Press; 1994. pp. 179–196. [Google Scholar]
- 6.Masliah E., Mallory M., Alford M., DeTeresa R., Iwai A., Saitoh T. Molecular mechanisms of synaptic disconnection in Alzheimer's disease. In: Hyman B., Duyckaerts C., Christen Y., editors. Connections, Cognition and Alzheimer's Disease. Berlin: Springer; 1997. pp. 121–140. [Google Scholar]
- 7.DeKosky S., Scheff S. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. doi:10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 8.Terry R., Peck A., DeTeresa R., Schechter R., Horoupian D. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann. Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. doi:10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- 9.Beach T., Walker R., McGeer E. Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia. 1989;2:420–436. doi: 10.1002/glia.440020605. doi:10.1002/glia.440020605. [DOI] [PubMed] [Google Scholar]
- 10.Rogers J., Luber-Narod J., Styren S., Civin W. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol. Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. doi:10.1016/S0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 11.Masliah E., Mallory M., Hansen L., Alford M., Albright T., Terry R., Shapiro P., Sundsmo M., Saitoh T. Immunoreactivity of CD45, a protein phosphotyrosine phosphatase, in Alzheimer disease. Acta Neuropathol. 1991;83:12–20. doi: 10.1007/BF00294425. doi:10.1007/BF00294425. [DOI] [PubMed] [Google Scholar]
- 12.Trojanowski J.Q., Lee V.M. ‘Fatal attractions’ of proteins. A comprehensive hypothetical mechanism underlying Alzheimer's disease and other neurodegenerative disorders. Ann. N. Y. Acad. Sci. 2000;924:62–67. doi: 10.1111/j.1749-6632.2000.tb05561.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Ann. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. doi:10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal K., Grundke-Iqbal I. Neurofibrillary pathology leads to synaptic loss and not the other way around in Alzheimer disease. J. Alzheimers Dis. 2002;4:235–238. doi: 10.3233/jad-2002-4313. [DOI] [PubMed] [Google Scholar]
- 15.Mandelkow E., Mandelkow E. Tau in Alzheimer's disease. Trends Cell. Biol. 1998;8:125–127. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 16.Crews L., Rockenstein E., Masliah E. APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct. Funct. 2010;214:111–126. doi: 10.1007/s00429-009-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boekhoorn K., Joels M., Lucassen P.J. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. doi:10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Li B., Yamamori H., Tatebayashi Y., Shafit-Zagardo B., Tanimukai H., Chen S., Iqbal K., Grundke-Iqbal I. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J. Neuropathol. Exp. Neurol. 2008;67:78–84. doi: 10.1097/nen.0b013e318160c5db. doi:10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen P.H., Hof P.R., Chen X., Gluck K., Austin G., Younkin S.G., Younkin L.H., DeGasperi R., Gama Sosa M.A., Robakis N.K., et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp. Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. doi:10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Chevallier N.L., Soriano S., Kang D.E., Masliah E., Hu G., Koo E.H. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am. J. Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donovan M.H., Yazdani U., Norris R.D., Games D., German D.C., Eisch A.J. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J. Comp. Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. doi:10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 22.Jin K., Galvan V., Xie L., Mao X.O., Gorostiza O.F., Bredesen D.E., Greenberg D.A. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl Acad. Sci. USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. doi:10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong H., Goico B., Martin M., Csernansky C.A., Bertchume A., Csernansky J.G. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. doi:10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. doi:10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 25.DeKosky S.T., Scheff S.W., Styren S.D. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. doi:10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- 26.Selkoe D. Amyloid b protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989;58:611–612. doi: 10.1016/0092-8674(89)90093-7. doi:10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- 27.Sisodia S.S., Price D.L. Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 1995;9:366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- 28.Tanzi R., Gusella J., Watkins P., Bruns G., St. George-Hyslop P., van Keuren M., Patterson D., Pagan S., Kurnik D., Neve R. Amyloid beta protein gene: cDNA, mRNA distribution and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. doi:10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 29.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H.J., Saido T.C. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. doi:10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 30.Kim J., Basak J.M., Holtzman D.M. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. doi:10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bendiske J., Bahr B.A. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis—an approach for slowing Alzheimer disease? J. Neuropathol. Exp. Neurol. 2003;62:451–463. doi: 10.1093/jnen/62.5.451. [DOI] [PubMed] [Google Scholar]
- 32.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J. Biol. Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. doi:10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 33.Selkoe D.J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 34.Walsh D.M., Selkoe D.J. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept. Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. doi:10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- 35.Klein W.L., Krafft G.A., Finch C.E. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. doi:10.1016/S0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 36.Association A.s. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Rocchi A., Pellegrini S., Siciliano G., Murri L. Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res. Bull. 2003;61:1–24. doi: 10.1016/s0361-9230(03)00067-4. doi:10.1016/S0361-9230(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 38.Cruts M., Van Broeckhoven C. Molecular genetics of Alzheimer's disease. Ann. Med. 1998;30:560–565. doi: 10.3109/07853899809002605. doi:10.3109/07853899809002605. [DOI] [PubMed] [Google Scholar]
- 39.Bertoli-Avella A.M., Oostra B.A., Heutink P. Chasing genes in Alzheimer's and Parkinson's disease. Hum. Genet. 2004;114:413–438. doi: 10.1007/s00439-004-1097-7. doi:10.1007/s00439-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 40.Pastor P., Goate A.M. Molecular genetics of Alzheimer's disease. Curr. Psychiatry Rep. 2004;6:125–133. doi: 10.1007/s11920-004-0052-6. doi:10.1007/s11920-004-0052-6. [DOI] [PubMed] [Google Scholar]
- 41.Hutton M., Hardy J. The presenilins and Alzheimer's disease. Hum. Mol. Genet. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. doi:10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 42.Masliah E., Sisk A., Mallory M., Mucke L., Schenk D., Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F b-amyloid precursor protein and Alzheimer's disease. J. Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Games D., Masliah E., Lee M., Johnson-Wood K., Schenk D. Neurodegenerative Alzheimer-like pathology in PDAPP 717V– > F transgenic mice. In: Hyman B., Duyckaerts C., Christen Y., editors. Connections, cognition and Alzheimer's disease. Berlin: Springer; 1997. pp. 105–119. [Google Scholar]
- 44.Price D.L., Wong P.C., Markowska A.L., Lee M.K., Thinakaren G., Cleveland D.W., Sisodia S.S., Borchelt D.R. The value of transgenic models for the study of neurodegenerative diseases. Ann. N. Y. Acad. Sci. 2000;920:179–191. doi: 10.1111/j.1749-6632.2000.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 45.Rockenstein E., Mallory M., Mante M., Sisk A., Masliah E. Early formation of mature amyloid-b proteins deposits in a mutant APP transgenic model depends on levels of Ab1–42. J. Neurosci. Res. 2001;66:573–582. doi: 10.1002/jnr.1247. doi:10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 46.Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemes J., Donaldson T., Gillespie F., et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F b-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. doi:10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 47.Mucke L., Masliah E., Yu G.Q., Mallory M., Rockenstein E.M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andra K., Abramowski D., Duke M., Probst A., Wiederholt K., Burki K., Goedert M., Sommer B., Staufenbiel M. Expression of APP in transgenic mice: a comparison of neuron-specific promoters. Neurobiol. Aging. 1996;17:183–190. doi: 10.1016/0197-4580(95)02066-7. doi:10.1016/0197-4580(95)02066-7. [DOI] [PubMed] [Google Scholar]
- 49.Borchelt D., Ratovitski T., van Lare J., Lee M., Gonzales V., Jenkins N., Copeland N., Price D., Sisodia S. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutamt presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. doi:10.1016/S0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 50.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Ab elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. doi:10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 51.Masliah E. Recent advances in the understanding of the role of synaptic proteins in Alzheimer's disease and other neurodegenerative disorders. J. Alzheimers Dis. 2001;3:121–129. doi: 10.3233/jad-2001-3117. [DOI] [PubMed] [Google Scholar]
- 52.Scheff S.W., Price D.A. Alzheimer's disease-related synapse loss in the cingulate cortex. J. Alzheimers Dis. 2001;3:495–505. doi: 10.3233/jad-2001-3509. [DOI] [PubMed] [Google Scholar]
- 53.Raff M.C., Whitmore A.V., Finn J.T. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. doi:10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 54.Jin K., Peel A.L., Mao X.O., Xie L., Cottrell B.A., Henshall D.C., Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. doi:10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H.G., Moreira P.I., Zhu X., Smith M.A., Perry G. Staying connected: synapses in Alzheimer disease. Am. J. Pathol. 2004;165:1461–1464. doi: 10.1016/S0002-9440(10)63404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haughey N.J., Liu D., Nath A., Borchard A.C., Mattson M.P. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolecular Med. 2002;1:125–135. doi: 10.1385/NMM:1:2:125. doi:10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- 57.Rockenstein E., Mante M., Adame A., Crews L., Moessler H., Masliah E. Effects of cerebrolysintrade mark on neurogenesis in an APP transgenic model of Alzheimer's disease. Acta Neuropathol. (Berl) 2007;113:265–275. doi: 10.1007/s00401-006-0166-5. doi:10.1007/s00401-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 58.Lazarov O., Marr R.A. Neurogenesis and Alzheimer's. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.08.009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. doi:10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 60.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. doi:10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 61.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. doi:10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 62.Rao M.S., Shetty A.K. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. doi:10.1111/j.0953-816X.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 63.Gage F.H., Kempermann G., Palmer T.D., Peterson D.A., Ray J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. doi:10.1002/(SICI)1097-4695(199808)36:2<249::AID-NEU11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 64.Olson A.K., Eadie B.D., Ernst C., Christie B.R. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. doi:10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 65.Bruel-Jungerman E., Laroche S., Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. doi:10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 66.Brown J., Cooper-Kuhn C.M., Kempermann G., Van Praag H., Winkler J., Gage F.H., Kuhn H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. doi:10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 67.Lie D.C., Colamarino S.A., Song H.J., Desire L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. doi:10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 68.Androutsellis-Theotokis A., Leker R.R., Soldner F., Hoeppner D.J., Ravin R., Poser S.W., Rueger M.A., Bae S.K., Kittappa R., McKay R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 69.Beatus P., Lendahl U. Notch and neurogenesis. J. Neurosci. Res. 1998;54:125–136. doi: 10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. doi:10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 70.Vassar R. β-Secretase, APP and Abeta in Alzheimer's disease. Subcell. Biochem. 2005;38:79–103. doi:10.1007/0-387-23226-5_4. [PubMed] [Google Scholar]
- 71.Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. doi:10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 72.Sinha S., Anderson J., John V., McConlogue L., Basi G., Thorsett E., Schenk D. Recent advances in the understanding of the processing of APP to beta amyloid peptide. Ann. N. Y. Acad. Sci. 2000;920:206–208. doi: 10.1111/j.1749-6632.2000.tb06923.x. [DOI] [PubMed] [Google Scholar]
- 73.Cuello A.C. Intracellular and extracellular Abeta, a tale of two neuropathologies. Brain Pathol. 2005;15:66–71. doi: 10.1111/j.1750-3639.2005.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walsh D., Tseng B., Rydel R., Podlisny M., Selkoe D. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. doi:10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 75.Selkoe D.J., Yamazaki T., Citron M., Podlisny M.B., Koo E.H., Teplow D.B., Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann. N. Y. Acad. Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. doi:10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 76.Sinha S., Anderson J.P., Barbour R., Basi G.S., Caccavello R., Davis D., Doan M., Dovey H.F., Frigon N., Hong J., et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. doi:10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 77.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. doi:10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 78.Cai H., Wang Y., McCarthy D., Wen H., Borchelt D.R., Price D.L., Wong P.C. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. doi:10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 79.Luo Y., Bolon B., Kahn S., Bennett B.D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., et al. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. doi:10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 80.Masliah E. The role of synaptic proteins in Alzheimer's disease. Ann. N. Y. Acad. Sci. 2000;924:68–75. doi: 10.1111/j.1749-6632.2000.tb05562.x. [DOI] [PubMed] [Google Scholar]
- 81.Volles M.J., Lansbury P.T., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. doi:10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 82.Volles M.J., Lee S.J., Rochet J.C., Shtilerman M.D., Ding T.T., Kessler J.C., Lansbury P.T., Jr Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. doi:10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 83.Glabe C.C. Amyloid accumulation and pathogensis of Alzheimer's disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell. Biochem. 2005;38:167–177. doi: 10.1007/0-387-23226-5_8. doi:10.1007/0-387-23226-5_8. [DOI] [PubMed] [Google Scholar]
- 84.Lansbury P.T., Jr Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc. Natl Acad. Sci. USA. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. doi:10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh D.M., Klyubin I., Fadeeva J.V., Rowan M.J., Selkoe D.J. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30:552–557. doi: 10.1042/bst0300552. doi:10.1042/BST0300552. [DOI] [PubMed] [Google Scholar]
- 86.Gylys K.H., Fein J.A., Yang F., Wiley D.J., Miller C.A., Cole G.M. Synaptic changes in Alzheimer's disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am. J. Pathol. 2004;165:1809–1817. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simon A.M., Schiapparelli L., Salazar-Colocho P., Cuadrado-Tejedor M., Escribano L., Lopez de Maturana R., Del Rio J., Perez-Mediavilla A., Frechilla D. Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Abeta levels. Neurobiol. Dis. 2009;33:369–378. doi: 10.1016/j.nbd.2008.11.005. doi:10.1016/j.nbd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Koffie R.M., Meyer-Luehmann M., Hashimoto T., Adams K.W., Mielke M.L., Garcia-Alloza M., Micheva K.D., Smith S.J., Kim M.L., Lee V.M., et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl Acad. Sci. USA. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. doi:10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sultana R., Banks W.A., Butterfield D.A. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer's disease. J. Neurosci. Res. 2010;88:469–477. doi: 10.1002/jnr.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roselli F., Hutzler P., Wegerich Y., Livrea P., Almeida O.F. Disassembly of shank and homer synaptic clusters is driven by soluble beta-amyloid(1–40) through divergent NMDAR-dependent signalling pathways. PLoS One. 2009;4:e6011. doi: 10.1371/journal.pone.0006011. doi:10.1371/journal.pone.0006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Almeida C.G., Tampellini D., Takahashi R.H., Greengard P., Lin M.T., Snyder E.M., Gouras G.K. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. doi:10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 92.Klein W.L. Abeta toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. doi:10.1016/S0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 93.Lin H., Bhatia R., Lal R. Amyloid beta protein forms ion channels: implications for Alzheimer's disease pathophysiology. FASEB J. 2001;15:2433–2444. doi: 10.1096/fj.01-0377com. doi:10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- 94.Lashuel H.A., Hartley D., Petre B.M., Walz T., Lansbury P.T., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. doi:10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 95.Arispe N., Pollard H.B., Rojas E. Zn2+ interaction with Alzheimer amyloid beta protein calcium channels. Proc. Natl Acad. Sci. USA. 1996;93:1710–1715. doi: 10.1073/pnas.93.4.1710. doi:10.1073/pnas.93.4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arispe N., Rojas E., Pollard H.B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl Acad. Sci. USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. doi:10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shankar G.M., Bloodgood B.L., Townsend M., Walsh D.M., Selkoe D.J., Sabatini B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. doi:10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. doi:10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. doi:10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. doi:10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura T., Lipton S.A. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. doi:10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- 102.Palop J.J., Chin J., Roberson E.D., Wang J., Thwin M.T., Bien-Ly N., Yoo J., Ho K.O., Yu G.Q., Kreitzer A., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. doi:10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakamura T., Lipton S.A. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases. Apoptosis. 2010 doi: 10.1007/s10495-010-0476-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin M.T., Beal M.F. Alzheimer's APP mangles mitochondria. Nat. Med. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. doi:10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 105.Nixon R.A., Cataldo A.M. Lysosomal system pathways: genes to neurodegeneration in Alzheimer's disease. J. Alzheimer's Dis,. 2006;9:277–289. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- 106.Ho G.J., Hashimoto M., Adame A., Izu M., Alford M.F., Thal L.J., Hansen L.A., Masliah E. Altered p59Fyn kinase expression accompanies disease progression in Alzheimer's disease: implications for its functional role. Neurobiol. Aging. 2005;26:625–635. doi: 10.1016/j.neurobiolaging.2004.06.016. doi:10.1016/j.neurobiolaging.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 107.Zhang C., Qiu H.E., Krafft G.A., Klein W.L. A beta peptide enhances focal adhesion kinase/Fyn association in a rat CNS nerve cell line. Neurosci. Lett. 1996;211:187–190. doi: 10.1016/0304-3940(96)12761-0. doi:10.1016/0304-3940(96)12761-0. [DOI] [PubMed] [Google Scholar]
- 108.Chin J., Palop J.J., Puolivali J., Massaro C., Bien-Ly N., Gerstein H., Scearce-Levie K., Masliah E., Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. doi:10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chin J., Palop J.J., Yu G.Q., Kojima N., Masliah E., Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J. Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. doi:10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rockenstein E., Torrance M., Adame A., Mante M., Bar-on P., Rose J.B., Crews L., Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. doi:10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rockenstein E., Torrance M., Mante M., Adame A., Paulino A., Rose J.B., Crews L., Moessler H., Masliah E. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. J. Neurosci. Res. 2006;83:1252–1261. doi: 10.1002/jnr.20818. doi:10.1002/jnr.20818. [DOI] [PubMed] [Google Scholar]
- 112.Baum L., Hansen L., Masliah E., Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol. Chem. Neuropathol. 1996;29:253–261. doi: 10.1007/BF02815006. doi:10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- 113.Hooper C., Markevich V., Plattner F., Killick R., Schofield E., Engel T., Hernandez F., Anderton B., Rosenblum K., Bliss T., et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur. J. Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 114.Cruz J.C., Kim D., Moy L.Y., Dobbin M.M., Sun X., Bronson R.T., Tsai L.H. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J. Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. doi:10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patrick G.N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 116.Cruz J.C., Tsai L.H. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr. Opin. Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. doi:10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Greenberg S.M., Qiu W.Q., Selkoe D.J., Ben-Itzhak A., Kosik K.S. Amino-terminal region of the beta-amyloid precursor protein activates mitogen-activated protein kinase. Neurosci. Lett. 1995;198:52–56. doi: 10.1016/0304-3940(95)11944-r. doi:10.1016/0304-3940(95)11944-R. [DOI] [PubMed] [Google Scholar]
- 118.Combs C.K., Johnson D.E., Cannady S.B., Lehman T.M., Landreth G.E. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J. Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Webster B., Hansen L., Adame A., Crews L., Torrance M., Thal L., Masliah E. Astroglial activation of extracellular-regulated kinase in early stages of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:142–151. doi: 10.1097/01.jnen.0000199599.63204.6f. doi:10.1097/01.jnen.0000199599.63204.6f. [DOI] [PubMed] [Google Scholar]
- 120.Zhu X., Lee H.G., Raina A.K., Perry G., Smith M.A. The role of mitogen-activated protein kinase pathways in Alzheimer's disease. Neurosignals. 2002;11:270–281. doi: 10.1159/000067426. doi:10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- 121.Zhu X., Sun Z., Lee H.G., Siedlak S.L., Perry G., Smith M.A. Distribution, levels, and activation of MEK1 in Alzheimer's disease. J. Neurochem. 2003;86:136–142. doi: 10.1046/j.1471-4159.2003.01820.x. doi:10.1046/j.1471-4159.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- 122.Zhu X., Raina A.K., Rottkamp C.A., Aliev G., Perry G., Boux H., Smith M.A. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer's disease. J. Neurochem. 2001;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. doi:10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 123.Ma Q.L., Harris-White M.E., Ubeda O.J., Simmons M., Beech W., Lim G.P., Teter B., Frautschy S.A., Cole G.M. Evidence of Abeta- and transgene-dependent defects in ERK-CREB signaling in Alzheimer's models. J. Neurochem. 2007;103:1594–1607. doi: 10.1111/j.1471-4159.2007.04869.x. doi:10.1111/j.1471-4159.2007.04869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma Q.L., Yang F., Rosario E.R., Ubeda O.J., Beech W., Gant D.J., Chen P.P., Hudspeth B., Chen C., Zhao Y., et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009;29:9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. doi:10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma Q.L., Yang F., Calon F., Ubeda O.J., Hansen J.E., Weisbart R.H., Beech W., Frautschy S.A., Cole G.M. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J. Biol. Chem. 2008;283:14132–14143. doi: 10.1074/jbc.M708034200. doi:10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sengupta A., Novak M., Grundke-Iqbal I., Iqbal K. Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett. 2006;580:5925–5933. doi: 10.1016/j.febslet.2006.09.060. doi:10.1016/j.febslet.2006.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sengupta A., Wu Q., Grundke-Iqbal I., Iqbal K., Singh T.J. Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol. Cell. Biochem. 1997;167:99–105. doi: 10.1023/a:1006883924775. doi:10.1023/A:1006883924775. [DOI] [PubMed] [Google Scholar]
- 128.Maccioni R.B., Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol. Rev. 1995;75:835–864. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- 129.Kosik K.S., Qiu W.Q., Greenberg S. Cellular signaling pathways and cytoskeletal organization. Ann. N. Y. Acad. Sci. 1996;777:114–120. doi: 10.1111/j.1749-6632.1996.tb34409.x. doi:10.1111/j.1749-6632.1996.tb34409.x. [DOI] [PubMed] [Google Scholar]
- 130.Cotman C.W. Apoptosis decision cascades and neuronal degeneration in Alzheimer's disease. Neurobiol. Aging. 1998;19:S29–S32. doi: 10.1016/s0197-4580(98)00042-6. doi:10.1016/S0197-4580(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 131.Cotman C.W., Su J.H. Mechanisms of neuronal death in Alzheimer's disease. Brain Pathol. 1996;6:493–506. doi: 10.1111/j.1750-3639.1996.tb00878.x. doi:10.1111/j.1750-3639.1996.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 132.Rohn T.T., Head E., Su J.H., Anderson A.J., Bahr B.A., Cotman C.W., Cribbs D.H. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer's disease. Am. J. Pathol. 2001;158:189–198. doi: 10.1016/S0002-9440(10)63957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nixon R.A. Calcium-activated neutral proteinases as regulators of cellular function. Implications for Alzheimer's disease pathogenesis. Ann. N. Y. Acad. Sci. 1989;568:198–208. doi: 10.1111/j.1749-6632.1989.tb12509.x. doi:10.1111/j.1749-6632.1989.tb12509.x. [DOI] [PubMed] [Google Scholar]
- 134.Lee M.S., Kwon Y.T., Li M., Peng J., Friedlander R.M., Tsai L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. doi:10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 135.Liu F., Su Y., Li B., Zhou Y., Ryder J., Gonzalez-DeWhitt P., May P.C., Ni B. Regulation of amyloid precursor protein (APP) phosphorylation and processing by p35/Cdk5 and p25/Cdk5. FEBS Lett. 2003;547:193–196. doi: 10.1016/s0014-5793(03)00714-2. doi:10.1016/S0014-5793(03)00714-2. [DOI] [PubMed] [Google Scholar]
- 136.Lee K.Y., Clark A.W., Rosales J.L., Chapman K., Fung T., Johnston R.N. Elevated neuronal Cdc2-like kinase activity in the Alzheimer disease brain. Neurosci. Res. 1999;34:21–29. doi: 10.1016/s0168-0102(99)00026-7. doi:10.1016/S0168-0102(99)00026-7. [DOI] [PubMed] [Google Scholar]
- 137.Cicero S., Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. doi:10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang X., Wang X., Gong X., Tong M., Park D., Xia Z., Mao Z. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 2005;25:4823–4834. doi: 10.1523/JNEUROSCI.1331-05.2005. doi:10.1523/JNEUROSCI.1331-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dhavan R., Tsai L.H. A decade of CDK5. Nat. Rev. Mol. Cell. Biol. 2001;2:749–759. doi: 10.1038/35096019. doi:10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 140.Shelton S.B., Johnson G.V. Cyclin-dependent kinase-5 in neurodegeneration. J. Neurochem. 2004;88:1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- 141.Smith D.S., Greer P.L., Tsai L.H. Cdk5 on the brain. Cell Growth Differ. 2001;12:277–283. [PubMed] [Google Scholar]
- 142.Tsai L.H., Delalle I., Caviness V.S., Jr, Chae T., Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. doi:10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 143.Ahlijanian M.K., Barrezueta N.X., Williams R.D., Jakowski A., Kowsz K.P., McCarthy S., Coskran T., Carlo A., Seymour P.A., Burkhardt J.E., et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl Acad. Sci. USA. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. doi:10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hirota Y., Ohshima T., Kaneko N., Ikeda M., Iwasato T., Kulkarni A.B., Mikoshiba K., Okano H., Sawamoto K. Cyclin-dependent kinase 5 is required for control of neuroblast migration in the postnatal subventricular zone. J. Neurosci. 2007;27:12829–12838. doi: 10.1523/JNEUROSCI.1014-07.2007. doi:10.1523/JNEUROSCI.1014-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohshima T., Ward J.M., Huh C.G., Longenecker G., Veeranna, Pant H.C., Brady R.O., Martin L.J., Kulkarni A.B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl Acad. Sci. USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. doi:10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chae T., Kwon Y.T., Bronson R., Dikkes P., Li E., Tsai L.H. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. doi:10.1016/S0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 147.Fischer A., Sananbenesi F., Pang P.T., Lu B., Tsai L.H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. doi:10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 148.Johansson J.U., Lilja L., Chen X.L., Higashida H., Meister B., Noda M., Zhong Z.G., Yokoyama S., Berggren P.O., Bark C. Cyclin-dependent kinase 5 activators p35 and p39 facilitate formation of functional synapses. Brain Res. Mol. Brain Res. 2005;138:215–227. doi: 10.1016/j.molbrainres.2005.04.014. doi:10.1016/j.molbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 149.Pigino G., Paglini G., Ulloa L., Avila J., Caceres A. Analysis of the expression, distribution and function of cyclin dependent kinase 5 (cdk5) in developing cerebellar macroneurons. J. Cell. Sci. 1997;110(Pt 2):257–270. doi: 10.1242/jcs.110.2.257. [DOI] [PubMed] [Google Scholar]
- 150.Nikolic M., Dudek H., Kwon Y.T., Ramos Y.F., Tsai L.H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. doi:10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 151.Jessberger S., Aigner S., Clemenson G.D., Jr, Toni N., Lie D.C., Karalay O., Overall R., Kempermann G., Gage F.H. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. doi:10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lagace D.C., Benavides D.R., Kansy J.W., Mapelli M., Greengard P., Bibb J.A., Eisch A.J. Cdk5 is essential for adult hippocampal neurogenesis. Proc. Natl Acad. Sci. USA. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. doi:10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gilmore E.C., Ohshima T., Goffinet A.M., Kulkarni A.B., Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haughey N.J., Nath A., Chan S.L., Borchard A.C., Rao M.S., Mattson M.P. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J. Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. doi:10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 155.Sahlgren C.M., Mikhailov A., Vaittinen S., Pallari H.M., Kalimo H., Pant H.C., Eriksson J.E. Cdk5 regulates the organization of Nestin and its association with p35. Mol. Cell. Biol. 2003;23:5090–5106. doi: 10.1128/MCB.23.14.5090-5106.2003. doi:10.1128/MCB.23.14.5090-5106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Matsubara M., Kusubata M., Ishiguro K., Uchida T., Titani K., Taniguchi H. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J. Biol. Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 157.Li G., Faibushevich A., Turunen B.J., Yoon S.O., Georg G., Michaelis M.L., Dobrowsky R.T. Stabilization of the cyclin-dependent kinase 5 activator, p35, by paclitaxel decreases beta-amyloid toxicity in cortical neurons. J. Neurochem. 2003;84:347–362. doi: 10.1046/j.1471-4159.2003.01526.x. doi:10.1046/j.1471-4159.2003.01526.x. [DOI] [PubMed] [Google Scholar]
- 158.Sobue K., Agarwal-Mawal A., Li W., Sun W., Miura Y., Paudel H.K. Interaction of neuronal Cdc2-like protein kinase with microtubule-associated protein tau. J. Biol. Chem. 2000;275:16673–16680. doi: 10.1074/jbc.M000784200. doi:10.1074/jbc.M000784200. [DOI] [PubMed] [Google Scholar]
- 159.Perry E.K., Tomlinson B.E., Blessed G., Bergmann K., Gibson P.H., Perry R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. doi:10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fleisher A.S., Sowell B.B., Taylor C., Gamst A.C., Petersen R.C., Thal L.J. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. doi:10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 161.van Dyck C.H., Tariot P.N., Meyers B., Malca Resnick E. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2007;21:136–143. doi: 10.1097/WAD.0b013e318065c495. doi:10.1097/WAD.0b013e318065c495. [DOI] [PubMed] [Google Scholar]
- 162.Rockenstein E., Adame A., Mante M., Moessler H., Windisch M., Masliah E. The neuroprotective effects of Cerebrolysin trade mark in a transgenic model of Alzheimer's disease are associated with improved behavioral performance. J. Neural Transm. 2003;110:1313–1327. doi: 10.1007/s00702-003-0025-7. doi:10.1007/s00702-003-0025-7. [DOI] [PubMed] [Google Scholar]
- 163.Ringman J.M., Frautschy S.A., Cole G.M., Masterman D.L., Cummings J.L. A potential role of the curry spice curcumin in Alzheimer's disease. Curr. Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. doi:10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lipton S.A. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr. Alzheimer Res. 2005;2:155–165. doi: 10.2174/1567205053585846. doi:10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 165.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. doi:10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 166.Hong M., Chen D.C., Klein P.S., Lee V.M. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. doi:10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 167.Andorfer C., Kress Y., Espinoza M., de Silva R., Tucker K.L., Barde Y.A., Duff K., Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. doi:10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]