Abstract
We present neuropsychological data from an 81-year-old individual who was followed over a six-year period, initially as a healthy control participant. She performed above age-adjusted cutoff scores for impairment on most neuropsychological tests, including learning and memory measures, until the final assessment when she received a diagnosis of probable Alzheimer's disease (AD). Despite generally normal scores on individual cognitive tests, her cognitive profile revealed increasingly large cognitive discrepancies when contrasting verbal versus visuospatial tasks, and complex versus basic-level tasks. The present case provides intriguing evidence that cognitive-discrepancy measures could improve our ability to detect subtle changes in cognition at the earliest, preclinical stages of AD.
Keywords: Alzheimer's disease, Neuropsychology, Cognition, Discrepancy, Preclinical
Introduction
Identifying cognitive markers of a preclinical phase of Alzheimer's disease (AD) has been a major research focus in neuropsychology. This line of study is important both scientifically for increasing our understanding of the earliest effects of this neurodegenerative process, and clinically for individuals who may benefit from pharmacological, environmental, behavioral, and long-term planning interventions (Akaike, 2006; Allain, Bentue-Ferrer, Gandon, Le Doze, & Belliard, 1997; Giovannetti et al., 2007; Miguel-Hidalgo, Alvarez, Cacabelos, & Quack, 2002; Morrison et al., 2005; Vajda, 2002). Memory deficits are arguably the most frequently identified cognitive markers for AD, and they also constitute a primary diagnostic criterion for amnestic mild cognitive impairment (Petersen, 2004). However, individuals in prodromal stages prior to a dementia diagnosis often demonstrate heterogeneous cognitive deficits sometimes without significant memory deficits (Backman, Jones, Berger, Laukka, & Small, 2004), which poses a diagnostic challenge for clinicians. Recent studies of preclinical AD (Pre-AD) have documented a range of subtle changes in cognitive domains in addition to learning and memory, including executive functions, processing speed, working memory and attention, and visuospatial skills (Chen et al., 2001; Greenwood, Sunderland, Friz, & Parasuraman, 2000; Jacobson, Delis, Bondi, & Salmon, 2005a; Jacobson et al., 2005b; Twamley, Ropacki, & Bondi, 2006). Other studies have failed to identify a distinct or consistent pattern of cognitive deficits in Pre-AD (Goldman et al., 2001) resulting in a closer examination of the characterization and classification of preclinical and prodromal periods of AD (Dubois et al., 2007). Even the hallmark signs of insidious onset and gradual cognitive decline have been questioned, as some studies describe a plateau period with largely preserved cognition, followed by a rapid decline just prior to an AD diagnosis (Small, Fratiglioni, & Backman, 2001).
Not surprisingly, no single test or cognitive domain has proven sufficient for identifying the majority of individuals in the earliest, preclinical stages of AD (Albert, Blacker, Moss, Tanzi, & McArdle, 2007; Backman et al., 2004; Chen et al., 2000; Lopez et al., 2006). Cognitive markers like delayed-recall that have been investigated in large-scale studies do not always translate into accurate diagnosis at the individual-patient level (Ivnik et al., 2000), particularly when ‘atypical’ cognitive profiles emerge. Consequently, case studies that characterize less frequent phenomenon (Godbolt et al., 2005) promise to make important contributions to identifying clinical characteristics of Pre-AD.
In the current study, we present an individual (DH) who was medically and cognitively healthy and followed annually as an elderly control participant over a six-year period before a rapid cognitive decline resulted in an AD diagnosis. The results of annual cognitive testing are inherently valuable, given the scarcity of comprehensive longitudinal data in a documented preclinical phase. This case also illustrates another potentially important feature of Pre-AD: the possibility that significant cognitive discrepancies exist during an asymptomatic period when overall cognitive performance is above a cutoff-level of impairment. In a recent series of studies, we have used cognitive-discrepancy analyses to identify normal-functioning elderly subgroups with subtle cognitive changes that may herald the onset of AD (Houston et al., 2005; Jacobson et al., 2005a, 2005b; Wetter et al., 2005, 2006). We have suggested that inconsistent findings in the search for preclinical cognitive markers could reflect the use of central-tendency analyses of single test scores that were not sensitive to the presence of subgroups within the larger, at-risk elderly group. For example, both AD and Pre-AD subgroups have been identified with disproportionate impairment in verbal relative to spatial skills (or vice versa) (Albert, Duffy, & McAnulty, 1990; Demadura, Delis, Jacobson, & Salmon, 2001; Finton et al., 2003; Martin, 1990; Massman & Doody, 1996; Strite, Massman, Cooke, & Doody, 1997). It follows that cognitive-discrepancy analyses contrasting two domains may be superior to central-tendency analyses of each task individually for identifying asymmetric deficits, particularly in Pre-AD.
For example, Jacobson, Delis, Bondi, and Salmon (2002) investigated verbal/spatial discrepancies in cognitively intact, community-dwelling elderly participants, half of whom showed a subsequent decline in cognition and conversion to an AD diagnosis. When test scores were examined two years prior to the cognitive decline, performance discrepancies between the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983) and Block Design subtest (Wechsler, 1981) revealed that the subgroup who would later convert to an AD diagnosis had significantly greater cognitive-discrepancy scores. Importantly, these discrepancies existed despite the groups obtaining comparable scores on each measure when examined individually. Similar results were also found in older adults genetically at-risk for AD because of the Apolipoprotein E4 allele (ApoE-e4) using discrepancy scores derived from verbal versus spatial tests of attention (Jacobson et al., 2005a), global versus local memory task (Jacobson et al., 2005b), and verbal versus design fluency (Houston et al., 2005). Discrepancy scores derived from higher-level versus more basic tasks within the same modality (i.e., verbal memory versus vocabulary) (Wetter et al., 2006) were found to be effective for distinguishing elderly individuals with a higher genetic risk for AD.
We present a case study that examines neuropsychological tests data from an elderly individual who had intact cognitive abilities initially, but whose test performances also suggested emerging cognitive discrepancies over the six-year period prior to a diagnosis of probable AD diagnosis. Our goal was to apply our previous methodologies to this individual's annual cognitive assessments to determine if cognitive-discrepancy scores would be sensitive to subtle declines in DH's cognitive functioning during a ‘plateau’ period in which most test scores were within normal limits. We highlight cognitive-discrepancy scores because they can potentially identify intra-individual changes that might not be apparent in a single, norm-referenced test score (Bondi et al., 2008).
Case Study
DH is a right-handed, Caucasian woman with two years of college education who was one of approximately 30 participants recruited as a healthy control participant through the UCSD Alzheimer's Disease Research Center (ADRC) for a study of executive functions in older adults. In addition to comprehensive neuropsychological assessments, she received annual neurologic and psychosocial evaluations to confirm stable physical and emotional health, as well as functional independence. We followed this participant for 6 years beginning at age 81. At the fifth assessment, DH reported symptoms consistent with mild depression, she received a working diagnosis of pseudodementia, and she was referred for neuroimaging studies. At the sixth assessment (age 86), DH complained of subjective changes in her memory ability and some functional impairment in activities of daily living. The assessment at this time revealed moderately impaired performance on memory tests and in multiple cognitive domains, and she subsequently received a consensus diagnosis of probable AD. Prior to this time, DH was living independently, had no obvious impairment in her daily activities, and met standard ADRC criteria for participation as a normal control subject, which excluded individuals with a history of significant neurologic or psychiatric disorder, alcohol or substance abuse or dependence, traumatic brain injury with loss of consciousness, stroke, learning disability, or use of medications that impair cognition. DH was initially in an asymptomatic, preclinical stage of AD according to recent diagnostic criteria (Dubois et al., 2007). Subsequent MRI confirmation of medial temporal lobe atrophy was followed by a brief, prodromal period when cognitive and functional deficits became apparent, until she finally met criteria for a probable AD diagnosis after the final, sixth assessment. Apolipoprotein E genotyping had been completed using the method described in previous studies (Corder et al., 1993) and indicated an Apolipoprotein E status of e3/3. She completed a UCSD IRB-approved informed consent process prior to the assessments.
Methods
Neuropsychological assessment
Trained psychometrists using standardized test procedures supervised by a neuropsychologist administered all cognitive tests. In addition to an annual neuropsychological test battery (see Salmon et al., 2002), we administered several additional tests to measure cognitive discrepancies based on our previous studies cited above. Table 1 provides a description of DH's cognitive test results, and Table 2 details specific test variables and normative information used to calculate cognitive-discrepancy scores in the present study. Specifically, we examined three cognitive discrepancies that contrast differential declines in tasks that are more verbally mediated relative to tasks that are more non-verbally or spatially mediated (Bisiacchi, Borella, Bergamaschi, Carretti, & Mondini, 2008; Jacobson et al., 2002). We also examined four discrepancies between a complex higher-level cognitive skill and a corresponding basic-ability skill in the same domain. Tests were selected to contrast an ability that is likely to be resilient (e.g., vocabulary ability) with an ability considered to be more vulnerable to a dementing process (e.g., verbal memory).
TABLE 1.
Tests and variable descriptions used for cognitive discrepancy analyses
| Verbal/spatial discrepancy | Verbal test | Non-verbal test |
|---|---|---|
| Verbal naming vs. visuoconstruction | Boston Naming Test1 30 item short form: total spontaneous correct | Block Design subtest1 (WAIS-R): total points including time bonus |
| Verbal vs. non-verbal attention | Digit Span subtest1 (WAIS-R) total correct forward/backward | D-KEFS Trail-making test2: seconds to completion |
| Verbal vs. non-verbal memory | California Verbal Learning1 Test: total words recalled on Long-delay free recall | Heaton Visual Reproduction1 Test: total points delayed recall of designs |
| Basic skill versus Complex task Discrepancy | Basic skill task | Complex skill task |
| Psychomotor speed vs. Sequencing/set-shifting (D-KEFS Trail-making test) 2 | motor speed condition (secs. to completion) |
|
| Color/Word naming vs. Inhibition & set-shifting (D-KEFS Color Word Interference Test)2 | average of color naming & color word reading (secs. to completion) |
|
| Phonemic vs. Semantic fluency (D-KEFS Verbal Fluency Test)2 | letter fluency: total words (‘F, A, S’) in 60 secs. |
|
| Vocabulary vs. verbal list learning | Vocabulary subtest1 (WAIS-R) total pts. | |
Notes: z-Scores based on normative information from these sources:
UCSD ADRC age-corrected norms (ages 80–87; n = 18); see Salmon et al. (2002).
D-KEFS age-corrected norms (80–89 years, n = 100). (Delis & Kaplan, 2001).
TABLE 2.
Diagnosis, age, and cognitive screening measures: Raw and z-scores for neuropsychological tests
| Assessment #1 | Assessment #2 | Assessment #3 | Assessment #4 | Assessment #5 | Assessment #6 | |
|---|---|---|---|---|---|---|
| Diagnosis | NC | NC | NC | NC | Pseud-Dem | Prob AD |
| Age | 81 | 82 | 83 | 84 | 85 | 86 |
| MMSE | 29 | n/a | 29 | n/a | 27 | 26 |
| DRS total/(SS) | 138 | 136 | 136 | 136 | 132 | 130 |
| 12 | 10 | 10 | 11 | 9 | 9 | |
| ANART errors/est IQ | 8/120+ | 6/120+ | 8/120+ | 5/120+ | 13/110–120 | 13/117 110–120 |
| Raw score (z-score) | Raw score (z-score) | Raw score (z-score) | Raw score (z-score) | Raw score (z-score) | Raw score (z-score) | |
| Vocabulary | 58 | 62 | 53 | 54 | 50 | 51 |
| 0.44 | 1.18 | −0.48 | −0.31 | −1.04 | −0.85 | |
| Digit Symbol | 47 | 49 | 47 | 43 | 43 | 40 |
| 0.34 | 0.54 | 0.34 | 0.07 | 0.07 | −0.38 | |
| Block Design | 58 | 62 | 53 | 51 | 40 | 51 |
| 1.65 | 0.91 | 1.19 | 1.00 | 0.25 | 1.00 | |
| WCST Categories | 6 | 5 | 6 | 6 | 5 | 6 |
| 0.56 | −0.19 | 0.56 | 0.56 | −0.19 | 0.56 | |
| WCST errors | 9 | 7 | 8 | 0 | 8 | 5 |
| 0.30 | −0.06 | 0.11 | −1.3 | 0.11 | −0.41 | |
| Boston Naming total pts. | 29 | 28 | 26 | 27 | 23 | 22 |
| 0.48 | −0.19 | −1.55 | −0.87 | −3.58 | −4.20 | |
| WMS LM LDR | 22 | 14 | 24 | 20 | 17 | 12 |
| 0.35 | −0.50 | 0.70 | 0.0 | −0.25 | −1.0 | |
| WMS LM % savings | 100 | 100 | 100 | 83 | 100 | 67 |
| 2.25 | 2.25 | 2.25 | 0.92 | 2.25 | −0.34 | |
| CVLT LD FR | 11 | 9 | 9 | 6 | 5 | 3 |
| 0.35 | 0.60 | 0.60 | −0.4 | −0.7 | −1.4 | |
| CVLT Recog Discrim | 0.91 | 0.91 | 0.89 | 0.80 | 0.84 | 0.93 |
| −0.15 | −0.15 | − 0.6 | −2.2 | −1.3 | 0.23 | |
| DRS Memory (SS) | 25 | 23 | 23 | 22 | 20 | 22 |
| 14 | 10 | 10 | 9 | 6 | 9 | |
| Clock drawing*/clock setting* | 3 of 3 | 3 of 3 | 3 of 3 | 3 of 3 | 3 of 3 | 3 of 3 |
| 12 of 12 | 12 of 12 | 12 of 12 | 12 of 12 | 12 of 12 | 12 of 12 | |
| CERAD list trial 3* | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 9/10 |
| CERAD recall* | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| CERAD recall* | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
Limited distribution of scores - z-score not calculated. Bold type indicates a score below a cutoff for impairment of 1.5 SD below comparison group mean.
Abbreviations: DRS, Dementia Rating Scale; ANART, American National Adult Reading Test; MMSE, Mini Mental State Exam; Pseu-Dem, pseudodementia; Prob AD, probable AD; WCST = Wisconsin Card Sorting Test; WMS-LR = Wechsler Memory Scale-Revised, Logical Memory subtest; CVLT, California Verbal Learning Test; CERAD, Consortium to Establish a Registry for Alzheimer's Disease (Verbal Memory task).
For most comparisons, cognitive-discrepancy scores were calculated by first converting raw scores to z-scores based on archival data from a cohort of older, normal control participants from the UCSD ADRC with a range of age and education similar to DH. This group met normal control criteria, and had a minimum of 4 consecutive annual neuropsychological and neurologic examinations without a significant cognitive decline or change in diagnosis. The resulting group (n = 28) had a mean age of 82.1 (SD = 2.1), and education range from 12 to 18 years, mean = 15.4 (SD = 2.5). Because DH received executive function tests that were not administered to the ADRC group, we calculated some z-scores from the D-KEFS normative database (Delis & Kaplan, 2001), using the age-adjusted scaled scores for calculation of z-scores [cohort age range was 80–89; n = 100; mean (SD) educational level of 12.06 (2.54) years].
Results
Overall cognitive functioning
Based on her ANART score, DH had an estimated premorbid Verbal IQ in the high average range, consistent with her previous level of education and occupation as a teacher. On a screening measure for dementia, her initial performance was in the above-average range (Mattis Dementia Rating Scale total = 138 points; age-scaled score 60–81%ile) and declined to average, then low-average range (132 points; 24–40%ile) at her final two assessments. Her MMSE total scores remained within normal limits (29–27 points) during all six assessments.
Table 2 summarizes DH's longitudinal neuropsychological test scores (raw and standardized z-scores). With a few exceptions (confrontation naming at the fourth assessment), her performances on individual tests in most domains (language, psychomotor speed, visuoconstruction, attention and working memory, executive functioning) were above typical cutoff levels for impairment (i.e., >1.5 SD below age-adjusted means) relative to the normative group. Her performances on the most commonly used indicators of Pre-AD such as the CVLT long-delay recall scores did not decline into an impaired range (−1.5 SD below the normative group mean) until the final assessment prior to her AD diagnosis. For all six evaluations, her scores were at or above the average range relative to the normative comparison group on all other memory measures including the Wechsler Memory Scale-Revised, Logical memory delayed recall, Heaton Visual Reproduction Test delayed recall, DRS memory subscale, and CERAD verbal recall.
Cognitive discrepancies: Verbal versus Spatial tasks
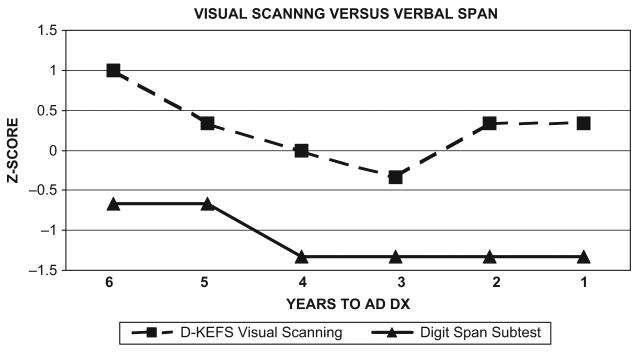
Figure 1 shows cognitive-discrepancy scores derived from an auditory attention test (WAIS-R Digit Span subtest) relative to a visual attention test (Visual Scanning condition of the DKEFS Trail Making Test). Her auditory attention span remained within the average to low-average ranges while her visual attention ability was within normal limits for her age. In contrast, there were cognitive discrepancies greater than 1 SD in four of the six assessments despite relatively unimpaired performance on each task individually.
Figure 1.
Visual Scanning versus Verbal Attention Span Discrepancies: D-KEFS Visual Scanning with Mildly Impaired Digit Span Scores.
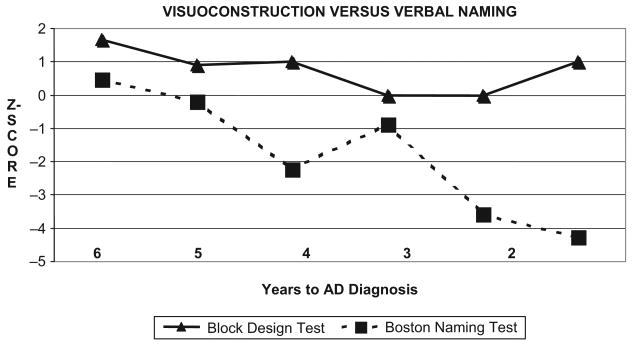
Figure 2 shows DH's performance on the Boston Naming Test (BNT) relative to the Block Design subtest of the WAIS-R (BD) across six years of testing. Although her naming ability showed a gradual decline, her performance did not reach a consistent level of impairment until the final two assessments. Her block construction skills remained stable, at or above the average range throughout this period. However, cognitive-discrepancy score analyses showed a greater than 1 SD discrepancy between BNT and BD z-scores in five of the six annual assessments prior to her diagnosis change, with increasing discrepancies over time.
Figure 2.
Visuoconstruction versus Verbal Naming Discrepancies: Stable Block Design Scores with Declining Boston Naming Scores.
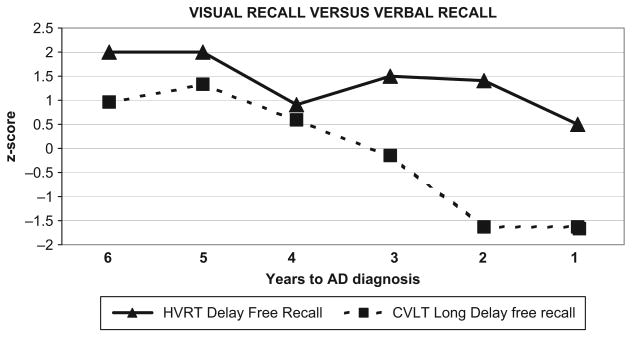
Figure 3 shows cognitive-discrepancy scores comparing the CVLT long-delay free recall condition relative to the delayed recall condition of the Visual Reproduction subtest (Heaton version [HVRT]). Her non-verbal recall remained in the average to high average range for all six assessments relative to a gradual decline in her verbal recall ability. However, her CVLT delayed-recall scores remained above a level of mild to moderate impairment until one year prior to her AD diagnosis. Her cognitive-discrepancy scores steadily increased over time, yielding a 1 SD difference between verbal/visual recall in four of six assessments.
Figure 3.
Visual Recall versus Verbal Recall Discrepancies: Increasing Discrepancy between HVRT and CVLT Long-delay Recall Scores.
Cognitive discrepancies: Executive function and Memory tasks
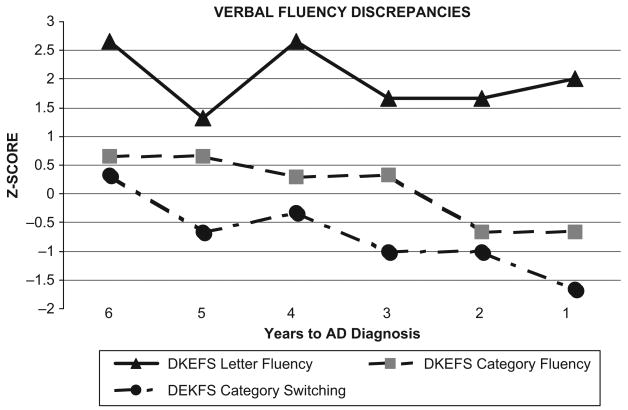
We examined verbal fluency discrepancies using three conditions of the D-KEFS Verbal Fluency test: Letter Fluency (average of F, A, and S), Category Fluency (animals and boy's names) and Category-Switching (alternating fruit and furniture words); see Figure 4. Her test scores demonstrated consistently above-average and stable performance on letter fluency relative to a gradual decline on the two types of category-fluency tasks. The cognitive-discrepancy scores between letter and category-switching fluency was greater than 1 SD in all six assessments.
Figure 4.
Verbal Fluency Discrepancies: Stable Letter Fluency Relative to Declining Category and Category Switching Scores.
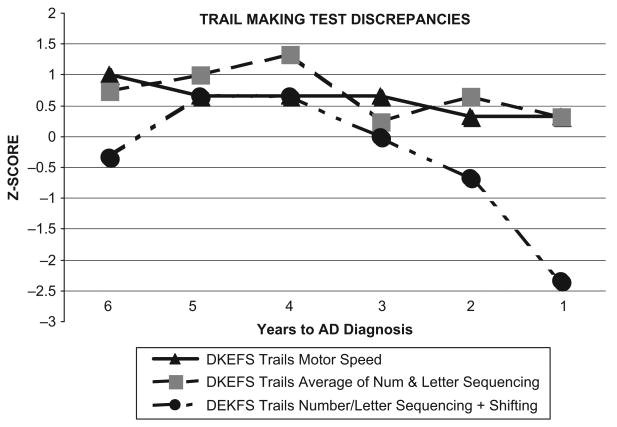
We used the D-KEFS Trail Making Test to examine discrepancies between complex visuomotor sequencing/shifting ability, and a basic-skill task requiring visuomotor speed (tracing a line between a series of circles with no letters or numbers; see Figure 5). While DH's performance on the basic-ability task remained stable (and within normal limits), her scores on the Number/Letter Switching showed considerable variability, eventually declining into the impaired range. Her cognitive-discrepancy scores reflected a 1 SD difference between complex and basic-skill tasks on three of six assessments.
Figure 5.
Trail Making Test Discrepancies: Stable Basic Motor, Sequencing Skills with Variable Performance and Deficits in Shifting Condition.
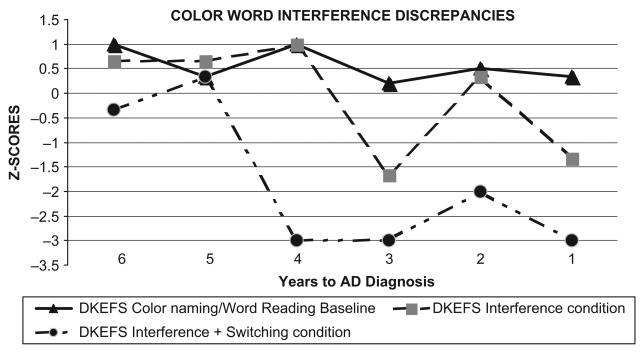
DH's performance on four conditions of the DKEFS Color/Word Interference Test (CWIT) is shown in Figure 6. A basic-skill composite measure was computed as the average of the Color Naming (CN) and the Word Reading (WR) conditions. The complex skills consisted of (a) interference-only condition (traditional Stroop test), and (b) the interference/shifting condition (switching between naming the dissonant ink-color and reading the dissonant word). Cognitive-discrepancy scores between basic-skill and interference-only measures were greater than 1 SD in two of six assessments. However, the cognitive discrepancy score using the more difficult interference/shifting paradigm resulted in a greater than 1 SD discrepancy relative to the basic-skill measure (mean of CN & WR) in five of six assessments, largely the result of declining scores on the two complex CWIT conditions.
Figure 6.
Stroop Discrepancies: Color/word Naming Scores and Traditional Stroop Scores with Impaired Interference/Switching Condition.
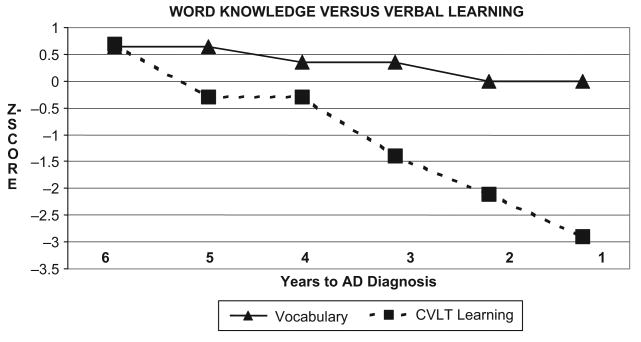
We examined the participant's discrepancy between basic word knowledge (WAIS-R Vocabulary subtest) and complex verbal learning ability (CVLT learning trials; total for trials 1–5) (see Figure 7). DH showed a significant cognitive discrepancy between her word knowledge and her total verbal learning ability on three of the six assessments prior to her diagnosis change. She exhibited a gradual decline in verbal learning ability, relative to stable and average to above-average performance on the Vocabulary task.
Figure 7.
CVLT Learning versus Vocabulary Discrepancies: Stable Basic-skill Word Knowledge Ability with Declining Verbal Learning Scores.
Figure 8 summarizes the pattern of the two types of cognitive-discrepancy scores across six assessments. We plotted the average of the combined cognitive discrepancy scores (z-score differences) for each assessment for all verbal/spatial discrepancies (Figure 8a), and for all complex/basic-skill discrepancies (Figure 8b). We fitted two linear trend lines to these data, which indicated positive slopes over time for verbal/spatial discrepancies (y = .391) and complex/basic-skill discrepancies (y = .387), suggesting an overall increase in cognitive-discrepancy scores as D.H. approached an AD diagnosis.
Figure 8.
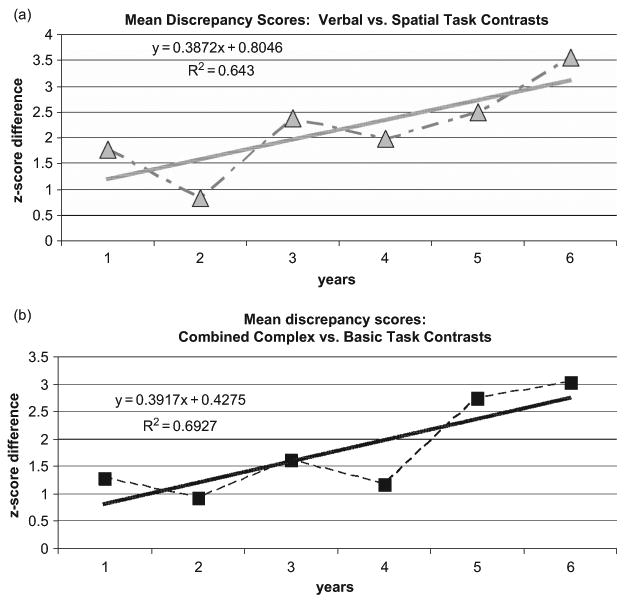
Scatterplot and Trend Lines: Increasing Longitudinal Discrepancies in (a) Verbal/Spatial Discrepancies and (b) Basic-ability/Complex Task Discrepancies.
Neuroimaging
The participant underwent structural magnetic resonance imaging (MRI) following the fifth assessment, one year prior to a change in diagnosis to probable AD. High-resolution anatomical images were acquired using a T1-weighted fast-spin echo (SPGR) at a 1.5-Tesla GE MRI scanner (TR = 24 ms, TE = 5 ms, flip angle = 45 degrees, FOV = 24 cm, 1.2 mm contiguous sections). Selected axial and coronal slices reveal asymmetric atrophy in medial temporal regions (Figure 9a), particularly in the left hemisphere. The neuroradiologist's impressions described generalized cortical atrophy greater in temporal lobe regions, ventricular enlargement, and no indication of significant ischemic changes, tumor or mass. A 3-dimensional reconstruction (Bigler et al., 2002) of the T1-weighted image is also depicted (Figure 9b) to illustrate cortical atrophy and ventricular enlargement.
Figure 9.
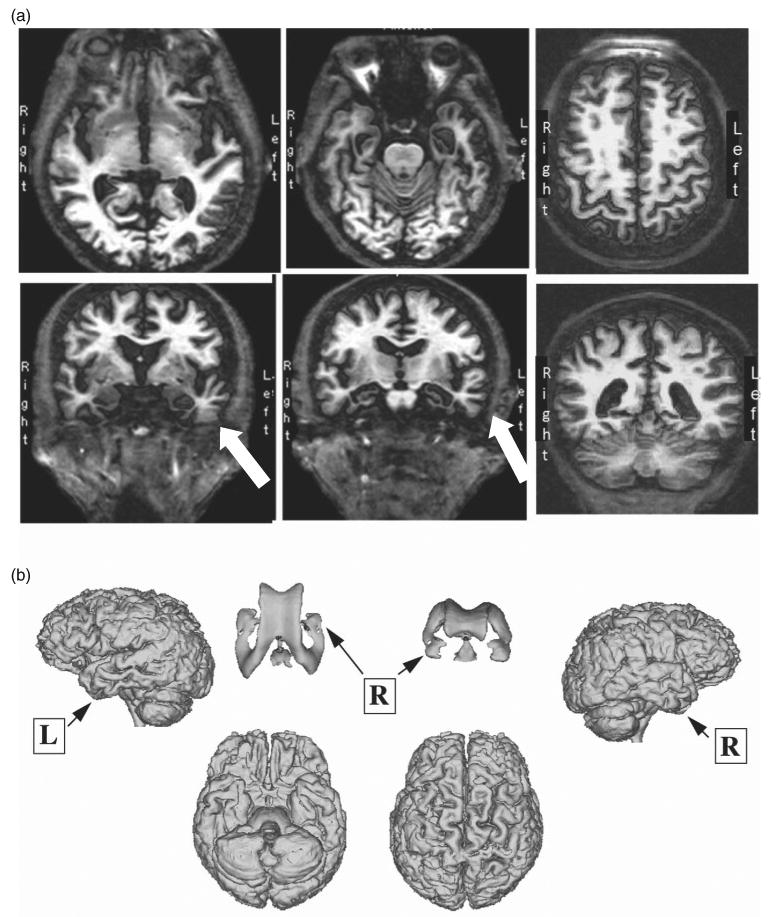
Selected T1-weighted MRI scan: (a) images highlighting left temporal atrophy and (b) 3D cortical reconstruction with ventricular system.
Discussion
The present case illustrates the diagnostic challenges inherent in identifying preclinical AD when an individual's cognitive profile lacks many of the typical hallmarks of this phase. Despite an eventual diagnosis of probable AD after the sixth year of participation, none of the first four assessments would justify an MCI diagnosis: her performances on most individual cognitive tasks, including memory tests, were within or above the average range, she was living independently, and she had no subjective memory complaints until the final assessment. With few exceptions (e.g., naming ability at the fourth assessment), her performances on individual neuropsychological tests remained above a level of impairment until the final year. Discrepancy scores have been used in many contexts as a means of identifying subtle declines in cognitive skills relative to those that are more resilient to a neurodegenerative process or dementia (Dori & Chelune, 2004; Finton et al., 2003; Ivnik et al., 2000; Wilde et al., 2001). Analysis of DH's intra-individual differences revealed a consistent pattern of cognitive discrepancies that may have signaled an impending decline despite generally intact performances on individual tasks. DH's complex versus basic-level task discrepancy scores reflected stable performance on more fundamental task components in the face of mild declines in executive function or memory abilities. Her verbal versus non-verbal discrepancies reflected an asymmetric decline in largely verbally mediated tasks.
Although the majority of DH's discrepancy scores reflected at least a 2 SD difference by the final assessment, the question arises as to what constitutes an atypical discrepancy? There is a considerable body of literature addressing the question of clinical versus statistical significance of cognitive discrepancies (Crawford & Garthwaite, 2005; Crawford, Garthwaite, & Gault, 2007; Lange & Chelune, 2006; Lange, Chelune, & Tulsky, 2006). A commonly used criteria considers a discrepancy ‘unusual’ or significant if it occurs in less than 10% of the population (Hawkins & Tulsky, 2001; Kramer et al., 2006), although methods for discrepancy score calculations vary. Fortunately, many tests now incorporate discrepancy measures (e.g., CVLT-II, WAIS-III, D-KEFS, Warrington Recognition Memory Test) as standardized variables, although base rate information is not always available. Chelune, Holdnack, and Levy (2006) examined D-KEFS normative data and found that a letter-category fluency scaled-score discrepancy of +3 occurs in 10% of the sample (with 13–15 years of education). By comparison, DH's letter-category fluency discrepancy (at age 82) showed a scaled score difference of 5, with a contrast scaled score of 15 (95th percentile rank) based on her same age peers in the D-KEFS sample. In addition to the discrepancy magnitude, the frequency of significant discrepancies is also a consideration. A pattern of large, recurring discrepancies that are internally consistent may provide more convincing evidence of impending cognitive decline than a single, isolated discrepancy. For example, three significant discrepancy measures occurred in 2% of the in the CVLT-II normative sample, while a single significant discrepancy occurred in 22% of the sample (Donders, 2006). Finally, discrepancy scores vary by IQ and education with higher functioning individuals exhibiting larger discrepancies, so the use of stratified norms will prove important for accurate interpretation (Hawkins & Tulsky, 2001).
A longitudinal review of DH's performance offers some evidence for a sub-clinical decline on certain tests, although neuropsychologists in clinical practice rarely have the benefit of examining six consecutive annual assessments. The current case underscores some of the benefits afforded by serial assessments in individuals who have high premorbid abilities. Cognitive impairment can be masked in these individuals because declining scores remain above norm-based cutoff scores (Mitrushina & Satz, 1991) as was seen in DH's case. Her test scores eventually indicated a very rapid decline in the two years prior to her conversion to a probable AD diagnosis, and may be representative of a subgroup of patients whose disease onset begins with a plateau period followed by a precipitous rate of cognitive decline (Small & Backman, 2007; Smith et al., 2007). The abrupt decline also was concurrent with subjective reports of emotional changes. The emergence of depression and anxiety has been documented as a common feature associated with symptoms of dementia (Potter & Steffens, 2007), perhaps contributing to both subjective complaints and objective deficits (van Norden et al., 2008).
DH's cognitive profile may be characteristic of individuals with greater ‘cognitive reserve’ who are less likely to exhibit obvious cognitive deficits on individual tests until neurodegenerative changes overwhelm ‘compensatory’ strategies (Riis et al., 2008; Tuokko, Garrett, McDowell, Silverberg, & Kristjansson, 2003). However, it is possible that her putative cognitive resilience was reinforced by repeated testing. Confirmation of significant change in neuropsychological test scores is a complex issue. Interpreting change in difference scores has additional considerations, as differential practice effects of the two component tasks could serve to either mask or accentuate performance discrepancies. Detailed studies of practice effects in older adults typically show either stable or improved performance on subsequent testings (Hickman, Howieson, Dame, Sexton, & Kaye, 2000; Paolo, Troester, & Ryan, 1997) with the most significant performance gains occurring at the second testing occasion (Ivnik et al., 1999). Thus, practice effects could have contributed to the attenuation of cognitive discrepancy scores in the second year. Drawing firm conclusions about significant change is ultimately enhanced with the use of reliable change indices (Ivnik et al., 1999) and confidence interval estimates which require information about measurement error, reliability estimates, standard error of difference, and test intercorrelations (that are not always readily available). Theoretically, verbal/spatial discrepancies would be more prone to practice effects than would complex/basic-skill discrepancies because many performance based tasks (e.g., Block Design) show greater improvement on re-test relative to verbal tasks (Theisen, Rapport, Axelrod, & Brines, 1998).
Additional limitations of discrepancy scores can be attributed to psychometric measurement issues, as the reliability of a contrast measure will typically be lower than either of the contributing test variables (Woods et al., 2005). Recently published data on D-KEFS contrast measures showed reliability ranging from r = .43 (color/word naming vs. switching/interference) to r = .66 (letter vs. category fluency (Crawford, Sutherland, & Garthwaite, 2008). Discrepancy scores also can be influenced by relative differences in the sensitivity and difficulty of the component tasks, differences in score distributions, and floor or ceiling effects (Cherry, Buckwalter, & Henderson, 2002; Orsini, Trojano, Chiacchio, & Grossi, 1988). Finally, the use of tests that quantify more discrete components of a cognitive domain (e.g., evaluating types of semantic memory rather than general semantic knowledge) (Ahmed, Arnold, Thompson, Graham, & Hodges, 2008) could improve sensitivity to prodromal changes compared to discrepancy scores that are based on variables from multi-factorial cognitive tests.
The presence of cognitive discrepancies in multiple domains could represent another early indicator of an incipient decline in functioning. Prior research has shown that cognitive discrepancies greater than 1 SD were more frequent in older individuals who ultimately developed AD (Jacobson et al., 2002), and in elderly groups at increased risk for AD (Jacobson et al., 2005a,b). Several of our previous studies examining cognitive discrepancies in older adults have shown that only 10–20% of older adults (over age 65) who do not have an increased genetic risk for AD (ApoE-e4 allele) show a greater than 1 SD discrepancy between verbal/spatial tasks or complex/basic-level tasks. In contrast, 40–55% of those with increased ApoE-e4 genetic risk had large discrepancies (Houston et al., 2005; Jacobson et al., 2005a,b; Wetter et al., 2005), a ratio that is consistent with other studies of AD subjects with asymmetric cognitive functioning (Massman & Doody, 1996; Strite et al., 1997). A significant caveat to the use of cognitive discrepancies is the possibility of premorbid asymmetries in individual examinees. However, a recent study of cognition in middle-aged twins who were at increased genetic risk for AD due to ApoE-E4 genotype found larger verbal/non-verbal cognitive discrepancies relative to those without the E4 genetic risk (Schultz et al., 2008). Cognitive discrepancies have been documented in the early stages of AD as well (Delis et al., 1992; Demadura et al., 2001; Finton et al., 2003; Massman & Doody, 1996).
Most of DH's discrepancy scores resulted from declining scores on tasks with a substantial verbal component, a profile often associated with greater left hemisphere compromise (Ott et al., 2000). Consistent with such profiles, DH's MRI findings after her fifth assessment revealed atrophy in brain regions most likely to be impacted by AD-related neurodegeneration, particularly in medial temporal regions of the left hemisphere. Some studies suggest that verbal/spatial discrepancies reflect asymmetric changes in brain functions (Delis et al., 1992; Haxby et al., 1990), brain structures (Finton et al., 2003; Soininen et al., 1994), or brain metabolism (Franceschi et al., 1995). It should be noted, however, that the intermediate stages of progression to an AD diagnosis are likely to be heterogeneous both in terms of cognition and sites of neuropathology, particularly in individuals with multiple deficits (Mariani, Monastero, & Mecocci, 2007; Troster, 2008). Rather than lateralized changes in brain structure and function, cognitive discrepancies—particularly complex versus basic-ability contrasts—could reflect a more general disruption of neural networks (Parasuraman & Martin, 1994). Recent studies suggest that early AD is characterized by the loss of cortico-cortical projections that facilitate interactions of multiple brain regions (Andrews-Hanna et al., 2007), which would likely result in greater deficits in higher-level, executive functions relative to more fundamental cognitive abilities. The base-rates for structural brain asymmetry in Pre-AD require sample sizes that are only possible with large, multi-site neuroimaging studies.
In conclusion, some individuals without significant memory impairment, and with generally normal performance on individual cognitive tests, may nevertheless be in a preclinical phase of AD. In some situations, cognitive-discrepancy scores may be more sensitive than individual test scores for identifying preclinical AD, because they highlight intra-individual changes in cognitive skills (Storandt, Grant, Miller, & Morris, 2006). The present case suggests that serial assessments and the unfolding of cognitive discrepancies over time may improve detection of subtle cognitive changes in individuals with high premorbid ability who are at the earliest, preclinical stages of AD.
TABLE 3.
Verbal vs. spatial discrepancies: Mean (SE), frequency and range over six years
| Mean (SE) z-score discrepancy | # Years with >1 SD z-score discrepancy | Minimum and maximum z-score discrepancy | |
|---|---|---|---|
| Cognitive Discrepancy: Verbal vs. Spatial Task Comparisons | |||
| Naming (BNT) vs. Visuoconstruction (BD) | 2.56 (.72) | 5/6 | 0.85–5.26 |
| Auditory Attention (DS) vs. Visuospatial Attention (VS) | 1.38 (.13) | 5/6 | 1.0–1.66 |
| Verbal Recall (CVLT) vs. Visuospatial Recall (HVRT) | 1.47 (.41) | 4/6 | 0.31–3.04 |
| Basic-level Task vs. Complex Skill Comparisons | |||
| Letter (LF) vs. Category Fluency (CF) | 1.89 (.31) | 5/6 | 0.67–2.66 |
| Letter vs. Category-Shifting Fluency (CSF) | 2.71 (.23) | 6/6 | 1.99–3.66 |
| Motor Speed (MS) vs. Number/Letter Sequencing & Shifting (SS) | 1.05 (.36) | 3/6 | 0.25–2.66 |
| Color Naming & Word Reading (CNWR) vs. Color/Word Inhibition only (CWIT) | 0.73 (.33) | 2/6 | 0–1.66 |
| Color Naming & Word Reading (CNWR) vs. Inhibition & Shifting (CWITS) | 2.39 (.60) | 5/6 | 0–3.33 |
| Vocabulary (VOC) vs. Verbal Learning Trials (CVLT1-5) | 1.40 (.42) | 3/6 | 0.05–2.90 |
Abbreviations: BNT, Boston Naming Test; BD, WAIS-R Block Design subtest; DS, WAIS-R Digit Span subtest; VS, Visual Scanning condition D-KEFS Trail Making; CVLT, California Verbal Learning Test Delayed Recall; HVRT, Heaton Visual Reproduction Test– Russell version (Russell, 1975); LF / CF / CSF, D-KEFS Verbal Fluency: Letter / Category / Category Shifting; MS / SS, D-KEFS Trail Making Motor Speed / Sequencing and Shifting; CNWR, D-KEFS Color Word Interference Test combined Color and Word reading; CWIT, D-KEFS Color Word Interference Test-Interference condition; CWITS / Interference Shifting condition; VOC, WAIS-R Vocabulary subtest; CVLT1-5, California Verbal Learning Test Learning Trials 1–5.
Acknowledgments
The authors wish to thank Dr Terry Jernigan, Sarah Archibald M.S., and Dr Christine Fennema-Notestine for their assistance with MRI image collection and presentation. We also thank the participant, as well as Jodessa Braga and the staff of the UCSD Alzheimer's Disease Research Center for their contributions to this research. Finally, we would like to thank the anonymous reviewers for their helpful comments. Dr Delis is a co-author of the D-KEFS and CVLT-II and receives royalties from them. Preparation of this article was supported in by a Veterans Administration Career Development Award (M.W.J.), a Veterans Administration Merit Review Award (D.D.), NIMH award R01MH063782 (G.M.P.); Alzheimer's Association Award IIRG-07-59343 (M.W.B.), National Institute on Aging awards K24 AG026431 and R01 AG012674 (M.W.B.), and NIA Grant P50 AG 05131 (UCSD ADRC).
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Ahmed S, Arnold R, Thompson SA, Graham KS, Hodges JR. Naming of objects, faces and buildings in mild cognitive impairment. Cortex. 2008;44(6):746–752. doi: 10.1016/j.cortex.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Akaike A. Preclinical evidence of neuroprotection by cholinesterase inhibitors. Alzheimer's Disease and Associated Disorders. 2006;20(2 Suppl. 1):S8–11. doi: 10.1097/01.wad.0000213802.74434.d6. [DOI] [PubMed] [Google Scholar]
- Albert MS, Duffy FH, McAnulty GB. Electrophysiologic comparisons between two groups of patients with Alzheimer's disease. Archives of Neurology. 1990;47(8):857–863. doi: 10.1001/archneur.1990.00530080039008. [DOI] [PubMed] [Google Scholar]
- Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21(2):158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Allain H, Bentue-Ferrer D, Gandon JM, Le Doze F, Belliard S. Drugs used in Alzheimer's disease and neuroplasticity. Clinical Therapeutics: The International Peer-Reviewed Journal of Drug Therapy. 1997;19(1):4–15. doi: 10.1016/s0149-2918(97)80068-9. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. Journal of Internal Medicine. 2004;256(3):195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF, Miller MJ, Rice SA, Hessel CD, Earl HD, et al. Dementia, asymmetry of temporal lobe structures, and Apolipoprotein E genotype: Relationships to cerebral atrophy and neuropsychological impairment. Journal of the International Neuropsychological Society. 2002;8(7):925–933. doi: 10.1017/s1355617702870072. [DOI] [PubMed] [Google Scholar]
- Bisiacchi PS, Borella E, Bergamaschi S, Carretti B, Mondini S. Interplay between memory and executive functions in normal and pathological aging. Journal of Clinical and Experimental Neuropsychology. 2008;30(6):723–733. doi: 10.1080/13803390701689587. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer's disease. Neuropsychology Review. 2008;18(1):73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ, Holdnack J, Levy J. Education effects and base-rate information for the Delis-Kaplan Executive Function System (DKEFS) fluency measures. Archives of Clinical Neuropsychology. 2006;21:B45. [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55(12):1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58(9):853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Cherry BJ, Buckwalter JG, Henderson VW. Better preservation of memory span relative to supraspan immediate recall in Alzheimer's disease. Neuropsychologia. 2002;40(7):846–852. doi: 10.1016/s0028-3932(01)00173-7. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Testing for suspected impairments and dissociations in single-case studies in neuropsychology: Evaluation of alternatives using Monte Carlo simulations and revised tests for dissociations. Neuropsychology. 2005;19(3):318–331. doi: 10.1037/0894-4105.19.3.318. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH, Gault CB. Estimating the percentage of the population with abnormally low scores (or abnormally large score differences) on standardized neuropsychological test batteries: A generic method with applications. Neuropsychology. 2007;21(4):419–430. doi: 10.1037/0894-4105.21.4.419. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Sutherland D, Garthwaite PH. On the reliability and standard errors of measurement of contrast measures from the D-KEFS. Journal of the International Neuropsychological Society. 2008;14(6):1069–1073. doi: 10.1017/S1355617708081228. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E. Manual for the Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, et al. Spatial cognition in Alzheimer's disease: Subtypes of global-local impairment. Journal of Clinical & Experimental Neuropsychology. 1992;14(4):463–477. doi: 10.1080/01688639208402838. [DOI] [PubMed] [Google Scholar]
- Demadura T, Delis DC, Jacobson M, Salmon D. Do subgroups of patients with Alzheimer's disease exhibit asymmetric deficits on memory tests? Journal of Clinical & Experimental Neuropsychology. 2001;23(2):164–171. doi: 10.1076/jcen.23.2.164.1207. [DOI] [PubMed] [Google Scholar]
- Donders J. Performance discrepancies on the California Verbal Learning Test – Second Edition (CVLT-II) in the standardization sample. Psychological Assessment. 2006;18(4):458–463. doi: 10.1037/1040-3590.18.4.458. [DOI] [PubMed] [Google Scholar]
- Dori GA, Chelune GJ. Education-stratified base-rate information on discrepancy scores within and between the Wechsler Adult Intelligence Scale –Third Edition and the Wechsler Memory Scale – Third Edition. Psychological Assessment. 2004;16(2):146–154. doi: 10.1037/1040-3590.16.2.146. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: Revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Finton MJ, Lucas JA, Rippeth JD, Bohac DL, Smith GE, Ivnik RJ, Petersen RC, Graff-Radford NR. Cognitive asymmetries associated with apolipoprotein E genotype in patients with Alzheimer's disease. Journal of the International Neuropsychological Society. 2003;9(5):751–759. doi: 10.1017/S1355617703950089. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Alberoni M, Bressi S, Canal N, Comi G, Fazio F, Grassi F, Perani D, Volonte MA. Correlations between cognitive impairment, middle cerebral artery flow velocity and cortical glucose metabolism in the early phase of Alzheimer's disease. Dementia. 1995;6(1):32–38. doi: 10.1159/000106919. [DOI] [PubMed] [Google Scholar]
- Giovannetti T, Bettcher BM, Libon DJ, Brennan L, Sestito N, Kessler RK. Environmental adaptations improve everyday action performance in Alzheimer's disease: Empirical support from performance-based assessment. Neuropsychology. 2007;21(4):448–457. doi: 10.1037/0894-4105.21.4.448. [DOI] [PubMed] [Google Scholar]
- Godbolt AK, Cipolotti L, Anderson VM, Archer H, Janssen JC, Price S, et al. A decade of pre-diagnostic assessment in a case of familial Alzheimer's disease: Tracking progression from asymptomatic to MCI and dementia. Neurocase. 2005;11(1):56–64. doi: 10.1080/13554790490896866. [DOI] [PubMed] [Google Scholar]
- Goldman WP, Price JL, Storandt M, Grant EA, McKeelJr DW, Rubin EH, Morris JC. Absence of cognitive impairment or decline in preclinical Alzheimer's disease. Neurology. 2001;56(3):361–367. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the epsilon 4 allele of the apolipoprotein E gene. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KA, Tulsky DS. The influence of IQ stratification on WAIS-III/WMS-III FSIQ-general memory index discrepancy base-rates in the standardization sample. Journal of the International Neuropsychological Society. 2001;7(7):875–880. [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Koss E, Horwitz B, Heston L, Schapiro M, et al. Longitudinal study of cerebral metabolic asymmetries and associated neuropsychological patterns in early dementia of the Alzheimer type. Archives of Neurology. 1990;47(7):753–760. doi: 10.1001/archneur.1990.00530070043010. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Howieson DB, Dame A, Sexton G, Kaye J. Longitudinal analysis of the effects of the aging process on neuropsychological test performance in the healthy young-old and oldest-old. Developmental Neuropsychology. 2000;17(3):323–337. doi: 10.1207/S15326942DN1703_3. [DOI] [PubMed] [Google Scholar]
- Houston WS, Delis DC, Lansing A, Jacobson MW, Cobell KR, Salmon DP, Bondi MW. Executive function asymmetry in older adults genetically at-risk for Alzheimer's disease: Verbal versus design fluency. Journal of the International Neuropsychological Society. 2005;11(7):863–870. doi: 10.1017/s1355617705051015. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Smith GE, Lucas JA, Petersen RC, Boeve BF, Kokmen E, Tangalos EG. Testing normal older people three or four times at 1- to 2-year intervals: Defining normal variance. Neuropsychology. 1999;13(1):121–127. doi: 10.1037//0894-4105.13.1.121. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Smith GE, Petersen RC, Boeve BF, Kokmen E, Tangalos EG. Diagnostic accuracy of four approaches to interpreting neuropsychological test data. Neuropsychology. 2000;14(2):163–177. doi: 10.1037//0894-4105.14.2.163. [DOI] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Bondi MW, Salmon DP. Do neuropsychological tests detect preclinical Alzheimer's disease: Individual-test versus cognitive-discrepancy score analyses. Neuropsychology. 2002;16(2):132–139. doi: 10.1037//0894-4105.16.2.132. [DOI] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Bondi MW, Salmon DP. Asymmetry in auditory and spatial attention span in normal elderly genetically at risk for Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology. 2005a;27(2):240–253. doi: 10.1080/13803390490515441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Lansing A, Houston W, Olsen R, Wetter S, et al. Asymmetries in global-local processing ability in elderly people with the apolipoprotein e-epsilon4 allele. Neuropsychology. 2005b;19(6):822–829. doi: 10.1037/0894-4105.19.6.822. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- Kramer JH, Nelson A, Johnson JK, Yaffe K, Glenn S, Rosen HJ, Miller BL. Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22(4):306–311. doi: 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange RT, Chelune GJ. Application of new WAIS-III/WMS-III discrepancy scores for evaluating memory functioning: Relationship between intellectual and memory ability. Journal of Clinical and Experimental Neuropsychology. 2006;28(4):592–604. doi: 10.1080/13803390590949304. [DOI] [PubMed] [Google Scholar]
- Lange RT, Chelune GJ, Tulsky DS. Development of WAIS-III General Ability Index Minus WMS-III memory discrepancy scores. Clinical Neuropsychology. 2006;20(3):382–395. doi: 10.1080/13854040590967586. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Jagust WJ, Fitzpatrick A, Carlson MC, DeKosky ST, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(2):159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: A systematic review. Journal of Alzheimer's Disease. 2007;12(1):23–35. doi: 10.3233/jad-2007-12104. [DOI] [PubMed] [Google Scholar]
- Martin A. Neuropsychology of Alzheimer's disease: The case for subgroups. In: Schwartz MF, editor. Modular deficits in Alzheimer-type dementia. Issues in the biology of language and cognition. Cambridge, MA: The MIT Press; 1990. pp. 143–175. [Google Scholar]
- Massman PJ, Doody RS. Hemispheric asymmetry in Alzheimer's disease is apparent in motor functioning. Journal of Clinical and Experimental Neuropsychology. 1996;18(1):110–121. doi: 10.1080/01688639608408267. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by beta-amyloid(1–40) Brain Research. 2002;958(1):210–221. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Satz P. Effect of repeated administration of a neuropsychological battery in the elderly. Journal of Clinical Psychology. 1991;47(6):790–801. doi: 10.1002/1097-4679(199111)47:6<790::aid-jclp2270470610>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Chichin E, Carter J, Burack O, Lantz M, Meier DE. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. Journal of American Geriatric Society. 2005;53(2):290–294. doi: 10.1111/j.1532-5415.2005.53116.x. [DOI] [PubMed] [Google Scholar]
- Orsini A, Trojano L, Chiacchio L, Grossi D. Immediate memory spans in dementia. Perceptual & Motor Skills. 1988;67(1):267–272. doi: 10.2466/pms.1988.67.1.267. [DOI] [PubMed] [Google Scholar]
- Paolo AM, Troester AI, Ryan JJ. California Verbal Learning Test: Normative data for the elderly. Journal of Clinical & Experimental Neuropsychology. 1997;19(2):220–234. doi: 10.1080/01688639708403853. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Martin A. Cognition in Alzheimer's disease: Disorders of attention and semantic knowledge. Current Opinion in Neurobiology. 1994;4(2):237–244. doi: 10.1016/0959-4388(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105–117. doi: 10.1097/01.nrl.0000252947.15389.a9. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR. Compensatory neural activity distinguishes different patterns of normal cognitive aging. Neuroimage. 2008;39(1):441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell EW. A multiple scoring method for the assessment of complex memory functions. Journal of Consulting and Clinical Psychology. 1975;43:800–809. [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, Thal LJ, et al. Alzheimer's Disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59(7):1022–1028. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]
- Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, et al. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology. 2008;70(19 Pt 2):1771–1777. doi: 10.1212/01.wnl.0000286941.74372.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer's disease: A growth mixture modeling analysis. Cortex. 2007;43(7):826–834. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Backman L. Canaries in a coal mine: Cognitive markers of preclinical Alzheimer disease. Archives of General Psychiatry. 2001;58(9):859–860. doi: 10.1001/archpsyc.58.9.859. [DOI] [PubMed] [Google Scholar]
- Smith GE, Pankratz VS, Negash S, Machulda MM, Petersen RC, Boeve BF, et al. A plateau in pre-Alzheimer memory decline: Evidence for compensatory mechanisms? Neurology. 2007;69(2):133–139. doi: 10.1212/01.wnl.0000265594.23511.16. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkaenen A, Vainio P, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: Correlation to visual and verbal memory. Neurology. 1994;44(9):1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Strite D, Massman PJ, Cooke N, Doody RS. Neuropsychological asymmetry in Alzheimer's disease: Verbal versus visuoconstructional deficits across stages of dementia. Journal of the International Neuropsychological Society. 1997;3(5):420–427. [PubMed] [Google Scholar]
- Theisen ME, Rapport LJ, Axelrod BN, Brines DB. Effects of practice in repeated administrations of the Wechsler Memory Scale Revised in normal adults. Assessment. 1998;5(1):85–92. doi: 10.1177/107319119800500110. [DOI] [PubMed] [Google Scholar]
- Troster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson's disease with dementia: Differentiation, early detection, and implications for ‘mild cognitive impairment’ and biomarkers. Neuropsychology Review. 2008;18(1):103–119. doi: 10.1007/s11065-008-9055-0. [DOI] [PubMed] [Google Scholar]
- Tuokko H, Garrett DD, McDowell I, Silverberg N, Kristjansson B. Cognitive decline in high-functioning older adults: Reserve or ascertainment bias? Aging and Mental Health. 2003;7(4):259–270. doi: 10.1080/1360786031000120750. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2006;12(5):707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda FJ. Neuroprotection and neurodegenerative disease. Journal of Clinical Neurosciences. 2002;9(1):4–8. doi: 10.1054/jocn.2001.1027. [DOI] [PubMed] [Google Scholar]
- van Norden AG, Fick WF, de Laat KF, van Uden IW, van Oudheusden LJ, Tendolkar I, et al. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 2008;71(15):1152–1159. doi: 10.1212/01.wnl.0000327564.44819.49. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wetter SR, Delis DC, Houston WS, Jacobson MW, Lansing A, Cobell K, et al. Deficits in inhibition and flexibility are associated with the APOE-E4 allele in nondemented older adults. Journal of Clinical and Experimental Neuropsychology. 2005;27(8):943–952. doi: 10.1080/13803390490919001. [DOI] [PubMed] [Google Scholar]
- Wetter SR, Delis DC, Houston WS, Jacobson MW, Lansing A, Cobell K, et al. Heterogeneity in verbal memory: A marker of preclinical Alzheimer's disease? Neuropsychology, Development and Cognition. Section B, Aging Neuropsychology and Cognition. 2006;13(3–4):503–515. doi: 10.1080/138255890969492. [DOI] [PubMed] [Google Scholar]
- Wilde N, Strauss E, Chelune GJ, Loring DW, Martin RC, Hermann BP, et al. WMS-III performance in patients with temporal lobe epilepsy: Group differences and individual classification. Journal of the International Neuropsychological Society. 2001;7(7):881–891. [PubMed] [Google Scholar]
- Woods SP, Scott JC, Conover E, Marcotte TD, Heaton RK, Grant I. Test-retest reliability of component process variables within the Hopkins Verbal Learning Test-Revised. Assessment. 2005;12(1):96–100. doi: 10.1177/1073191104270342. [DOI] [PubMed] [Google Scholar]