INTRODUCTION
Catechol-O-methyltransferase (COMT) is an enzyme which metabolizes catecholamines and catechol-estrogens in both the CNS and periphery. The discovery of a common functional genetic variant at codon 158 (val158met) (Lachman et al.,1996; Lotta et al.,1995) led to the observation that individuals who were homozygous for the val allele performed more poorly than other genotypic groups on tasks of executive function (Egan et al., 2001). The differences in performance have been attributed to localized function of COMT in the prefrontal system. Further these differences appear to contribute to individual differences in responses to stimulant drugs, such as d-amphetamine (Mattay et al, 2003). However, given the complex modulation and functional heterogeneity of frontal lobe systems, further evaluation of COMT val158met-related phenotypes is needed. Here, we examined additional measures of cognition, including lapses in attention and general measures of visuo-spatial-motor speed of processing, as well as self-reports of mood, both without a drug and in response to acute administration of d-amphetamine.
Dopamine (DA) is removed from the synapse in most parts of the brain by the dopamine transporter, but in the frontal cortex, the DA is cleared mainly by the catabolic enzyme COMT (Karoum et al., 1994). The substitution of methionine (met) for valine (val) at codon 158 in the COMT gene (COMT) leads to a lower enzymatic activity so that met/met carriers have higher synaptic levels of DA in the frontal cortex, which appears to improve their performance on measures of executive function and working memory (Mattay et al., 2003; Egan et al., 2001; Turnbridge et al., 2006). Both environmental factors and pharmacologic manipulations modify the effects of COMT val158met genotype on cognition. For example, years of education – one possible marker of socioeconomic status – interacts with the COMT val158met genotype such that met/met carriers' cognitive scores improve markedly with increasing years of education, whereas the scores of val/val individuals are only marginally influenced by years of education (Enoch et al., 2009). Interestingly, in one study (Mattay et al., 2003) the psychostimulant d-amphetamine, which increases synaptic DA levels, worsened executive function and working memory of met/met carriers, whereas it improved performance among val/val carriers. This was explained as an inverted “U” functional response curve so that performance is improved by modest increases in synaptic DA levels, but impaired when levels exceed a certain optimal level. The goal of the present study was to further characterize the function of COMT val158met by analyzing 1. Whether genotypic groups differ in drug free condition on measures of motor processing and attention, and 2. Whether genotypic groups differ on their responses to these measures following administration of d-amphetamine. We used two measures of cognition - the Digit Symbol Substitution Test (DSST; Wechsler, 1958), which provides a non-specific measure of visuo-spatial and motor speed-of-processing, and Deviation from the Mode (DevMod), a new measure of lapses in attention (de Wit 2009), derived from a simple reaction time task. We used a task measuring lapses in attention to obtain important information about moment-to-moment fluctuations in task performance.
In addition to these measures of cognitive function, we also evaluated mood states using Profile of Mood States (McNair et al., 1971) and personality, using Multidimensional Personality Questionnaire (Tellegen, 1982).
We hypothesized that the val/val carriers would perform more poorly than met/met carriers on lapses in attention and visuo-spatial-motor speed of processing tasks in the absence of pharmacologic manipulation, consistent with what has been reported on other measures of cognition. In addition, we hypothesized that val/val carriers would exhibit a greater improvement in performance after d-amphetamine than met/met carriers. We did not expect that these genotypic groups would differ in the mood-altering effects of d-amphetamine because these effects are not believed to be mediated in brain regions where COMT plays a major role (Volkow, 1997). Studies of this kind, investigating the relationships between genotype and responses to drugs, will help to explain inter-individual variability in responses to drugs, including drugs such as stimulants that are used in clinical settings. These studies will also help to identify the separate brain processes that mediate the cognitive and mood-altering effects of drugs.
METHODS
Participants
Healthy Caucasian male and female volunteers (N=161), ages 18-35 years were recruited by posters, advertisement and word-of-mouth referrals. In order to reduce variability related to tolerance or withdrawal from nicotine or caffeine, we excluded subjects who smoked more than 10 cigarettes per week or consumed more than three cups of coffee per day. All subjects completed a psychiatric screening interview based on DSM-IV criteria (American Psychiatric Association 1994), a psychiatric symptom checklist (SLC-90; Derogatis 1983), the Michigan Alcoholism Screening Test (MAST; Selzer 1971), and a health questionnaire with a detailed section on current and lifetime drug use. Subjects currently taking prescription medication, or who had an Axis I psychiatric disorder, a history of treatment for substance use disorder or a history of personal or legal problems related to drug use, or any current or past medical condition considered to be a contraindication to d-amphetamine (such as abnormal EKG or hypertension) were excluded from the study. Candidates had to speak English and have at least high school education. Body mass index limitations were 19 to 26kg/m2. Because women show a dampened response to d-amphetamine during the luteal phase of the menstrual cycle (White et al., 2002) they were scheduled to participate during the follicular phase only. Women who were pregnant or lactating, or planning to become pregnant during the study were excluded.
Design
This within-subject design study consisted of three sessions which were separated by at least 48 hours. Subjects received capsules containing placebo, d-amphetamine 10 mg and d-amphetamine 20 mg in counterbalanced order under double-blind conditions. A smaller subset of subjects also received a 5 mg dose, but these data are not reported here in order to maximize power to detect genotypic differences. d-amphetamine (Mallinkrodt, MO) was placed in size 00 capsules with dextrose filler. Placebo capsules contained dextrose only. The study was approved by The University of Chicago Institutional Review Board and was performed in accordance with the Helsinki Declaration of 1975.
Volunteers first completed an orientation session in which the study procedures were explained. They signed the consent form and then provided a blood sample for genotyping purposes. They completed self-questionnaires and practiced computerized tests used in the study. Subjects were instructed to abstain from taking drugs, including alcohol, 24 hours before each session and to fast from midnight the night before the sessions. In addition, they were instructed not to consume more nicotine or caffeine than usual 24 hours before and 12 hours after the start of each session.
The three experimental sessions were conducted from 09:00am to 1 pm and were separated by at least 48 hours. Before the start of every session, subjects gave urine and breath samples to verify their abstinence from alcohol and other drugs. They received a light breakfast and at 9:00 am their baseline cognitive (Digit Symbol Substitution Test - see below) and mood (Profile of Mood States -see below) states were assessed. Subjects were tested individually, and remained in a comfortably furnished room with television and reading material for the four-hour session. They could watch emotionally neutral movies and read during the sessions when measurements were not being taken but they were not allowed to study. At 09:30 am, subjects ingested a capsule containing d-amphetamine (10 or 20 mg) or placebo with a glass of water. For blinding purposes, they were informed the capsule might contain a stimulant, sedative, or placebo. Self-reported drug effect questionnaires and Digit Symbol Substitution Test (see below) were obtained 30, 60, 90, 150, and 180 min after ingestion of the capsule. They completed a simple reaction time task once (Deviation from the Mode – see below), 90 minutes following capsule administration, when d-amphetamine is expected to have the highest concentration in the blood. At 1:00 pm subjects left the laboratory. After completing all three sessions subjects were debriefed and paid.
Dependent Measures
The Profile of Mood States (POMS)
(McNair et al., 1971) is an adjective checklist that is sensitive to the effects of psychoactive drugs. We use a version of the POMS consisting of 72 adjectives commonly used to describe momentary mood states. Subjects indicate how they feel at the moment in relation to each of the 72 adjectives on a 5-point scale from not at all (0) to extremely (4). Eight clusters (scales) of items have been separated empirically using factor analysis (anxiety, depression, anger, vigor, fatigue, confusion, friendliness, elation). The value of each scale is determined by averaging the scores for the adjectives in that cluster. Two additional (non-validated) scales are derived from the other scales as follows: arousal = (anxiety + vigor) − (fatigue + confusion); positive mood = elation – depression.
Digit Symbol Substitution Test
(DSST; Wechsler 1958) is a pencil and paper test in which subjects are required to substitute a series of numbers and symbols within 90 seconds. The number of correct responses within 90 seconds is reported. One point is given for each correctly drawn symbol. DSST is a test of visuo-spatial and motor speed-of-processing and it has a considerable executive function component. It is frequently used as a sensitive measure of frontal lobe executive functions (Vilkki et al., 1991; Parkin et al., 1999). We used 8 versions of this task, in mixed order, to reduce practice effects.
Deviation from the Mode
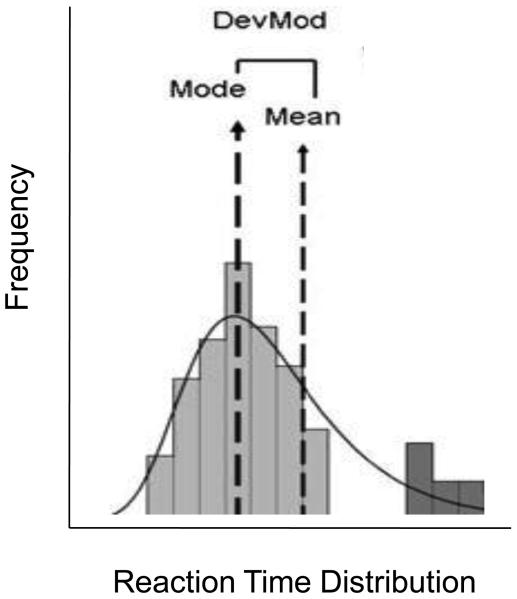
(DevMod; Leth-Steensen et al, 2000; de Wit, 2009; Spencer et al, 2009) is a measure of lapses in attention determined from the distribution of reaction times of a simple visual reaction time task (See Figure 1). A simple stimulus (a star) is presented briefly on the computer screen and the subject is required to press a mouse button as quickly as possible each time the stimulus appears. This is repeated 100 times. On average, the task takes 2-3 minutes to complete. Three summary measures are derived from the distribution of each individual's reaction times: the mean, the mode and the median. The measure of lapses of attention corresponds to the mean Deviation from the Mode (DevMod), or the mean of the difference between each RT and the mode (See Figure 1 for details). It provides a measure of skew, or unusually long RT's, because the mean is more sensitive to the outliers (i.e. lapses in attention) than the mode, which is generally unaffected by outliers. The mean DevMod is equivalent to the difference between the mean and the mode of a reaction time distribution. The larger the DevMod, the greater the proportion of long reaction times. The task has been validated in previous studies under both physiologic and pharmacologic challenge conditions (Child and de Wit, 2008; Acheson and de Wit, 2008. Acheson, Richards and de Wit, 2007; Spencer et al, 2009).
Figure 1.
Schematic of DevMod measuring lapses of attention. This figure shows the separation of the mode and the mean when there are long reaction times or ‘lapses in attention’. It shows that long reaction times change the mean while leaving the mode relatively unaffected. The difference between the mean and the mode provides a measure of the skew, and the deviation from the mode (DevMod) is considered a measure of inattention. Text and figure printed with permission from de Wit (2009).
Multidimensional Personality Questionnaire
(MPQ; Tellegen, 1982) is a self-report personality instrument designed to assess three broad traits: Positive Emotionality (Extraversion), Negative Emotionality (Neuroticism) and Constraint (Constraint-Impulsivity). In this analysis we had a priori hypothesis that COMT genotypic groups would differ on Extraversion scale (Positive Emotionality) of MPQ due to a finding by Stein et al (2005) that individuals who are homozygous for the met allele are more likely to score low on extraversion in comparison to individuals with the val allele. Analysis of the MPQ in twins suggests that scores on all three higher-order scales are influenced by moderate to strong genetic factors (Tellegen et al., 1988).
Genotyping
Genotyping was performed using the Addictions Array (Hodgkinson CA., et al 2008) based on the Illumina GoldenGate platform. Arrays were imaged using an Illumina Beadstation GX500 and the data analyzed using GenCall v6.2.0.4 and GTS Reports software v5.1.2.0 (Illumina). Criteria for sample exclusion and classification as genotyping failure were previously described (Hodgkinson CA et al., 2008).
Population Stratification
To examine potential population stratification, we genotyped all subjects participating in the study for 186 ancestry markers (AIMs) that were included on an Illumina array (Hodgkinson et al. 2008). We then ran STRUCTURE (Prichard et al., 2000) which identifies subpopulations of individuals who are genetically similar through a Markov chain Monte Carlo sampling procedure using markers selected across the genome.
Statistical Analysis
Subjects were categorized into three COMT val158met groups: met/met carriers, met/val carriers or val/val carriers. The three genotypic groups were compared on demographic and personality measures assessed in this study including gender, BMI, education in years, age, current and lifetime substance use, and personality, using ANOVA for continuous measures or X2 tests for categorical measures. If we found that possible confounding variables of demographic factors were associated with outcome measures in this analysis their effect was removed by including them as covariates in further statistical analyses.
Comparison of genotypic groups on measures obtained during experimental sessions, without drug
We calculated the mean baseline mood and DSST score for individual genotypic group by averaging precapsule scores for each session (ie. placebo, d-amphetamine 10mg and d-amphetamine 20mg). Since DevMod was assessed only once during each session (at 90 minutes following d-amphetamine administration), genotypic groups were compared using data from the placebo session. The three genotypic groups were compared using One Way ANOVA. Post hoc analyses were conducted using t-tests and the p-value was set at p≤0.05 (two-tailed) for all analyses.
Comparison of genotypic groups, in response to d-amphetamine
Responses to d-amphetamine in the three genotypic groups were compared by calculating the area under the curve (AUC) for the placebo, 10 and 20 mg d-amphetamine sessions, using two way ANOVAs or ANCOVAs (if we found a significant effect of covariates). Area under the curve was calculated by multiplying the average of each pair of consecutive observations by the corresponding time interval and then summing all such values starting with the first time point and ending with the last, as described in Matthews et al (1990).
When significant gene-drug interactions were obtained post hoc analyses were conducted using t-tests to determine which groups differed, at which drug doses. The p-value was set at p≤0.05 (two-tailed) for all analyses.
Relationship between DSST and DevMod
In order to investigate how brief lapses in attention relate to more general measures of cognitive performance according to the genotype, we examined correlations between DSST and DevMod 1.) in the drug free condition (ie placebo condition) and 2.) in response to d-amphetamine (10 mg and 20 mg).
RESULTS
Subjects
Table 1 summarizes subject demographics and self-reported personality for the overall sample. On average, subjects were in their early twenties, with either some college education or a college degree. They consumed moderate amounts of caffeine and alcohol, and their lifetime illicit drug use was typical for individuals of college age. Despite similarities on these measures, the val/val carriers were younger than either val/met or met/met carriers. Age was included as a covariate in all analyses. Caucasian ancestry was confirmed in all participants. COMT val158met differed on Positive Emotionality scale (Extraversion) (F(2,147)=4.2, p≤0.05). Post hoc comparisons revealed that val/val carriers scored higher than met/met carriers and val/met carriers (p≤0.01 for both; Table 1).
Genotype frequencies
This sample of subjects consisted of 36 val/val carriers, 72 val/met carriers and 53 met/met carriers. This genotype frequency is in Hardy Weinberg Equilibrium (HWE).
Profile of Mood States
COMT val158met genotypic groups did not differ in their rating of mood in the drug-free condition on either of the POMS composite scales (Arousal: F(2,153)=2.61, ns; Positive Mood F(2,153)=2.41, ns). Further, although d-amphetamine produced typical effects on mood in the group as a whole (Arousal: F(2,300)=56.1, p<0.001; Positive Mood F(2,300)=38.1, p<0.001), the genotypic groups did not differ on these responses (Arousal x COMT genotype interaction F(4,296)=0.15, p=ns; Positive Mood x COMT genotype interaction F(4,296)=0.96, p=ns).
Digit Symbol Substitution Test
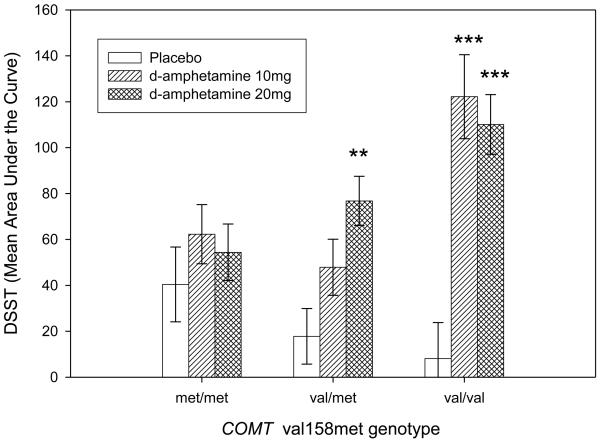
One subject's DSST data were lost. Three subjects' data were outliers. Their AUC values on the placebo session and their genotypes were: 445-met/met, 461.5-val/met and -366.5-met/met. After their removal the data were normally distributed. Important demographic factors such as age, gender and body mass index did not influence performance on DSST either in a drug free state or in response to d-amphetamine. Table 2 lists mean (SD) for all the timepoints across all three sessions. Genotypic groups did not differ on DSST in the drug free state (F(2, 146)=.589, ns). However, there was an interaction between genotype and d-amphetamine on DSST (F(4,290) = 3.2; p≤0.05) as shown in Figure 2. The interaction reflects a significant improvement in performance after d-amphetamine administration in the val/val and val/met carriers, but no similar improvement in the met/met group.
Figure 2.
Mean Area Under the Curve±SEM of subjects' performance on the Digit Symbol Substitution Test according to COMT val158met genotype. The groups did not differ significantly on the placebo session, but d-amphetamine 10 mg (***p≤0.001) and d-amphetamine 20 mg (***p≤0.001) improved performance in the val/val carriers (N=36). D-Amphetamine (20 mg) improved performance in the val/met (N=72) carriers (**p≤0.01) whereas the drug did not change performance in the met/met carriers (N=53).
Deviation from the Mode
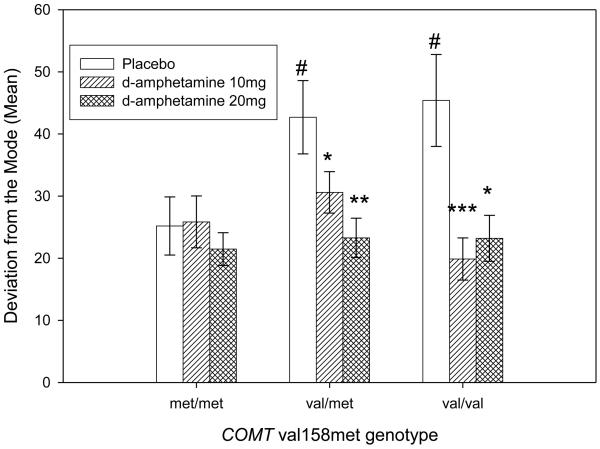
This group of subjects consisted of a smaller sample size including 22 val/val carriers, 49 val/met carriers and 28 met/met carriers due to the fact that DevMod was only tested in a subset of the subjects. Allele frequencies were in HWE for this subset of the sample as well. One subject's score was excluded for being an extreme outlier in the d-amphetamine 20mg session (z score=7.4). The groups were similar on all demographic measures except that val/val carriers were slightly younger (mean age=21.2, SEM=0.5) than the val/met carriers (mean age =23.5, SEM=0.5). We included age as a covariate in analyses of DevMod. Of all the demographic variables studied, only caffeine use was associated with DevMod; therefore caffeine use was included as a covariate. Table 3 provides mean (SD) reaction times for each genotype in each of the three conditions. We first evaluated the performance of each genotypic group in the drug free state (i.e. placebo condition). The genotypic groups differed in their performance on DevMod (F(2,94)=3.21; p≤0.05). Post hoc comparisons indicate that, in comparison to met/met carriers, val/met carriers and val/val carriers had higher DevMod in the placebo condition (p≤0.05 for both). We detected an interaction between amphetamine dose and genotype (F(4,188) = 2.83; p≤0.05). Post hoc comparisons showed that both doses of d-amphetamine improved performance in val/met carriers (10mg p≤0.05; 20 mg p≤0.01) and val/val carriers (10mg p≤0.001; 20mg p≤0.05), but neither dose of d-amphetamine improved performance for the met/met carriers.
Thus, like the DSST, we observed an improvement in performance in the val/val and val/met but not in the met/met groups following administration of d-amphetamine (Figure 3).
Figure 3.
Mean±SEM of subjects' performance on the DevMod according to COMT val158met genotype. In the absence of any drug, val/met carriers and val/val carriers had higher DevMod in comparison to met/met carriers (#p≤0.05 for both). D-Amphetamine (10 mg and 20 mg) decreased lapses in attention in the val/val carriers (N=22; ***p≤0.001 and *p≤0.05 respectively). D-Amphetamine (10 and 20 mg) also decreased lapses in attention for val/met carriers (N=49) *p≤0.05 and **p<.01 respectively). The drug did not change DevMod performance in the met/met carriers (N=28).
Relationship between DSST and DevMod
In most comparisons DSST and DevMod were not related. However, in the val/met genotype group, DSST and DevMod scores were negatively correlated in the drug free condition (r2=−0.323, p=0.025).
CONCLUSIONS
In summary, we found that val/val and val/met carriers of COMT val158met polymorphism showed more lapses in attention in a drug-free state and a greater improvement in general cognition following administration of d-amphetamine. We also found that val/val carriers scored higher on the personality trait of extraversion compared to val/met and met/met carriers. The groups did not differ in mood states, either in a drug free state or following administration of d-amphetamine.
Our finding that homozygotes for the met allele of the COMT val158met genotypic performed better in the drug-free condition on the measure of attention (i.e. lapses in attention) is consistent with previous research. Mattay (2003) reported that healthy volunteers with the met/met alleles performed better on measures of working memory and executive function, and Egan (2001) reported that schizophrenic met/met patients had better executive function. In our study met/met carriers of COMT val158met had fewer lapses in attention on DevMod in comparison to val/val and val/met carriers. On the other hand, the three genotypic groups did not perform differently on a general measure of cognition (DSST) despite previous reports that working memory and executive function, also dependent on prefrontal cortex functioning, are mediated by the COMT val158met genotype. This suggests that the DSST may not be sensitive to the deficits related to COMT function, but also that the impairments in individuals with the val allele may be relatively modest.
Most cognitive tasks involve more than a single underlying process, and brief lapses in attention, such as those measured here, might contribute to more general measures of cognitive performance. For example, it has been proposed that momentary lapses in attention can disrupt goal-oriented behavior (Czeisler et al., 2005) in both healthy individuals (Dockree et al, 2006) and clinical syndromes such as Attention Deficit Hyperactivity Disorder (Castellanos et al., 2005; Reimer et al., 2005). Our results provide some support for this idea. Although the DevMod was not related to DSST performance in most subjects, the two measures were inversely correlated in the val/met group. In that group, individuals who exhibited more lapses in attention performed worse on our measure of general psychomotor performance, supporting the idea that attention can affect general cognitive function. Further studies are needed to provide more information about how lapses in attention relate to more general measures of cognition.
Consistent with previous studies, we found that d-amphetamine improved performance on the DSST and the DevMod measures only in the val/val and val/met individuals. Mattay et al (2003) also showed that amphetamine preferentially improved working memory and executive function in val/val carriers. However, in the Mattay et al (2003) study, amphetamine did not improve performance in the heterozygotes (val/met), whereas in our study amphetamine reduced lapses in attention and improved performance on DSST in val/met carriers. In addition, met/met carriers in the Mattay et al (2003) study exhibited decrements in performance after amphetamine, whereas amphetamine did not affect either DSST or DevMod in met/met carriers in our study. It may be that the larger sample size (27 versus 161) in our study accounts for these differences.
It is notable that the genotypic groups in our study differed on measures of cognition but not on mood-altering effects of d-amphetamine. Previous studies have not focused on the COMT val158met genotype in relation to subjective ratings of mood, either in drug-free condition or following stimulant administration. However, the dissociation between cognitive and mood effects of the drug, in relation to COMT suggests that these effects might be mediated by different brain areas. Catechol-O-methyltransferase might be expected to have a greater impact on cognition, which is dependent on cortical function, but not mood, which is thought to depend more on the actions of DA in the striatum. Consistent with this, we have previously reported genotypic differences in subjective ratings of amphetamine in relation to polymorphism in function of the dopamine transporter (Lott et al., 2005) and norepinephrine transporter (Dlugos et al., 2007), which are thought to have greater influence on dopaminergic function in striatal relative to prefrontal brain regions.
We found that the COMT val158met genotype is associated with extraversion, but not neuroticism or constraint-impulsivity. In our analysis we used Multidimensional Personality Questionnaire – a self-reported measure of personality known to be influenced by moderate to strong genetic factors (Tellegen et al., 1988). Our results showed that val/val carriers scored higher on a measure of extraversion, compared to the met/met and val/met carriers. This is consistent with the work published by Stein et al (2005) who found that among female college students, met/met carriers scored lower on extraversion compared to val/met or val/val carriers. Although we did not observe sex differences in our sample, the direction of the genotypic-personality association was the same as the Stein et al (2005) study.
Although we appear to have had enough power to detect significant effects of COMT val158met, the sample may not have been powerful enough to detect more subtle differences between the groups. For example, previous research indicates that val/val carriers perform more poorly than other genotypes on tasks measuring executive function and working memory in a drug-free state (for a review see Turnbridge et al., 2006), whereas we failed to observe group differences on the DSST, a task of general cognition (Vilkki et al., 1991; Parkin et al., 1999). It is possible that we would have detected an effect of genotype on with a larger number of participants. Similarly, insufficient power might have prevented us from observing previously reported sex differences in extraversion or emotionality (Stein et al, 2005; Hettema et al, 2008). In our analyses we failed to observe sex differences, either in the relationship between the MPQ and genotype or mood changes in response to drug and genotype. Finally, the sensitivity of the DSST task may have been influenced by practice effects from administering the task repeatedly. Although there was some evidence of improvement across administrations of the task within the placebo session, performance was stable across the three sessions.
The results of this study extend our knowledge of how the COMT val158met polymorphism affects behavior, both in the drug free state and after administration of amphetamine. While previous research focused on measures of cognition, including working memory and executive function, this is the first study, to our knowledge, which showed that inattention, or lapses in attention, is dependent on COMT val158met genotype in both the drug-free state and in response to amphetamine. In addition, our results show beneficial effects of amphetamine on sustained attention as well as visuo-spatial-motor speed of processing for val/val and val/met carriers, but do not support the idea that the stimulant has detrimental effects in met/met carriers. Finally, the results indicate that the cognitive effects of d-amphetamine may involve different brain mechanisms than the mood-altering effects of the drug, since we failed to detect associations between COMT genotype and amphetamine-induced mood states. Our results extend our understanding of the mechanisms involved in individual differences in sustained attention in the absence of any drug. They also add to our understanding of individual differences in responses to a stimulant drug that is used clinically to inhibit inappropriate behavior.
Acknowledgments
We would like to thank Patricia Kriegel, Margo Meverden and Christian Peters for their skillful technical assistance. This work was supported by F32DA024920-02, DA021336, DA02812 and MO RR00055.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Exp Clin Psychopharmacol. 2008;16(2):113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–587. doi: 10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Castellanos F, Sonuga-Barke E, Scheres A, et al. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H. Enhanced mood and psychomotor performance by a caffeine-containing energy capsule in fatigued individuals. Exp Clin Psychopharmacol. 2008;16(1):13–21. doi: 10.1037/1064-1297.16.1.13. [DOI] [PubMed] [Google Scholar]
- Czeisler C, Walsh J, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorders. N Engl J Med. 2005;353:347–486. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L. SLC-90-R Manual II. Clinical Psychometric Research; Towson, MD: 1983. [Google Scholar]
- Dlugos A, Freitag C, Hohoff C, McDonald J, Cook EH, Deckert J, et al. Norepinephrine transporter gene variation modulates acute response to d-amphetamine. Biol Psychiatry. 2007;61:1296–1305. doi: 10.1016/j.biopsych.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Dockree P, Bellgrove M, O'Keeffe F, et al. Sustained attention in traumatic brain injury (TBI) and healthy controls: enhanced sensitivity with dual task load. Exp Brain Res. 2006;168:218–229. doi: 10.1007/s00221-005-0079-x. [DOI] [PubMed] [Google Scholar]
- Egan M, Goldberg T, Kolachana B, Callicott JH, Mazzanti C, Straub R, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. COMT Val158Met and cognition: main effects and interaction with educational attainment. Genes Brain Behav. 2009;8(1):36–42. doi: 10.1111/j.1601-183X.2008.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, An SS, Bukszar J, Van den Oord E, Neale MC, Kendler KS, et al. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol Psychiatry. 2008;64(4):302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan QP, Xu K, Shen PH, Heinz E, Lobos EA, et al. Addictions biology: Haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43(5):505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoum F, Chrapusta S, Egan M. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Lachman H, Papolos D, Saito T, Yu Y, Szumlanski L, Weinshilboum M. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz Z, Douglas V. Mean response times, variability and skew in the responding of ADHD children: A Response time distributional approach. Acta Psycho. 2000;104:167–90. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lott D, Kim S, Cook E, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Mele'n K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay V, Goldberg T, Fera F, Hariri A, Tessitore A, Egan MF, et al. COMT val158met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Parkin A, Java R. Deterioration of frontal lobe function in normal aging: influences of fluid intelligence versus perceptual speed. Neuropsychology. 1999;13:539–545. doi: 10.1037//0894-4105.13.4.539. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer B, D'Ambrosio L, Gilbert J, et al. Behavior differences in drivers with attention deficit hyperactivity disorder: The driving behavior questionnaire. Accid Anal Prev. 2005;37:996–1004. doi: 10.1016/j.aap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Selzer M. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Spencer S, Hawk L, Richards J, Shiels K, Pelham W, Waxmonsky J. Stimulant Treatment Reduces Lapses in Attention among Children with ADHD: The Effects of Methylphenidate on Intra-Individual Response Time Distributions. J Abnorm Child Psychol. 2009;37(6):805–816. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Fallin M, Schork N, Gelernter J. COMT Polymorphisms and Anxiety-Related Personality Traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Tunbridge E, Harrison P, Weinberger D. Catechol-o-Methyltransferase, Cognition,and Psychosis: Val158Met and Beyo. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Multidimensional Pesonality Questionnaire manual. University of Minnesota Press; Minneapolis, MN: 1982. [Google Scholar]
- Tellegen A. The analysis of consistency in personality assessment. J Person. 1988;56:621–663. [Google Scholar]
- Turnbridge E, Harrison P, Weinberger D. Catechol-o-methyltransferase: Cognition and psychosis Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Vilkki J, Holst P. Mental programming after frontal lobe lesions: results on digit symbol performance with self-selected goals (1991) Cortex. 1991;27:203–11. doi: 10.1016/s0010-9452(13)80124-4. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154:50–65. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- White T, Justice A, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Measure and Appraisal of Adult Intelligence. Williams and Wilkins; Baltimore: 1958. [Google Scholar]