Abstract
Very little remains known about the regulation of human organ stem cells (in general, and during the aging process), and most previous data were collected in short-lived rodents. We examined whether stem cell aging in rodents could be extrapolated to genetically and environmentally variable humans. Our findings establish key evolutionarily conserved mechanisms of human stem cell aging. We find that satellite cells are maintained in aged human skeletal muscle, but fail to activate in response to muscle attrition, due to diminished activation of Notch compounded by elevated transforming growth factor beta (TGF-β)/phospho Smad3 (pSmad3). Furthermore, this work reveals that mitogen-activated protein kinase (MAPK)/phosphate extracellular signal-regulated kinase (pERK) signalling declines in human muscle with age, and is important for activating Notch in human muscle stem cells. This molecular understanding, combined with data that human satellite cells remain intrinsically young, introduced novel therapeutic targets. Indeed, activation of MAPK/Notch restored ‘youthful’ myogenic responses to satellite cells from 70-year-old humans, rendering them similar to cells from 20-year-old humans. These findings strongly suggest that aging of human muscle maintenance and repair can be reversed by ‘youthful’ calibration of specific molecular pathways.
Keywords: satellite cell, muscle, aging, Notch, MAPK/ERK
INTRODUCTION
The rate of metabolism and cumulative oxidative damage to DNA and proteins, as well as genomic instability and mutations to mitochondrial DNA, have all been implicated in determining the intrinsic rate of cell aging and ultimately, species' life-span (Cevenini et al, 2008; Vijg & Campisi, 2008). Interestingly, recent studies have delineated that the aging process in organ stem cells is largely caused by age-specific changes in the differentiated niches, and that regenerative outcomes often depend on the age of the niche, rather than on stem cell age (Grounds, 1998). It was further established that, despite the deteriorated repair of old tissues (such as muscle), old tissue organ stem cells are capable of productive regenerative responses when exposed to young extrinsic milieu (myofibres or blood sera) (Carlson & Conboy, 2007; Conboy et al, 2005).
At the mechanistic level, our work in the mouse model defined that injury to myofibres, induces expression of the Notch ligand Delta, which thereby acts as a positional cue to activate Notch in satellite cells and causes them to break quiescence and to proliferate (Conboy & Rando, 2002; Conboy et al, 2003). Activation of Notch also prevents premature differentiation of satellite cells into fusion-competent myoblasts, through inhibition of the Wnt pathway, which promotes myogenic differentiation (Brack et al, 2008). With age, however, Notch activation becomes lacking due to diminished Delta expression in myofibres and in satellite cells. Such decline in Notch activation is further compounded by excessive TGF-β/phospho-Smad (pSmad), causing an accumulation of cyclin-dependant kinase (CDK) inhibitors in muscle stem cells and preventing their regenerative responses (Carlson et al, 2008a; Conboy et al, 2003).
Age-specific muscle atrophy and lack of old tissue repair are common between mice and humans, but virtually nothing is known about the cellular and molecular determinants of human muscle stem cell behaviour in general, or with respect to aging. Additionally, most animal models study muscle repair after extreme types of injury (e.g. cardiotoxin injection), which is different from physiological attrition and regeneration of human muscle, such as through physical activity.
In this work, we sought to identify the molecular determinants of muscle regeneration, and their age-specific changes in humans, via a physiological model of acute exercise following muscle atrophy. The results shown here define, in cellular and molecular terms, how muscle stem cell responses are regulated in young individuals and which age-specific changes account for the attrition, diminished regeneration and poor muscle function manifested in old age. Our findings reveal that while a diversity of changes are caused by the aging process, the mechanisms controlling muscle stem cell responses and their age-specific alternations are evolutionarily conserved between humans and mice. Specifically, the decline in human satellite cell performance with age is extrinsic, and is imposed by their myofibre niches through Notch and TGF-β/pSmad imbalance. Confirming and extrapolating these data further, we established that mitogen-activated protein kinase (MAPK)/phosphate extracellular signal-regulated kinase (pERK) pathway is both important for activation of Notch in human satellite cells, and becomes down-regulated in human muscle with age. These findings provide a previously unknown molecular explanation to the age-specific decline of Notch activation in the muscle compartment. In its sum, this work identifies key mechanisms responsible for healthy maintenance and repair of human skeletal muscle, and clarifies in molecular terms, why organ repair becomes inadequate in older individuals. This work has theoretical, as well as translational significance for understanding human aging and for enhancing old human organ repair.
RESULTS
In order to compare muscle regeneration success and functional recovery between young (∼20 year old) and aged (∼70 year old) individuals, myofibre atrophy was induced by immobility (cast application for two weeks). This followed by acute exercise (loading) of skeletal muscle after cast removal (for 3 days and for 4 weeks), which aimed to promote muscle regeneration and functional improvement in strength and agility. Muscle biopsies were collected prior to immobility (basal level), after 2 weeks of immobility (induced atrophy), 3 days after cast removal (initiation of regeneration and functional recovery) and at 4 weeks after cast removal (ongoing regeneration and functional recovery). The scheme of this experimental setup is depicted in Fig 1A.
Figure 1. Immobility-induced muscle atrophy causes an age-specific increase in degeneration and lack of myogenic recovery.
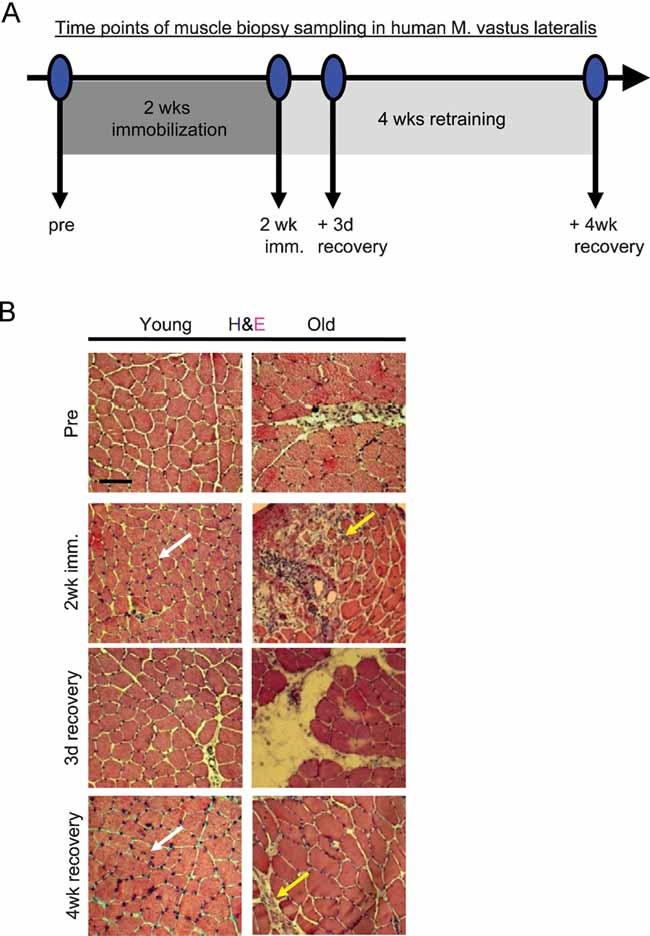
- Scheme of experimental setup, as described in text.
- Muscle histology from resting state (pre), immobility-atrophy (2 week imm.) and loading-recovery (3 day recovery, 4 week recovery) was analysed by haematoxylin and eosin (H & E) of 10-µm skeletal muscle cryosections. During immobilization phase of the study, areas of severe degeneration and scar tissue formation were evident in old muscles (yellow arrows) versus healthy maintenance of young immobilized muscles (white arrows). Scale bar = 100 µm. n = 10.
To determine whether muscle maintenance was age-dependent under the conditions of mobility, immobility-atrophy and loading-recovery, we analysed 10 µm cryosections derived from the young and old muscle biopsies at the indicated time points. As shown in Fig 1B, the muscle histology was markedly different between young and aged individuals, in the basal (‘Pre’) state (prior to immobility) and was particularly different during the immobility and recovery periods of immobility-induced atrophy. As compared to young, the old human muscle fibres were uneven in size and less numerous before immobility (Pre). Old myofibres underwent severe degeneration during immobility, as compared to mild degeneration of young myofibres (2 weeks). Additionally, old myofibres, but not young ones, exhibited a persistent inflammatory response and scar formation at both 3 days and 4 weeks of recovery (Fig 1B). The immobility-caused myofibre degeneration in old individuals was highly pronounced and similar to pathological degenerating muscle, with its typical clusters of new embryonic myosin heavy chain (eMyHC+) myofibres and broken sarcolemma, evidenced by uneven pattern of dystrophin, e.g. in cases of congenital myopathies (Renault et al, 2002; Straub & Bushby, 2006) (Fig S1A and B of Supporting Information). In contrast, acutely deteriorating muscle clusters were absent, and intact dystrophin+ sarcolemma was typical in young muscles, thus suggesting better tissue maintenance during immobility (Fig S1 of Supporting Information). These data determine that both maintenance of immobilized skeletal muscle and regeneration of atrophic myofibres after cast removal, become inefficient in older individuals, manifested as the replacement of functional tissue by fibrotic scar tissue (Fig S1C–F of Supporting Information).
In accordance with these findings, based on the quantification of quadricep muscle cross-sectional area (MRI), old muscle fibres were much smaller than young in the ‘pre’ state, and as compared to young, the size of old muscle fibres was not efficiently recovered, following cast removal and exercise (Fig S2A of Supporting Information). These observations are consistent with published literature and animal studies (Brown & Hasser, 1996; Degens & Alway, 2003; Machida & Booth, 2005; Pistilli et al, 2007). The histology and muscle size data were further confirmed and extrapolated by functional studies on muscle concentric/isometric strength and total muscle contraction work (Fig S2B–D of Supporting Information)–establishing that while both young and aged individuals recovered close to basal levels of these functional parameters, by exercising after immobility, old muscles always remained weaker than young.
The age-specific decline in muscle fibre maintenance, repair, size and strength under all studied conditions (i.e. during normal muscle use and during recovery from the immobility-induced atrophy) could result from known age-specific alterations in many parameters, such as innervation and vascularization, as well as the lack of muscle fibre regeneration (Grounds, 1998; Thomas, 2001; Wagers & Conboy, 2005). Based on our work in the animal model, we hypothesized that a decline in the maintenance and repair of the muscle functional unit (myofibre maintenance via resident satellite cells) is a main factor causing the lack of old human muscle regeneration, strength and agility. Thus, we next examined whether the diminished size of aged myofibres, and their lack of regeneration, could be caused by an age-specific physical loss of muscle stem cells and/or by an age-specific decline in satellite cell activation.
Certain controversy exists in the published literature, with respect to the age-specific decline in numbers of satellite cells. Some studies report diminished numbers of these cells in older animals and humans, while other published data argues against such a decline (Collins et al, 2007; Conboy et al, 2003; Renault et al, 2002; Schultz & Lipton, 1982; Shefer et al, 2006). Our data suggest that in mice, there is no significant decline in the number of quiescent satellite cells with age, but the ability of these cells to expand in response to traumatic muscle injury (induced by cardiotoxin or dry ice) declines due to the lack of Notch activation (Carlson et al, 2008a; Conboy et al, 2003). Consequentially, the numbers of satellite cells that are activated by injury to expand and produce proliferating fusion-competent myoblasts dramatically decline in old mouse muscle (Carlson & Conboy, 2007; Carlson et al, 2008a; Conboy et al, 2003).
Different experimental systems and standards of what is considered to be a satellite cell are used in the field. Additionally, cardiotoxin and dry ice are not physiological agents of human muscle repair and remodelling. We therefore decided to clarify the situation by using our model of human myofibre regeneration after atrophy, and compare the number of quiescent muscle satellite cells associated with undamaged myofibres with the number of satellite cells activated by myofibre deterioration. Satellite cell numbers were quantified, using immuno-detection of three different markers: paired box gene 7 (Pax7), neural cell adhesion molecule (NCAM) and muscle-cadherin (M-Cadherin), in young and old human skeletal muscle cryosections.
As shown in Fig 2A–C, aging produces a ∼2-fold decline in the number of satellite cells, endogenous to old muscle in the basal state. The number of old myogenic cells increases slightly during old muscle immobility (2 weeks), which is consistent with the ongoing degeneration and attempts at regeneration of old tissue, shown in Fig 1B and Fig S1 of Supporting Information. Importantly, there was a very pronounced, ∼4-fold age-specific decline in the expansion of satellite cells in response to exercise after the immobility-induced atrophy (3 days and 4 weeks), Fig 2A–C. The age-specific decline in numbers of Pax7 myogenic cells during exercise after immobility was also confirmed by Western blotting (Fig 2D, E). These data establish that stem cell activation significantly declines with age in humans, which may contribute to the lack of muscle maintenance and repair, and to the replacement of myofibres by fibrous scar tissue in old people (Fig 1, Fig S1 of Supporting Information).
Figure 2. Pronounced lack of myogenic cell expansion is detected in old human muscle that undergoes exercise after immobility.
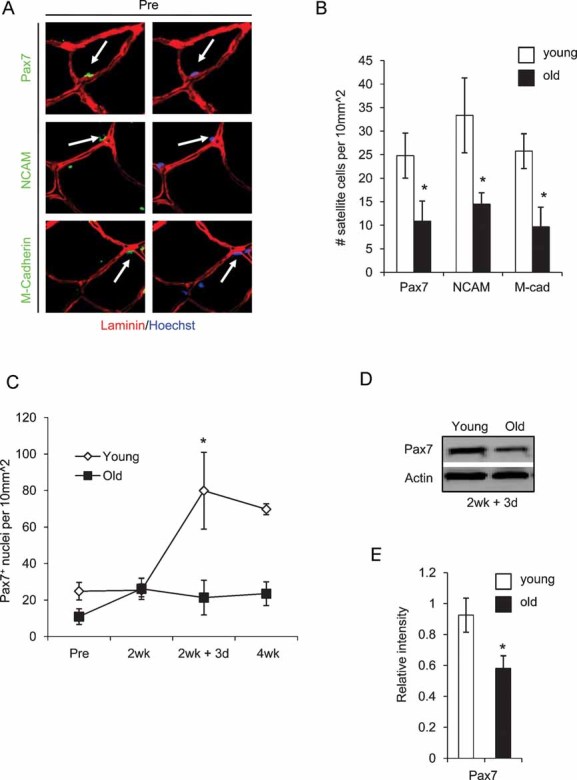
A. Skeletal muscle cryosections were immunostained for Pax7, M-Cadherin and NCAM (green). Laminin immuno-detection is shown in red and Hoechst labels nuclei (blue). Sublaminar mono-nucleated cells expressing these markers were identified in association with both young and old myofibres.
B. The total numbers of Pax7, NCAM and M-Cadherin+ satellite cell nuclei were quantified per 10 mm2 for both young and old muscles. *P ≤ 0.05 (old compared to young at ‘pre’ condition).
C. Total number of Pax7+ nuclei were analysed at the pre and 2 week (resting and immobilization phase) and the 2 week + 3 day and 4 week time points (regeneration phase). *, P ≤ 0.05 (young compared to old at 2 week + 3 day).
D, E.Western blotting for Pax7 was performed on whole muscle protein isolates (quantified in E), using actin as a loading control. *P ≤ 0.05, old compared with young. Data are means ± s.d. n = 10 for immunostained cryosections, n = 6 for Western blot analysis.
Previously, activation of Notch was determined to be indispensable for productive regeneration of young muscle, and capable of rescuing the repair of aged muscle in a mouse model of acute tissue injury (Carlson et al, 2008a, 2008b; Conboy et al, 2003; Conboy & Rando, 2002). Therefore, we set to determine whether (1) active Notch is present in young human satellite cells and becomes down-regulated in old satellite cells and (2) Notch ligand Delta is expressed at higher levels in young human regenerating muscle, as compared to old.
To establish whether Notch activation is lacking in old human satellite cells associated with aged muscle in vivo, we performed Notch and Pax7 co-immunodetection experiments in cryosections of human muscle biopsies. As shown in Fig 3A (and Fig S3 of Supporting Information), nuclear active Notch is eagerly detected in Pax7+ myofibre-associated cells. Furthermore, we also found that levels of the Notch ligand Delta are diminished in old myofibres, as compared to young myofibres (Fig 3B). In agreement with these data, decline in active Notch and its ligand Delta is observed in Western blot analysis of young and old human muscle (Fig 3C, D).
Figure 3. Notch activation and Delta upregulation is diminished in regenerating old human skeletal muscle.
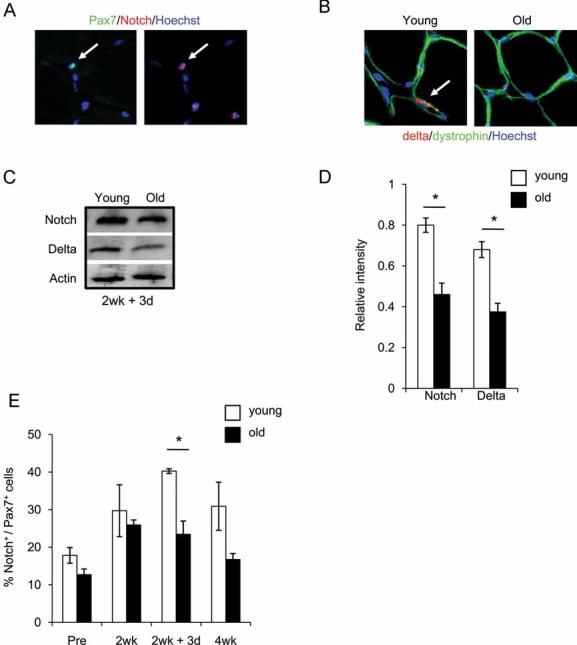
A. Cryosections were analysed by immunostaining for co-expression of nuclear Pax7+/active Notch in resident satellite cells.
B. Delta (red) and dystrophin (green) immuno-detection is shown for 10 µm skeletal muscle cryosections. Hoechst labels nuclei (blue).
C, D. Western blot of Notch and Delta levels on whole muscle protein isolates for 2 week + 3 day; quantified in D. *P ≤ 0.05, old compared with young for both Notch and Delta.
E. Quantification of Notch/Pax7 double-positive myofibre-associated cells from cryosections. Data are means ± s.d., n = 10–15 for immunodetection of cryosections. n = 6 for Western blotting analysis. As compared to young tissue, in old muscle loaded after immobility, there is significant down-regulation of Delta, active Notch and decline in numbers of myofibre-associated myogenic cells that co-express Pax7 and active Notch.
Notably, the expansion of myogenic cells in regenerating human muscle (during exercise after immobility) positively correlated with the levels of active Notch. The numbers of Pax7+/Notch active cells were low in the basal state and during immobility, but greatly increased at 3 days post-immobility in young, but not in old human muscle (Fig 3E). The numbers of Pax7+/Notch active human satellite cells declined after several weeks of regeneration, when the differentiation process typically follows initial cell expansion (Fig 3E (Collins et al, 2005; Wagers & Conboy, 2005)). Accordingly, while there were still more Pax7+/Notch active cells in the young, as compared to old human muscle at 4 weeks post-immobility, the total number of activated myogenic cells declined in both young and old tissue (Fig 3E). These results demonstrate that Notch regulation becomes altered during human aging in skeletal muscle, and suggests the importance of Notch for the expansion of human satellite cells.
To deepen the molecular understanding of the age-specific lack of organ stem cell responses in humans, we examined the activity of TGF-β/pSmad pathway in resting and regenerating young and old human skeletal muscle. In mice, the lack of Notch activation is compounded by an increase of TGF-β/pSmad3 that results in the regulation of CDK inhibitors, thus assuring loss of satellite cell regenerative capacity and deteriorated repair of old muscle (Carlson et al, 2008a; Conboy et al, 2003).
Remarkably, as shown in Fig 4, these molecular signatures of aging within the muscle stem cell compartment are conserved between mouse and human that suggests the fundamental significance of uncovered regulatory mechanisms. As compared to young, old human muscle fibres contain higher levels of TGF-β, which associates with the laminin-rich basement membrane of the satellite cell microniche (Fig 4A). Accordingly, levels of nuclear pSmad3 (the transcriptional factor that is activated by TGF-β signalling) are excessive in old human satellite cells, as compared to youngs (Fig 4B). To further confirm these results in a more quantitative way, we also performed Western blot analysis of young and old human muscles. As shown in Fig 4C (quantified in Fig 4D), the levels of TGF-β, pSmad3 and CDK inhibitors, p15 and p21 (known to be induced by TGF-β signalling and reduced by active Notch) are all higher in the old, as compared to young human muscle. Interestingly, p27 and p16 were undetectable in either young or old tissue, suggesting that these CDK inhibitors do not play a major role in studied processes. Efficient immuno-detection of p27 and p16 with the same antibodies was performed using positive control protein extracts (not shown).
Figure 4. Mechanisms of muscle stem cell aging are conserved between mice and humans, with respect to TGF-β signalling imbalance.
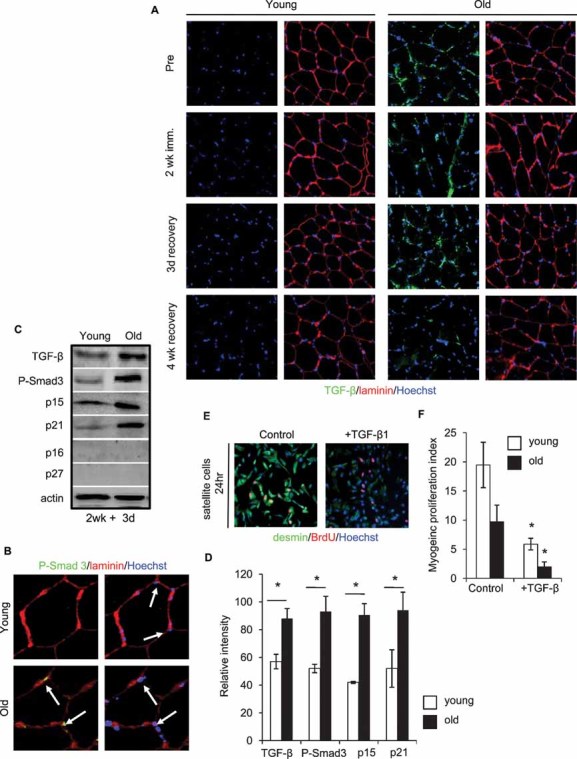
Immunodetection of:
A. TGF-β (green) and laminin (red),
B. P-Smad3 (green) and laminin (red) is shown for 10 µm skeletal muscle cryosections. Hoechst labels nuclei (blue).
C, D. Western blotting for TGF-β, P-Smad3, p15, p21, p16 and p27 from whole muscle protein lysates; quantified in D. Actin was used as loading control. *P ≤ 0.05, old compared with young. Significant age-specific elevation of TGF-β/pSmad and of CDK inhibitors, p15 and p21 was detected in old muscle as compared to young.
E, F. Activated satellite cells were cultured for 24 h in OPTI-MEM containing age-matched human sera in the presence of 25 ng/ml recombinant TGF-β1. Myogenic responses were analysed and quantified F, based on the co-expression of desmin/BrdU. Data are means ± s.d., n = 10–15 for immunodetection of cryosections, n = 6 for Western blotting analysis, n = 6 for myogenic culture experiments.
To examine the effects of TGF-β on myogenic properties of human muscle stem cells, exogenous molecule was added to young and old human satellite cells in culture. Myogenic capacity was determined, based on number of fusion-competent, proliferating myoblasts, e.g. cells that rapidly (in 2 h) incorporate bromodeoxyuridine (BrdU), co-express desmin and myogenic differentiation (MyoD) and fuse into eMyHC + myotubes when transferred to mitogen-low medium. As shown in Fig 4E, F and Fig S4 of Supporting Information, the myogenic regenerative potential was dramatically reduced by TGF-β1, thus confirming its role as a conserve between mouse and human negative regulator of muscle regeneration. Satellite cells isolated from young humans, exhibited higher myogenic potential as compared to the satellite cells derived from old people in these 24 h isochronic cultures, where the age of cells is matched with the age of sera (Fig 4E, F). An even higher magnitude of age-specific deficiency in myogenic responses was observed in 7 day isochronic human satellite cell cultures (Fig S5 and Table S1 of Supporting Information). Additionally, similar to findings in the mouse model, young human satellite cells had diminished regenerative responses, when cultured in the presence of old sera in heterochronic co-culture assays (Fig S5 and Table S1 of Supporting Information) (Carlson & Conboy, 2007). Conversely, the myogenicity of old satellite cells was improved when cultured in young sera. These data are the first to demonstrate that cellular and molecular mechanisms of muscle stem cell aging are highly conserved between mouse and human, with respect to the age-specific decline in satellite cell activation and to the biochemical imbalance in TGF-β and Notch.
The molecular causes for the age-specific decline in Delta expression and Notch activation in mouse muscles remains unknown. However, it is well established that the expression of Delta and subsequent activation of Notch are positively regulated by MAPK during embryonic development of several distinct organs in Drosophila and Caenorhabditis elegans (Carmena et al, 2002; Shaye & Greenwald, 2002). Exploring the evolutionary and developmental conservation of Notch and MAPK cross-talk, we examined whether (1) MAPK pathway strength becomes diminished in old human muscles, as compared to youngs and (2) whether MAPK signalling intensity is causal for Notch activation and myogenic properties of human satellite cells. Quite interestingly, Western blot analysis of young and old human muscles, demonstrated that the MAPK signalling strength is indeed significantly down-regulated with age (Fig 5A, B). Extrapolating the functional significance of these findings, we examined the levels of Delta, amounts of active Notch and the efficiency of myogenic responses in human satellite cells cultured in the presence of agonists and antagonists of MAPK pathway. Remarkably, MAPK agonist fibroblast growth factor 2 (FGF-2) induced Delta and active Notch, while specific inhibitor of MAPK (MEK inhibitor) significantly attenuated Delta and active Notch levels (Fig 5C, D). As expected, the levels of pERK (a key downstream effector of MAPK) were induced by FGF-2 and reduced by MEK inhibitor, thus validating the success of experimental modulation of MAPK (Fig 5C, D). Furthermore, the myogenic regenerative capacity of young and importantly, old satellite cells was significantly enhanced through forced activation of MAPK, and even young satellite cells failed to produce proliferating fusion-competent myoblasts when MAPK was experimentally inhibited (Fig 5E, F, Fig S6 of Supporting Information). Consistent with the data shown above in control isochronic cultures, young satellite cells outperformed the old satellite cells (Fig 5E, F).
Figure 5. MAPK signalling strength becomes diminished in old human muscle, and is causal for Notch activation and myogenic properties of human satellite cells.
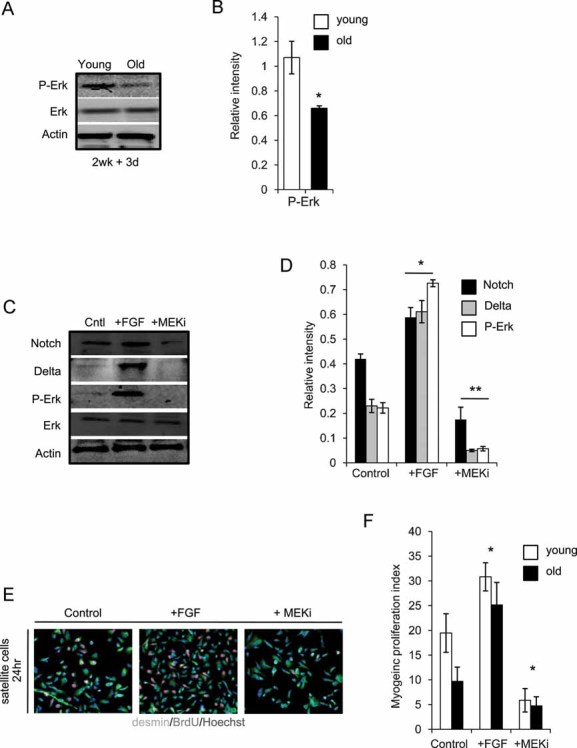
A, B. Western blot on whole muscle protein isolates was performed for P-Erk and Erk, quantified in B where *, P ≤ 0.05, old compared with young. Similar levels of Erk, but lower levels of pErk were detected in old muscle as compared to young.
C, D. Western blot of Notch, Delta, P-Erk and Erk was performed (quantified in D), following 24 h exogenous addition of 10 ng/ml FGF or 10 µM MEK inhibitor (MEKi) to isolated human satellite cell cultures *, **P ≤ 0.05, +FGF compared to Control and +MEKi compared to Control. Experimental attenuation of MAPK decreases levels of Delta and active Notch, while induction of MAPK up-regulates Delta and active Notch.
E, F. Activated satellite cell, cultured as in C, were analysed for myogenic responses and quantified F, based on the co-expression of desmin/BrdU. Data are means ± s.d., n = 6 for all panels indicated. Myogenic responses of both young and old human satellite cells were enhanced by forced activation of MAPK and were diminished by attenuation of MAPK.
To confirm that the main effect of MAPK on human satellite cell responses was through Notch activation, we activated MAPK in the presence of a Notch antagonist, gamma secretase inhibitor (GSI). As shown in Fig S7 of Supporting Information, inhibition of Notch by GSI precluded satellite cell regenerative responses even when MAPK was induced by FGF-2, thus suggesting that the positive regulation of satellite cell myogenicity by MAPK acts up-stream of Notch activation. These data establish that MAPK pathway is an age-responsive positive regulator of Notch in human muscle, and that the MAPK/Notch cross-talk is evolutionarily conserved between invertebrate embryogenesis and postnatal human muscle stem cell activation and aging.
Revealed cellular and molecular mechanisms of human muscle stem cell aging, prompted us to examine whether forced Notch activation would be able to restore myogenic responses to old human satellite cells cultured with aged human sera (old isochronic cultures). At the same time, we examined whether the regenerative responses of young human satellite cells cultured with young human sera would be incapacitated when Notch activation is inhibited (young isochronic cultures). Experimental activation of Notch receptor by exogenous ligand, Delta and forced inhibition of Notch by a GSI were performed for seven days of culture (control activation and inhibition of Notch shown in Fig S8 of Supporting Information; additional experiments on control young and old isochronic cultures are shown in Fig S5 of Supporting Information). Myogenic responses were measured, as described above, based on the generation of proliferating fusion-competent myoblasts. These experiments demonstrated that Notch is indeed a necessary and sufficient molecular determinant of human myogenic responses—required for productive myoblast generation by young stem cells, and capable of rescuing myogenesis of aged human satellite cells even in old systemic milieu (Fig 6A, B). To extrapolate these findings with higher molecular definition, we compared the levels of CDK inhibitors, p15 and p21 in satellite cells cultured in control isochronic conditions and those cells with forced activation and inhibition of Notch. The results of Western blotting shown in Fig 6C, D demonstrate that the levels of p15 and p21 are higher in old satellite cells as compared to young (in agreement with the age-specific elevation of these CDK inhibitors in human muscle in vivo, Fig 4C). Importantly, Notch activation diminished the levels of these CDK inhibitors in human satellite cells, while Notch inhibition resulted in the up-regulation of p15 and p21 (Fig 6C, D). These data establish the functional significance of Notch activation for regeneration and maintenance of human muscle, and demonstrate that Notch is an important negative regulator of CDK inhibitors in human satellite cells.
Figure 6. Notch is a necessary and sufficient molecular determinant of human myogenic responses in vitro, which rescues productive regeneration in the presence of aged sera and attenuates expression levels of p15 and p21 in human satellite cell cultures.
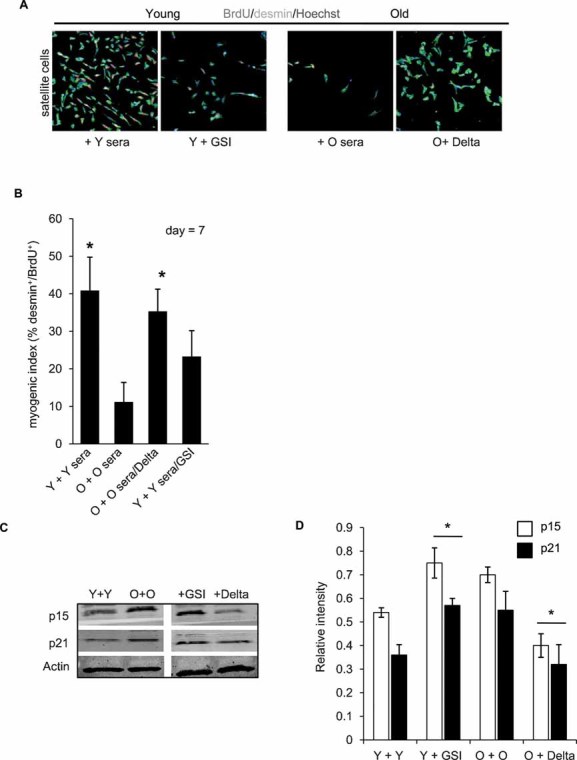
A, B. Young and old satellite cells were isolated and cultured in the presence of young or old sera, with or without experimentally induced Notch activation (immobilized Delta ligand for old cells) or Notch inhibition (treatment with GSI for young cells). After seven days of culture, cells were fixed and analysed for myogenic responses (generation of desmin/BrdU co-stained cells). Quantification is shown in B. *P ≤ 0.05 (Y + Y sera compared to O + O sera and Y + Y sera/GSI, O + O sera/Delta compared to O + O sera). n = 6. Representative immunostaining is depicted in A; desmin (green) BrdU (red), Hoechst (blue) labels nuclei. Activation of Notch is required for myogenic properties of young satellite cells and rescues myogenic potential of old human satellite cells even in the presence of aged human sera. Young satellite cells did not have significantly higher myogenic potential when Notch was experimentally activated, as compared to control young cells, suggesting that young myogenic responses are at the optimal high (not shown).
C, D. Treated cells were analysed for the expression of p15 and p21 by Western blot; quantified in D. Actin serves as loading control. *P ≤ 0.05, Y + GSI compared to Y + Y and O + Delta compared to O + O. Data are means ± s.d., n = 6 for immunodetection assays and Western blot analyses. Forced activation of Notch reduces levels of p15 and p21, while experimental attenuation of Notch activity increases the levels of these CDK inhibitors in satellite cells.
DISCUSSION
This work is the first to identify the molecular interactions between satellite cells and their young versus aged niches in humans, and to demonstrate that mechanisms of muscle stem cell regulation are evolutionary and developmentally conserved (a summary of our experimental design and approach is provided in Fig S9 of Supporting Information). Our data demonstrate that the age-specific shift from active Notch to excessive TGF-β/pSmad acts as the ‘effector’ of muscle regenerative decline in mice and humans (Fig. 3, 4, 6). Notably, we found that insufficient activation of Notch in old human satellite cells results from diminished MAPK signalling, which explains why Notch becomes lacking in old muscles, and establishes that canonical cross-talk between Notch and MAPK is to some degree conserved between invertebrate organogenesis and human muscle regeneration (Fig 5). Future work might establish that the age-specific deregulation of the studied, and other canonical organogenic pathways, is a common trend that might explain the fundamental failure of old tissue repair across mammalian species.
While vascularization, innervation, immune response and other attributes of organ repair become inefficient with aging (Grounds, 1998; Thomas, 2001; Wagers & Conboy, 2005), and certainly contribute to declined strength and agility of old muscles (Fig 1, Fig S1 and S2 of Supporting Information), the data presented here uncover that in old individuals muscle stem cells fail to activate for tissue repair (Fig 2). Moreover, this work is the first to establish that the intrinsic capacity of human satellite cells remains largely intact for at least 70 years, and can be rejuvenated by specific molecular cues (Fig 3, 5, 6, Fig S3 and S5 of Supporting Information). Since satellite cells physically persist in old humans, but their responses are acutely inhibited by biochemical changes in aged niches, stem-cell based transplantation therapy is unlikely to be effective in older individuals unless the herein identified inhibitory changes are neutralized.
The paper explained
PROBLEM
The capacity of tissues to regenerate declines with age and eventually fails, leading to degenerative disorders and catastrophic organ failure. A textbook example is muscle wasting, accompanied by the loss of strength and agility in older individuals. Despite extensive mice studies, the mechanisms regulating human skeletal muscle stem cells and the changes caused by aging, are not entirely understood. This precludes rational clinical intervention such as attempts to enhance the regenerative capacity of muscle stem cells, or promoting the successful transplantation of young healthy cells into an aged body.
RESULTS
This work uncovers the molecular culprits responsible for the lack of tissue maintenance and repair seen in old humans, and demonstrates that, as seen in mice, old human muscle stem cells are actually capable of productive regeneration, but are inhibited by their own muscle to do so. However, particular molecular cues or exposure to young human serum restores ‘youthful’ responses to muscle stem cells isolated from 70 year old humans, rendering them similar to cells from 20 year olds. Interestingly, young human muscle stem cells are ‘aged’ instantly, by the aged tissues, and thus are unlikely to work upon transplantation into the old.
IMPACT
These findings suggest clear therapeutic targets for boosting regenerative capacity, rejuvenating tissue maintenance and forestalling tissue aging.
Summarily, uncovering the mechanisms of molecular aging improves our understanding of stem cell behaviour and reveals tempting approaches for rejuvenating human tissue repair. The modulation of Notch, for example, and other signalling pathways is a logical pursuit—especially considering the growing evidence that such evolutionarily conserved pathways, critical to both mammalian organogenesis and postnatal tissue maintenance, become incapacitated by the aging process themselves.
MATERIALS AND METHODS
Human subjects
Young (22.6 years, range 21–24 years) and old (71.3 years, range 68–74 years) male subjects volunteered to participate in the study. Before inclusion, a physician screened the subjects to exclude persons with cardiovascular disease, diabetes, neural- or musculoskeletal disease, inflammatory or pulmonary disorders and any known predisposition to deep venous thrombosis. Only healthy, non-medicated individuals were included in the study. All subjects were moderately active (O: 5.2 ± 1.4 h/week, Y: 5.0 ± 0.9 h/week) and none of the subjects had previously participated in systematic strength training. The local Ethics Committee approved the conditions of the study (KF01-322606) and all experimental procedures were performed in Dr. Michael Kjaer's laboratory in accordance with the Declaration of Helsinki.
Assessment of contractile muscle function
Maximal muscle contraction strength was measured (1 KHz) for the quadriceps femoris muscle, in vivo, as the peak knee extension torque exerted during maximal voluntary concentric muscle contraction in an isokinetic dynamometer (KinCom) (Suetta et al, 2004a). Total contractile work in the range of motion (90° to 10°, 0° = full knee extension) was determined as the time-integral of contractile power production, where power was calculated by the instantaneous product of muscle torque and joint angular speed, the latter expressed in radians (Crameri et al, 2007).
Muscle biopsy sampling and analyses
Bilateral muscle samples from 21 individuals (10 young, 11 old) were obtained from the middle portion of Musculus vastus lateralis, utilizing the percutaneous needle biopsy technique of Bergström (Bergstrom et al, 1976). After dissecting the muscle samples of all visible blood, adipose and connective tissue, the muscle samples were oriented in embedding medium (Tissue Tec) frozen in isopentane cooled with liquid nitrogen, and stored at −80°C. Subsequently serial transverse sections (10 µm) were cut in a cryotome at −20°C and stained for myofibrillar ATPase at pH 9.4, after both alkaline (pH 10.3) and acid (pH 4.3 and 4.6) preincubations (Brooke & Kaiser, 1970). All samples of each individual person, were stained in the same batch to avoid inter-assay variation. For the determination of muscle fibre size, only truly horizontal fibres were used, with a minimum of 100 fibres included for the analysis. A videoscope consisting of a microscope (Olympus BX 50) and colour video camera (Sanyo high resolution CCD), in combination with Tema Image-analyses System (Scanbeam Denmark) were used to calculate the mean fibre area of the muscle fibres.
Isolation and culture of human muscle stem cells
Myofibre and satellite cell cultures were isolated from basal state human biopsies, in a manner similar to those previously described for the mouse model (Carlson & Conboy, 2007; Conboy et al, 2003). Whole muscle biopsies were prepared for myofibre fragments by enzymatic digestion—37°C in Dulbecco's modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA)/Penicillin–Streptomycin (Pen–Strep, Invitrogen)/0.2% Collagenase Type IIA (Sigma), trituration and multiple sedimentation and washing procedures. Isolated satellite cells and myofibres were resuspended in growth medium (Ham's F10 nutrient mixture (Mediatech, Inc., Herndon, VA), 10% bovine growth sera (BGS), 5 ng/ml human basic fibroblast growth factor (hbFGF) (Invitrogen) and 1% Pen–Strep, and cultured on Matrigel (diluted 1:250). Additionally, blood was collected from subjects for sera isolation. Briefly, blood cells were coagulated, followed by centrifugation at 8000 rpm at 4°C in a microfuge for 3 min.
For isochronic and heterochronic systemic cultures, myofibre explants or isolated satellite cells were cultured in the presence of 10% young or old sera. Isochronic cultures were performed by culturing isolated satellite cells with their respective donor serum. Heterochronic cultures used pooled sera, derived from the specific donor cell lines examined. Following specific time-point incubations, cells were fixed in 70% ethanol (EtOH)/phosphate buffered saline (PBS) for immuno-detection (see the Section below).
Immunocytochemistry and histological analysis
Isolated muscle tissue was frozen in OCT compound (Tissue Tek) and cryosectioned as 10-µm slices (Thermo Shandon Cryotome E). Immunostaining and haematoxylin and eosin staining were performed as previously described (Carlson et al, 2008a; Conboy et al, 2003, 2005). For indirect immunoflourescence assays, sections were permeabilized in (PBS, +1% foetal bovine serum (FBS), +0.25% Triton X-100) and incubated with primary antibodies overnight at 4°C, PBS, +1% FBS. Secondary staining with fluorophore-conjugated, species-specific antibodies was performed for 1 h at room temperature (1:500 in PBS, +1% FBS). Nuclei were visualized by Hoechst staining, and samples were analysed at room temperature with a Zeiss Axio Imager A1, and imaged with an Axiocam MRc camera/AxioVision software.
Western blot analysis
Whole skeletal muscle lysates were prepared in lysis buffer (50 mM Tris, 150 mM NaCl, 1% NP40, 0.25% sodium deoxycholate and 1 mM EDTA, pH 7.4), with addition of protease inhibitor cocktail (Sigma) and 1 mM phenylmethanesulphonylfluoride (PMSF). Phosphatase activity was inhibited by 1 mM sodium fluoride and 1 mM sodium orthovanadate. For most assays, 30 µg protein extracts were run on pre-cast SDS PAGE gels (Bio-Rad). Primary antibodies were diluted in 5% non-fat milk/1 × PBS Tween-20 (1 × PBST), and nitrocellulose membranes were incubated with antibody mixtures overnight at 4°C. HRP-conjugated secondary antibodies (Santa Cruz Biotechnologies) were diluted 1:1000 in 1 × PBST/1% BSA and incubated for 1 h at room temperature. Blots were developed using Western Lightning enhanced chemiluminescence (ECL) reagent (PerkinElmer), and analysed with Bio-Rad Gel Doc/Chemi Doc Imaging System and Quantity One software. Results were quantified by digitizing the data and normalizing pixel density of examined protein by actin-specific pixel density.
Reagents
Antibodies to activated Notch1 (ab8925), M-Cadherin, NCAM, MyoD1 and BrdU (ab6326) were purchased from Abcam. Antibody to developmental eMyHC (clone RNMy2/9D2) was acquired from Vector Laboratories. Antibodies to dystrophin, actin, laminin and desmin (clone DE-U-10) were acquired from Sigma. Recombinant mouse Delta-like 4 (DLL4) (RD 1389; (2 µg/ml)), recombinant TGF-β1, recombinant human FGF, TGF-β antibody and Pax7 antibody (MAB1675) were obtained from R&D Systems. Erk and P-Erk antibodies, as well as MEK inhibitor were purchased from Cell Signalling. Antibodies to p15 (sc613), p21 (sc756), p16, p27, P-Smad2/3 and Delta were acquired from Santa Cruz Biotechnologies. GSI was purchased from CalBiochem (EMD). Fluorophore-conjugated secondary antibodies (Alexa Fluor) were supplied by Invitrogen. HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnologies. GSI X [50 nM] (no. 565771) was purchased from Calbiochem.
Statistical analysis
Quantified data are expressed as mean ± s.d. Significance testing was performed using one-way analysis of variance, with an alpha level of 0.01–0.05, to compare data from different experimental groups. A minimum of three replicates were performed for each described experimental condition. In vitro experiments typically analysed five young and old individuals per assay, unless otherwise noted in figure legend.
Acknowledgments
This work was supported by NIH RO1 AG 027252, CIRM RN1-00532, Glenn Research Foundation grants to IMC and CIRM training grant to MEC. Support was also provided by the Danish Medical Research Council, NOVO-Nordisk Foundation and Lundbeck Foundation to MK, and by grants from the Danish National Research Council, the Faculty of Health Sciences, University of Copenhagen and the Danish Ministry of Culture to CS. We thank Monika Bayer for her technical assistance with muscle isolation and establishing myogenic cultures.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author's contributions
MEC and MJC performed experiments and analysed data on the age-related molecular regulators of human muscle stem cells. CS and MK designed and directed the age-dependent human subject study, conducted human subject experiments and contributed the data on the human muscle physiology and histology with the assistance of PA and AM (Fig S2 of Supporting Information). IC designed and directed the study on the age-related molecular regulators of human muscle stem cells, integrated the data and wrote the manuscript.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Bergstrom J, Alvestrand A, Furst P, Hultman E, Sahlin K, Vinnars E, Widstrom A. Influence of severe potassium depletion and subsequent repletion with potassium on muscle electrolytes, metabolites and amino acids in man. Clin Sci Mol Med Suppl. 1976;51:589–599. doi: 10.1042/cs0510589. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brown M, Hasser EM. Differential effects of reduced muscle use (hindlimb unweighting) on skeletal muscle with aging. Aging (Milano) 1996;8:99–105. doi: 10.1007/BF03339562. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008a;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Silva HS, Conboy IM. Aging of signal transduction pathways, and pathology. Exp Cell Res. 2008b;314:1951–1961. doi: 10.1016/j.yexcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena A, Buff E, Halfon MS, Gisselbrecht S, Jiménez F, Baylies MK, Michelson AM. Reciprocal regulatory interactions between the Notch and Ras signaling pathways in the Drosophila embryonic mesoderm. Dev Biol. 2002;244:226–242. doi: 10.1006/dbio.2002.0606. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Invidia L, Lescai F, Salvioli S, Tieri P, Castellani G, Franceschi C. Human models of aging and longevity. Expert Opin Biol Ther. 2008;8:1393–1405. doi: 10.1517/14712598.8.9.1393. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjaer M. Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol. 2007;583:365–380. doi: 10.1113/jphysiol.2007.128827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve. 2003;27:339–347. doi: 10.1002/mus.10314. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann NY Acad Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Machida S, Booth FW. Changes in signalling molecule levels in 10-day hindlimb immobilized rat muscles. Acta Physiol Scand. 2005;183:171–179. doi: 10.1111/j.1365-201X.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Siu PM, Alway SE. Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292:C1298–1304. doi: 10.1152/ajpcell.00496.2006. [DOI] [PubMed] [Google Scholar]
- Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- Schultz E, Lipton BH. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Aging Dev. 1982;20:377–383. doi: 10.1016/0047-6374(82)90105-1. [DOI] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I. Endocytosis-mediated downregulation of LIN-12/Notch upon Ras activation in Caenorhabditis elegans. Nature. 2002;420:686–690. doi: 10.1038/nature01234. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Bushby K. The childhood limb-girdle muscular dystrophies. Semin Pediatr Neurol. 2006;13:104–114. doi: 10.1016/j.spen.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, Magnusson SP. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol. 2004a;97:1954–1961. doi: 10.1152/japplphysiol.01307.2003. [DOI] [PubMed] [Google Scholar]
- Thomas DR. Age-related changes in wound healing. Drugs Aging. 2001;18:607–620. doi: 10.2165/00002512-200118080-00005. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises and a cure for aging. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


