Abstract
Hearing loss is a global health problem with profound socioeconomic impact. We contend that acquired hearing loss is mainly a modern disorder caused by man-made noise and modern drugs, among other causes. These factors, combined with increasing lifespan, have exposed a deficit in cochlear self-regeneration that was irrelevant for most of mammalian evolution. Nevertheless, the mammalian cochlea has evolved from phylogenetically older structures, which do have the capacity for self-repair. Moreover, nonmammalian vertebrates can regenerate auditory hair cells that restore sensory function. We will offer a critical perspective on recent advances in stem cell biology, gene therapy, cell cycle regulation and pharmacotherapeutics to define and validate regenerative medical interventions for mammalian hair cell loss. Although these advances are promising, we are only beginning to fully appreciate the complexity of the many challenges that lie ahead.
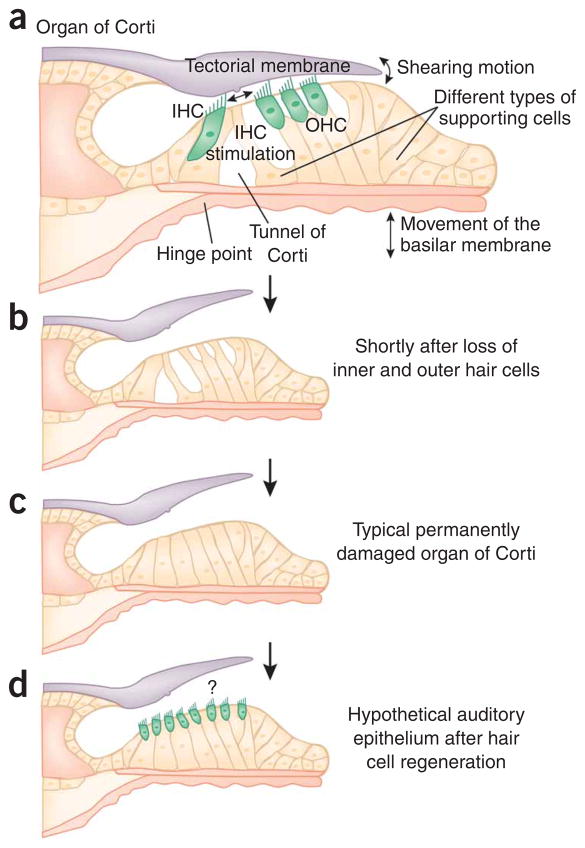
Of all the cochlear structures and cell types, the sensory hair cells, fewer than 15,000 at birth, are the Achilles’ heel of the auditory system. Loss of hair cells accounts for most hearing loss, affecting substantial proportions of the population (see Box 1). Generally, outer hair cells (OHCs) are more sensitive than inner hair cells (IHCs) to insults that damage hearing and to the effects of aging1. OHC loss, usually starting at the high-frequency basal region of the cochlea, leads to a substantial auditory threshold shift. More severe forms of hearing impairment generally involve the loss of IHCs. After hair cell loss, the organ of Corti undergoes gradual cytomorphological dedifferentiation of supporting cells; this is sometimes followed by a collapse of the tunnel of Corti, resulting in a structure that often features an unorganized mound of inconspicuous cells2–4 (Box 2 and Fig. 1).
Box 1. Hearing loss and deafness in the United States and United Kingdom.
United States
Men are more likely to experience hearing loss than women.
About 2 to 3 children out of every 1,000 children are born deaf or hard of hearing.
Approximately 36 million American adults (17%) report some degree of hearing loss.
Approximately 26 million Americans (15%) between the ages of 20 and 69 have high-frequency hearing loss due to loud sounds or noise at work or in leisure activities.
About 4,000 new cases of sudden deafness occur each year.
Only 1 out of 5 people who could benefit from a hearing aid actually wears one.
About 23,000 adults and 15,000 children have received cochlear implants.
Source: National Institute on Deafness and Other Communication Disorders, National Institutes of Health (http://www.nidcd.nih.gov/)
United Kingdom
There are more than 34,000 deaf children in the UK.
Approximately 840 children are born every year with moderate to profound deafness.
About 1 in every 1,000 children is deaf at 3 years of age. This rises to 2 per 1,000 children aged 9 to 16.
There are approximately 9 million deaf and hard-of-hearing people in the UK, of which about 698,000 are severely or profoundly deaf.
About 3.5 million people of working age (16–65 years) are deaf or hard of hearing.
About 55% of people over the age of 60 are deaf or hard of hearing.
Approximately 2 million people have hearing aids, but only 1.4 million use them regularly.
There are approximately 4 million people who could benefit from hearing aids but do not have them.
Sources: Royal National Institute for the Deaf (http://www.rnid.org.uk/) and National Deaf Children’s Society (http://www.ndcs.org.uk/)
Box 2. Anatomy and physiology of the cochlea.
Of all sensory hair cell–bearing organs, the mammalian cochlea, which converts sound waves into neural signals, is probably the most specialized and most complex. The organ of Corti within the cochlea (Fig. 1a) harbors a single row of approximately 3,500 inner hair cells (IHCs), the primary auditory transducers responsible for conversion of sound into neural signals. The IHC row is separated from the three rows of outer hair cells (OHCs) by highly specialized pillar cells that enclose the tunnel of Corti, which delineates a mechanical hinge whose movement is important for proper stimulation of the IHC stereocilia. The OHCs are situated on top of phalangeal supporting cells called Deiters cells. Other, morphologically distinct supporting cell types embrace the hair cell-bearing part of the organ of Corti. The OHCs play a key role in the frequency-specific amplification of basilar membrane motion; they connect with their stereociliary bundles to the tectorial membrane, an acellular structure that covers the organ of Corti along its entire length.
When sound travels through the middle ear, it is transmitted to the base of the cochlea via the stapes, which causes displacement of perilymph in the scala vestibuli, leading to movement of the basilar membrane (Fig. 1a). This displacement results in a traveling wave that moves from base to apex and reaches a maximum at a specific place along the basilar membrane corresponding to the frequency of the stimulus46. High frequencies—up to 20,000 Hz in humans—are represented at the cochlear base, and low frequencies—down to 20 Hz in humans—correspond to apical locations. This spatial arrangement of frequency representation and detection is called tonotopy. The sharp frequency tuning at a specific basilar membrane location is governed by the properties of an active system, the cochlear amplifier, that is attributed to OHCs. Nevertheless, frequency tuning also depends on other components, such as extracellular, cellular and molecular structures. Examples of these are the tonotopic changes of mass, composition and stiffness of the tectorial membrane47,48; differences in hair cell morphology, such as cell and stereociliary length and compliance49; and physiological differences, such as the changing conductances of the mechanoelectrical transduction channels along the tonotopic axis, which has been observed in nonmammalian cochleae50.
Figure 1.
Conceptual drawings of the normal, damaged and repaired organ of Corti. (a) In the normal organ of Corti, movements of the basilar membrane are relayed by means of hinging near the tunnel of Corti and shearing motions between outer hair cells (OHCs) and the tectorial membrane. The connections of OHC stereocilia with the tectorial membrane are essential for proper function of the cochlear amplifier. All these parts of the organ of Corti must be in appropriate mechanical correlation with one another to ensure proper stimulation of the inner hair cells (IHCs). (b) Complete loss of IHCs and OHCs, shortly after a toxic insult. (c) Collapse of the tunnel of Corti and dedifferentiation of the supporting cells. (d) Hypothetical reseeding of generic hair cells in the damaged organ of Corti epithelium. It is unclear whether a connection with the tectorial membrane will re-form, how far it will stretch over the new hair cell area and whether the hair cells will be appropriately stimulated by movements of the basilar membrane.
Regeneration of cochlear hair cells is considered the ultimate remedy for hearing loss. Nevertheless, what would seem to be a simple replacement of a single cell type turns out to be a remarkably complex endeavor when one takes into account the very different functions of IHCs and OHCs, as well as their precise integration into accessory structures, such as the tectorial membrane, and the restoration of organ of Corti micromechanics. This situation is aggravated by the morphological dedifferentiation of the cellular components of the organ of Corti, particularly in cases of progressive hearing loss. Consequently, hair cell replacement cannot be viewed as simply seeding new hair cells and getting them connected to the afferent auditory nerve. For proper restoration, hair cell regeneration needs to be conducted in the context of extensive cochlear restoration, either back to the original morphological configuration or into an alternative design featuring sensitivity and tonotopy, combined with longevity of the newly introduced cells (Fig. 1d).
We divide this perspective into two parts: in the first part, we discuss current basic research in inner ear cell regeneration, as well as the unraveling of key genes involved in cochlear development and cell proliferation control. In the second part, we focus on therapeutic approaches and roadblocks, including delivery of cells, genes and small compounds.
Laboratory-based results
Nonmammalian vertebrate hair cell regeneration reveals existence of inner ear stem cells
In 1988, Corwin and Cotanche5 and, independently, Ryals and Rubel6 reported that cochlear hair cells regenerate after acoustic trauma in birds. The two reports hypothesized similarly: “that regenerated hair cells originate from mitotic divisions of supporting cells or some unidentified latent stem cells”5 and “that the regenerative potential is retained in adult animals, suggesting that a dormant stem cell population is retained throughout life”6. In contrast, the mammalian cochlea is unable to regenerate hair cells after ototoxic insults7,8. However, in 1993 it was reported that hair cell regeneration in response to aminoglycoside ototoxicity occurs in the vestibular sensory epithelia of adult mammals, albeit to a far less impressive extent than had been seen in birds9,10. Hair cell regeneration in all these instances seems to originate from supporting cells that reenter the cell cycle when neighboring hair cells are dying. Mitotic supporting cells subsequently divide asymmetrically, generating new hair cells and supporting cells. In some instances, a phenotypic conversion also described as transdifferentiation into hair cells has also been observed. This mechanism is potentially faster in generating hair cells than the asymmetric division of supporting cells, but it also requires subsequent divisions to replenish the supporting cell pool.
Asymmetric division of supporting cells to generate replacement hair cells and to make identical copies of themselves is a defining feature of somatic stem cells. Other regenerating sensory systems—for example, the olfactory neuroepithelium—use resident precursors for cell replenishment during natural turnover; in addition, they harbor a quiescent adult stem cell population for complete regeneration after massive injury11. It is compelling to argue that with the exception of the mammalian organ of Corti, hair cell-bearing organs too are bona fide stem cell systems in which all or a subpopulation of supporting cells serves as dormant or latent stem cells.
This concept was explicitly tested by Li and colleagues12, who demonstrated that the adult vestibular sensory epithelia of mice do harbor cells that proliferate in vitro. When subjected to low density and nonadherent culture conditions, these inner ear stem cells were able to generate clonal floating colonies, so-called spheres. This adapted version of the neurosphere assay13 demonstrated capacity for self-renewal, which is the defining feature of stem cells. Sphere cells were able to differentiate in vitro into cells expressing markers for mature hair cells, supporting cells, or neurons, and they were able to differentiate into cells positive for hair cell markers and having hair cell–like morphology after grafting into inner ears of chicken embryos. More confirmation of the concept that supporting cells can serve as stem or progenitor cells came from experiments in which supporting cells from the embryonic chicken utricle were progressively expanded and subsequently differentiated into hair cells14.
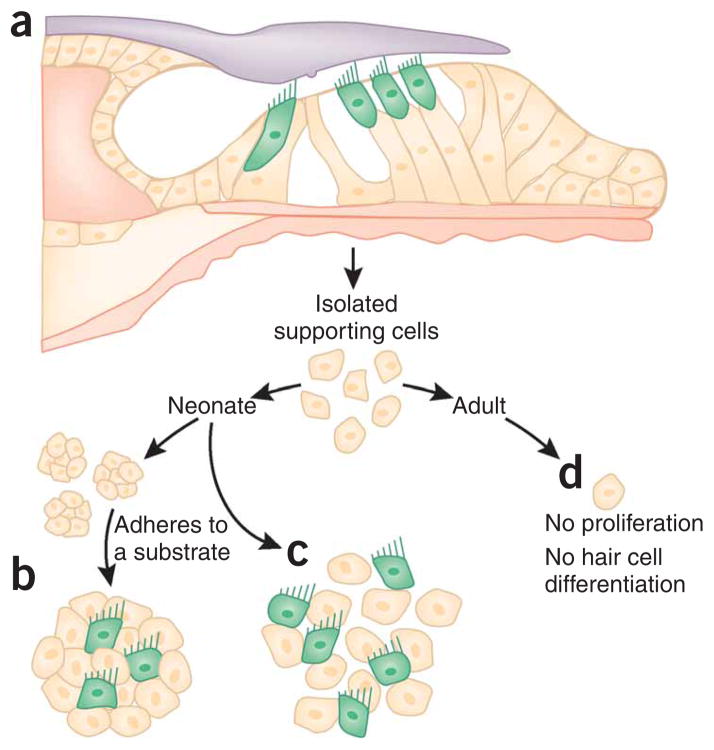
It was not surprising that the adult mammalian vestibular system harbors cells with stem cell features, because vestibular sensory epithelia have regenerative capacity7,8. Nevertheless, it was unexpected that cells isolated from neonatal organ of Corti tissue showed stem cell features such as the ability to generate clonal spheres and subsequent ability to differentiate into cells resembling hair cells15–17 (Fig. 2a,b). Other experiments, discussed in more detail below, also revealed that neonatal cochlear supporting cells feature proliferative potential and the ability to generate cells expressing hair cell markers in vitro18 (Fig. 2a,c). Both studies found that this proliferative potential is lost between the second and third week after birth in mice15,18 (Fig. 2d). The identity of the specific supporting cell type(s) that have sphere-forming capacity is unknown, but it is conceivable that the identification of more cell type–specific markers will provide the necessary tools to answer this important question. Because cells do not disappear from the neonatal organ of Corti, it was hypothesized that the extensive cytomorphological specialization and the maturation of organ of Corti supporting cells lead to a loss of regenerative competence, which may be an explanation for the inability of the organ of Corti to replace lost hair cells16.
Figure 2.
Proliferation and hair cell differentiation from supporting cells. (a) Isolation of stem cells and supporting cells from the organ of Corti. (b) Hair cell differentiation in sphere-derived cultures happens when spheres attach onto substrate, forming islands of cells. Hair cell marker–positive cells (green) differentiate in these islands and appear to sit on top of cells that express supporting cell markers. (c) Alternatively, proliferating supporting cells, cultured with embryonic mesenchymal cells, exit the cell cycle and differentiate into hair cell marker–positive cells. (d) Supporting cells of the adult organ of Corti do not proliferate.
From an evolutionary viewpoint, it is puzzling that the adult mammalian organ of Corti has lost its capacity for self-repair. We argue that, in general, acquired hearing loss is a modern-day disorder exposing an apparent deficit in mammalian evolution whereby the ability for cochlear self-regeneration was lost. Nevertheless, it is a reasonable assumption that the organ of Corti has evolved from a structure with the capacity of self-repair, because all other hair cell-bearing organs do replace lost hair cells. The existence of limited hair cell repair potential in the developing and neonatal mammalian cochlea also supports this idea15,18. In the next section, we explore how the reexpression of genes usually expressed early on in development may be one potential way of replacing hair cells and restoring hearing.
Generating replacement hair cells by reexpressing Atonal homolog 1 (Atoh1)
The basic helix-loop-helix transcription factor Atoh1 (also known as Math1) has emerged as a candidate for gene-based treatment of auditory and vestibular disorders19,20. Atoh1 is expressed in the prosensory patches that give rise to the auditory and vestibular sensory epithelia21–23. The Atoh1 gene is essential for hair cell development as its targeted disruption results in the absence of auditory and vestibular hair cells22. Moreover, misexpression of Atoh1 in cultures of the embryonic and postnatal organ of Corti generates cells with morphological and molecular correlates of hair cell identity23,24. These data have served as rationale for a series of recent studies to test the potential of Atoh1 misexpression to generate functional hair cells in vivo.
Kawamoto and colleagues misexpressed Atoh1 by adenovirus-mediated gene transfer in the adult guinea pig inner ear19. The mechanical infusion of viral particles into the endolymphatic space causes a hydrodynamic lesion that damages hair cells while simultaneously inoculating the epithelium (comprising of the organ of Corti) with the Atoh1-encoding virus. Atoh1 expression was detected in nonsensory cells in the organ of Corti 4 d after inoculation, and the hair cell marker Myosin7a was present in some infected cells at 60 d. The newly formed cells attracted neurofilament-bearing processes and seemed to display immature stereociliary bundles. The authors therefore suggest that Atoh1 misexpression converts nonsensory cells into hair cells. In subsequent work, Izumikawa and colleagues showed that adenovirus-mediated expression of Atoh1 in the organ of Corti of deafened, adult guinea pigs can generate hair cells, which seem to contribute to improvement in auditory brainstem response thresholds20. The cellular mechanism responsible for this de novo production of hair cells is not known, though the authors indicate that an increase in the number of nuclei in the organ of Corti of Atoh1-transfected ears could be explained by migration of transfected cells into, or clonal expansion of cells within, the damaged regions. Although the improvement of auditory brainstem response thresholds was detected in a limited number of guinea pigs and the effect was variable, the data suggest it may be possible to manipulate the fate of nonsensory cells to generate sensory cells with the potential to improve auditory function.
A fundamental prerequisite for restoring hearing in the hair cell–compromised inner ear is to generate functional hair cells. Gubbels and colleagues established an in utero gene transfer technique to conduct gain-of-function studies in the developing mouse inner ear that permits postnatal analysis of sensory hair cells25. They microinjected an expression plasmid encoding Atoh1 and green fluorescent protein (GFP) through the uterus into the early otic vesicles of embryos. Otic epithelial progenitor cells that give rise to the organ of Corti were subsequently transfected by in vivo electroporation. Supernumerary and ectopic cells were generated that expressed Myosin7a and displayed stereociliary bundles at their apical surfaces. These putative hair cells associated with neurofilament-bearing processes that traced back to the cochlear nucleus, and they expressed the ribbon synapse marker carboxy-terminal binding protein-2 in their basolateral domain. Moreover, postnatal electrophysiological analyses indicated that the Atoh1-transfected cells showed mechanoelectrical transduction currents and age-appropriate basolateral conductances. Although these data demonstrate that it is formally possible to generate functional hair cells by Atoh1 misexpression in the developing inner ear, auditory brainstem response thresholds at postnatal days 28–35 still indicated elevated auditory thresholds. These data are consistent with the observation of altered auditory function in mouse mutants that produce extra rows of hair cells26,27.
Atoh1-based strategies to restore auditory function face inherent limitations. Hearing loss caused by a genetic mutation affecting the function of the sensory hair cell itself cannot be corrected by Atoh1-based intervention because new, equally defective hair cells would result. In this case, a co-therapeutic would need to be delivered with Atoh1 that corrects the primary defect in the induced hair cells. The principal constraint on such a therapeutic modality is the inability to sufficiently control the temporal and spatial expression of multiple genes simultaneously. However, hearing loss caused by aminoglycoside antibiotics, antitumor drugs, loud sound or aging may be amenable to Atoh1-based strategies. On the other hand, Atoh1 probably generates hair cells by the transdifferentiation of supporting cells, whose subsequent depletion could destabilize the structurally intricate cytoarchitecture of the organ of Corti (Fig. 1). The reported increase in cell nuclei in the damaged organ of Corti in response to Atoh1 expression20 leaves an interesting open question about the mechanism by which Atoh1 could function in restoration of the overall cell count.
Generating replacement hair cells by disrupting Notch signaling
In the normally quiescent bird auditory epithelium, hair cell regeneration seems to reactivate developmental programs, and the Notch signaling pathway is probably the one we know most about. During development, Notch receptor activation suppresses hair cell differentiation by means of upregulating Hes and Hey genes, which are potent inhibitors of Atoh1. Interference with Notch function, for example by elimination of Notch ligands28, results in more than the usual number of hair cells (supernumerary hair cells). Likewise, Notch inhibition during zebrafish lateral line hair cell regeneration and in the regenerating bird auditory epithelium increases the production of new hair cells, without showing effects on undamaged sensory epithelia29,30. The latter experiments were done using γ-secretase inhibitors, which suppress the activation-dependent cleavage of the Notch intracellular domain that is needed for functional signaling. Application of γ-secretase inhibitors to embryonic and neonatal organ of Corti explants results in robust production of supernumerary hair cells31,32, an effect that was initially also observed by antisense oligonucleotide knockdown of Notch 1 and Jag 1 (ref. 33). Prolonged pharmacological γ-secretase inhibition also seems to induce mitogenic proliferation of supporting cells in embryonic organ of Corti cultures32, an effect that has also been observed in the regenerating zebrafish lateral line30. However, recent experiments on damaged adult guinea pig organs of Corti are less encouraging: local application of γ-secretase inhibitors resulted in formation of a few—less than one per 200 μm cochlear segment—ectopic hair cells and no recurrence of outer and inner hair cells34. These results point to a possible loss of competence of the adult organ of Corti to respond to interference with Notch signaling inhibitors. It seems that further investigation is needed, particularly of other signaling pathways that are interwoven with Notch signaling, before one should draw conclusions about possible pharmacological approaches to hair cell regeneration, particularly in adult mammals.
Generating replacement hair cells by inhibiting cell cycle inhibitors
In birds and many nonmammalian vertebrates, supporting cells are able to produce new hair cells through two mechanisms: transdifferentiation and mitotic regeneration. In the latter process, normally postmitotic supporting cells reenter the cell cycle, divide and give rise to both hair cells and supporting cells. An essential question is whether the mammalian supporting cell is terminally postmitotic, having permanently lost the capacity to reenter the cell cycle, or whether manipulation of genes that regulate cell cycle progression could induce the supporting cell to reenter mitosis. The answer may lie with the cyclin-dependent kinase inhibitor p27Kip1 (also known as Cdkn1b), which occurs in the developing organ of Corti from embryonic days 12–14 and correlates with cessation of cell division of the otic epithelial progenitors that give rise to hair cells and supporting cells. In the maturing inner ear, p27Kip1 is downregulated in hair cells but its expression persists in all classes of supporting cells. Targeted deletion of p27Kip1 in mice generates supernumerary hair and supporting cells, suggesting that p27Kip1 expression in differentiated supporting cells may be critically relevant to the incapacity of the mammalian inner ear to regenerate35,36.
In a strategy that takes cognizance of these findings, White and colleagues addressed the regenerative capacity of mammalian supporting cells in an elegant series of experiments18. They isolated supporting cells from the neonatal cochleae of mice expressing GFP from the p27Kip1 promoter using fluorescence-activated cell sorting. The p27Kip1-GFP+ cells were cultured with periotic mesenchymal cells known to supply the necessary factors to support growth and differentiation of embryonic cochlear sensory progenitors. Sixty percent of the GFP+ supporting cells downregulated expression of p27Kip1, and 40% incorporated the thymidine analog BrdU, indicating that the cells reentered the cell cycle. After 6 d in vitro, about 3% of the cells were immunopositive for the hair cell marker MyosinVI, and 20% of these cells had incorporated BrdU. Fourteen-day cultures showed cells expressing phalloidin-labeled protrusions that were immunopositive for the hair bundle marker espin. These data suggest that neonatal mouse supporting cells are capable of generating sensory hair cells by both transdifferentiation and mitotic regeneration after p27Kip1 downregulation. Interestingly, the regenerative features of isolated supporting cells were only detectable in neonates and not in adult animals, an observation that parallels the finding that sphere-forming stem cells also occur only in the neonatal organ of Corti15 (Fig. 2). The failure of older supporting cells to reenter the cell cycle was shown to correlate with a failure to downregulate p27Kip1, which provides clues about the potential mechanisms leading to lack of regenerative potential in the mammalian cochlea18.
Mitotic regeneration is of particular interest as a therapeutic modality because restoration of auditory function in the damaged inner ear is almost certain to require the concomitant replacement of both hair cells and supporting cells. p27Kip1 is an attractive control point, though its permanent inactivation is likely to lead to uncontrolled growth of cells. Temporal control of p27Kip1 function, perhaps through pharmacotherapeutics, would need to be spatially restricted to the inner ear to sidestep deleterious effects in other organs. Clearly, identification of candidate regulatory proteins that may be suitable targets for therapeutic intervention is only a first—although critical—step in defining translational approaches to restoring auditory function.
The enormity of the challenge in manipulating cell cycle progression to stimulate functional hair cell regeneration is highlighted by recent work with the tumor suppressor gene retinoblastoma (Rb1). The retinoblastoma (Rb) protein it encodes is a member of the pocket protein family that is crucially involved in regulation of cell cycle exit, differentiation and survival. Rb is upregulated in embryonic and postnatal hair cells, leading to the idea that its function is required to maintain their quiescent state and that its manipulation could lead to the production of functional hair cells.
Conditional inactivation of the Rb1 gene in developing otic sensory epithelia leads to the overproduction of vestibular and auditory hair cells37,38. Sage and colleagues showed incorporation of BrdU into progenitors in the zone of nonproliferating cells defined by p27kip1 expression at E13.5, indicating that Rb-deficient sensory progenitors had reentered the cell cycle38. Notably, BrdU incorporation in the organ of Corti after hair cell specification seemed to label Myosin7a+ cells, suggesting that Rb-null hair cells reentered the cell cycle. Rb-deficient utricular hair cells at E18.5 showed weak mechanoelectrical transduction currents, suggesting that they were functional. Mantela and colleagues37, however, identified pathological changes within and outside the organ of Corti in Rb-null mice, including apoptosis and polyploidy in Rb-null hair cells.
The subsequent generation of an inner ear–conditional Rb-null allele permitted postnatal analyses of auditory function39. This confirmed previous findings that proliferating hair cells reenter the cell cycle and show mechanoelectrical transduction. However, at 3 months of age the Rb-null mice show total hair cell loss, and brainstem audiometry indicates that they are profoundly deaf, suggesting that Rb plays further roles in hair cell maturation and survival. Weber and colleagues acutely inactivated Rb in postnatal cochlear hair cells using an inducible Cre under Atoh1 regulatory control40. About 40% of the Rb-null hair cells reentered the cell cycle by P4 though they did not progress through cell division. Rb-null hair cells were rapidly lost beginning at P4–P15, no supernumerary hair cells were detected, and click brainstem audiometry indicated profound deafness. These postnatal studies indicate that permanent inactivation of Rb function alone will not permit functional mitotic regeneration in the diseased inner ear.
Therapeutic approaches
State-of-the-art treatment for hearing loss consists of devices such as hearing aids for mild to profound hearing loss, and cochlear implants for profound hearing loss and deafness. Hearing aids amplify sound, thereby counteracting threshold shifts caused by the loss of cochlear amplification, generally a result of OHC loss. They require intact IHCs for sound transduction to the cochlear nerve. A viable option for patients suffering from IHC loss is the cochlear implant, which consists of a curled linear array of up to two dozen electrodes that is placed into the scala tympani. The electrode array directly stimulates auditory neurons and preserves some cochlear tonotopy. Despite their successes, hearing aids and cochlear implants are not perfect. In particular, the efficacy of cochlear implants differs greatly among patients; frequency discrimination and performance in noisy environments is a prevailing issue. The advent of regenerative medicine has kick-started the search for biological treatment strategies to restore cochlear function, and the advances we described above are beginning to be translated into potential treatment strategies. These strategies are discussed in the next paragraphs.
Stem cells and cell delivery
Several laboratories have begun to explore the implantation of stem cell–derived precursor cells into the damaged cochlea. These studies reported limited survival of the cell grafts, and only a few cells were found integrated into the host tissue. Despite some evidence for differentiation into mature cell types, no robust and unequivocal occurrence of new hair cells was observed (ref. 41 and references cited therein). However, these experiments were done with neural stem cells whose capacity to generate inner ear cell types is unknown. Consequently, it remains to be determined how inner ear stem cells or embryonic stem cell–derived otic progenitors42 will perform in transplantation studies; these cells have competence to differentiate into hair cell–like cells, and they are probably a suitable choice for cochlear hair cell replacement studies.
Still, the prospect of cell transplantation being able to regenerate hair cells (and possibly other cell types of the organ of Corti) remains dim unless several technical and conceptual issues are addressed. These cochlea-specific roadblocks exist in addition to general safety considerations, such as prevention of tumor formation and graft-versus-host disease. First, it is unclear how grafted cells would be able to reach the whole expanse of the cochlear scalae, particularly along a wide frequency range. In humans, the stretched-out organ is longer than an inch, and it is difficult to imagine repopulating the organ from a single injection site. Second, one potential target compartment, the scala media (Fig. 1), is filled with an extracellular solution rich in K+ (~ 150 mM), providing a challenging environment for transplanted cells. Third, integration of cells into the damaged organ of Corti requires breaking the tight and adherens junctions at the organ’s apical side. No studies thus far have investigated the potential of stem or progenitor cells to overcome adult organ of Corti cell junctions. Fourth, the grafted cells must be able to efficiently home in on the organ of Corti. Only new hair cells situated in correct locations on the basilar membrane have a chance of being activated by basilar membrane movement; grafts integrated at other locations may not work, and could even compromise cochlear function. The goal should be to generate the correct number of hair cells in the correct location, as small changes in hair cell numbers or supernumerary hair cells have been shown to result in hearing loss25–27. Fifth, signaling pathways to direct differentiation into the correct hair cell types with correct hair bundle orientation would have to be active in the damaged adult organ of Corti. Finally, even repopulating the damaged organ of Corti with correctly innervated hair cells might not be sufficient because of the potential lack of support structures such as the tunnel of Corti or disconnection from the tectorial membrane (Fig. 1d). It is unlikely that grafted otic progenitors have the potential to magically convert a damaged organ of Corti back to the undamaged state. It is more reasonable to assume that a repaired hearing organ will probably still be somewhat compromised in its function.
Gene delivery to the inner ear
Effective therapies to ameliorate the effects of inner ear disease are likely to involve not only manipulation of suitable target genes and/or transplantation of cells, but also the equally complex task of defining atraumatic delivery of these therapeutic agents. The inner ear maintains an ionic asymmetry between endolymph and perilymph that is essential for mechanoelectrical transduction43. Physical entry into the membranous labyrinth to deliver bioactive reagents to the sensory epithelium of the organ of Corti must not permanently compromise this delicate ionic homeostasis. In addition, viral vectors used for gene delivery vary widely in the cell types they infect, the size of promoter and coding region they can accommodate, and their potential to trigger an undesirable immune response20,44. At present, there is no ideal vector system and delivery method for therapeutic, exogenous gene transfer to the mature inner ear. A crucial need in the field is to pair otolaryngologists, basic science researchers, and engineers to address these issues collaboratively.
Small molecule modulators of gene expression and pharmacotherapeutics
Many of the difficulties specifically associated with progenitor cell transplantation and gene delivery can be overcome with small molecule activators or inhibitors of gene expression. It is conceivable that local delivery of a compound that temporarily inhibits p27Kip1, retinoblastoma, or other cyclin-dependent kinase inhibitors expressed in the organ of Corti45 would lead to cell proliferation. Suppression of cell cycle inhibitors, however, could be playing with fire, because unregulated cell proliferation carries the danger of tumor formation. It is therefore imperative to focus on compounds that specifically target the damaged organ of Corti. For example, cell cycle inhibitors that are specifically expressed in the organ of Corti, such as p27Kip1, would be a much safer target than those ubiquitously expressed in the cochlea, such as retinoblastoma. Atoh1-activating compounds, on the other hand, would be useful in initiating hair cell differentiation in the organ of Corti, where, at least shortly after hair cell loss, supporting cells seem to be competent to respond to Atoh1 expression. The Notch pathway is also involved in Atoh1 regulation, and γ-secretase inhibitors seem to be effective initiators of hair cell formation in the embryonic and neonatal organ of Corti, but they lack efficacy in the adult organ. The encapsulation of the cochlea offers an advantage for localized drug treatment, which would potentially allow the use of drugs that cannot be applied systemically. At present, however, no effective compounds that directly address appropriate cell cycle inhibitors or Atoh1 gene expression are known, and manipulations of Notch signaling seem to be not very effective in the adult organ of Corti.
Conclusions and outlook
Substantial progress has been made in recent years to accumulate tools that potentially can be used, alone or in combination, to develop strategies for hair cell regeneration. Grafting of stem or progenitor cells, viral gene delivery, and manipulations that affect Atoh1 and cell cycle genes have all reached the proof-of-principle stage. At the same time, the accumulated knowledge of organ of Corti function—the integral interplay of OHC-based cochlear amplification, micromechanical movements and the importance of accessory structures such as the tectorial membrane—revealed a plethora of new challenges for cochlear hair cell regeneration. A systematic elaboration of these roadblocks is needed before it will be possible to judge whether mammalian cochlear hair cell regeneration will become a viable treatment option for future generations of people suffering from profound hearing loss.
Nevertheless, these challenges also bring opportunities for new discoveries. For basic science studies, we expect that the next decade will reveal many more aspects of the developmental principles underlying cochlear cell type specification. Exploration of new technologies—for example, the direct conversion of supporting cells in the damaged cochlea into a progenitor cell state—will require more knowledge about transcription factors that define the otic lineage. Likewise, we need to study in much more detail the molecular and cytohistological changes that happen in supporting cells in the organ of Corti after damage. These cells are the key targets of all the manipulations that we have discussed in this perspective. On the translational side, we expect that some of the proof-of-principle studies that we discussed will be refined—for example, by combination of specific strategies such as Atoh1 expression and temporary interference with cell cycle genes. Finally, the ball is in the court of bioengineers and clinician/scientists to develop appropriate devices and methods that will allow us to deliver progenitor cells, gene therapy vectors and drug candidates to the appropriate locations inside the cochlea, so that it will be possible to develop better animal models for regenerative studies. Overall, the task of regenerating cochlear sensory hair cells has not become less challenging, but recent advances now allow us to define the issues that still persist much better than ever before, which is providing a much-needed framework allowing the systematic study of potential treatment options.
Acknowledgments
We gratefully acknowledge support by the US National Institutes of Health (DC008595 to J.V.B. and DC06167 to S.H.), the McKnight Endowment Fund for Neuroscience (J.V.B. and S.H.) and the California Institute for Regenerative Medicine (RC1-00119 to S.H.).
Footnotes
Published online at http://www.nature.com/natureneuroscience/
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Dallos P, Billone MC, Durrant JD, Wang C, Raynor S. Cochlear inner and outer hair cells: functional differences. Science. 1972;177:356–358. doi: 10.1126/science.177.4046.356. [DOI] [PubMed] [Google Scholar]
- 2.Merchant SN, Adams JC, Nadol JB., Jr Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2005;26:151–160. doi: 10.1097/00129492-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Nadol JB, Jr, Merchant SN. Histopathology and molecular genetics of hearing loss in the human. Int J Pediatr Otorhinolaryngol. 2001;61:1–15. doi: 10.1016/s0165-5876(01)00546-8. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt KA, Liberman MC, Nadol JB., Jr Morphometric analysis of age-related changes in the human basilar membrane. Ann Otol Rhinol Laryngol. 2001;110:1147–1153. doi: 10.1177/000348940111001212. [DOI] [PubMed] [Google Scholar]
- 5.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 6.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins JE, Jr, Johnsson LG, Stebbins WC, Moody DB, Coombs SL. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol (Stockh) 1976;81:337–343. doi: 10.3109/00016487609119971. [DOI] [PubMed] [Google Scholar]
- 8.Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton. 1991;18:215–227. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- 9.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 10.Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 11.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds BA, Rietze RL. Neural stem cells and neurospheres—re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Corwin JT. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc Natl Acad Sci USA. 2007;104:16675–16680. doi: 10.1073/pnas.0704576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima K, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savary E, et al. Distinct population of hair cell progenitors can be isolated from the postnatal mouse cochlea using side population analysis. Stem Cells. 2007;25:332–339. doi: 10.1634/stemcells.2006-0303. [DOI] [PubMed] [Google Scholar]
- 17.Zhai S, et al. Isolation and culture of hair cell progenitors from postnatal rat cochleae. J Neurobiol. 2005;65:282–293. doi: 10.1002/neu.20190. [DOI] [PubMed] [Google Scholar]
- 18.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumikawa M, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 21.Lumpkin EA, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 22.Bermingham NA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 23.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 25.Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- 27.Mansour SL, et al. Hearing loss in a mouse model of Muenke syndrome. Hum Mol Genet. 2009;18:43–50. doi: 10.1093/hmg/ddn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 29.Daudet N, et al. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto N, et al. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- 32.Takebayashi S, et al. Multiple roles of Notch signaling in cochlear development. Dev Biol. 2007;307:165–178. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 33.Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- 34.Hori R, et al. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–1914. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- 35.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 36.Lowenheim H, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of Corti. Proc Natl Acad Sci USA. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantela J, et al. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sage C, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 39.Sage C, et al. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci USA. 2006;103:7345–7350. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber T, et al. Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proc Natl Acad Sci USA. 2008;105:781–785. doi: 10.1073/pnas.0708061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heller S, Raphael Y. Emerging strategies for restoring the cochlea. In: Schacht J, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research vol. 31, Auditory Trauma, Protection, and Repair. Springer; New York: 2008. pp. 321–338. [Google Scholar]
- 42.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nin F, et al. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci USA. 2008;105:1751–1756. doi: 10.1073/pnas.0711463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med. 2008;10:610–618. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- 45.Chen P, et al. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- 46.Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richter CP, Emadi G, Getnick G, Quesnel A, Dallos P. Tectorial membrane stiffness gradients. Biophys J. 2007;93:2265–2276. doi: 10.1529/biophysj.106.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell IJ, et al. Sharpened cochlear tuning in a mouse with a genetically modified tectorial membrane. Nat Neurosci. 2007;10:215–223. doi: 10.1038/nn1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flock A, Strelioff D. Graded and nonlinear mechanical properties of sensory hairs in the mammalian hearing organ. Nature. 1984;310:597–599. doi: 10.1038/310597a0. [DOI] [PubMed] [Google Scholar]
- 50.Ricci AJ, Crawford AC, Fettiplace R. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron. 2003;40:983–990. doi: 10.1016/s0896-6273(03)00721-9. [DOI] [PubMed] [Google Scholar]