Abstract
Objective:
To identify factors associated with timely initiation of antibiotic therapy for patients hospitalized with pneumonia.
Design:
Secondary analysis of a cluster-randomized, controlled trial.
Setting:
32 emergency departments (ED) in Pennsylvania and Connecticut.
Subjects:
Patients with a clinical and radiographic diagnosis of community acquired pneumonia.
Interventions:
From January to December 2001, EDs were randomly allocated to guideline implementation strategies of low (n=8), moderate (n=12), and high intensity (n=12) to improve the initial site of treatment and the performance of evidence-based processes of care. Our primary outcome was antibiotic initiation within 4 hours of presentation, which at that time was the recommended process of care for inpatients.
Results:
Of the 2076 inpatients enrolled, 1632 (78.6%) received antibiotic therapy within 4 hours of presentation. Antibiotic timeliness ranged from 55.6% to 100% (p<0.001) by emergency department and from 77.0% to 79.7% (p=0.2) across the three guideline implementation arms. In multivariable analysis, heart rate ≥ 125 per minute (OR=1.6, 95% CI 1.1-2.3), respiratory rate ≥30 per minute (OR=2.3, 95% CI 1.6-3.4), and aspiration pneumonia (OR=3.7, 95% CI 1.1-12.7) were positively associated with timely initiation of antibiotic therapy, while a hematocrit <30% (OR=0.6, 95% CI 0.4-1.0) was negatively associated with this outcome.
Conclusions:
Timely initiation of antibiotic therapy is associated primarily with patient-related factors that reflect severity of illness at presentation. Although this study demonstrates an opportunity to improve performance on this quality measure in nearly one quarter of inpatients with pneumonia, we failed to identify any modifiable patient, provider, or hosptial level factors to target in such quality improvement efforts.
Keywords: pneumonia, quality of care, process of care
Introduction
Community-acquired pneumonia is a common, costly, and potentially life-threatening illness. To improve the quality and efficiency of care of this illness, national medical societies, such as the Infectious Disease Society of America and the American Thoracic Society, as well as foreign counterparts such as the European Respiratory Society have developed medical practice guidelines with specific treatment recommendations.[1,2]
Rapid initiation of antibiotic therapy is a process of care commonly recommended by these guidelines that is associated with decreased mortality and shortened length-of-stay for patients hospitalized with pneumonia.[3-5] While the performance of timely antibiotic therapy has been associated with improved pneumonia patient outcomes, compliance with this performance measure remains suboptimal.[4,6-8] Although prior studies identified factors that are associated with this recommended process of care, such as patient race, geographic location, and hospital size, these studies are limited by their retrospective design, focus on older populations, and failure to assess provider level factors.[9-11]
Better understanding of the factors associated with timely initiation of antibiotic therapy could help focus quality improvement efforts to increase compliance with this quality indicator and could potentially lead to improved short-term patient outcomes. The purpose of this study is to identify the patient, provider, and hospital level factors associated with timely initiation of antibiotic therapy in the Emergency Department Triage of Community-Acquired Pneumonia (EDCAP) Trial.[12,13]
Methods
We studied inpatients enrolled in the EDCAP Trial, a multicenter, cluster-randomized controlled trial carried out at 32 hospital emergency departments (EDs) between January 2001 and December 2001. In EDCAP, we randomized study sites to low, moderate, or high-intensity intervention strategies to implement the Pneumonia Severity Index (PSI) to guide the initial site of treatment [12,13] and to perform four evidence-based initial processes of care for inpatients and outpatients with pneumonia. In addition to comparing the effectiveness and safety of varying intensities of intervention strategies, a secondary aim of the EDCAP Trial was to identify factors associated with individual process of care outcomes in the treatment of pneumonia. Institutional review boards responsible for all sites approved the trial and all enrolled patients provided informed consent for participation. Detailed methods and the primary study findings are reported elsewhere.[12,13]
Patient Eligibility and Study Sites
Eligible patients were 18 years of age or older with a clinical diagnosis of pneumonia and evidence of a new pulmonary infiltrate identified on a chest radiograph by the ED provider or an on-site radiologist. We excluded patients if they had hospital-acquired pneumonia, immunosuppression, specified comorbid conditions (e.g., cystic fibrosis or pulmonary tuberculosis), or psychosocial conditions/substance abuse that were incompatible with outpatient treatment, enrollment, or follow-up. We also excluded incarcerated, homeless, pregnant, or previously enrolled patients, and those enrolled in another research protocol.
The 32 EDs participating in the EDCAP Trial were evenly distributed between Pennsylvania and Connecticut. Of these EDs, 29 (90.6%) were in urban locations and 16 (50.0%) were in teaching hospitals.
Practice Guideline
The practice guideline utilized in the EDCAP Trial consisted of recommendations for four initial processes of care and the initial site of treatment following presentation to the ED.(13) It was based on a systematic review of the literature, developed using expert consensus, and approved by medical providers at all participating EDs. The processes of care recommended for inpatients were: 1) Initiation of antibiotic therapy within 4 hours of initial presentation; 2) Appropriate use of antibiotics; 3) Obtaining 2 blood cultures prior to antibiotic administration; and 4) Assessment of arterial oxygenation upon presentation. All four processes of care were recommended by medical specialty society guidelines published prior to the trial.[1,2]
Baseline Data Collection
Research staff collected baseline patient, provider, and hospital characteristics. We documented demographic data (age, sex, race, nursing home status, and medical insurance status), comorbid medical conditions (neoplastic disease, chronic liver disease, congestive heart failure, cerebrovascular disease, renal disease, cognitive impairment, coronary artery disease, chronic pulmonary disease, and diabetes mellitus), physical examination findings (temperature, heart rate, respiratory rate, and systolic blood pressure), laboratory and radiographic findings (arterial oxygenation, arterial pH, BUN, serum sodium, serum glucose, hematocrit, and pleural effusion by x-ray), and recent medical treatments (home oxygen therapy, oral or inhaled corticosteroid therapy, and antibiotic therapy within seven days of presentation).
We collected the following ED medical provider characteristics: provider type (physician, physician's assistant, or nurse practitioner), demographics (sex, age, race), number of shifts worked per month, and year of medical school graduation and specialty (emergency medicine, internal medicine, family practice, other) for physicians. We also collected the following ED characteristics: teaching status, annual volume, availability of observation unit(s), use of a pneumonia pathway, and use of a standardized pneumonia order form. Additional ED practice patterns were ascertained from a questionnaire distributed to all participating ED Directors, including their assessments of the success of systematic implementation of guidelines promoting timely antibiotic initiation and site-specific tracking and auditing of timely antibiotic therapy at their practice site.
We quantified severity of illness using the pneumonia severity index (PSI), which is a validated prediction rule for prognosis that stratifies patients with pneumonia into five classes of risk for mortality and other adverse medical outcomes.[14] Variables included in the PSI include patient medical history, physical examination findings, and selected laboratory and radiographic findings readily available at the time of presentation. Patients without any of the 11 prognostic factors in the first step of the PSI were assigned to risk class I. The remaining patients were assigned to risk classes II-V based on total risk points for the 20 PSI variables.
Primary Outcome
The primary outcome for this study was timeliness of antibiotic treatment for inpatients, defined as the time from presentation first charted in the ED to delivery of the first dose of antibiotic therapy. We defined inpatient treatment as hospital admission, transfer from an ED to an inpatient hospital observation unit, or admission to an ED observation unit with discharge to any setting more than 24 hours after initial presentation. To quantify this outcome, we assessed the proportion of patients who received antibiotic therapy within four hours of presentation, as defined by the study practice guideline.
Methods of Analysis
We used chi-square tests to assess univariate associations between patient characteristics and the primary study outcome. We used 3-level logistic regression modeling to assess univariate associations between provider or hospital level factors and the primary outcome, accounting for the clustering of patients at the provider and hospital levels. We then included those patient, provider, and hospital level factors associated with the primary outcome at p≤0.10 in univariate analyses in a multilevel logistic regression model of antibiotic timeliness, controlling for study intervention arm. We created a parsimonious model by using a backward stepwise approach to remove variables from the main effects model with p-values greater than 0.05. We present odds ratios (ORs) and 95% confidence intervals (CIs) for all variables associated with antibiotic timeliness. For all statistical analyses, we used Stata version 9.0 (StataCorp, College Station, Texas). The multilevel multivariable regression models were fitted using the GLLAMM macro.[15]
Results
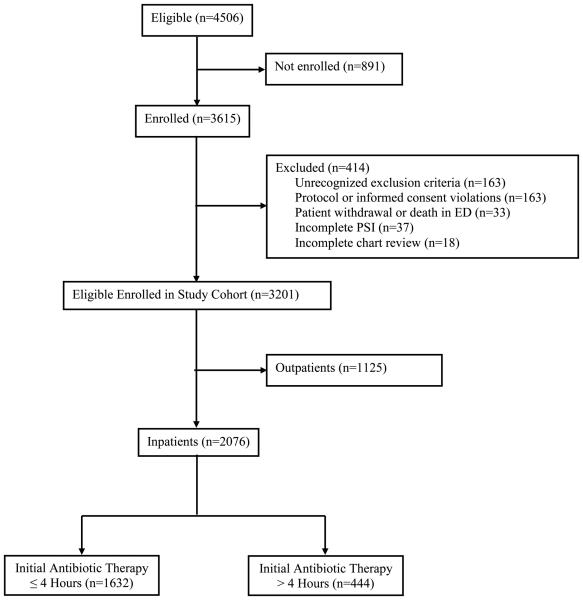
In the EDCAP Trial, 3615 (80.2%) of 4506 eligible patients were enrolled, with a median of 113 patients enrolled per site (interquartile range, 101 to 123; Figure 1). From the enrolled patients, 414 patients were subsequently excluded, most frequently due to the discovery of an exclusion criterion post-enrollment or protocol or consent violations. This analysis focuses on the 2076 inpatients in the final EDCAP study cohort; 1632 (78.6%) received initial antibiotic therapy within four hours of presentation to the hospital.
Figure 1. Patient Enrollment for the EDCAP Trial and Resulting Guideline Compliance with Timely Antibiotic Administration.
In the EDCAP Trial, a total of 4506 patients were eligible, 3615 were initially enrolled, and 414 were subsequently excluded. Reasons for exclusion included: (1) discovery of one or more exclusion criteria not recognized at presentation (n=163); (2) enrollment protocol or informed consent violations (n=163); (3) patient withdrawal or death in the emergency department before assignment of initial site of treatment (n=33); (4) incomplete information for PSI risk class (n=37); and (5) incomplete medical record review (n=18)., The most common reasons for protocol violations were: (1) enrollment of patients managed by a study investigator (n=47); (2) previous study enrollment (n=32); and (3) participation in a competing research protocol (n=29). Overall, of the 3201 patients who comprised the entire study cohort to assess initial processes of care, 2076 were treated as inpatients, of whom 1632 (78.6%) received initial antibiotic therapy within 4 hours of presentation.
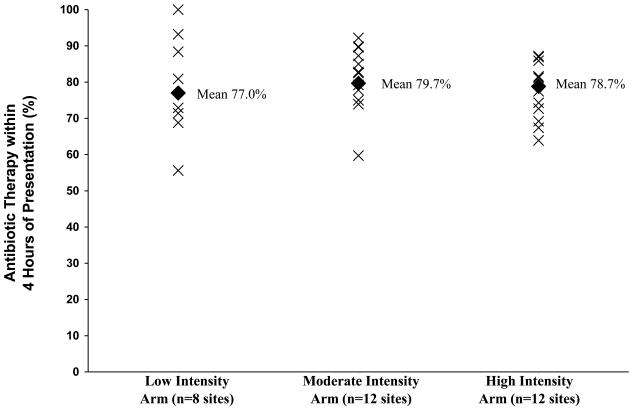
Across the 32 study sites, rates of compliance with our primary outcome ranged from 55.6% to 100% (p< 0.001), with no statistically significant differences observed across the three intervention arms (low intensity 77.0%, moderate intensity 79.7%, high intensity 78.8%; p=0.2). The site-specific proportions stratified by intervention arm are shown in Figure 2.
Figure 2. Site Specific Timeliness of Antibiotic Therapy by Intervention Arm.
This graph shows the site-specific proportions (X's) of patients who received initial antibiotic therapy within 4 hours of presentation of all 32 sites randomized to the low intensity (n=8), moderate intensity (n=12), and high intensity (n=12) intervention arms of the EDCAP Trial. The diamonds denote the corresponding proportions for all inpatients within each intervention arm.
Baseline Patient, Provider, and Site Characteristics
The majority of inpatients were older than 51 years of age (86.1%), 8.0% were non-white, 51.2% were female, and 5.8% resided in nursing homes (Table 1). Less than half (46.4%) of patients were in the two highest PSI risk classes (class IV and V), and almost 75% had at least one of the comorbid conditions listed in Table 1.
Table 1.
Prevalence of Baseline Patient Characteristics by Timeliness of Initial Antibiotic Therapy*
| Patient Characteristics | % Patients Receiving Antibiotics ≤ 4 Hours (N=1632) |
% Patients Receiving Antibiotics > 4 Hours (N=444) |
% Total Patients (N=2076) |
|---|---|---|---|
| Demographics | |||
| Age ≥51 years | 86.2 | 85.6 | 86.1 |
| Non-white race | 8.1 | 7.9 | 8.0 |
| Female sex | 51.0 | 51.4 | 51.2 |
| Medical insurance | |||
| Medicare or commercial | 84.4 | 82.4 | 84.0 |
| Medicaid | 11.7 | 13.5 | 12.1 |
| Uninsured | 3.9 | 4.1 | 3.9 |
| Living alone | 19.5 | 20.9 | 19.8 |
| Nursing home resident | 6.1 | 4.7 | 5.8 |
| Pneumonia severity index (PSI) | |||
| Class I | 7.3 | 7.9 | 7.4 |
| Class II | 18.9 | 22.7 | 19.7 |
| Class III | 26.7 | 25.7 | 26.5 |
| Class IV | 37.5 | 35.6 | 37.1 |
| Class V | 9.7 | 8.1 | 9.3 |
| Comorbid conditions in PSI | |||
| Neoplastic disease | 3.5 | 4.1 | 3.6 |
| Liver disease | 0.7 | 1.6 | 0.9 |
| Congestive heart failure | 20.3 | 16.4 | 19.5 |
| Cerebrovascular disease | 11.6 | 9.2 | 11.1 |
| Renal disease | 4.5 | 5.6 | 4.8 |
| Other comorbid conditions | |||
| Cognitive impairment | 7.8 | 6.3 | 7.5 |
| History of coronary artery disease | 27.5 | 28.2 | 27.7 |
| Chronic pulmonary disease | 38.4 | 39.9 | 38.7 |
| Diabetes mellitus | 25.0 | 23.0 | 24.6 |
| One or more of the above conditions | 74.6 | 75.5 | 74.8 |
| Vital signs | |||
| Temperature <35°C or ≥40°C | 2.3 | 2.3 | 2.3 |
| Pulse ≥125 per minute | 13.2 | 8.6 | 12.3 |
| Respiratory rate ≥ 30/minute (N=2068) | 17.2 | 7.9 | 15.2 |
| Systolic BP <90 mmHg (N=2074) | 2.3 | 2.0 | 2.3 |
| Laboratory findings | |||
| Arterial pH <7.35 (N=2076) | 3.6 | 2.9 | 3.4 |
| BUN ≥ 30mg/dL (N=2023) | 21.7 | 21.5 | 21.7 |
| Sodium <130mEq/L (N=2023) | 5.9 | 4.7 | 5.6 |
| Glucose ≥250 mg/dL (N=2010) | 7.9 | 3.6 | 7.7 |
| Hematocrit <30% (N=2061) | 6.0 | 9.3 | 6.7 |
| Treatments prior to presentation | |||
| Home oxygen therapy | 9.0 | 11.3 | 9.5 |
| Oral or inhaled corticosteroid therapy | 15.4 | 16.7 | 15.7 |
| Antibiotic therapy within 7 days | 14.6 | 18.7 | 15.5 |
| Radiographic findings | |||
| Bilateral radiographic infiltrates (N=2054) | 18.9 | 18.7 | 18.9 |
| Multilobar radiographic infiltrates (N=2053) | 24.3 | 21.9 | 23.8 |
| Pleural effusion (N=2054) | 18.9 | 21.6 | 19.5 |
| Other | |||
| Antibiotic allergy or intolerance | 23.5 | 22.7 | 23.4 |
| Suspected aspiration | 2.0 | 0.7 | 1.2 |
| Physician order limiting resuscitative efforts | 4.2 | 3.6 | 4.1 |
Denominators for frequency calculations were based on the entire inpatient population (N=2076) except for vital signs, laboratory, and radiographic findings where, due to missing data, specific denominators are listed.
The providers who treated inpatients in this cohort were predominantly male (81.0%), white (86.9%), and graduates of medical school in the 1990's (38.9%) or the 1980's (38.1%); 67.0% worked >11 ED shifts/month (Table 2). Half of all EDs had an annual volume <34,000 patient visits, and almost half (46.9%) had an observation unit. More than 80% of EDs reported systematic institutional implementation of guidelines to initiate antibiotic therapy within 4 hours, and 53.1% of sites performed some form of site-specific tracking or auditing of compliance with the 4 hour antibiotic initiation guideline recommendation.
Table 2.
Prevalence of Baseline Provider and Site Characteristics
| Provider Characteristics (n=378 Providers)* | Frequency | % |
|---|---|---|
| Male | 306 | 81.0 |
| Race (n=375) | ||
| White, non-Hispanic | 326 | 86.9 |
| Black, non-Hispanic | 4 | 1.1 |
| Hispanic | 2 | 0.5 |
| Asian or Pacific Islander | 33 | 8.8 |
| Other | 10 | 2.7 |
| Year of medical school graduation (n=375) | ||
| ≥1990 | 146 | 38.9 |
| 1980-89 | 143 | 38.1 |
| 1960-79 | 86 | 22.9 |
| Number of emergency department shifts per month | ||
| ≥12 | 252 | 67.0 |
| 5-11 | 65 | 17.3 |
| 1-4 | 59 | 15.7 |
| ED director | 31 | 8.2 |
| Emergency Department Characteristics (n=32 EDs) | Frequency | % |
|
| ||
| Intervention arm | ||
| Low intensity | 8 | 25.0 |
| Moderate intensity | 12 | 37.5 |
| High intensity | 12 | 37.5 |
| State | ||
| Pennsylvania | 16 | 50.0 |
| Connecticut | 16 | 50.0 |
| Teaching hospital | 16 | 50.0 |
| Annual volume < 34,000 visits | 16 | 50.0 |
| Presence of an observation unit | 15 | 46.9 |
| ED Practice Patterns | ||
| Systematic institutional implementation of within 4 hours antibiotic initiation guidelines |
26 | 81.2 |
| Site-specific tracking/auditing of within 4 hours initiation of antibiotics | 17 | 53.1 |
| Distribution of patient specific data provided to ED provider | 13 | 40.6 |
| ED aggregate data about first dose antibiotics within 4 hours for pneumonia inpatients was distributed to ED providers |
15 | 46.9 |
| ED providers were asked to identify barriers to giving pneumonia inpatients the first dose of antibiotics within 4 hours |
14 | 43.8 |
Denominators for frequency calculations were based on all providers who treated inpatients (n=378), except for year of medical school graduation and number of emergency department shifts per month where, due to missing data, specific denominators are listed.
Factors Associated with Timely Antibiotic Therapy
Of the 35 patient factors examined, 7 had a univariate association with initiation of antibiotic therapy within 4 hours of presentation at the p≤0.10 level (Table 3). History of congestive heart failure, pulse ≥125 beats per minute, respiratory rate ≥30 per minute, and suspected aspiration were associated with increased odds of timely antibiotic therapy, while history of chronic liver disease, hematocrit <30%, and antibiotic therapy within the past 7 days were associated with decreased odds of timely antibiotic therapy. No physician or hospital characteristics had a univariate associations with antibiotic timeliness.
Table 3.
Factors Associated with Timely Antibiotic Therapy for Inpatients (within 4 hours)
| Patient Characteristics* |
Timely Antibiotic Therapy (%) |
Univariate Association* * OR (95% CI) |
Multivariable Association OR (95% CI) |
|---|---|---|---|
| Liver disease | |||
| Yes | 61.1 | 0.4 (0.2, 1.3) | --- |
| No | 78.8 | ||
| Hematocrit < 30% | |||
| Yes | 70.3 | 0.6 (0.4, 0.9) | 0.6 (0.4, 1.0) |
| No | 78.6 | ||
| Antibiotic therapy within 7 days | |||
| Yes | 74.1 | 0.7 (0.6. 1.0) | --- |
| No | 85.4 | ||
| Congestive heart failure | |||
| Yes | 81.9 | 1.3 (0.9, 1.7) | --- |
| No | 77.8 | ||
| Pulse ≥ 125 per minute | |||
| Yes | 85.0 | 1.6 (1.1, 2.4) | 1.6 (1.1, 2.3) |
| No | 77.7 | ||
| Respiratory rate ≥ 30/minute |
|||
| Yes | 88.8 | 2.5 (1.7, 3.7) | 2.3 (1.6, 3.4) |
| No | 76.4 | ||
| Suspected aspiration | |||
| Yes | 91.4 | 2.9 (0.9, 15.1) | 3.7 (1.1, 12.7) |
| No | 78.3 |
The multivariable analysis was based on 2051 (98.8%) of inpatients who met the eligibility criteria and had no missing data on the relevant predictor or study outcome variables [i.e., 25 patients (1.2%) had at least 1 missing data element].
Defined as p<0.10
In multivariable analyses, four factors were independently associated with timely antibiotic therapy (Table 3). Patients with a pulse ≥125 beats per minute (OR=1.6; 95% CI 1.1-2.3), a respiratory rate ≥30 per minute (OR=2.3; 95% CI 1.6-3.4), and suspected aspiration (OR=3.7; 95% CI 1.1-12.7) were associated with significantly increased odds of receiving timely antibiotic therapy. Hematocrit <30% was inversely associated with this outcome (OR=0.6; 95% CI 0.4-1.0).
Discussion
In our study involving 2076 patients hospitalized for community-acquired pneumonia, initiation of antibiotic therapy within 4 hours of presentation varied significantly by site of treatment and was associated primarily with patient factors that reflect severity of illness at the time of presentation. Tachycardia (pulse ≥125/minute) and tachypnea (respiratory rate ≥ 30/minute), both identified as independent predictors of mortality for patients with pneumonia,[14] were independently associated with initiation of timely antibiotic therapy. Suspected aspiration pneumonia, also associated with an increased risk of short-term mortality and frequently representing a marker of underlying neurologic impairment,[16] was also an independent predictor of this outcome. These findings suggest that ED medical providers are appropriately using their clinical judgment to identify more severely ill patients likely to benefit most from rapid initiation of antibiotic therapy.
In our study, anemia (hematocrit <30%) was negatively associated with timely initiation of antibiotic therapy. This unexpected finding is inconsistent with our other observations that markers of severity of illness are postively associated with timely initiation of antibiotic therapy. Although the association between hematocrit and the study outcome could be explained if anemia was a confounder of factors previously shown to have a negative association with timeliness of antibiotic therapy (e.g., race and hospital size) or was associated with factors that could potentially impede timely initiation of therapy (e.g., time of presentation), we found no association between anemia and these types of variables.
Our observations that patient factors reflecting severity of illness are positively associated with timely initiation of antibiotic therapy are consistent with prior studies, which reported that tachycardia, tachypnea, and fever were independently associated with timely antibiotic therapy.[9] Although not found to be associated with timely initiation of antibiotic therapy in our analysis, prior studies have identified patient race, prior receipt of antibiotics within 48 hours, and history of cerebrovascular disease as having independent associations with this process of care.[9,10]
Our study failed to identify any provider or hospital level factors associated with timely inititation of antibiotic therapy. Prior studies have suggested that geographic location, time of initial presentation (7 am – 3 pm, 11 pm – 7 am), admission to a major teaching hospital, emergency department crowding, and larger hospital size were negatively associated with initiation of antibiotic therapy within 4 hours of presentation to the hospital.[9,10,17] In addition, high nurse-to-bed ratios and receipt of first dose antibiotics within the ED have been previously found to be positively associated with timely initiation of antibiotic therapy.[9-11]
Even the most intensive multi-faceted guideline implementation strategy used in the original EDCAP Trial did not significantly improve the performance rate for rapid initiation of antibiotic therapy, which was nearly 80% across all study sites.[13] Of 12 previously published interventional studies designed to improve performance on this quality measure, 8 resulted in significant improvements in antibiotic timeliness.[13,18-28] Interventions that focused exclusively on a single process of care, were multi-faceted in design, and involved multiple stakeholders in patient care (e.g., physicians, nurses, pharmacists) were most likely to be successful.
The limitations of our study should be acknowledged. First, demographic differences between patients who were and were not enrolled in the EDCAP trial and the fact that the majority of study sites were from urban areas may diminish the generalizability of our findings. However, the 80% enrollment rate was laudable for a large multicenter trial. Second, the associations between patient, provider, or hospital factors and timeliness of initial antibiotic therapy examined in our study might have been altered by the design of the EDCAP Trial to test the effectiveness of three intervention arms of incremental intensity in increasing the proportion of low-risk patients treated as outpatients. However, we did not observe any statistically significant differences in our outcome across intervention arm or any first-order interactions between intervention arm and the independent predictors of our study outcome. Third, because antibiotic timeliness as a quality measure has been hotly debated due to potential unintended consequences of this recommendation, the Center for Medicare and Medicaid Services and the Joint Commission now use a 6 hour benchmark to define antibiotic timeliness.[1] Finally, that provider and system-level factors were not independently associated with this outcome in our analysis may reflect a lack of statistical power in this 32-site study, or a failure to collect the most relevant provider and hospital level factors.
In conclusion, in a cohort of inpatients at 32 hospitals participating in a quality improvement trial for pneumonia, we found that timely initiation of antibiotic therapy is related primarily to patient factors that reflect severity of illness at presentation. Although this study demonstrates an opportunity to improve performance on this quality measure in nearly one quarter of patients hospitalized for pneumonia, we failed to identify any provider or hospital level factors to target in such quality improvement efforts.
Acknowledgements
This research was supported by the Agency for Healthcare Research and Quality (RO1 HS10049). Dr. M.J. Fine was supported in part by a career developmental award from the National Institute for Allergy and Infectious Diseases (K24 AI001769). We thank Kelly H. Burkitt, PhD for her assistance with critical revisions of the manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ERS Task Force Report Guidelines for management of adult community-acquired lower respiratory tract infections. European Respiratory Society. Eur Respir J. 1998;11:986–91. doi: 10.1183/09031936.98.11040986. [DOI] [PubMed] [Google Scholar]
- 3.Battleman DS, Callahan M, Thaler HT. Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community-acquired pneumonia: link between quality of care and resource utilization. Arch Intern Med. 2002;162:682–8. doi: 10.1001/archinte.162.6.682. [DOI] [PubMed] [Google Scholar]
- 4.Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–44. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 5.Meehan TP, Fine MJ, Krumholz HM, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–4. [PubMed] [Google Scholar]
- 6.Feagan BG, Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK. Treatment and outcomes of community-acquired pneumonia at Canadian hospitals. CMAJ. 2000;162:1415–20. [PMC free article] [PubMed] [Google Scholar]
- 7.Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to Medicare beneficiaries: A profile at state and national levels. JAMA. 2000;284:1670–6. doi: 10.1001/jama.284.13.1670. [DOI] [PubMed] [Google Scholar]
- 8.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 9.Fine JM, Fine MJ, Galusha D, Petrillo M, Meehan TP. Patient and hospital characteristics associated with recommended processes of care for elderly patients hospitalized with pneumonia: results from the Medicare quality indicator system pneumonia module. Arch Intern Med. 2002;162:827–33. doi: 10.1001/archinte.162.7.827. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen EM, Cornell J, Whittle J. Racial variations in processes of care for patients with community-acquired pneumonia. BMC Health Serv Res. 2004;4:20. doi: 10.1186/1472-6963-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schouten JA, Hulscher ME, Kullberg BJ, Cox A, Gyssens IC, van der Meer JW, Grol RP. Understanding variation in quality of antibiotic use for community-acquired pneumonia: effect of patient, professional and hospital factors. J Antimicrob Chemother. 2005;56:575–82. doi: 10.1093/jac/dki275. [DOI] [PubMed] [Google Scholar]
- 12.Yealy DM, Auble TE, Stone RA, et al. The emergency department community-acquired pneumonia trial: Methodology of a quality improvement intervention. Ann Emerg Med. 2004;43:770–82. doi: 10.1016/j.annemergmed.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med. 2005;143:881–94. doi: 10.7326/0003-4819-143-12-200512200-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 15.Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear mixed models using adaptive quadrature. The Stata Journal. 2002;2:1–21. [Google Scholar]
- 16.Leroy O, Georges H, Beuscart C, et al. Severe community-acquired pneumonia in ICUs: prospective validation of a prognostic score. Intensive Care Med. 1996;22:1307–14. doi: 10.1007/BF01709543. [DOI] [PubMed] [Google Scholar]
- 17.Fee C, Weber EJ, Maak CA, Bacchetti P. Effect of emergency department crowding on time to antibiotics in patients admitted with community-acquired pneumonia. Ann Emerg Med. 2007;50:501–9. doi: 10.1016/j.annemergmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Barlow G, Nathwani D, Williams F, et al. Reducing door-to-antibiotic time in community-acquired pneumonia: Controlled before-and-after evaluation and cost-effectiveness analysis. Thorax. 2007;62:67–74. doi: 10.1136/thx.2005.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benenson R, Magalski A, Cavanaugh S, Williams E. Effects of a pneumonia clinical pathway on time to antibiotic treatment, length of stay, and mortality. Acad Emerg Med. 1999;6:1243–8. doi: 10.1111/j.1553-2712.1999.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 20.Meehan TP, Weingarten SR, Holmboe ES, et al. A statewide initiative to improve the care of hospitalized pneumonia patients: The Connecticut Pneumonia Pathway Project. Am J Med. 2001;111:203–10. doi: 10.1016/s0002-9343(01)00803-8. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence SJ, Shadel BN, Leet TL, Hall JB, Mundy LM. An intervention to improve antibiotic delivery and sputum procurement in patients hospitalized with community-acquired pneumonia. Chest. 2002;122:913–9. doi: 10.1378/chest.122.3.913. [DOI] [PubMed] [Google Scholar]
- 22.Chu LA, Bratzler DW, Lewis RJ, Murray C, Moore L, Shook C, Weingarten SR. Improving the quality of care for patients with pneumonia in very small hospitals. Arch Intern Med. 2003;163:326–32. doi: 10.1001/archinte.163.3.326. [DOI] [PubMed] [Google Scholar]
- 23.Metersky ML, Galusha DH, Meehan TP. Improving the care of patients with community-acquired pneumonia: a multihospital collaborative QI project. Conn Med. 1999;63:425–31. [PubMed] [Google Scholar]
- 24.Halm EA, Horowitz C, Silver A, Fein A, Dlugacz YD, Hirsch B, Chassin MR. Limited impact of a multicenter intervention to improve the quality and efficiency of pneumonia care. Chest. 2004;126:100–7. doi: 10.1378/chest.126.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capelastegui A, Espana PP, Quintana JM, Gorordo I, Ortega M, Idoiaga I, Bibao A. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis. 2004;39:955–63. doi: 10.1086/423960. [DOI] [PubMed] [Google Scholar]
- 26.Schouten JA, Hulscher ME, Trap-Liefers J, Akkermans RP, Kullberg BJ, Grol R, van der Meer JW. Tailored interventions to improve antibiotic use for lower respiratory tract infections in hospitals: a cluster-randomized, controlled trial. Clin Infect Dis. 2007;44:931–41. doi: 10.1086/512193. [DOI] [PubMed] [Google Scholar]
- 27.Rollins D, Thomasson C, Sperry B. Improving antibiotic delivery time to pneumonia patients: continuous quality improvement in action. J Nurs Care Qual. 1994;8:22–31. doi: 10.1097/00001786-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Quality of care improvements for patients with pneumonia. Florida Medical Quality Assurance, Inc. Eval Health Prof. 1998;21:514–24. doi: 10.1177/016327879802100409. [DOI] [PubMed] [Google Scholar]