Abstract
Objectives
The objective of this study was to investigate the effect of acupuncture administered during myelosuppressive chemotherapy on white blood cell (WBC) count and absolute neutrophil count (ANC) in patients with ovarian cancer.
Design
This study is a pilot, randomized, sham-controlled clinical trial. Patients received active acupuncture versus sham acupuncture while undergoing chemotherapy. A standardized acupuncture protocol was employed with manual and electrostimulation. The frequency of treatment was 2–3 times per week for a total of 10 sessions, starting 1 week before the second cycle of chemotherapy.
Setting
The setting was two outpatient academic centers for patients with cancer.
Subjects
Twenty-one (21) newly diagnosed and recurrent ovarian cancer patients were the subjects.
Outcome measures
WBC count, ANC, and plasma granulocyte colony-stimulating factor (G-CSF) were assessed weekly.
Results
The median leukocyte value in the acupuncture arm at the first day of the third cycle of chemotherapy was significantly higher than in the control arm after adjusting for baseline value (8600 cells/μL, range: 4800–12,000 versus 4400 cell/μL, range: 2300–10,000) (p = 0.046). The incidence of grade 2–4 leukopenia was less in the acupuncture arm than in the sham arm (30% versus 90%; p = 0.02). However, the median leukocyte nadir, neutrophil nadir, and recovering ANC were all higher but not statistically significantly different (p = 0.116–0.16), after adjusting for baseline differences. There were no statistically significant differences in plasma G-CSF between the two groups.
Conclusions
We observed clinically relevant trends of higher WBC values during one cycle of chemotherapy in patients with ovarian cancer, which suggests a potential myeloprotective effect of acupuncture. A larger trial is warranted to more definitively determine the efficacy of acupuncture on clinically important outcomes of chemotherapy-induced neutropenia.
Introduction
Chemotherapy-induced neutropenia (CIN) is significant in gynecologic cancer patients who receive myelosuppressive chemotherapy,1 which can lead to a dose reduction of chemotherapy agents, delayed treatment, hospitalization, and intravenous antibiotic use.2 The occurrence of neutropenia during chemotherapy often compromises the intended clinical outcome.3
Following surgical debulking, patients with ovarian cancer are treated with chemotherapy, which typically includes platinum and a taxane. The reported incidence of neutropenia associated with ovarian cancer varies depending on the type of chemotherapy, prior therapy, and doses used. A representative study found that carboplatin and paclitaxel doublet chemotherapy resulted in 28% risk of grade 4 neutropenia after the first cycle and 68% of grade 2–4 neutropenia in all six cycles for patients with newly diagnosed gynecologic malignancies.4 The reported rate of febrile neutropenia (FN) in this population was 4%.4
Currently, the standard treatment of CIN is to use hematopoietic colony-stimulating factors (CSF), which includes granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) for attenuating white blood cell (WBC) and absolute neutrophil counts (ANC).2,5 However, CSF use is expensive and the frequently reported adverse effects of G-CSF include musculoskeletal pain and fever.6 Myeloid growth factors could also initiate or accelerate the development of myelodysplasia or acute myeloid leukemia.7,8
Chinese researchers have observed that acupuncture may affect leukocyte number and activity such as phagocytosis in animal and human studies.9–11 A meta-analysis on this topic suggests that acupuncture could increase WBC count by, on average, 1221 (95% confidence interval: 636–1807) cell/μL.12 However, conclusions in this meta-analysis were limited by the poor methodological quality of the included studies. A suggested mechanism is that acupuncture may increase serum CSF and promote the maturation of granulocytes.13,14
We conducted a pilot, sham-controlled randomized clinical trial to investigate the effects of acupuncture on CIN in patients with ovarian cancer. With the goal of informing a future, more definitive trial, our primary objectives were (1) to collect preliminary data on the effects of acupuncture on WBC count and ANC at the nadir, the lowest value of blood counts after chemotherapy, of cycle 2 and the second recovery day, which is the first day of cycle 3; and (2) to evaluate the feasibility of patient recruitment and acupuncture protocols. A secondary goal of this study was to determine whether acupuncture influences endogenous G-CSF levels.
Patients and the Methods
Selection of patients
This study was approved by the institutional review boards of Dana-Farber/Harvard Cancer Center, Harvard Medical School, and the New England School of Acupuncture. Written informed consent was obtained from all patients before study enrollment. Women were eligible for inclusion as follows: newly diagnosed or recurrent ovarian cancer, regardless of stage; receiving myelosuppressive chemotherapy (Table 1); Eastern Cooperative Oncology Group performance status of 0–2; platelet count >100,000 cell/μL; ANC >1000 cell/μL; no regular use of acupuncture within 120 days prior to enrollment; ability to give informed consent; and ≥ 18 years of age. Exclusion criteria were the following: current use of filgrastim (Neupogen™) or pegfilgrastim (Neulasta™); history of symptomatic cardiac or psychiatric disorder; and use of a pacemaker (due to electro-acupuncture).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Active acupuncture (n = 11) | Sham acupuncture (n = 10) |
|---|---|---|
| Demographics | ||
| Age ± SD | 50.8 ± 10.6 | 50 ± 9.9 |
| Race | ||
| White | 11 (52.4%) | 9 (42.9%) |
| Black | 0 (0%) | 1 (4.8%) |
| ECOG performance status | ||
| 0 | 3 | 0 |
| 1 | 8 | 7 |
| New diagnosis | 4 (19%) | 5 (23.8%) |
| Stage | ||
| I | 1 | 3 |
| II | 2 | 0 |
| III | 0 | 2 |
| IV | 1 | 0 |
| Unknown | 0 | 1 |
| Recurrent disease | 7 (33.3%) | 5 (23.8%) |
| No. (%) of patients on specific drug treatment | ||
| Carboplatin/paclitaxel | 6 (28.6%) | 5 (23.8%) |
| Gemcitabine | 3 (14.3%) | 3 (14.3%) |
| Topotecan | 1 (4.8%) | 2 (9.5%) |
| Vinorelbine | 1 (4.8%) | 0 (0%) |
| Drug doses/course of chemotherapy, mg | ||
| Carboplatin, mean ± SD | 527.8 ± 120 | 632.2 ± 49.9 |
| Paclitaxel, mean ± SD | 257.3 ± 81.4 | 280 ± 24.3 |
| Gemcitabine, mean ± SD | 1809.5 ± 886 | 1873.3 ± 534.9 |
| Topotecan, mean ± SD | 6.3 | 9.2 ± 3.6 |
| Vinorelbine, mean | 67 | – |
| Dexamethasone, mean ± SD | 13.7 ± 4.8 | 16 ± 12.6 |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group.
Study design
All patients enrolled were evaluated at baseline. Treatment assignments were generated using permuted block randomization with a variable block size by the study statistician who had no patient contact. Patients were randomized into one of two groups: active acupuncture or sham acupuncture (control group). The treating acupuncturists were not aware of the treatment assignment until the first acupuncture procedure. All other study personnel, including the study patients, were blinded to randomization assignments. Randomization assignments were revealed to the patient at the completion of their tenth acupuncture session.
Patients in both groups received 10 sessions of acupuncture, 2–3 times per week, beginning 1 week prior to cycle 2 of chemotherapy and ending at the beginning of cycle 3 of chemotherapy (Fig. 1). At the conclusion of the intervention, patients in the study arm continued their remaining chemotherapy cycles without any acupuncture treatment until day 78. Patients in the control arm were offered the active acupuncture protocol immediately after they completed the sham protocol as a courtesy.
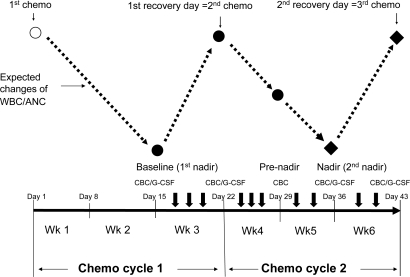
FIG. 1.
Study schema outlining a 21-day cycle of chemotherapy and timing of acupuncture treatments. Black solid dots: time points of outcome measurements. Open circle: first chemotherapy day. Black diamonds: the primary endpoints of the study. Dashed lines: the expected changes of white blood cells (WBC)/absolute neutrophil counts (ANC) during chemotherapy. Short, thick down arrows: acupuncture treatments. Thin down arrows: days of chemotherapy and the recovery days. CBC, complete blood counts; G-CSF, granulocyte colony-stimulating factor.
A validated expectation and treatment credibility scale was administered to assess the success of patient blinding in both groups after the second and tenth acupuncture treatments before unblinding.15–17
Chemotherapy
Newly diagnosed patients received standard carboplatin and paclitaxel chemotherapy, which consisted of paclitaxel 175 mg/m2 intravenously (IV) over 3 hours, followed by carboplatin area under the curve (AUC) 5 IV over 30–60 minutes.
For patients with recurrent cancers, all chemotherapy regimens were eligible, with the exception of liposomally encapsulated doxorubicin hydrochloride (Doxil™), including platinum-based treatment combinations or weekly single agents (gemcitabine, vinorelbine, topotecan, paclitaxel, or docetaxel) administered in 21- or 28-day cycles.
Based upon previous trials,18 a nadir was defined at day 15 for a 21-day cycle chemotherapy (Fig. 1) or day 22 for a 28-day cycle regimen, respectively. A recovery day was defined around day 22 for a 21-day cycle regimen, or day 29 for a 28-day cycle regimen.
Acupuncture protocol
All acupuncture treatments were performed at Dana-Farber Cancer Institute and Massachusetts General Hospital. Patients were considered protocol compliant if they completed at least 8 of the 10 treatments.
Five (5) acupuncturists from two sites administered all the treatments. All acupuncturists had at least 10 years clinical experience. Among them, 4 were licensed acupuncturists in Traditional Chinese Medicine (TCM) and 1 was a physician acupuncturist.
A standardized acupuncture protocol was developed, which was based on a review of TCM literature, and a consensus process among treating acupuncturists.19
The active acupuncture protocol
Acupuncture points and their anatomical locations were as follows: lower extremity (LR3, K3, SP6, ST36, SP10); upper extremity (LI4, PC6, LI11); and the top of head (GV20).20 Disposable acupuncture needles (VINCO™ MicroClean™, Helio Medical Supplies, Inc., USA) with a size of 36 gauge, 1 inch were used (0.20×25 mm). The depth of needling was at approximately 10 mm. A de qi sensation was required.21 After insertion, an AWQ-104L electro-acupuncture machine (Mayfair Medical Supplies Ltd., Hong Kong) was connected to needles on ST36 and SP6 bilaterally, at a frequency of 20–25 Hz. Then, a TDP CQ-27 infrared heat lamp (Lhasa OMS, Inc., USA) was placed at approximately 30 cm directly above the feet of the patient. Each session was 30 minutes.
The sham acupuncture protocol
The goal of the sham intervention was to provide a credible patient–practitioner interaction while minimizing acupuncture-induced physiologic effects. Seven (7) pre-defined sham needling sites were chosen to be in the same general region of active acupuncture points, but away from regular TCM acupuncture points. The thinnest possible acupuncture needles (Seirin®, J type, Seirin Corporation, Japan), size 02 (0.12 mm×30 mm) were used, and the depths of needle insertions were limited to 0.2 mm. No needle manipulation was performed. An identical but deactivated electro-acupuncture machine was used. An identical heat lamp was placed at approximately 75 cm above the feet of the patient, but the direction of the lamp head was tilted away from the feet to minimize a warming effect. An instrument stand covered with table sheets was placed over the chest of the patient to form a visual barrier. Interaction of acupuncturists with patients was scripted.
Clinical and laboratory evaluation
Complete blood counts were collected at baseline (first nadir), the nadir of cycle 1, and then once every 7 days at 5 time points during the study period (Fig. 1). Plasma level of human G-CSF was assessed at 4 time points (Fig. 1) using the QuantiGlo G-CSF Chemiluminescent Immunoassay (R&D System Inc., Minnesota, USA).
Acupuncture safety was evaluated by monitoring the overall adverse events according to National Cancer Institute toxicity criteria.22
Statistical analysis
This study was designed to provide preliminary data to inform the design and analysis of a subsequent large-scale, fully powered study to evaluate the effects of acupuncture on CIN in patients with cancer. As such, the trial did not have sufficient statistical power to detect differences of the magnitude as expected. A sample size of 50 patients (25 per group) was initially determined to obtain preliminary data on the outcome measures, with only 62% and 34% power to detect differences in nadir of 500 neutrophils and 500 WBC, respectively. Nonparametric Wilcoxon rank sum tests were used to assess between-group comparisons as unadjusted analyses. Analysis of covariance was used to examine group differences after adjusting for baseline differences. Fisher's exact test was used for comparing toxicity frequency and the validation of assignment blinding. Descriptive frequency analyses of missing visits, attrition, and their reasons were performed. The analysis was performed in accordance with the intention-to-treat principle. Missing data were handled based on available data approach. SAS for Windows (SAS Institute Inc., Cary, NC) was used for statistical computation. STATA for Windows (StataCorp LP, College Station, TX) was used for graphic illustrations. All p values were two-sided and a p value <0.05 was considered statistically significant.
Results
Patient recruitment and enrollment
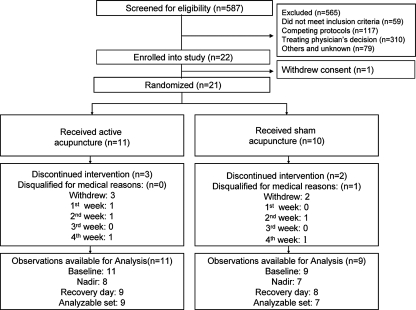
During a 24-month recruitment period, from January 2005 to December 2006, 565 of 587 screened patients were excluded as ineligible or declined enrollment. The two main reasons for exclusion were treating physician's decisions (54.8%) and existing competing protocols (20.7%). Figure 2 shows a CONSORT diagram and reasons for exclusion. One (1) patient withdrew from the study before randomization. The remaining 21 patients were randomized to receive either active acupuncture or sham acupuncture. Eighteen (18; 85.7%) of the 21 completed more than eight acupuncture sessions. Five (5; 23.8%) withdrew at different stages of the study due to disease progression (n = 4), side-effects of chemotherapy, or time commitment (n = 1). Another patient who completed 10 sessions of acupuncture was later disqualified due to use of G-CSF before the nadir day. Some of the participants were not naïve to acupuncture but were not undergoing any other acupuncture during the study as required by the inclusion criteria.
FIG. 2.
CONSORT flow of participants through the trial.
Baseline characteristics
Patients' baseline characteristics are listed in Table 1. Seventeen (17; 81%) of 21 patients received a 21-day cycle of chemotherapy and 4 (19.1%) received a 28-day cycle protocol. At baseline, the patients and the controls shared similar demographic and clinical characteristics (Table 1). Similarly, the intensity and dosages of chemotherapy agents and glucocorticosteroids administered were essentially equivalent for both groups with no statistical difference. One (1) or 2 acupuncturists at each study site treated each participant during the study.
A total of 20 (95%) patients completed the questionnaire regarding the assessment of masking of study arm assignment. Six (6; 60%) of 10 patients in the active arm did not correctly guess the treatment they received, which was similar in the sham acupuncture group (70%) (p = 1.00).
Effect of acupuncture on WBC and ANC
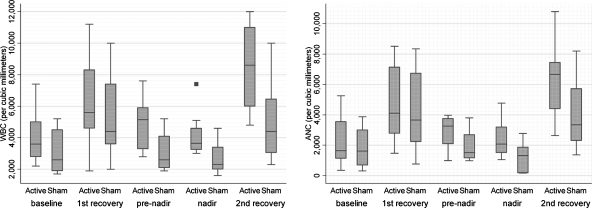
The effects of acupuncture treatment on WBC counts and ANC in study patients are shown in Tables 2 and 3. The difference in WBC counts between the two groups became statistically significant after patients received 6 sessions of acupuncture (p = 0.04, unadjusted). WBC values then remained consistently higher in the patients receiving active versus sham acupuncture at nadir (p = 0.05, unadjusted) and the second recovery day (p = 0.03, unadjusted). At the second recovery day, this difference was statistically significant even after adjusting for baseline differences (p = 0.046; Fig. 3).
Table 2.
Median WBC Counts Over a Timecourse of Chemotherapy for Participants Receiving Either Active or Sham Acupuncture
|
Median WBC (cell/μL) (range) | |||||
|---|---|---|---|---|---|
| |
Acupuncture |
Sham control |
|
||
| Time points | No. of patients | No. of patients | p-value | ||
| Baseline | 11 | 3600 (2200–7400) | 9 | 2600 (1700–5200) | |
| First recovery | 10 | 5600 (1900–11,200) | 10 | 4400 (2000–10,000) | 0.686 |
| Pre-nadir | 8 | 5150 (2800–7600) | 7 | 2600 (1900–5200) | 0.108 |
| Nadir | 8 | 3650 (3000–7400) | 7 | 2300 (1600–4600) | 0.16 |
| Second recovery | 9 | 8600 (4800–12,000) | 8 | 4400 (2300–10,000) | 0.046 |
p-values were calculated after adjusting for baseline difference using analysis of covariance.
WBC, white blood cells.
Table 3.
Median ANC Over a Timecourse of Chemotherapy for Participants Receiving Either Active or Sham Acupuncture
|
Median ANC count (cell/μL) (range) | |||||
|---|---|---|---|---|---|
| |
Acupuncture |
Sham control |
|
||
| Time points | No. of patients | No. of patients | p-value | ||
| Baseline | 11 | 1640 (350–5250) | 9 | 1610 (50–3870) | |
| First recovery | 10 | 4110 (1470–8510) | 10 | 3660 (760–8340) | 0.619 |
| Pre-nadir | 8 | 3260 (990–3970) | 7 | 1510 (980–3800) | 0.110 |
| Nadir | 8 | 2080 (1050–4770) | 7 | 1310 (160–2770) | 0.115 |
| Second recovery | 9 | 6670 (2630–10,800) | 8 | 3345 (1360–8200) | 0.099 |
p-values were calculated after adjusting for baseline difference using analysis of covariance.
ANC, absolute neutrophil counts.
FIG. 3.
Effects of active and sham acupuncture on white blood cells (WBC) counts and absolute neutrophil counts (ANC) during chemotherapy. The x-axis denotes the number of WBC and ANC. The y-axis indicates the study arms and the corresponding time of measurements. The ends of the boxes represent the 25th and 75th percentiles, the bars indicate the 10th and 90th percentiles, and a line shows the median. The solid box indicates an outlier value.
Differences in ANC values between active and sham groups showed a pattern similar to that of WBC counts but were not statistically significant. At the pre-nadir time, average ANC values in the active acupuncture group were almost twofold greater than in the sham arm (p = 0.127, unadjusted). This effect persisted through the nadir and the second recovery day, but were not statistically significant, respectively (p = 0.17 and p = 0.094, unadjusted).
Effect of acupuncture on G-CSF
G-CSF value changes during the study period are shown in Table 4 No statistically significant difference between the two groups was found at any time point. Interestingly, although statistically not significant, G-CSF values in the patients receiving active acupuncture showed a fourfold increase (p = 0.15, unadjusted), as compared to the sham acupuncture group, at the first recovery day after three acupuncture sessions.
Table 4.
Median G-CSF Values Over a Timecourse of Chemotherapy for Participants Receiving Either Active or Sham Acupuncture
|
Median G-CSF pg/mL (range) | |||||
|---|---|---|---|---|---|
| |
Acupuncture |
Sham control |
|
||
| Time points | No. of patients | No. of patients | p-value | ||
| Baseline | 11 | 37.3 (28.6–393.4) | 10 | 31.95 (11.8–211.3) | |
| First recovery | 10 | 120.8 (23.5–207.3) | 9 | 27.9 (14–158.5) | 0.15 |
| Nadir | 8 | 50.9 (19.6–84.9) | 6 | 37.55 (31.7–146.7) | 0.62 |
| Second recovery | 8 | 33.35 (15.5–177.4) | 7 | 66.3 (14.7–128.3) | 0.78 |
p-values were calculated after adjusting for baseline difference using analysis of covariance.
G-CSF, granulocyte colony-stimulating factor.
Adverse events during the study period
Study-related toxicity and side-effects were minimal. Two (2) adverse events occurred during the study period. A sham needle was mistakenly not removed from a subject at the completion of acupuncture. This caused some patient distress, but no physical harm. Another patient developed a local port infection and was removed from the study; this was unrelated to acupuncture treatment.
Over the study period, a total of 55 nonhematologic toxicity events were reported. The most reported side-effects were fatigue (32.7%) and bruising (21.8%). Fatigue was reported more commonly in the sham group than in the active group (43.8% versus 17.4%) while bruising was higher in the active group than in the sham arm (34.8% versus 12.5%). Twelve (12; 21.8%) of 55 occurrences were adjudged as possibly related to acupuncture. Of 20 patients with data available, the number of patients with grade 2–4 hematologic toxicity was less in the active group than the sham group (30% versus 90% in leukopenia and 40% versus 70% in neutropenia) (p = 0.02 and p = 0.37). Grade 3–4 toxicity was also less frequent in the active group than in the sham group: 10% versus 20% in leukopenia and 10% versus 40% in neutropenia (p = 1.00 and p = 0.30). No febrile neutropenia was observed in either arm.
Discussion
To our knowledge, this is the first sham-controlled clinical trial on acupuncture for CIN conducted in the West. Although our results must be interpreted cautiously, due to small samples sizes and other limitations discussed below, we found consistent trends of higher WBC counts in active acupuncture relative to sham acupuncture in a group of patients with ovarian cancer undergoing myelosuppressive chemotherapy. Specifically, recovering WBC counts were statistically significantly higher in active versus sham acupuncture patients. Our results are consistent with the results of other clinical trials recently summarized in a metaanalysis,12 and suggest that acupuncture could result in a significant reduction in CIN, which may meaningfully impact the complications of neutropenia in patients receiving chemotherapy for cancer. This study also demonstrates that a valid sham-controlled study evaluating acupuncture for CIN is feasible, and that further investigation is warranted.
Clinical trials have reported varying rates of neutropenia and leukopenia in patients with ovarian cancer undergoing chemotherapy. In trials with chemotherapy protocols similar to those in our trial,4,23 grade 3–4 neutropenia was reported to be between 37% and 58%. Another similar study reported rates of grade 3–4 leukopenia and neutropenia of 21% and 28%, respectively.24 These are higher than the approximate 10% rate of leukopenia and neutropenia observed in the study arm in our study. The lower rates of leukopenia and neutropenia in the active acupuncture arm in our current study suggest a potential myeloprotective effect of acupuncture in this group of patients.
It is of note that on the second recovery day, the day that determines the subsequent cycle of chemotherapy, none of the patients in the active arm developed grade 2, 3, or 4 neutropenia. Overall, active acupuncture resulted in approximately a twofold reduction in both leukopenia and neutropenia rates compared to the sham control. According to current practice, treatment modifications such as cycle delay, dose reduction, or additional use of G-CSF would be needed if a patient had an ANC count less than 1500 cell/μL (grade 2 neutropenia or greater). Our findings suggest that acupuncture might be able to reduce the incidence of treatment delays, modifications, and the cost of supportive care, if these observations are confirmed in future larger studies. Currently, the American Society of Clinical Oncology updated guideline in 2006 recommends prophylactic use of CSF when the risk of FN is in the range of approximately 20% or higher.5 In addition, the National Comprehensive Cancer Network in its recent practice guideline (version 1. 2007) recommends considering use of CSF when FN risk is between 10% and 20% and no CSF use when FN risk is below 10%.2 No specific therapy currently exists for CIN when FN risk is below 10%, which counts for much current standard chemotherapy. Acupuncture might be particularly relevant for this type of CIN.
The effects of acupuncture on peripheral circulatory blood cells have had a long history of interest for many clinicians and researchers.25–27 Some of them stressed that repeated acupuncture treatments are an important feature for the final outcome of treatment.28 This may explain the pattern of responses along with a time course in our current study as numbers of acupuncture sessions accumulated. The different statistically significant levels detected between WBC and ANC count in our study could be explained by the small sample size of the study, since the change of ANC counts was approximately proportional to the increase of WBC. At least 30 patients would be needed to reach statistical significance at nadir for ANC count.
Regarding possible biological mechanisms of neutrophil cell responses to acupuncture, we found no statistical differences between the acupuncture arm and sham arm in terms of plasma G-CSF level. However, a fourfold increase of G-CSF was observed in active acupuncture at the earlier stage of the trial. We speculate that there may be a priming effect of G-CSF. Administration of the hematopoietic growth factors before chemotherapy (priming) has been clinically studied for various hematologic conditions with mixed results.29,30 It was suggested that growth-factor priming could increase the number of myeloid precursors in the bone marrow and decease the number of stem cells killed by chemotherapy to produce a myeloprotective effect.31 In our study, acupuncture was initiated at the nadir day of cycle 1 chemotherapy, which could be seen as a priming intervention to cycle 2 chemotherapy, which may explain the clinical effects on peripheral blood counts we observed in the study. In addition, a study also found that acupuncture could significantly increase segmented neutrophils and the function of the spleen in a cyclophosphamide-induced neutropenic rabbit model,32 which suggests nonmarrow factors involvement in the effect of acupuncture on neutropenia. Therefore, additional research is needed to evaluate these hypotheses.
One might argue that the stress-induced cortisol level elevation during acupuncture procedure might contribute to our findings; however, several clinical studies of clinically stressed subjects (i.e., due to surgical trauma) suggested that acupuncture was actually down-regulating rather than up-regulating cortisol levels.33
The acupuncture protocol used in the study was implemented easily by our study acupuncturists without difficulty. Meanwhile, the results of this acupuncture pilot trial indicate that it is safe to conduct an acupuncture clinical trial in patients with cancer undergoing chemotherapy. The only apparent side-effect in our study was mild bruising at needling sites. No serious acupuncture-related adverse events were observed. This observation parallels other studies that have observed that acupuncture is safe for patients undergoing chemotherapy.34–36
There are a number of limitations in our study that must be considered. Because of the small sample size we employed, there is a possibility that the results we observed may simply be due to chance. A larger study is required to more definitively evaluate the effects of acupuncture on CIN. Although this pilot study was only intended to recruit a small cohort that was adequate for assessing trial feasibility and giving preliminary estimates of efficacy, it is important to note that recruitment of the 20 participants in this trial proved to be more difficult than anticipated. Difficulties were mainly related to competition with several new and existing clinical trials in our academic centers, and also to practical issues associated with coordinating acupuncture and chemotherapy sessions, and the extra travel burden imposed on participants. Many potential participants were deterred due to the need for frequently traveling into hospitals during the study. Future studies should consider more aggressive and inclusive recruitment strategies, alternative acupuncture treatment schedules, and reducing patient travel, thus reducing burden on patients. A second limitation of our study was that it evaluated a heterogeneous population, including cancer patients with new and recurrent diseases and with different myelosuppressive chemotherapy protocols. This heterogeneity may reduce the study's internal validity. Finally, it is possible that the sham acupuncture protocol used in this study was not fully inert and that the shallow needling we employed had a small physiologic effect. There is currently no “gold standard” for a sham acupuncture control, and shallow needling has been recommended as one of the sham acupuncture controls,37–39 and even recently developed noninsertive acupuncture devices are believed to have some physiologic effect.40–43 Consequently, while our sham treatment provided a valid and credible control, it may have resulted in an underestimate of effect sizes.
Conclusions
In conclusion, our small pilot study suggests that acupuncture may improve WBC counts in patients with ovarian cancer undergoing concurrent myelosuppressive chemotherapy, and may also reduce the severity of leukopenia. Future, larger trials are warranted to confirm and fully evaluate the potential clinical benefits of acupuncture for chemotherapy-induced neutropenia.
Acknowledgments
This project described was supported by grant number 1U19AT002022-01 from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM, or the National Institutes of Health. Presented in part as abstracts at American Society of Clinical Oncology 43rd Annual Meeting, Chicago, Illinois, June 1–5, 2007; The American Society of Hematology 49th Annual Meeting, Atlanta, Georgia, December 8–11, 2007; and The Society for Integrative Oncology 4th International Conference, San Francisco, California, November 15–17, 2007.
Collaborating investigators: Dana-Farber Cancer Institute: Ross Berkowitz, M.D., Elizabeth Garner, M.D., Susana Campos, M.D., Sue Berlin, D.O., Karen Anderson, M.D., Ph.D., Craig Bunnell, M.D., M.P.H., Harold Burstein, M.D., Ph.D., Wendy Chen, M.D., M.P.H., Judy Garber, M.D., M.P.H., Michael Hassett, M.D., Hanna Yoko Irie, M.D., Ph.D.
Massachusetts General Hospital: Michael Seiden, M.D., Carolyn Krasner, M.D., Marcella del Carmen, M.D., Arlan Fuller, M.D., Linda Duska, M.D., Neil Horowitz, M.D.
Study acupuncturists: Zhi Ping Li, M.B., Lic. Ac., Grant Hou, M.B., Lic. Ac., Joy Zhang, M.B., Lic. Ac. Qunhao Zhang, PhD., Lic., Ac. Research staff: Maria Roche, N.P., Tina Atkinson, Julie Lee, R.N., Andrea Hrbek, Ellen Connors, M.A.
Disclosure Statement
The authors state that no competing financial interests exist.
References
- 1.Finberg RW. Talcott JA. Fever and neutropenia: How to use a new treatment strategy. NEJM. 1999;341:362–363. doi: 10.1056/NEJM199907293410509. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Guidelines Network. Myeloid growth factors. www.nccn.org. 2007. www.nccn.org/professionals/physician_gls/PDF/myeloid_growth.pdf www.nccn.orgwww.nccn.org/professionals/physician_gls/PDF/myeloid_growth.pdf
- 3.Bonadonna G. Valagussa P. Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. NEJM. 1995;332:901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 4.Matulonis U. Campos S. Duska L, et al. A phase ii trial of three sequential doublets for the treatment of advanced mullerian malignancies. Gynecol Oncol. 2003;91:293–298. doi: 10.1016/s0090-8258(03)00496-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith TJ. Khatcheressian J. Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 6.Papaldo P. Lopez M. Marolla P, et al. Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J Clin Oncol. 2005;23:6908–6918. doi: 10.1200/JCO.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 7.Touw IP. Bontenbal M. Granulocyte colony-stimulating factor: Key (f)actor or innocent bystander in the development of secondary myeloid malignancy? J Natl Cancer Inst. 2007;99:183–186. doi: 10.1093/jnci/djk057. [DOI] [PubMed] [Google Scholar]
- 8.Hershman D. Neugut AI. Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LZ. New Edition Chinese Acupuncture and Moxibustion, 7. 1. Shanghai: Shanghai Science and Technology Publishing House; 1992. The effect of acupuncture and moxibustion on blood system; pp. 947–949. [Google Scholar]
- 10.Zhou RX. Huang FL. Jiang SR. Jiang JC. Acupuncture effects on phagocytosis of white blood cells in human. Chin Acupuncture Moxibustion. 1987;6:31–33. [Google Scholar]
- 11.Zhu X. Zhao HX. Liu H. Gan WM. Point block therapy for post-chemo leukopenia, 100 cases report. Chin Acupuncture Moxibustion. 1995;15:3–4. [Google Scholar]
- 12.Lu W. Hu D. Dean-Clower E, et al. Acupuncture for chemotherapy-induced leukopenia: Exploratory meta-analysis of randomized controlled trials. J Soc Integr Oncol. 2007;5:1–10. doi: 10.2310/7200.2006.035. [DOI] [PubMed] [Google Scholar]
- 13.Yan DH. Effects of acupuncture and moxibustion on the contents and activity of serum colony stimulating factor (csf) in cyclophosphamide-treated mice. Zhen Ci Yan Jiu (Acupuncture Res) 1997;22:304–306. [Google Scholar]
- 14.Zhao XX. Wang HP. Tian KY. Huang XM. Effect of acupuncture and moxibustion on dynamic changes of bone marrow granulocyte series in chemotherapy-treated mice. Chin Acupuncture Moxibustion. 2000;20:172–174. [Google Scholar]
- 15.Borkovec TD. Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 16.Wayne PM. Krebs DE. Macklin EA, et al. Acupuncture for upper-extremity rehabilitation in chronic stroke: A randomized sham-controlled study. Arch Phys Med Rehab. 2005;86:2248–2255. doi: 10.1016/j.apmr.2005.07.287. [DOI] [PubMed] [Google Scholar]
- 17.Macklin EA. Wayne PM. Kalish LA, et al. Stop hypertension with the acupuncture research program (sharp): Results of a randomized, controlled clinical trial. Hypertension. 2006;48:838–845. doi: 10.1161/01.HYP.0000241090.28070.4c. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto R. Kaneuchi M. Nishiya M, et al. Clinical trial and pharmacokinetic study of combination paclitaxel and carboplatin in patients with epithelial ovarian cancer. Cancer Chemother Pharmacol. 2002;50:137–142. doi: 10.1007/s00280-002-0471-1. [DOI] [PubMed] [Google Scholar]
- 19.Lu W. Dean-Clower E. Doherty-Gilman A, et al. Using exploratory meta-analysis to quantify acupuncture parameters: A novel approach in designing clinical trials. Proceedings of The Society for Integrative Oncology 2nd International Conference San Diego, California; 2005. [Google Scholar]
- 20.Cheng XN. Chinese Acupuncture and Moxibustion. 2000. Beijing: Foreign Languages Press; 2000. [Google Scholar]
- 21.Langevin HM. Churchill DL. Fox JR, et al. Biomechanical response to acupuncture needling in humans. J Appl Physiol. 2001;91:2471–2478. doi: 10.1152/jappl.2001.91.6.2471. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v3.0 (ctcae) Washington: National Cancer Institute; [Dec 12;2003 ]. [Google Scholar]
- 23.Matulonis UA. Campos S. Krasner CN, et al. Three sequential chemotherapy doublets for the treatment of newly diagnosed advanced mullerian malignancies: The modified triple doublet regimen. Gynecol Oncol. 2006;103:575–580. doi: 10.1016/j.ygyno.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Hilpert F. du Bois A. Greimel ER, et al. Feasibility, toxicity and quality of life of first-line chemotherapy with platinum/paclitaxel in elderly patients aged > or = 70 years with advanced ovarian cancer: A study by the AGO OVAR Germany. Ann Oncol. 2007;18:282–287. doi: 10.1093/annonc/mdl401. [DOI] [PubMed] [Google Scholar]
- 25.Lackner J. Lackner F. Semsroth M. Observations on the course of leucocytes in cardiac surgery under acupuncture-analgesia (author's transl) Anaesthesist. 1976;25(5):246–47. [PubMed] [Google Scholar]
- 26.Lau BH. Wong DS. Slater JM. Effect of acupuncture on allergic rhinitis: Clinical and laboratory evaluations. Am J Chin Med (Gard City NY) 1975;3:263–70. doi: 10.1142/s0192415x7500027x. [DOI] [PubMed] [Google Scholar]
- 27.Ionescu-Tirgoviste C. Stroiescu F. Changes in eosinophils after acupuncture of the meridian of the liver. Stud Cercet Endocrinol. 1968;19:405–411. [PubMed] [Google Scholar]
- 28.Kou W. Bell JD. Gareus I, et al. Repeated acupuncture treatment affects leukocyte circulation in healthy young male subjects: A randomized single-blind two-period crossover study. Brain Behav Immun. 2005;19:318–324. doi: 10.1016/j.bbi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Lowenberg B. van Putten W. Theobald M, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. NEJM. 2003;349:743–752. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 30.Thomas X. Raffoux E. Botton S, et al. Effect of priming with granulocyte-macrophage colony-stimulating factor in younger adults with newly diagnosed acute myeloid leukemia: A trial by the acute leukemia french association (alfa) group. Leukemia. 2007;21:453–461. doi: 10.1038/sj.leu.2404521. [DOI] [PubMed] [Google Scholar]
- 31.Janik JE. Miller LL. Korn EL, et al. A prospective randomized phase ii trial of gm-csf priming to prevent topotecan-induced neutropenia in chemotherapy-naive patients with malignant melanoma or renal cell carcinoma. Blood. 2001;97:1942–1946. doi: 10.1182/blood.v97.7.1942. [DOI] [PubMed] [Google Scholar]
- 32.Sun P. Zhou Y. Mao H, et al. Relationship between electro-acupuncture and spleen function on leukocyte. J Acupuncture Tuina Sci. 2007;5:336–340. [Google Scholar]
- 33.Akimoto T. Nakahori C. Aizawa K, et al. Acupuncture and responses of immunologic and endocrine markers during competition. Med Sci Sports Exerc. 2003;35:1296–1302. doi: 10.1249/01.MSS.0000078934.07213.25. [DOI] [PubMed] [Google Scholar]
- 34.Melchart D. Ihbe-Heffinger A. Leps B, et al. Acupuncture and acupressure for the prevention of chemotherapy-induced nausea: A randomised cross-over pilot study. Support Care Cancer. 2006;14:878–882. doi: 10.1007/s00520-006-0028-7. [DOI] [PubMed] [Google Scholar]
- 35.Shen J. Wenger N. Glaspy J, et al. Electroacupuncture for control of myeloablative chemotherapy-induced emesis: A randomized controlled trial. JAMA. 2000;284:2755–2761. doi: 10.1001/jama.284.21.2755. [DOI] [PubMed] [Google Scholar]
- 36.Reindl TK. Geilen W. Hartmann R, et al. Acupuncture against chemotherapy-induced nausea and vomiting in pediatric oncology: Interim results of a multicenter crossover study. Support Care Cancer. 2006;14:172–176. doi: 10.1007/s00520-005-0846-z. [DOI] [PubMed] [Google Scholar]
- 37.MacPherson H. White A. Cummings M, et al. Standards for reporting interventions in controlled trials of acupuncture: The stricta recommendations. Complement Ther Med. 2001;9:246–249. doi: 10.1054/ctim.2001.0488. [DOI] [PubMed] [Google Scholar]
- 38.White AR. Filshie J. Cummings TM. Clinical trials of acupuncture: Consensus recommendations for optimal treatment, sham controls and blinding. Complement Ther Med. 2001;9:237–245. doi: 10.1054/ctim.2001.0489. [DOI] [PubMed] [Google Scholar]
- 39.Lundeberg T. Lund I. Are reviews based on sham acupuncture procedures in fibromyalgia syndrome (FMS) valid? Acupunct Med. 2007;25:100–106. doi: 10.1136/aim.25.3.100. [DOI] [PubMed] [Google Scholar]
- 40.Streitberger K. Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352:364–365. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 41.Lund I. Lundeberg T. Are minimal, superficial or sham acupuncture procedures acceptable as inert placebo controls? Acupunct Med. 2006;24:13–15. doi: 10.1136/aim.24.1.13. [DOI] [PubMed] [Google Scholar]
- 42.Dhond RP. Kettner N. Napadow V. Do the neural correlates of acupuncture and placebo effects differ? Pain. 2007;128:8–12. doi: 10.1016/j.pain.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pariente J. White P. Frackowiak RS. Lewith G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage. 2005;25:1161–1167. doi: 10.1016/j.neuroimage.2005.01.016. [DOI] [PubMed] [Google Scholar]