Abstract
A new porous polymer monolithic capillary column modified with gold nanoparticles that enables the selective capture of cysteine-containing peptides has been developed to reduce the complexity of peptide mixtures generated in bottom-up proteomic analysis. The column is prepared from a poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith through reaction of some of its epoxide moieties with cysteamine to affording a monolith rich in surface thiol groups. In situ reduction of chloroauric acid within the column is then used to form gold nanoparticles attached to the surface of the pores of the monolith. This process preserves the excellent hydrodynamic properties of the monolithic column while providing a means to selectively retain cysteine-containing peptides from an analyte due to their high affinity for gold. Release of the retained peptides is subsequently achieved with an excess of 2-mercaptoetanol. The loading capacity determined for L-cysteine using frontal elution is 2.58 μmol/m. Since the gold-thiol link is less stable at elevated temperatures, the adsorption capacity is recovered by washing the column at 80 °C for 2 h. While regeneration is easy, the multiplicity of bonds between the monolithic support and the gold nanoparticles prevents their elution even under harsh conditions such as treatment with pure 2-mercaptoethanol or treatment with boiling water for 5 hours. Application of the gold modified monolith in tandem with a packed C18 capillary column is demonstrated with baseline separation of a peptide mixture achieved in a two step process. The first involves retention of cysteine-containing peptides in monolith with reversed phase separation of all other peptides, while the retained peptides are released from monolith and separated in the second step.
Keywords: polymer monolith, gold nanoparticles, cysteine-containing peptides, micro-HPLC-MS, proteomics
The concept of proteomics involves a comprehensive tool to study the structures, localizations, post-translational modifications, functions and interactions of all proteins expressed by an organism at a certain time and under certain conditions1. Current estimates are that the proteome of eukariots contains thousands of proteins with up to 20,000 expressed at any time. Such complexity represents a serious challenge to separation techniques. The limited speed and extensive manual manipulation required by today’s top-down two-dimensional slab gel electrophoresis introduced by O’Farrell 35 years ago 2-4 is unlikely to match the future needs of rapid screening techniques.5 Therefore, ever more efficient separation steps will always be needed and new analytical approaches must be developed and studied.6-8 Another strategy in proteome analysis is the bottom-up approach, which involves the simultaneous digestion of all proteins in the sample followed by the separation of peptides and their identification using LC-MS/MS.9-12 Because this process nearly always generates a very large number of peptides, approaches that reduce sample complexity are desirable. One of the options available for this purpose is the isolation of peptides containing a specific amino acid residue.13-15 For example, cysteine-containing peptides have been selectively separated using either covalent chromatography based on thiol-disulfide exchange with thiopropyl sepharose as the support,16-17 or after derivatization with an isotope-coded affinity tag.18 While useful, these methods require several reaction steps in addition to the separation. Therefore, straightforward and entirely chromatographic methods would represent an attractive alternative since they enable high speed, ease of automation, and simple coupling with a mass spectrometer.
The use of nanoparticles smaller than 100 nm in analytical chemistry with their unique size-related physical and chemical properties has been first suggested almost 30 years ago.19 However, implementation of their use in electrophoretic and chromatographic separation techniques lags behind the rapid development of other nanotechnologies despite the numerous advantages the nanoparticles can offer. For example, their large surface-to-volume ratio can potentially facilitate mass transfer and increase efficiency of the separation.20-21 Their large surface area also provides a platform for the attachment of the large number of functionalities needed for separations using interactive separation modes.22-23 In addition, many nanoparticles show chemical stability through a much wider pH range than silica, which is the most common packing material used in chromatography.24-25 Nanomaterials, mostly including fullerenes, carbon nanotubes, metal oxide nanoparticles, and polymer nanoparticles have been used either directly, or for the modification of packings in columns and microchip devices for application in gas and liquid chromatography, capillary electrophoresis and electrochromatography.20-21,26-28
Monolithic columns prepared from synthetic polymers with low resistance to flow enabling rapid HPLC of proteins and other large molecules emerged in the early 1990s as an alternative to packed chromatographic columns.29-34 An important feature of the porous polymer monoliths is the simple access to multiplicity of surface chemistries using direct copolymerization of functional monomers, chemical modification of preformed monoliths, and grafting.33,35-38 Functionalization of the pore surface with polymer nanoparticles is the newest contribution to the arsenal of approaches leading to monoliths with specific chemical properties. For example, using Coulombic interactions, Hilder et al. have attached polystyrene latex nanoparticles bearing quaternary amine groups to a monolith containing sulfonic acid functionalities and used the column for the separation of saccharides.39 Haddad and coworkers have significantly extended this concept with the preparation of columns for ion chromatography and capillary electrochromatography.40-44
While gold nanoparticles (GNPs) are readily available, stable, and compatible with biomolecules, they have not been used extensively in separation science.45 The surface of GNPs can readily be modified by taking advantage of the high affinity between gold and thiol ligands.26,46 The few known examples of use of GNPs include their addition to the running buffer in capillary and microchip electrophoresis where they serve as the pseudostationary phase improving the separation23,47-49 or the coating of the inner wall of capillaries in open tubular gas chromatography50-51 and capillary electrochromatography.52-54 In contrast, few reports mention the application of self-assembled monolayers of GNPs as coatings for silica-gel and nonporous polystyrene particles and their use in capillary HPLC.55 We indicated previously that GNPs can be attached to the surface of monolithic capillary columns with specifically designed surface chemistry.56,57 Very recently, short communication by Connoly at al. described immobilization of gold nanoparticles on a poly(butyl methacrylate-co-ethylene dimethacrylate) monolith with photografted poly(4,4-dimethyl-2-vinylazlactone) chains modified to bear amine groups.58 However, their work does not present any application of the conjugate.
This report describes in detail the preparation of a novel monolithic stationary phase coated with gold nanoparticles, which confer unique selectivity to the monolithic column for use in reducing the complexity of analytes in proteomic studies. The GNP-coated monoliths can selectively capture cysteine-containing peptides from their mixtures with other peptides and subsequently release them for micro-HPLC-MS analysis when all other peptides have been analyzed or eluted.
EXPERIMENTAL SECTION
Materials
Glycidyl methacrylate (GMA), ethylene dimethacrylate (EDMA), cyclohexanol, 1-dodecanol, azobisisobutyronitrile (AIBN), trimethoxysilylpropyl methacrylate, 2-mercaptoethanol, cysteamine, L-cysteine, sodium hydrogen sulfide, chloroauric acid, sodium borohydride, sodium dihydrogenphosphate, trisodium citrate, sodium hydroxide, hydrochloric acid, 2,2′-dipyridyl disulfide, phosphoric acid, formic acid, formamide, N,N-dimethylformamide, toluene, HPLC-grade solvents (acetonitrile, methanol, acetone), MS-grade solvents (water and acetonitrile), peptides (His-Cys-Lys-Phe-Trp-Trp, Tyr-Gly-Gly-Phe-Leu, Phe-Gly-Phe-Gly, Tyr-Gly) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Additional peptides including Asp-Ala-Glu-Phe-Arg-His-Asp-Ser- Gly-Tyr-Glu-Val-His-His-Gln-Lys- Leu (β-amyloid (1-17)), Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-Tyr-Glu-Val-His-His-Gln-Lys-Leu-Cys ([Cys17]-β–amyloid (1-17)), human luteinizing hormone releasing hormone (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly, LH-RH,), and His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Cys were obtained from AnaSpec (San Jose, CA, USA). GNP colloid (15 nm, 1.4×1012 particles/mL) was purchased from Ted Pella Inc. (Redding, CA, USA). EDMA and GMA were purified by passing them through an inhibitor remover column (Sigma-Aldrich, St. Louis, MO, USA) followed by distillation in vacuum. All other reagents were used as received. Water delivered by an EASYpure® II system (Barnstead, Boston, MA, USA) was used for micro-HPLC experiments, while commercial MS-grade water was used for LC-MS experiments. The peptides were dissolved in MS-grade water at a concentration of 0.1 mg/mL each. Teflon-coated and polyimide coated fused silica capillaries (375 μm o.d. ×100 μm I.D.) were purchased from Polymicro Technologies (Phoenix, AZ, USA). An Acclaim PepMap™ C18 Nanocolumn (3 μm particle size, 100 Å pore size, 15 cm × 75 μm I.D.), a product of Dionex Corp. (Sunnyvale, CA, USA) was used in this study.
Instrumentation
An Agilent 1200 Series Nanoflow LC system, which consists of a micro vacuum degasser, a nano pump, a microwell-plate Autosampler, and a multiple wavelength detector, was used for the micro-HPLC and micro-HPLC-MS experiments. The latter included interfacing the separation column with a Bruker microTOF-Q system (Bruker Daltonic, Bremen, Germany) using an online NanoElectrospray ion source operated in positive ion mode. The sample solution entered the spray chamber through a grounded distal coated silica tip (360/50 μm tubing o.d., 15 μm I.D., New Objective, Woburn, MA, USA) with an electrospray voltage of 1500 V and a drying gas flow of 3.0 L/min at 150 °C to facilitate the solvent evaporation.
A Hitachi S-4300 SE/N Schottky emission scanning electron microscope (SEM) integrated with backscattered electron (BSE) detector (Hitachi, Pleasanton, CA, USA) and energy dispersive X-ray spectrometer (EDS, Thermo Electron, Waltham, MA, USA) was used to obtain SEM/BSE images and EDS data. EDS data were used for element identification and semi-quantitative compositional estimates. Monolithic columns were cut lengths of 2~3 mm and coated with a very thin-layer of conductive carbon to diminish charging and heating of the sample from the electron beam, a requisite for both imaging and compositional analysis under high vacuum.
Preparation of generic poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolithic column
In order to ensure attachment of the monoliths to the fused-silica capillary, the inner wall was vinylized.59 A polymerization mixture containing 24% (w/w) GMA (functional monomer), 16% (w/w) EDMA (crosslinker), 30% (w/w) cyclohexanol and 30% (w/w) 1-dodecanol (porogens), and 1% AIBN initiator (w/w with respect to the total monomer content) was sonicated to obtain a homogenous solution, and then purged with nitrogen for 10 min. The vinylized capillaries were filled with the polymerization mixture using a syringe and sealed with rubber septa at both ends. The polymerization was carried out in a water bath at 60°C for 24 h. The column was then rinsed with acetonitrile to remove the porogens; this column is designated as “GMA column” in the following text.
Thiol-modified monolithic column
Two reactions (Figure 1) were used to introduce thiol groups in the poly(glycidyl methacrylate-co-ethylene dimethacrylate) monoliths. The reaction with sodium-hydrogen sulfide was carried out as reported by Preinerstorfer et al.56 To achieve the reaction with cysteamine, 2.5 mol/L aqueous solution was pumped through the glycidyl methacrylate-based column at a flow rate of 0.5 μL/min for 20 min.60 The columns were then rinsed with water to remove excess reagents. The resulting thiol-modified monolithic columns are referred to as “SH columns” in the text below.
Figure 1.
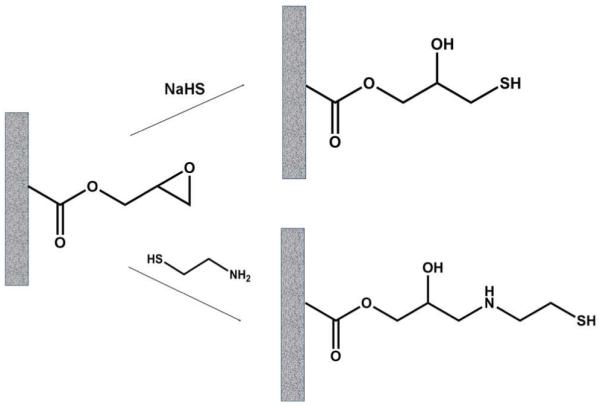
Reaction of poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith with sodium hydrogen sulfide and cysteamine affording polymer with thiol functionalities.
To assess the amount of thiol groups, we prepared a larger monolith in a vial using conditions that paralleled those used to prepare the capillaries. The content of thiol groups was then determined using disulfide exchange reaction with excess dipyridyldisulfide at room temperature.59,61 The mixture was centrifuged after the 1 h reaction, and the supernatant liquid containing the reaction product - pyridine-2-thione and excess dipyridyldisulfide was collected, diluted, and subjected to quantitative reversed phase HPLC analysis.
Gold nanoparticles modified monolithic columns
The apparatus used for the in situ method consisted of a T-piece with two inlets connected to two syringe pumps for the delivery of individual reagents: an aqueous solutions of HAuCl4 and a reducing agent sodium borohydride or sodium citrate, respectively. The SH column was attached to the third outlet. Both reagents were mixed within the T-piece before entering the SH column. Best results were obtained using 50 mmol/L HAuCl4, 200 mmol/L trisodium citrate each pumped in the monolith at a flow rate of 1.0 μL/min at a temperature of 100 °C for 30 min. The column was then rinsed with water to remove excess reagents, and stored in the refrigerator at 4°C. The monoliths functionalized in this fashion are referred to as “GNP column” in the text below.
RESULTS AND DISCUSSION
Preparation of the thiol-modified monolith
Because thiol-containing compounds are known to bind strongly to GNPs62,63 the first step towards the GNP column involves the preparation of a thiol-bearing monolith. Since thiols containing polymer monoliths are not readily accessed via free radical polymerization of monomers containing free sulfhydryl groups due to issues of chain transfer,59 we opted for the modification of the oxirane moieties of a simple poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith64. In separate experiments, two nucleophilic reagents - sodium hydrogen sulfide and cysteamine – were explored for the opening of oxirane rings as shown in Figure 1. Monoliths containing 0.58 and 1.05 mmol/g thiol groups were obtained using reaction condition optimized for sodium hydrogen sulfide and cysteamine, respectively. Overall, the reaction with neat liquid cysteamine was selected as it proved most suitable, affording monoliths with a higher content of thiol groups.
Preparation of monolith modified with gold nanoparticles
Although the polymerization of a mixture of GNP and monomers within the confines of a capillary to afford a monolith is technically possible, most of the GNPs would likely be buried within the monolithic matrix and not be available at the pore surface for the desired interactions. Therefore, we tested two other approaches involving the loading of pre-formed GNPs onto the monolith, or the in-situ preparation of the GNPs within the monolith.
In an initial study, the GNP column was prepared by attaching the preformed nanoparticles onto the thiol rich pore surface of the 10 cm long SH column. A commercial red colloid solution containing 15 nm GNP was pumped through the column. The color of the monolith turned red while the liquid leaving the capillary was colorless. Both these observations suggest retention of GNPs in the monolith as a control experiment in which the GNP colloid was pumped through the generic GMA column with no thiol functionalities did not afford change in color for the monolith while the eluent remained red. Unfortunately, 20 h were needed to modify the entire length of the column as use the low pressure limit of the syringe pump does not allow fast flow of the colloid solution through the column.
An alternative approach involving the in situ generation of GNPs within the thiolated monolith proved more convenient. GNPs generated in situ by reduction of chloroauric acid were immediately captured by the thiol groups of the monolith. In an initial approach the monolith was saturated with an aqueous solution of chloroauric acid pumped through the SH column. The color of the monolith turned from white to faint-yellow but turned to red when an aqueous solution of sodium borohydride was pumped through the column to generate the GNPs. This approach enabled the preparation of a GNP column within 1 h. However, the amount of GNPs formed inside the monolith is limited to the quantity of HAuCl4 adsorbed at the pore surface. Thus, this techniques leads to a monolith that contains only 5.5±0.6 (n=3) atom% Au.
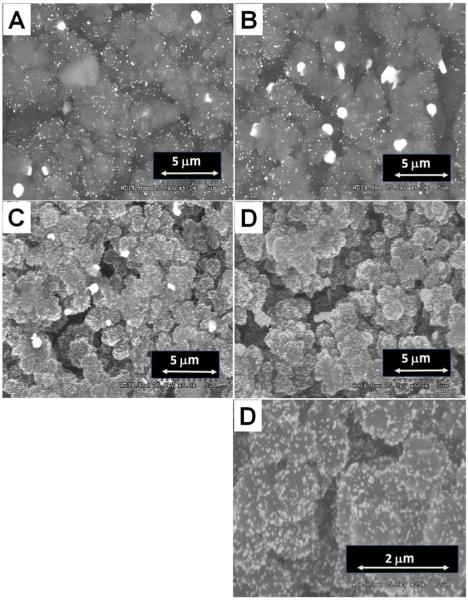
Better results were obtained when a mixture of HAuCl4 and a reducing agent was pumped through the monolith. With sodium borohydride as the powerful reducing agent the reaction can be carried out at room temperature but it is hampered by the concurrent release of bubbles of hydrogen which tend to stick to the pore surface within the monolith leading to low surface coverage and the formation of rather large GNPs. To avoid this problem trisodium citrate was used as it can effect the reduction if a higher temperature is used. Several parameters including the concentration of reagents, the flow rate, and the reaction time affect both the quantity and size of the GNPs that are formed. For example, a higher concentration of HAuCl4 leads to the formation of more GNPs. However, overly concentrated HAuCl4 solutions lead to aggregated GNPs as do long reaction times. The ratio of HAuCl4 to trisodium citrate also affects the size and uniformity of the GNPs. Higher flow rate enabling shorter residence time of the reacting mixture in the monolith leads to smaller and more uniform GNPs. Yet, the flow rate is once again limited by the back pressure in the system. Figure 2 displays SEM micrographs, taken with a BSE detector, of monoliths containing GNPs prepared under different conditions. The best GNP column contained 15.4±1.6 (n=3) atom% Au with a nanoparticle size of 40~50 nm.
Figure 2.
SEMs of monoliths modified with in situ prepared GNPs. Conditions: Stream 1 - 50 mmol/L HAuCl4, stream 2 - 80 mmol/L (A) or 200 mmol/L trisodium citrate (C-D), flow rate of the streams 1 μL/min (A, C, D) and 0.5 μL/min (B), reaction time 20 min (A, C, D) and 25 min (B).
Column permeability
Figure 3 shows that the back pressure in monolithic columns does not change significantly both after the modification with cysteamine and attachment of the GNPs. The slight increase can be assigned to the partial filling the pores with the additional material deposited in the pores and/or to swelling of the monolith. This finding is supported by the slight decrease in apparent porosity εT also shown in Figure 3 that is estimated using the equation: εT = f.t0/A.L, where f is the flow rate, t0 is the elution time of the unretained marker acetone, A is the cross section area of the column, and L is its length.
Figure 3.
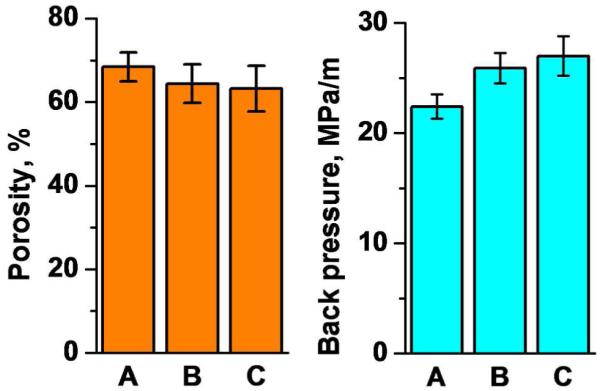
Resistance to flow and apparent porosity of the original poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolithic column (1), its cysteamine modified counterpart (B), and this columns with attached GNPs (C). Conditions: Pressure drop measured with acetonitrile at a flow rate of 1 μL/min; Porosity measured using unretained marker acetone, mobile phase 75% aqueous acetonitrile, flow rate, 1 μL/min, UV detection at 280 nm.
Retention of small molecules
Simple experiments using mixture of formamide, dimethylformamide, and toluene injected in all three types of monolithic columns under study (GMA, SH, and GNP) and eluted with 75% aqueous acetonitrile demonstrate changes in hydrophobicity of the separation media. While formamide and dimethylformamide are not retained in any of the columns and elute at the same time as the non-retained acetone, the more hydrophobic toluene is slightly retained. For example, a retention factor k = 0.22 was calculated for the original glycidyl methacrylate monolith, while the k values for the column modified with cysteamine and the column with attached GNPs were only 0.11 and 0.09, respectively. This is not surprising since reaction of the oxirane groups with cysteamine generates hydrophilic amine and hydroxyl functionalities and the citrate, which stabilizes the nanoparticles, then further contributes to the overall polarity.
Loading capacity
Increased surface area compared to the plain monolith is one of the advantages of the GNPs loaded monolithic column. This larger surface area also translates into enhanced loading capacity. Frontal elution of L-cysteine reveals a loading capacity of 2.58 μmol/m at 5% breakthrough for the 100 μm I.D. capillary column modified with GNPs. Considering that the typical amount of sample injected in micro-HPLC separations is about 10−5 μmol, the loading capacity of our 10 cm long column is 26,000 times greater than the sample size. Thus, the overloading of our column is largely eliminated.
Regeneration of the column
Because of the dynamic nature of the bond between gold and thiol groups, thiol containing compound bound to the gold surface can be exchanged using a solution of different thiol compound.65,66 This enables the use of the GNP column for separations since the retained compounds can be eluted using aqueous 2.0 mol/L solution of 2-mercaptoethanol (vide infra). However, once the compounds originally retained by the GNPs are released by action of the thiolated eluent no sites remain available for reuse of the column. Regeneration of the binding capacity of the GNP column can be carried out by heating the column as elevated temperatures weaken the adhesion of mercaptoethanol onto gold.67,68 in contrast, the GNPs held onto the surface of the monolith by more robust multivalent linkages can resist this regeneration process.
Figure 4 shows the effect of washing the column with water at different temperatures. Most of the loading capacity is recovered within 2 h at temperatures exceeding 80°C. Therefore, the loading capacity of the GNP column for cysteine after treatment at 80°C is 2.16 μmol/m or 84% of the original capacity. A shorted thermal treatment of 1 h at 80°C is less effective as only 75% of the original capacity is recovered.
Figure 4.
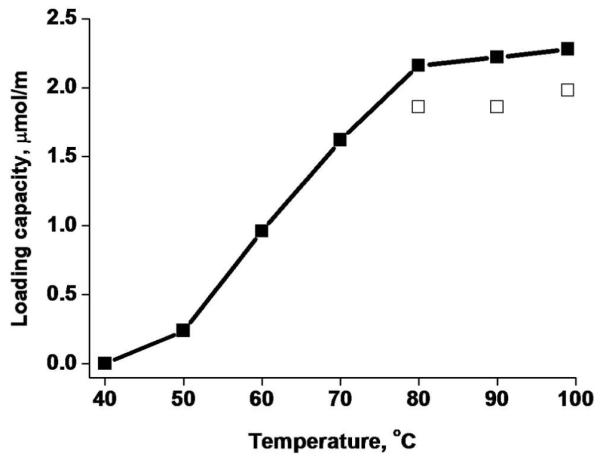
Loading capacity of GNP column regenerated for 1 (open squares) and 2 h (closed squares) at different temperatures. Conditions: 2.0 mol/L 2-mercaptoethanol, flow rate 0.5 μL/min.
The SEM/BSE image of the regenerated monolith shown Figure 5 confirms that GNPs remain attached to the surface with no appreciable aggregation. EDS analysis indicates 13.8 atom %Au. This value is close to that found for the column before use and lies within the experimental error of the measurement.
Figure 5.
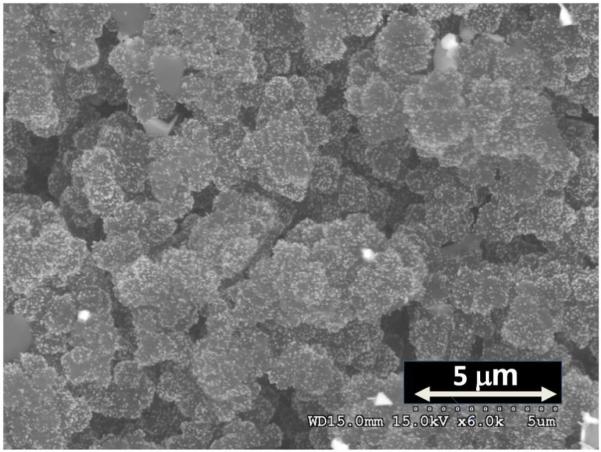
SEM micrograph of regenerated monolith containing GNPs.
Stability
As mentioned earlier the elution process involving thiol exchange using 2-mercaptoethanol solution does not cause loss of nanoparticles from the solid support or decrease in coverage. This is confirmed in stability tests in which, pure 2-mercaptoethanol and water heated to 100°C were pumped through the the GNPs modified column for 5 h. During the entire washing period the column kept its red color while the eluent from the column remained colorless. SEM/BSE images demonstrated again presence of significant number of GNPs attached to the surface. The actual contents of gold as determined using EDS after the treatment with 2-mercaptoethanol and hot water were 13.8 and 13.5 atom%, respectively. These tests indicate that the GNPs are strongly bound to the monolith through multiple gold-thiol bonds thus making it unlikely that all interacting functionalities can be substituted simultaneously to release the nanoparticle.
Capture of cysteine containing peptides
The primary function of the GNP column is the retention of thiol containing peptides based on its selectivity towards SH groups. Figure 6 demonstrates that out of four peptides tested, three that do not contain cysteine residues were eluted as a peak at the time corresponding to the dead volume of the column. In contrast, the pentapeptide His-Cys-Lys-Phe-Trp-Trp with one cysteine residue was not eluted and was retained by the column.
Figure 6.
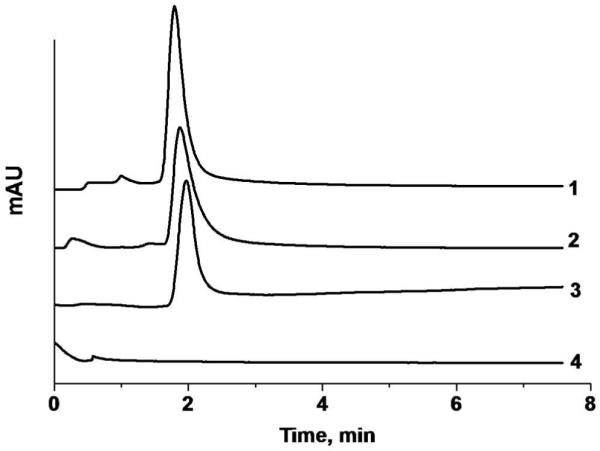
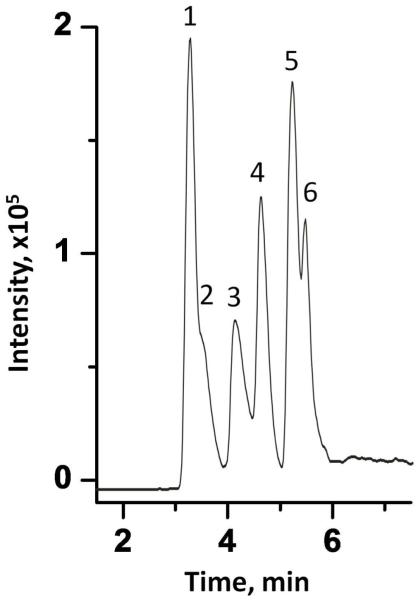
Elution of peptides using monolithic column containing GNPs. Condition: Column length 10 cm, concentration of peptide solution 0.1 mg/mL, injection volume 100 nL, mobile phase 0.1% (v/v) formic acid in aqueous 90% acetonitrile, flow rate 1 μL/min, UV detection at 214 nm. Traces: Tyr-Gly (1), Phe-Gly-Phe-Gly (2), Tyr-Gly-Gly-Phe-Leu (3), His-Cys-Lys-Phe-Trp-Trp (4).
Separation of peptides using tandem columns
The commercial C18 capillary column Acclaim PepMap™ is widely used for the separation of peptides. Figure 7 shows the separation of a mixture of six peptides, three of which contained a cysteine residue. Despite optimization of the elution conditions and use of a gradient, two pairs, [Cys17]-β-amyloid (1-17) and β-amyloid (1-17) as well as His-Cys-Lys-Phe-Trp-Trp and Tyr-Gly-Gly-Phe-Leu, coelute and baseline separation cannot be achieved.
Figure 7.
Separation of peptide mixture using a packed Acclaim PepMap™ C18 Nanocolumn. Conditions: column 15 cm × 75 μm I.D., concentration of each peptide in solution 0.1 mg/mL, injection volume 100 nL, flow rate 0.5 μL/min, mobile phase A, aqueous 0.1% (v/v) formic acid, B, 0.1% (v/v) formic acid in acetonitrile, gradient 0-60 % B in A in 8 min, UV detection at 214 nm. Peaks: [Cys17]-β-aamyloid (1-17) (1); β-amyloid (1-17) (2), His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Cys (3), LH-RH (4), His-Cys-Lys-Phe-Trp-Trp (5), Tyr-Gly-Gly-Phe-Leu (6).
Due to its low hydrophobicity, the GNP column itself cannot be used to separate peptides in reversed phase mode. However, its affinity for thiol functionalities can be used to selectively capture cysteine-containing peptides thus separating them from those that do not contain this amino acid residue, and effectively simplifying the complexity of the mixture.
To demonstrate this application, we combined the GNP and Acclaim PepMap C18 columns in a tandem arrangement. After injection of an aqueous solution of the peptide mixture, the cysteine-containing peptides are retained in the GNP column while the other peptides pass through only to be retained at the top of the hydrophobic C18 column. Their elution is then achieved using a gradient of acetonitrile with baseline separation being achieved as shown in the left panel of Figure 8. The acetonitrile gradient cannot liberate the peptides held in the GNP column. To achieve this, the mobile phase is changed to 2.0 mol/L aqueous 2-mercaptoethanol pumped through the columns for 5 min to release the peptides from the GNP column and carry them to the top of the C18 column where they are absorbed as 2-mercaptoethanol does not affect the adsorption of peptides on the C18 column. Finally, a gradient of acetonitrile elutes the second group of peptides with baseline separation as shown in the right panel of Figure 8.
Figure 8.
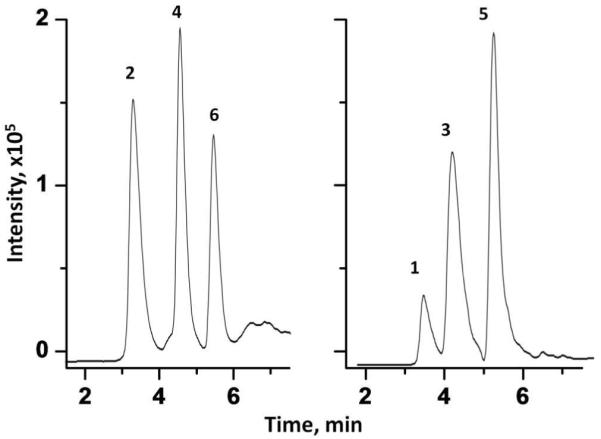
Separation of peptide mixture using a tandem system consisting of 10 cm × 100 μm I.D. monolithic column containing GNPs and 15 cm × 75 μm I.D. C18 packed column. Conditions: concentration of each peptide in solution 0.1 mg/mL, injection volume 100 nL, flow rate, 0.5 μL/min, mobile phases, step 1: aqueous 0.1% (v/v) formic acid; step 2: A, aqueous 0.1% (v/v) formic acid; B, 0.1% (v/v) formic acid in acetonitrile, gradient 0-60 % B in A in 8 min; step 3: 2.0 mol/L aqueous 2-mercaptoethanol; step 4: A, aqueous 0.1% (v/v) formic acid; B, 0.1% (v/v) formic acid in acetonitrile, gradient 0-60 % B in A in 8 min, UV detection at 214 nm. For peak assignment see Figure 7.
CONCLUSION
The capillary column containing gold nanoparticles bound to a porous polymer monolith should prove useful in proteomics analysis for the capture of cysteine containing peptides. This new type of monolithic column enables isolation of thiol containing peptides from mixtures with other peptides. The retained compounds are then released and separated. In future implementations, it may be desirable to increase coverage of the pores of the monolith with gold nanoparticles to further enhance the binding capacity of the column and accommodate larger samples. Finally, our experiments also suggest that using the facile thiol exchange process it may be possible to apply the gold nanoparticles as an “universal” reversible intermediate ligand enabling changes in the surface chemistry of the monolith via attachment of thiol containing compounds containing the desired surface functionality.
ACKNOWLEDGEMENT
Financial support of this research by a grant of the National Institute of Health (GM48364) is gratefully acknowledged. Q.C. thanks the China Scholarship Council (Ministry of Education of China) for support. Analytical nanostructural work performed at the Molecular Foundry, Lawrence Berkeley National Laboratory, was supported by the Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract No. DE-AC02-05CH11231.
References
- 1.Hamdan M, Righetti PG. Proteomics Today. Wiley; Hoboken, NJ: 2005. [Google Scholar]
- 2.O’Farrell PH. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 3.Righetti PG. Electrophoresis. 2004;25:2111–2127. doi: 10.1002/elps.200305808. [DOI] [PubMed] [Google Scholar]
- 4.Rabilloud T. Proteomics. 2002;2:3–10. [PubMed] [Google Scholar]
- 5.Gygi SP, Corthals GL, Zhang Y, Rochon Y, Aebersold R. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9390–9395. doi: 10.1073/pnas.160270797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aebersold R. J. Am. Soc. Mass Spectrom. 2003;14:685–695. doi: 10.1016/S1044-0305(03)00289-7. [DOI] [PubMed] [Google Scholar]
- 7.Hu L, Ye M, Jiang X, Feng S, Zou H. Anal. Chim. Acta. 2007;598:193–204. doi: 10.1016/j.aca.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Jmeian Y, El Rassi Z. Electrophoresis. 2009;30:249–261. doi: 10.1002/elps.200800639. [DOI] [PubMed] [Google Scholar]
- 9.Peterson DS, Rohr T, Svec F, Fréchet JMJ. Anal. Chem. 2003;75:5328–35. doi: 10.1021/ac034108j. [DOI] [PubMed] [Google Scholar]
- 10.Hunter TC, Andon NL, Koller A, Yates JR, Haynes PA. J. Chromatogr., B. 2002;782:165–181. doi: 10.1016/s1570-0232(02)00570-6. [DOI] [PubMed] [Google Scholar]
- 11.Mitulovic G, Stingl C, Smoluch M, Swart R, Chervet JP, Steinmacher I, Gerner C, Mechtler K. Proteomics. 2004;4:2545–2557. doi: 10.1002/pmic.200300806. [DOI] [PubMed] [Google Scholar]
- 12.Geiser L, Eeltink S, Svec F, Fréchet JMJ. J. Chromatogr., A. 2008;1188:88–96. doi: 10.1016/j.chroma.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger SR, Viner RI, Ho P. Electrophoresis. 2002;23:3182–3192. doi: 10.1002/1522-2683(200209)23:18<3182::AID-ELPS3182>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Foettinger A, Leitner A, Lindner W. J. Proteome Res. 2007;6:3827–3834. doi: 10.1021/pr0702767. [DOI] [PubMed] [Google Scholar]
- 15.Ren DY, Penner NA, Slentz BE, Inerowicz HD, Rybalko M, Regnier FE. J. Chromatogr., A. 2004;1031:87–92. doi: 10.1016/j.chroma.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Wang SH, Regnier FE. J. Chromatogr., A. 2001;924:345–357. doi: 10.1016/s0021-9673(01)00961-x. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Qian WJ, Strittmatter EF, Camp DG, Anderson GA, Thrall BD, Smith RD. Anal. Chem. 2004;76:5345–5353. doi: 10.1021/ac049485q. [DOI] [PubMed] [Google Scholar]
- 18.Gygi SP, Rist B, Griffin TJ, Eng J, Aebersold R. J. Proteome Res. 2002;1:47–54. doi: 10.1021/pr015509n. [DOI] [PubMed] [Google Scholar]
- 19.Stevens TS, Langhorst MA. Anal. Chem. 1982;54:950–953. [Google Scholar]
- 20.Zhang ZX, Wang ZY, Liao YP, Liu HW. J. Sep. Sci. 2006;29:1872–1878. doi: 10.1002/jssc.200600154. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson C, Birnbaum S, Nilsson S. J. Chromatogr., A. 2007;1168:212–224. doi: 10.1016/j.chroma.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Jinno K, Tanabe K, Saito Y, Nagashima H. Analyst. 1997;122:787–791. [Google Scholar]
- 23.Neiman B, Grushka E, Lev O. Anal. Chem. 2001;73:5220–5227. doi: 10.1021/ac0104375. [DOI] [PubMed] [Google Scholar]
- 24.Cintron JM, Colon LA. Analyst. 2002;127:701–704. doi: 10.1039/b203236h. [DOI] [PubMed] [Google Scholar]
- 25.Dun HJ, Zhang WQ, Wei Y, Song XQ, Li YM, Chen LR. Anal. Chem. 2004;76:5016–5023. doi: 10.1021/ac030389j. [DOI] [PubMed] [Google Scholar]
- 26.Guihen E, Glennon JD. Anal. Lett. 2003;36:3309–3336. [Google Scholar]
- 27.Nilsson C, Nilsson S. Electrophoresis. 2006;27:76–83. doi: 10.1002/elps.200500535. [DOI] [PubMed] [Google Scholar]
- 28.Zhang ZX, Yan B, Liao YP, Liu HW. Anal. Bioanal. Chem. 2008;391:925–927. doi: 10.1007/s00216-008-1930-2. [DOI] [PubMed] [Google Scholar]
- 29.Svec F, Fréchet JMJ. Anal. Chem. 1992;64:820–822. doi: 10.1021/ac00035a008. [DOI] [PubMed] [Google Scholar]
- 30.Wang QC, Svec F, Fréchet JMJ. J. Chromatog. A. 1994;669:230–235. doi: 10.1016/0021-9673(94)80352-8. [DOI] [PubMed] [Google Scholar]
- 31.Svec F, Fréchet JM. J. Science. 1996;273:205–211. doi: 10.1126/science.273.5272.205. [DOI] [PubMed] [Google Scholar]
- 32.Sýkora D, Svec F, Fréchet JMJ. J. Chromatogr. A. 1999;852:297–304. doi: 10.1016/s0021-9673(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 33.Irgum K, Viklund C, Svec F, Fréchet JMJ. Biotech. Prog. 1997;13:597–600. doi: 10.1021/bp9700667. [DOI] [PubMed] [Google Scholar]
- 34.Guiochon G. J. Chromatogr., A. 2007;1168:101–168. doi: 10.1016/j.chroma.2007.05.090. [DOI] [PubMed] [Google Scholar]
- 35.Svec F. J. Sep. Sci. 2004;27:1419–1430. doi: 10.1002/jssc.200401825. [DOI] [PubMed] [Google Scholar]
- 36.Meyer U, Svec F, Fréchet JMJ, Hawker CJ, Irgum K. Macromolecules. 2000;33:7769–75. [Google Scholar]
- 37.Rohr T, Hilder EF, Donovan JJ, Svec F, Fréchet JMJ. Macromolecules. 2003;36:1677–1684. [Google Scholar]
- 38.Eeltink S, Hilder EF, Geiser L, Svec F, Fréchet JMJ, Rozing GP, Schoenmakers PJ, Kok WT. J. Sep. Sci. 2007;30:407–13. doi: 10.1002/jssc.200600316. [DOI] [PubMed] [Google Scholar]
- 39.Hilder EF, Svec F, Fréchet JMJ. J. Chromatogr., A. 2004;1053:101–106. [PubMed] [Google Scholar]
- 40.Hutchinson JP, Zakaria P, Bowie AR, Macka M, Avdalovic N, Haddad PR. Anal. Chem. 2005;77:407–416. doi: 10.1021/ac048748d. [DOI] [PubMed] [Google Scholar]
- 41.Zakaria P, Hutchinson JP, Avdalovic N, Liu Y, Haddad PR. Anal. Chem. 2005;77:417–423. doi: 10.1021/ac048747l. [DOI] [PubMed] [Google Scholar]
- 42.Hutchinson JP, Hilder EF, Macka M, Avdalovic N, Haddad PR. J. Chromatogr., A. 2006;1109:10–18. doi: 10.1016/j.chroma.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 43.Hutchinson JP, Macka M, Avdalovic N, Haddad PR. J. Chromatogr., A. 2006;1106:43–51. doi: 10.1016/j.chroma.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Glenn KM, Lucy CA, Haddad PR. J. Chromatogr., A. 2007;1155:8–14. doi: 10.1016/j.chroma.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 45.Sykora D, Kasicka V, Miksik I, Rezanka P, Zaruba K, Matejka P, Kral V. J. Sep. Sci. 2010;33 doi: 10.1002/jssc.200900677. in press. [DOI] [PubMed] [Google Scholar]
- 46.Zhong ZY, Male KB, Luong JHT. Anal. Lett. 2003;36:3097–3118. [Google Scholar]
- 47.Huang MF, Kuo YC, Huang CC, Chang HT. Anal. Chem. 2004;76:192–196. doi: 10.1021/ac034908u. [DOI] [PubMed] [Google Scholar]
- 48.Yu CJ, Su CL, Tseng WL. Anal. Chem. 2006;78:8004–8010. doi: 10.1021/ac061059c. [DOI] [PubMed] [Google Scholar]
- 49.Pumera M, Wang J, Grushka E, Polsky R. Anal. Chem. 2001;73:5625–5628. doi: 10.1021/ac015589e. [DOI] [PubMed] [Google Scholar]
- 50.Gross GM, Nelson DA, Grate JW, Synovec RE. Anal. Chem. 2003;75:4558–4564. doi: 10.1021/ac030112j. [DOI] [PubMed] [Google Scholar]
- 51.Gross GM, Grate JW, Synovec RE. J. Chromatogr., A. 2004;1029:185–192. doi: 10.1016/j.chroma.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Guihen E, Holmes JD, Loughran M, O’Sullivan GP, Glennon JD. Anal. Chem. 2005;77:1840–1846. doi: 10.1021/ac048544x. [DOI] [PubMed] [Google Scholar]
- 53.Liu FK, Hsu YT, Wu CH. J. Chromatogr., A. 2005;1083:205–214. doi: 10.1016/j.chroma.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 54.Qu QS, Zhang XX, Shen M, Liu Y, Hu XY, Yang GJ, Wang CY, Zhang YK, Yan C. Electrophoresis. 2008;29:901–909. doi: 10.1002/elps.200700409. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K, Kitagawa S, Ohtani H. J. Chromatogr., A. 2006;1110:95–101. doi: 10.1016/j.chroma.2006.01.094. [DOI] [PubMed] [Google Scholar]
- 56.Svec F. J. Sep. Sci. 2009;32:3–9. doi: 10.1002/jssc.200800530. [DOI] [PubMed] [Google Scholar]
- 57.Svec F. Electrophoresis. 2009;30:S68–S82. doi: 10.1002/elps.200900062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connolly D, Twamley B, Paull B. Chem. Com. doi: 10.1039/b924152c. DOI: 10.1039/b924152c. [DOI] [PubMed] [Google Scholar]
- 59.Preinerstorfer B, Bicker W, Lindner W, Lammerhofer M. J. Chromatogr., A. 2004;1044:187–199. doi: 10.1016/j.chroma.2004.04.078. [DOI] [PubMed] [Google Scholar]
- 60.Burfield DR, Gan SN, Smithers RH. J. Chem. Soc., Perkin Trans. 1. 1977:666–671. [Google Scholar]
- 61.Sevcikova P, Glatz Z, Tomandl J. J. Chromatogr., A. 2003;990:197–204. doi: 10.1016/s0021-9673(03)00048-7. [DOI] [PubMed] [Google Scholar]
- 62.Kim YJ, Kim JW, Lee JE, Ryu JH, Kim J, Chang IS, Suh KD. J. Polym. Sci., Polym Chem. 2004;42:5627–5635. [Google Scholar]
- 63.Liu W, Yang XL, Xie L. J. Colloid Interface Sci. 2007;313:494–502. doi: 10.1016/j.jcis.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 64.Svec F, Fréchet JMJ. J. Chromatogr. A. 1995;702:89–95. doi: 10.1016/0021-9673(94)01021-6. [DOI] [PubMed] [Google Scholar]
- 65.Heister K, Allara DL, Bahnck K, Frey S, Zharnikov M, Grunze M. Langmuir. 1999;15:5440–5443. [Google Scholar]
- 66.Xu H, Hong R, Wang XY, Arvizo R, You CC, Samanta B, Patra D, Tuominen MT, Rotello VM. Adv. Mater. 2007;19:1383. + [Google Scholar]
- 67.Garg N, Carrasquillo-Molina E, Lee TR. Langmuir. 2002;18:2717–2726. [Google Scholar]
- 68.Sharma J, Chhabra R, Yan H, Liu Y. Chem. Comm. 2008:2140–2142. doi: 10.1039/b800109j. [DOI] [PubMed] [Google Scholar]