SUMMARY
We have previously demonstrated that the EP1 subtype of PGE2 receptor is expressed in the differentiated compartment of normal human epidermis and is coupled to intracellular calcium mobilization. We therefore hypothesized that the EP1 receptor is coupled to keratinocyte differentiation. In in vitro studies, radioligand binding, RT-PCR, immunoblot and receptor agonist-induced second messenger studies demonstrate that the EP1 receptor is up-regulated by high cell density in human keratinocytes and this up-regulation precedes corneocyte formation. Moreover, two different EP1 receptor antagonists, SC51322 and AH6809, both inhibited corneocyte formation. SC51322 also inhibited the induction of differentiation-specific proteins, cytokeratin K10 and epidermal transglutaminase. We next examined the immunolocalization of the EP1 receptor in non-melanoma skin cancer in humans. Well differentiated SCCs exhibited significantly greater membrane staining, while spindle cell carcinomas and BCCs had significantly decreased membrane staining compared with normal epidermis. This data supports a role for the EP1 receptor in regulating keratinocyte differentiation.
INTRODUCTION
The ability of keratinocytes to undergo differentiation and form detergent-insoluble squamous cells or corneocytes is critical to maintaining the skin's permeability barrier. This permeability barrier is essential for the normal functioning of the body's largest organ, as disruption of this barrier results in fluid loss and increased susceptibility to environmental and microbial insults. Moreover, escape from differentiation-induced growth arrest is a hallmark of non-melanoma skin cancer (NMSC). However, NMSC exhibits striking differences in the “squamous” cell phenotype, with basal cell carcinomas (BCC) recapitulating the phenotype of the undifferentiated basal cell compartment and well differentiated squamous cell carcinoma (SCC) exhibiting the full spectrum of differentiation-associated cellular changes. Moreover, the degree to which SCCs retain the differentiated phenotype often inversely correlates with the aggressiveness of the tumor, with poorly differentiated SCCs and spindle cell carcinomas exhibiting a more aggressive course. However, the mechanisms that regulate epidermal differentiation are poorly understood.
Prostaglandins (PG) are formed sequentially by cleavage of arachidonic acid (AA) from cellular phospholipids, conversion of AA to PGH2 by one of two cyclooxygenases (COX-1 and COX-2), and finally metabolism of PGH2 to the major prostaglandin species by specific PG synthases [1]. The major prostaglandin species released by epidermal keratinocytes is PGE2 [2]. PGE2 acts by binding to one of four heterotrimeric G-protein coupled receptors, termed E-series prostaglandin receptors (EP1-EP4)(reviewed in [3, 4]). These receptors differ in their G protein alpha subunit binding specificity and the second messenger pathways that are activated upon ligand binding. In addition, the four receptor subtypes also exhibit differences in PGE2 binding affinities. The EP3 and EP4 exhibit binding affinities for PGE2 in the subnanomolar range, while the EP1 and EP2 receptors are lower affinity PGE2 receptors, with binding affinities of 9.1 and 4.9 nM, respectively [5].
Several studies indicate that cyclooxygenase products are involved in regulating keratinocyte differentiation. Alterations in epidermal differentiation have been described in COX-1 and COX-2 knockout mice, as well as with transgenic mice overexpressing COX-2 in the epidermis [6, 7]. Moreover, in primary human keratinocytes in vitro, inhibition of cyclooxygenase activity has also been shown to inhibit calcium-dependent cornified envelope production [8]. Significantly, exogenous PGE2, but not prostacyclin (PGI2), was able to restore normal calcium-induced keratinocyte differentiation [8]. While the specific receptor subtype(s) mediating PGE2-induced differentiation are unknown, the concentration of PGE2 which restored calcium-induced differentiation was 100 nM, suggesting activation of low-affinity PGE2 receptors [8]. The idea that high levels of PGE2 are necessary for keratinocyte differentiation is also supported by the observation that COX-2 expression is up-regulated in the differentiated, suprabasalar compartment of normal human epidermis by immunohistochemistry [9]. In addition, COX-2 expression, but not COX-1 expression, is markedly up-regulated by high extracellular calcium concentrations in cultured human keratinocytes [9].
We have previously shown that the EP2 receptor, and possibly the EP4 receptor, act to stimulate growth of primary human keratinocytes via production of cyclic AMP [10]. Moreover, loss of EP2 receptor expression may play a role in the invasive behavior in SCC [11, 12]. In contrast, EP3 receptor activation stimulates diacylglycerol and ceramide production and results in keratinocyte growth inhibition [13]. However, the role of the EP1 receptor in keratinocyte biology is unknown. Several clues to the function of the EP1 receptor in keratinocytes can be inferred by its localization within the epidermis as well as the intracellular signaling pathway that is stimulated by EP1 receptor activation [14]. In this previous study, immunohistochemistry demonstrated that EP1 receptor expression was seen throughout the epidermis, but was markedly increased in the granular layer. In another study, it was noted that the EP1 receptor was highly expressed in human squamous cell carcinoma and actinic keratoses, but was weakly expressed or absent in BCCs [15]. Moreover, we have previously demonstrated that normal human keratinocytes express the EP1 receptor and that this receptor is coupled to intracellular calcium mobilization [14]. Since calcium is a potent inducer of keratinocyte differentiation, this suggests a potential role for the EP1 receptor in regulating keratinocyte differentiation. In this current study, we examine how the EP1 receptor is regulated during calcium and density-dependent induction of human keratinocyte differentiation in vitro. We then examine the role that the EP1 receptor plays in regulating the differentiated phenotype in vitro. Finally, we examine EP1 receptor expression by immunohistochemistry in pre-malignant human actinic keratoses (AKs), well differentiated SCCs, poorly differentiated SCCs (PD-SCCs), and spindle cell carcinomas and BCCs.
MATERIALS & METHODS
Materials
PGE2 receptor agonists, PGE2, and rabbit polyclonal antibodies against the human EP1 receptor were purchased from Cayman Chemical (Ann Arbor, MI). Peroxoblock, CAS block, and Picture Plus broad spectrum immunohistochemical staining kits were purchased from Zymed Laboratories (South San Francisco, CA). High pH antigen retrieval solution was purchased from Dako (Carpinteria, CA). Isolated membrane preparations from human embryonic kidney cells (HEK-293) stably expressing the human EP1 and EP3A1 receptors were the generous gift of Dr. Kathleen Metters, (Merck-Frosst Centre for Therapeutic Research; Quenbec, Canada) [16]. The stable platelet activating factor (PAF) ligand, carbamyl-PAF was kindly supplied by Dr. Jeffrey Travers (Indiana University, Indianapolis, IN).
Isolation and culture of primary human keratinocytes
Adult primary human keratinocytes were prepared from discarded epidermis obtained from reductive mammoplasties and panniculectomies as previously described [10]. Cells were cultured on tissue culture plasticware precoated with collagen (Vitrogen 100, Collagen Corp., Palo Alto, CA). Cells were grown in either Dulbecco minimal Eagle's medium (DMEM) containing 10% fetal bovine serum and 15 mM HEPES buffer (FBS-DMEM), or in low calcium, serum-free medium (Keratinocyte-SFM (K-SFM), Invitrogen Life Technologies) containing 0.06 mM calcium chloride. Media were supplemented with 40 IU per ml penicillin, 40 μg per ml streptomycin, and 0.1 μg per ml amphotericin B. The cells were cultured in 95% air, 5% CO2 at 37°C. All studies with human skin have been approved by the Indiana University-Purdue University at Indianapolis and the University of Rochester Institutional Review Boards.
[3H]-PGE2 competitive binding
Freshly isolated primary human keratinocytes were cultured in FBS-DMEM on 12-well plates. To assess specific [3H]-PGE2 binding activity, PHK's were plated to reach 80% confluence after an overnight incubation. Both specific PGE2 binding and cornified envelope quantitation (see below) was performed daily once cells had reached > 90% confluence, or one day prior to reaching 100% confluence (Day-1). On each day, starting at day -1 confluence and continuing until 2 days post-confluence (Day +2), duplicate wells were washed twice with ice-cold HEPES buffered saline (HBS): 15 mM HEPES, pH 7.4 containing 140 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, and 11 mM glucose. After washing, 200 μl of cold HBS containing 3 nM of [3H]-PGE2 (154 Ci/mmol, Perkin Elmer Life Sciences, Boston, MA) was added in the presence and absence of 1000-fold molar excess of PGE2 or specific receptor agonists. After incubating with rocking at 4°C for 1 hour, the wells were washed four times with 2 ml cold HBS and the cells trypsinized and radioactive incorporation quantitated by liquid scintillation counting. On the same day, separate wells were processed for cornified envelope formation as detailed below.
Preparation of total cell lysates from primary human keratinocyte cultures
PHK's were grown to the desired cell densities in serum-free media containing 0.06 mM calcium, bovine pituitary extract, and epidermal growth factor (K-SFM, Invitrogen, Carlsbad, California). Pre-confluent cultures were established under low power microscopic exam as monolayer cultures occupying less than 60 % of the available surface area at the time of total cell lysate preparation. Confluent cultures were defined as cultures in which the cell monolayer covered 100% of the tissue culture surface. Total cell lysates were then prepared as previously described [17]. Total protein was determined prior to addition of reducing agent and tracking dye using the DC Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Western blot analysis
Western blot analysis of EP1 and EP2 receptor expression was done using rabbit polyclonal anti-EP1 and anti-EP2 receptor antibodies (Cayman Chemical) on heat denatured (EP2) preparations or non-heat denatured (EP1) preparations as previously described [14]. EP1 immunoblots were performed using total cell lysates prepared as described above. In contrast, EP2 immunoblots were performed using cellular membranes prepared as previously described [11].
Calcium mobilization studies
PHKs were grown to low cell density, <60% confluence (pre-confluent), or high cell density, 100% confluent (post-confluent). Prior to Fura PE3/AM loading, confluent monolayers were pre-treated for 48 hours with indomethacin (3 μg per mL) to block endogenous PGE2 production. Cells were then loaded with 5 μM Fura PE3/AM-ester (Fisher Scientific, Hanover Park, Illinois) in growth supplement-free K-SFM containing 3 μg per mL indomethacin, 2.5mM probenecid (Sigma, St. Louis, Missouri), and 1 mg/ml Bovine Serum Albumin (BSA) for 1 hour at 37°C. After loading, the monolayer was washed 2 times with Hank's balanced salt solution (HBSS) containing 1.26 mM calcium (Sigma, St. Louis, Missouri), 2.5 mM Probenecid, and 1 mg/ml BSA balanced to pH 7.6. The cells were then detached by trypsinization, centrifuged, and resuspended in the HBSS solution, pH 7.6. Resuspended cells were incubated at room temperature for 30 min. For intracellular Ca2+ measurements, 2.5 mL of cells in the HBSS solution were loaded into a standard cuvette containing a magnetic stirring device. The cuvette was placed into a thermostatically controlled magnetic stirring chamber at 37°C in a Hitachi F-2000 fluorescence spectrophotometer. After establishing a baseline calcium level, agonists or vehicle (ethanol) was injected into the cuvette using an injection port. For a positive control, carbamyl-PAF (16 μg per mL) was added. Excitation wavelengths were 340 nm and 380 nm, and emitted light measured at 510 nm. Ratiometric recordings were produced using F-2000 software (Hitachi Instruments, Naperville, Illinois).
cAMP production
For pre-confluent versus post-confluent PGE2-induced cAMP, freshly isolated PHKs were grown in 6 or 12-well plates in FBS-DMEM. Pre-confluent cells were 50-70% confluent while post-confluent cultures were 1-2 days post-confluent. The cells were pretreated with 3 μg/ml indomethacin overnight to block endogenous PGE2 formation. The cells were then stimulated with 100 nM PGE2 for 1 minute in media pre-equilibrated to 37°C in the absence of phosphodiesterase inhibitors to measure the peak cAMP response as previously described [10]. To examine the ability of PGE2 receptor antagonists to block EP2 agonist-induced cAMP, PHK's were plated in 24-well plates at a density of 25,000 cells/well in low calcium, serum-free K-SFM media. Indomethacin was added as above to block endogenous PGE2 production. The cells were then stimulated with 10 nM of the specific EP2 receptor agonist (CAY10933, Cayman Chemical, Ann Arbor, MI) in the absence or presence of AH6809 (12.5 μM) and SC51322 (500 nM) for 15 minutes. In this case, isobutylmethylxanthine (IBMX) was added at a concentration of 4 mM to inhibit cAMP phosphodiesterase activity. Cyclic AMP levels were quantitated as above. In all cases, cAMP responses were normalized to total monolayer protein following hydrolysis in 1 N NaOH using a BCA assay kit (Pierce Biotechnology, Rockford, IL).
Cornified envelope quantitation and envelope competency assay
For cornified envelope quantitation (Fig 1A), PHKs growing in FBS-DMEM as described in the figure legend were trypsinized, pelleted and resuspended in 1 ml of FBS-DMEM. For total cell counts, 0.1 ml of the cell suspension was removed and counted. For cornified envelope quantitation, 100 μl of 20 % sodium dodecyl sulfate (SDS) with 20 mg/ml dithiothreitol was added to the remainder of the cell suspension. The cell suspensions were then placed in boiling water for 5 min and then allowed to cool to room temperature. DNAse I (5 U) was then added and the tubes incubated at 37°C for 15 minutes. Cornified envelopes were then counted using a hemocytometer and expressed as a percent of total cell counts. For the enumeration of cells competent to form cornified envelopes (Fig 4A), PHKs growing in K-SFM media were trypsinized and plated at a cell density of 80,000 cells/well into 6-well plates. At approximately 50-60% confluent growth, fresh media was added containing either dimethylsulfoxide (vehicle) or EP receptor antagonist (SC51322 or AH6809) as described in the figure legend. Media was changed every other day until 3-4 days after reaching confluence. The cells were then trypsinized, and an aliquot counted for total cell counts. The remaining cells were centrifuged, and the pellet resuspended in K-SFM containing 1.2 mM calcium and 5 μg/ml of the ionophore, A23187 to induce envelope formation as previously described [18]. Cornified envelopes were then quantitated by counting SDS-insoluble corneocytes using a hemocytometer at least 10 times per sample.
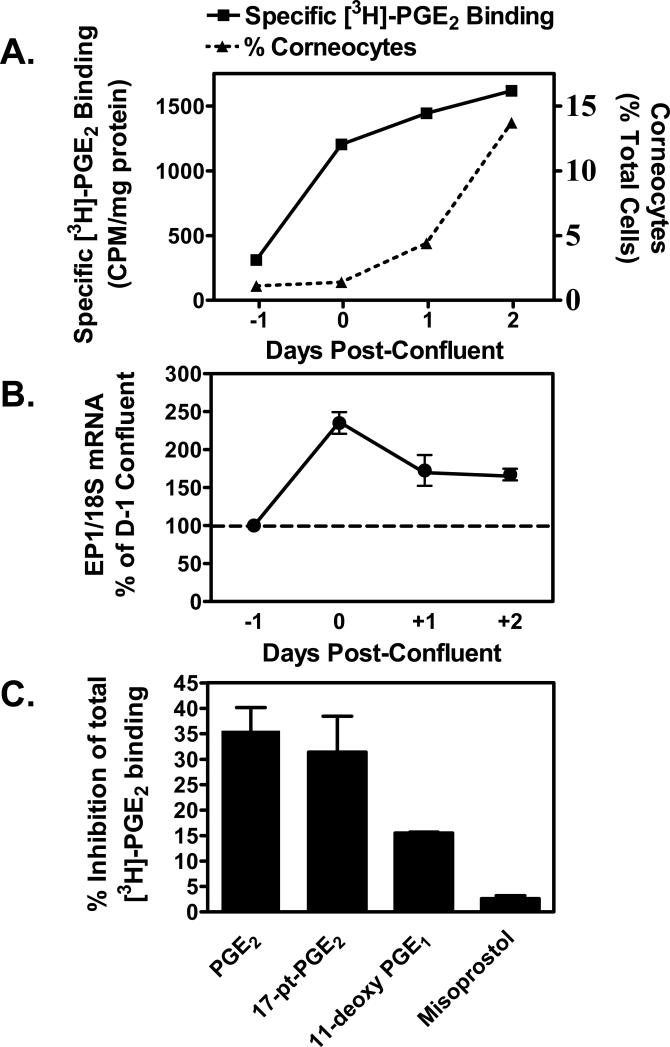
Figure 1. Up-regulation of the EP1 receptor occurs with high cell density and precedes the appearance of corneocytes in primary human keratinocytes (PHKs).
(A). Specific PGE2 binding activity is induced and precedes cornified envelope formation with increasing cell density. Freshly isolated primary human keratinocytes were plated onto collagen-coated 12-well plates in DMEM with 10% fetal bovine serum and antibiotics. One day prior to 100% confluence (day -1), and daily thereafter until the cells were 2 days post-confluent (Day +2), duplicate wells were incubated with [3H]-PGE2 in the presence or absence of a 1000-fold molar excess of unlabeled PGE2. Specific radiolabeled PGE2 binding was then determined and normalized to total cellular protein. On the same days, duplicate wells were trypsinized and terminally differentiated SDS-insoluble cornified cells were counted. Corneocytes are shown as a total cells present per well. (B). EP1 receptor mRNA is induced as PHKs acquire a confluent monolayer. PHKs were plated onto collagen-coated 6-well plates as described in panel A above. Starting one day prior to confluence, and daily thereafter through 2 days post-confluent, the cells were lysed and total RNA prepared for real-time PCR analysis of EP1 receptor expression. Results were normalized to 18S ribosomal RNA. The results represent the mean ± SEM of two experiments done in duplicate. (C). EP1 specific agonists compete for radiolabeled PGE2 binding in confluent PHKs. Two day post-confluent keratinocytes were treated with radiolabeled PGE2 as in panel A. Competition for radioligand binding is shown using 100-fold excess of unlabeled PGE2 and receptor agonists. Results shown are the mean and SEM of two experiments done in duplicate.
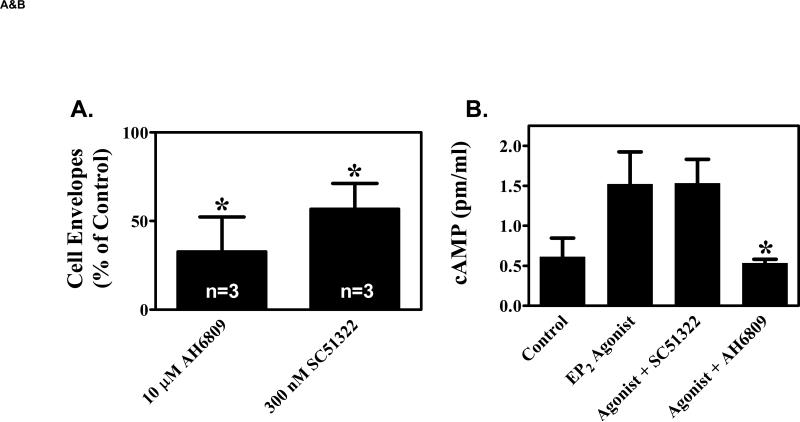
Figure 4. EP1 receptor antagonists inhibit keratinocyte differentiation.
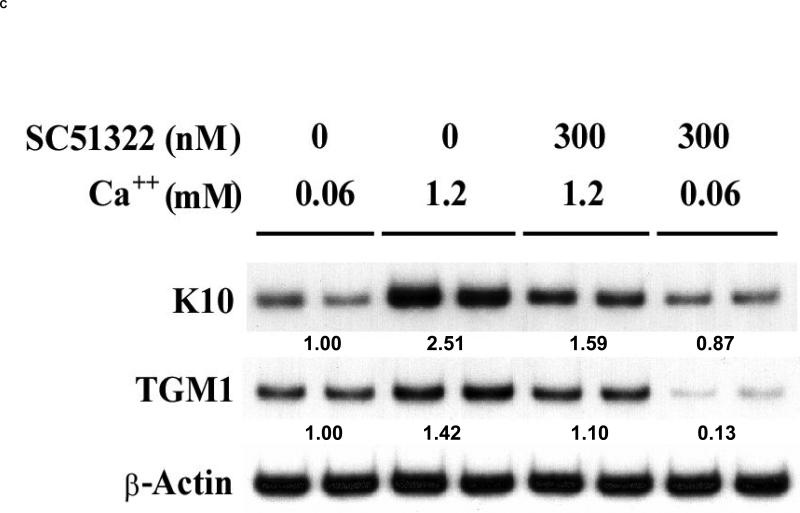
(A). Primary human keratinocytes were grown in K-SFM (0.06 mM Ca2+). Addition of vehicle (0.1% DMSO), 10 μM of the non-specific EP1, EP2, EP3 receptor antagonist, AH6809, or 300 nM of the EP1 selective antagonist, SC51322 was begun when the cells were 50-60% confluent and every other day thereafter. At 3-4 days after reaching confluence, the capacity of the cells to form SDS-insoluble cornified cell envelopes was determined after trypsinizing the cells, pelleting the cells, and treating the cells for 3 hours with a calcium ionophore and high calcium media to stimulate envelope formation (envelope competence). The results were normalized to total cell counts and expressed as a percent of vehicle control cells. The results represent the mean and SEM of three experiments done in single or duplicate wells, with corneocytes from each well counted at least 10 times using a hemocytometer. * Results significantly different from control cells (P < 0.05; one sample t-test). (B). The non-specific EP1, EP2, EP3 antagonist (AH6809), but not the EP1 specific antagonist (SC51322), inhibits EP2 receptor stimulated cAMP production. Primary human keratinocytes were first incubated with 3 μg/ml indomethacin to block endogenous PGE2 formation. The cells were then stimulated with a highly selective EP2 receptor agonist (CAY10933, 10 nM) in the absence or presence of AH6809 (12.5 μM) and SC51322 (500 nM) for 15 minutes. Cyclic AMP was measured using a commercial EIA kit. (C). The EP1 specific agonist SC51322 inhibits calcium-dependent up-regulation of the differentiation specific markers, cytokeratin K10 and epidermal transglutaminase (TGMI). Duplicate wells of primary human keratinocytes were grown in K-SFM (0.06 mM Ca2+) until they reached near confluence (1 day prior to attaining confluence). At this time, the cells were treated with vehicle or 300 nM SC51322. One hour later, additional Ca2+ (1.2 mM), was added to the wells as indicated. After an additional 48 hours (1 day post-confluent), the cells were processed for isolation of total RNA. Semi-quantitative RT-PCR was then performed on each of the duplicate samples using specific primers for K10, TGM1, or β-actin (loading control). All PCR reactions were stopped during the exponential phase of PCR amplification and visualized by agarose gel electrophoresis and autoradiography. The mean normalized band intensity for both K10 and TGM1 (normalized to β-Actin) is shown under each duplicate radiographic image. In each case, the band intensity is shown as a ratio compared with the low calcium (0.06 mM) vehicle control cells (assigned a value of 1.00). Band intensity was determined by area integration using NIH Image J software.
Quantitative real-time PCR
Total RNA was prepared from cellular monolayers using a commercial kit per the manufacturer's instructions (RNeasy ®, Qiagen, Valencia, CA). Reverse transcription was performed using the Superscript III kit (Invitrogen, Carlsbad, CA) and was followed by real-time quantitative PCR using a Cepheid Smart-Cycler real-time PCR instrument (Fisher Scientific, Pittsburgh, PA). Primers and probes for the human EP1 receptor were as follows: EP1 forward primer: 5’-AGCTCGCGCCCACGA-3’; EP1 reverse primer: 5’-ATGCACGACACCACCATGAT-3’; EP1 probe: 5’-[DTET]-TGGAGATGGTGGGCCAGCTTGTC-3’. Primers and probe for the EP2 receptor were as follows: EP2 forward primer: 5’-GGAGAGGAGGGCGCATCT-3’; EP2 reverse primer: 5’-GGGAGTCATTGGAGGCATTG-3’; EP2 probe: 5’-[DTET]-TTTTCCAGGCACCCCACCATGG-3’]. 18S forward primer: 5’-ACATCCAAGGAAGGCAGCAG-3’; 18S reverse primer: 5’-TCGTCACTACCTCCCCGG-3’; 18S probe: 5’-[DFAM]-CGCGCAAATTACCCACTCCCGA-3’]. All primers and probes were synthesized by Sigma-Genosys (St. Louis, Mo). Following real-time PCR, the results were converted to numeric values utilizing a standard curve generated from serial dilutions of a gel-purified PCR fragment of each target. Results for EP1 and EP2 receptor are normalized to 18S rRNA expression for each sample.
Semi-quantitative PCR for epidermal transglutaminase (TGMI) and keratin K10
First to third passage neonatal human keratinocytes were plated in 6-well dishes (35 mm dishes) in K-SFM (0.06 mM Ca2+). After allowing the cells to attach for 16-20 hours, cells grown to a nearly confluent monolayer (day -1) were treated with 300 nM SC51322 or DMSO (0.05% final concentration). In selected wells, 1.2 mM calcium chloride was added 1 hour after SC51322 addition. Total RNA was prepared from duplicate wells at 48 hours after treatment. cDNA was prepared by heat denaturing 0.75 ug RNA at 68°C for 5 minutes, followed by reverse transcription (Superscript II, Invitrogen) for 60 minutes at 42°C, using 2 μM oligo-dT(12-18) primer (Invitrogen), 1.25 mM each dNTP, 0.5 U RNase inhibitor (Promega), and 10 mM DTT. Relative message levels of transglutaminase 1 (TGM1), keratin 10 (K10), and β-actin were determined by PCR amplification of cDNA, diluted 1:10 with H2O and added at 1/10th volume to a 25 μl reaction comprising 2.5 U Taq DNA polymerase (Promega), PCR buffer (Sigma), 1.8 mM MgCl22, 400 nM primers, 400 μM each dNTP (Roche Molecular Biochemicals), and 5 μCi α-32P-dCTP. Samples were removed after 22 cycles (TGM1 and K10) or 18 cycles (β-actin) of PCR amplification which corresponded to the midpoint of exponential amplification as determined in previous experiments. The thermal profile was 94°C for 20 seconds, 57°C for 20 seconds, 72°C for 30 seconds. PCR products were then electrophoresed in 6% acrylamide, 0.5X TBE gels and autoradiographed. Primers of the following sequence were used: TGM1 forward: 5’-TCTGTGGGTCCTGTCCCATCCATCCTGACC-3’, and reverse: 5’-CCCCAACGGCCCACATCGGAACGTGGCCCA-3’; human β-actin forward: 5’-CAGGCTGTGCTATCCCTGTAC-3’ and reverse: 5’-CACGCACGATTTCCCGCTCGG-3’; human cytokeratin K10 forward: 5’-GGCTCTGGAAGAATCAAACTATGAGC-3’ and reverse: 5’-GGATGTTGGCATTATCAGTTGTTAGG-3’.
Tumor sections
Formalin-fixed, paraffin-embedded non-melanoma skin cancer sections were obtained from Department of Pathology archival tissue storage at the Indiana University School of Medicine. Procurement was done following approval by the Indiana University at Indianapolis Institutional Review Board.
Immunohistochemistry
Immunohistochemistry was done on human epidermis acquired from reductive mammoplasties and panniculectomiess. Human tumors were acquired from archival formalin-fixed, paraffin-embedded tumors. Immunolabeling was done using a monoclonal anti-EP1 receptor antibody, clone 5F12 and the SuperPicture broad spectrum IHC staining kit (Zymed Laboratories, South San Francisco) [14]. Briefly, after deparaffinization and rehydration, heat-induced antigen retrieval was done in 75% glycerol: 25% Dako high pH antigen retrieval buffer as described previously [14], incubating the sections with the primary antibody at a concentration of 10 μg/ml.
Scoring and statistical analysis of human tumors
Following IHC staining, each tumor was scored for both membrane and nuclear staining intensity by three different pathologists (SB, NCP, TMK). For both nuclear and membrane staining, a score of 0 indicated no staining; 1+ = weak staining; 2+ = moderate staining; and 3+ = strong staining. The absence of cytoplasmic granular layer staining with loss of keratohyaline granules was also noted. The distributions of nuclear and membrane scores were compared between each tumor type and normal tissue using Cochran-Armitage exact trend tests.
EP1 receptor immunolocalization in hyperplastic mouse epidermis and in EP1 knockout mice
EP1 knockout mice and C57Bl/6 syngeneic wild-type control mice were housed as previously described [19]. Dorsal skin from wild-type and EP1 knockout mice was excised Hairless, albino, female SKH1 mice were purchased from Charles Rivers Laboratories (SKH1-Hrhr; Wilmington, MA). The mice were housed in standard microisolator cages under a simulated 12 hour day/night cycle and were fed and watered ad libitum. UVB-induced hyperplasia in an SKH1 mouse was elicited by 1500 J/m2 of UVB irradiation using two Westinghouse FS40 sunlamps (National Biological Corp., Twinsberg, OH). A control mouse received no UVB irradiation. At 72 hours post UVB irradiation, the dorsal skin was excised from the euthanized irradiated and non-irradiated control mice. This time was chosen as it corresponds to the peak time point for UVB-induced hyperplasia[20]. The epidermis was then formalin-fixed and paraffin-embedded. To verify the specificity of the immunohistochemical response, formalin-fixed tissue from wild-type and EP1 knockout mice was also utilized. The fixed skin was then cut into sections, deparaffinized, and heat-induced antigen retrieval was performed as described for the human tissue. The EP1 monoclonal antibody was applied at the same concentration and for the same period as that done for the human studies. However, due to the fact that we were utilizing a mouse monoclonal antibody on mouse tissue, this required the use of a specialized kit that blocks non-specific detection of endogenous mouse immunoglobulin (Mouse on Mouse Kit, Vector Laboratories, Burlingame, CA). The addition of blocking reagent, primary and secondary antibodies, and detection reagents were performed in kit buffers and done as detailed by the manufacturer except that CAS block (Zymed Laboratories) was added at a 10% final concentration to primary and secondary antibody buffers. All mouse studies were approved by the Indiana University-Purdue University at Indianapolis Animal Care and Use Committee or the University of Ottawa Animal Care Committee.
RESULTS
Increased EP1-receptor expression precedes the onset of density-dependent corneocyte formation
Given our hypothesis that the EP1 receptor regulates keratinocyte differentiation, we first examined whether EP1 receptor expression is up-regulated before or during keratinocyte differentiation in vitro. Previous studies by others have demonstrated that high cell density and the attainment of cell-cell adhesion is key to the initiation of keratinocyte differentiation in vitro [2, 21, 22]. We therefore examined how EP1 receptor expression was altered during the period in which primary human keratinocytes (PHKs) attain a confluent monolayer and begin the process of corneocyte formation. In figure 1, we processed PHKs at daily intervals beginning at 1 day prior to reaching confluence to 2 days post-confluence for PGE2 binding activity and SDS-insoluble corneocyte formation (Fig 1A), as well as EP1 receptor mRNA expression (Fig 1B). In figures 1A & 1B, we demonstrate that both specific [3H]-PGE2 binding activity and EP1 receptor mRNA expression increase at the time PHKs attained a confluent monolayer (day 0). Moreover, this increased PGE2 binding activity and increased EP1 receptor mRNA expression precede a parallel increase in the formation of terminally differentiated corneocytes beginning 1 day post-confluence. To further characterize the binding activity seen in figure 1A, we next turned to competitive binding studies. In figure 1C, competition studies demonstrate that the EP1, EP2 and EP3 receptor agonist, 17-phenyl-ω-trinor-PGE2 (17-pt-PGE2), and PGE2 showed equivalent potencies for blocking [3H]-PGE2 binding. In contrast, the EP2, EP3, EP4 agonist, 11-deoxy-PGE1 (11d-PGE1), was approximately half as potent as PGE2 or 17-pt-PGE2, while the EP3 receptor agonist misoprostol was unable to compete for radioligand binding. Thus, this data is in agreement with the RT-PCR data in figure 1B and demonstrates that the EP1 receptor appears to be at least partially responsible for the increased PGE2 receptor binding activity that preceded corneocyte formation. However, the competitive binding data indicates that EP2 receptors likely represent a significant portion of the PGE2 receptors expressed in post-confluent keratinocytes. The potential involvement of EP4 receptors is less likely, as we have previously shown that only trace amounts of EP4 receptor mRNA is present in PHKs in culture [10].
EP1 receptor expression and EP1-mediated calcium signaling are up-regulated by high cell density
The data in figures 1A & B indicate that EP1 receptor expression is up-regulated with increasing cell density and this receptor up-regulation precedes density-induced differentiation. However, to rule out the possibility that the EP1 receptor is regulated by days in culture rather than high cell density, we next performed a series of experiments to verify that the EP1 receptor expression and function are dependent on cell density. In this case, we plated cells at high and low cell densities so that we could assess EP1 receptor expression and function in pre-confluent and post-confluent cultures after the same time in culture. We then examined EP1 receptor-mediated calcium signaling, mRNA expression, and protein expression.
In a previous study, we demonstrated that the EP1 receptor is coupled to intracellular calcium mobilization by the EP1 receptor agonists iloprost, 17-pt-PGE2 and sulprostone [14]. Given the importance of calcium in regulating differentiation-specific gene expression in keratinocytes, we first examined whether EP1-mediated intracellular calcium mobilization is cell density dependent. In figures 2A & B, we demonstrate that the ability of iloprost to induce calcium mobilization is restricted to post-confluent keratinocytes. While iloprost is also a potent agonist for the prostacyclin (IP) receptor [5], the possibility that iloprost stimulated calcium mobilization through activation of the IP receptor is remote, as this receptor is known to be coupled to Gs alpha subunit signaling and adenylate cyclase activation [23]. Moreover, two other EP1 receptor agonists, sulprostone and 17-pt-PGE2 [5], also induced calcium mobilization in post-confluent, but not pre-confluent PHKs (data not shown). In this case, the rank potency for calcium mobilization in confluent PHKs was: iloprost ≥ 17-pt-PGE2 > sulprostone. As a positive control, keratinocytes are known to express the platelet activating factor receptor (PAF-R), which is coupled to intracellular calcium signaling [24]. Thus, it is noteworthy that a PAF-R agonist, carbamyl-PAF (CPAF), was able to induce a calcium transient regardless of cell density.
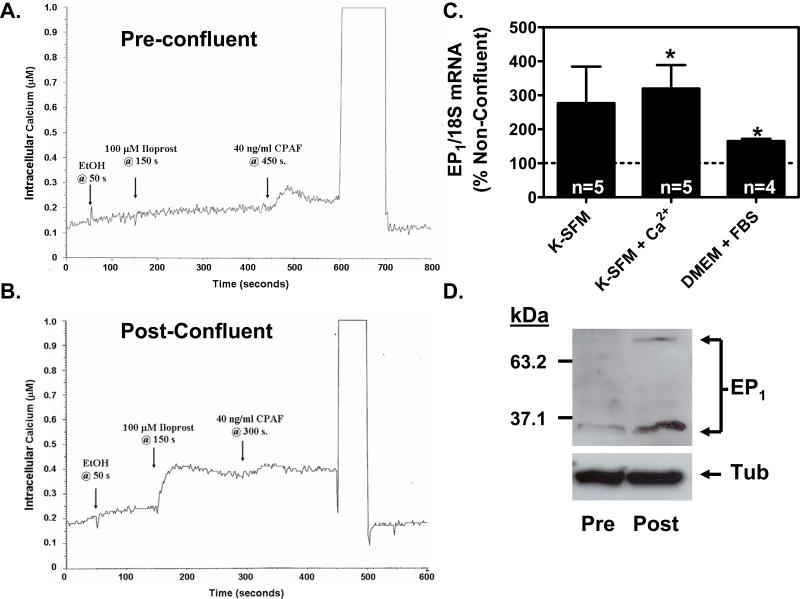
Figure 2. EP1 receptor signaling and expression are up-regulated in PHKs following the attainment of a confluent monolayer.
(A & B). Agonist induced calcium mobilization is restricted to PHKs grown to a post-confluent monolayer. Cells at low cell density (pre-confluent) or high cell density (post-confluent) were loaded with the fluorescent calcium indicator, Fura PE3/AM. After trypsinization, the cells were stimulated with ethanol (EtOH), 100 μM iloprost in ethanol, or 40 ng/ml of the positive control platelet activating factor receptor (PAF-R) agonist, carbamyl-PAF (CPAF). (A). The EP1 receptor agonist, iloprost, is unable to induce measurable calcium mobilization in pre-confluent PHKs. In contrast, the positive control PAF-R agonist, CPAF, is shown to induce a calcium mobilization response. (B). The EP1 receptor agonist, iloprost, induces a robust calcium mobilization response in post-confluent keratinocytes. (C). EP1 receptor mRNA expression is up-regulated in post-confluent PHKs compared with pre-confluent PHKs under differing culture conditions. RNA was prepared from both pre-confluent and post-confluent cells and EP1 expression was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA. Results represent the mean ± SEM for n=4-5 experiments done in duplicate; * p < 0.05, one-sample t-test relative to 100 % for pre-confluent controls. (D). EP1 receptor protein expression is up-regulated in post-confluent PHKs compared with pre-confluent PHKs. An immunoblot was performed on total cell lysates from pre-confluent PHKs and post-confluent PHKs grown in serum free media (K-SFM; 0.06 mM Ca2+). EP1 immunoreactive bands are seen at approximately 35 kDa and 70 kDa. The blot was stripped and reprobed with anti-α-tubulin antibody as a loading control (bottom panel).
We next sought to verify that EP1 receptor mRNA and protein expression are up-regulated in post-confluent compared to pre-confluent PHKs. We first examined EP1 receptor mRNA expression by qRT-PCR. In this case, we also examined whether differing culture conditions could also alter EP1 receptor expression. In particular, high extracellular calcium is a potent stimulus for the induction of differentiation-specific proteins. We therefore utilized serum-free defined media (K-SFM) with both low and high calcium concentrations, as well as the culture conditions utilized in the studies described in figure 1 (FBS-DMEM). In figure 2C, we show that EP1 receptor mRNA expression is up-regulated in post-confluent PHKs compared with pre-confluent PHKs under all culture conditions. Interestingly, varying the extracellular calcium concentration (K-SFM vs K-SFM + Ca2+) did not significantly alter EP1 receptor mRNA expression. In contrast, density-dependent up-regulation of the EP1 receptor was less robust in the serum-containing culture conditions utilized in figure 1 (for comparison, see figure 1B for cells at 1-2 days post-confluence; in figure 2C, the confluent cells were 2-3 days post-confluent). Finally, in figure 2D, we show by immunoblot that the EP1 receptor is up-regulated in post-confluent PHKs relative to pre-confluent PHKs. As expected, two different sized bands were detected [14] (see also supplemental figure 2). Under non-confluent conditions, only the lower molecular weight band was seen. Upon reaching confluence, the lower molecular weight band was increased and the high molecular weight band became apparent. It should be noted that G-protein coupled receptors (GPCR) are frequently observed to exhibit multiple bands by western blot [11, 25-27]. In fact, the large number of GPCR receptors exhibiting multiple banding by immunoblot preparations, along with other lines of evidence, have led to the general belief that these multiple bands represent GPCR homo- and hetero-dimers or higher order oligomers and that this oligomerization is involved in GPCR activity (reviewed in, [28, 29]). Thus, it is possible that these two immunoreactive bands represent monomeric and dimeric forms of the EP1 receptor. This idea is supported by the apparent molecular weights of the two immunoreactive bands, with the smaller band seen at approximately 35 kDa and the larger band seen at approximately 70 kDa. This is consistent with our previous study, in which the major immunoreactive bands were seen at 35-40 and 61-70 kDa [14]. This minor variability in protein size determinations could be due to a number of factors, including differences in protein MW standards or sample to sample variability in solubilization and/or SDS-dependent denaturation of this highly hydrophobic protein. These results are also consistent with our previous study showing the immunolocalization of the EP1 receptor within human epidermis [14]; in this case, the EP1 receptor exhibits greater staining in the more differentiated spinous and granular layers, with a marked increase in staining observed in the granular layer.
EP2 receptor expression and functional coupling to cAMP are not induced by high cell density
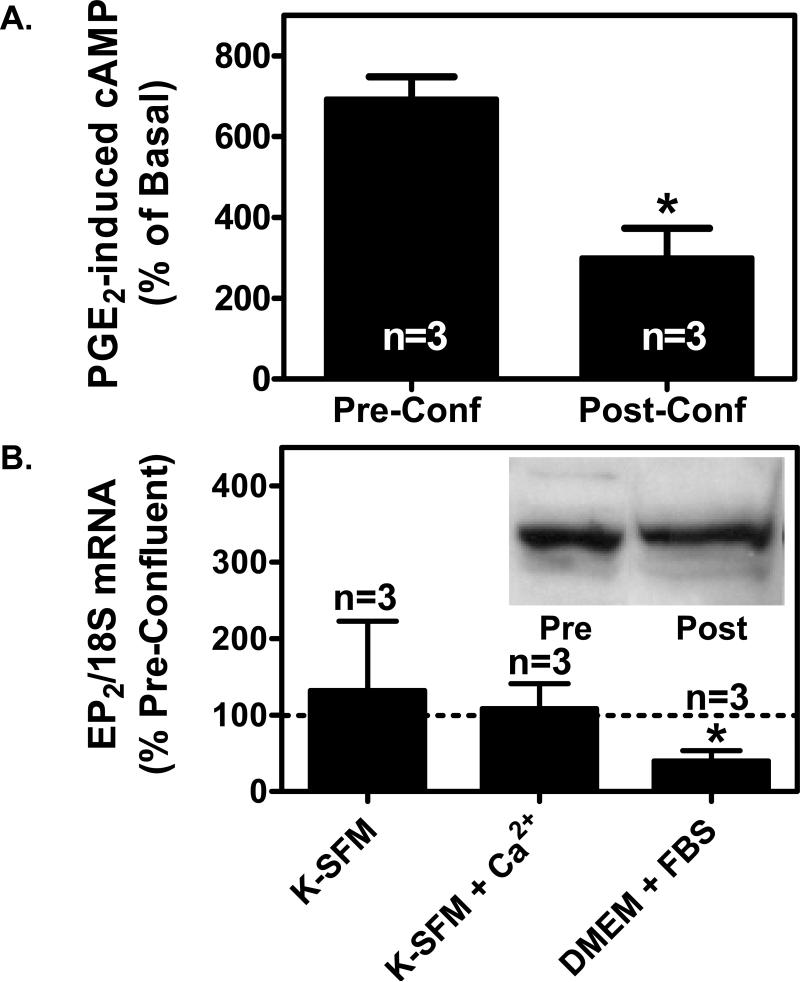
In figure 1C, we show that 17-pt-PGE2 was as effective as PGE2 in blocking radiolabeled PGE2 binding to post-confluent keratinocytes. Moreover, 11d-PGE1 also competed for binding to membranes from post-confluent keratinocytes. Given that 17-pt-PGE2 is also an EP2 receptor agonist and 11d-PGE1 exhibits weak binding affinity with the EP1 receptor [30], this suggests that EP2 receptors are present at relatively high levels in post-confluent keratinocytes. We therefore sought to determine whether EP2 receptor expression is also altered by high cell density under the serum-containing growth conditions utilized in figure 1. In figure 3A, we show that PGE2 receptors coupled to cyclic AMP (cAMP) production (EP2 and/or EP4) exhibit decreased functional activity in PHKs at high cell density (Post-Conf) versus low cell density (Pre-Conf) growth conditions. In figure 3B, EP2 mRNA expression was unaltered by cellular confluence in defined media containing both low and high calcium concentrations. In contrast, EP2 mRNA expression was significantly decreased under the serum containing culture conditions utilized in figures 1C and 3A. Similarly, EP2 protein expression was not increased by high cell density when assessed by immunoblot (insert in Fig 3B). These studies indicate that, depending on the culture conditions, EP2 receptor expression and activity is either unaffected or suppressed as keratinocytes achieve a confluent monolayer and begin the process of differentiation. However, while EP2 receptors are not up-regulated by high-cell density, it is clear that EP2 receptor function and expression at the RNA and protein level is still relatively high in post-confluent keratinocytes. This is consistent with our previous report utilizing immunohistochemistry [14]. In this case, while the EP2 receptor is expressed in the superficial spinous and granular layers of the epidermis, it exhibits greater staining in the less differentiated keratinocytes at or near the basal layer.
Figure 3. Depending on the culture media, high cell density either has no effect on EP2 receptor expression or suppresses EP2 receptor expression in PHKs.
(A). PGE2-induced cyclic AMP production is reduced in PHKs at high cell density. Pre-confluent PHKs or post-confluent PHKs cultured in DMEM + 10% FBS were treated with 3 μg/ml indomethacin overnight to block endogenous PGE2 formation. The cells were then stimulated with 100 nM PGE2 for 1 minute. Cyclic AMP was measured using a commercial EIA kit as described in the methods section. The results represent the mean and SEM of three experiments. (B). Quantitative real-time PCR was performed for EP2 receptor mRNA expression as described for the EP1 receptor in figure 2C above. Inset: Immunoblot for EP2 receptor expression in pre-confluent (Pre) and post-confluent (Post) PHKs grown in DMEM with 10% fetal bovine serum. Membrane preparations were produced from pre-confluent and 2 days post-confluent PHKs. In each case, 40 μg of the membrane preparation was separated by SDS-PAGE electrophoresis and immunoblot performed using a polyclonal anti-EP2 receptor antibody as described in the methods section.
Receptor antagonists demonstrate a role for the EP1 receptor in keratinocyte differentiation
The above data indicates that the EP1 receptor is up-regulated in PHKs during density-dependent differentiation and that this up-regulation precedes the formation of terminally differentiated SDS-insoluble corneocytes. Moreover, this up-regulation is associated with the ability of EP1 receptor agonists to stimulate intracellular calcium signaling, which is a well-known inducer of PHK differentiation. Thus, we hypothesized that the EP1 receptor is involved in density-dependent PHK differentiation. We therefore examined whether EP1 receptor antagonists would block density-dependent corneocyte formation. In figure 4A, we demonstrate that the capacity to form corneocytes is inhibited by 67% using the EP1 receptor antagonist AH6809 and by 43% using the more selective EP1 receptor antagonist SC51322 [5]. Given that EP2 receptors are expressed in post-confluent keratinocytes, we next show that SC51322 has no effect on EP2 receptor-mediated signaling through cAMP (figure 4B). It should also be noted that the inability of SC51322 to alter EP2 receptor activation was seen at a concentration (500 nM) that is nearly two-fold greater than that utilized to assess the role of the EP1 receptor in keratinocyte differentiation (figures 4A & C). This result confirms a previous report indicating that SC51322 has essentially no binding affinity for the EP2 receptor [5]. As expected, the non-specific EP1-3 receptor antagonist, AH6809, was highly effective in blocking EP2 agonist-stimulated cAMP production (*, p<0.05 compared with EP2 agonist-stimulated cAMP).
We next examined the ability of SC51322 to inhibit the expression of the differentiation markers cytokeratin K10 (K10) and epidermal transglutaminase (TGM1) [31, 32]. In this case, SC51322 was applied to PHKs over a 48 hour period that corresponded to 1 day pre-confluent to 1 day post-confluent. The ability of SC51322 treatment to inhibit extracellular calcium-induced up-regulation of these differentiation markers was then assessed. In figure 4C, we show that K10 expression is increased approximately 2.5-fold in PHKs by treatment with high concentrations of extracellular calcium. Interestingly, SC51322 inhibited calcium-induced expression of K10 by approximately 60%. SC51322 also inhibited the expression of epidermal transglutaminase (TGM1), the calcium-dependent enzyme that catalyzes the final cross-linking of envelope precursors to form the detergent-insoluble cell envelope [32]. In this case, the EP1 receptor antagonist inhibited TGM1 expression in both low and high calcium conditions.
The intensity of EP1 receptor plasma membrane localization correlates with a differentiated phenotype in human non-melanoma skin cancers
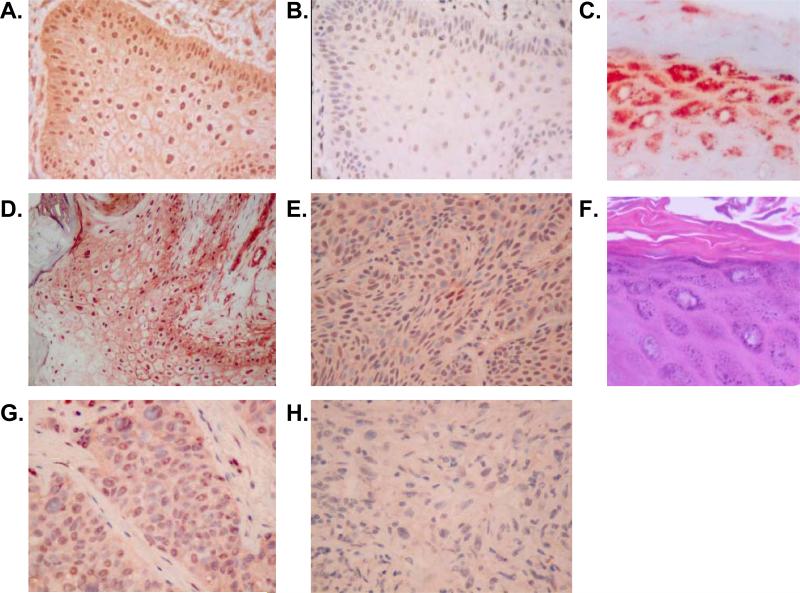
In studies by other investigators, it has been noted that the EP1 receptor is highly expressed in human squamous cell carcinomas (SCCs) and actinic keratoses (AKs), but is weakly expressed or absent in basal cell carcinomas (BCCs) [15]. We therefore extended these studies by examining the immunohistochemical localization of the EP1 receptor in these lesions as well as poorly differentiated SCCs (PD-SCCs) and spindle cell carcinomas. We have previously demonstrated by IHC that normal human epidermis exhibits a weak cell membrane-associated staining pattern for the EP1 receptor [14]. In our new studies shown in table 1 and figure 5, we demonstrate that the membrane staining pattern was markedly and significantly increased in areas of well-differentiated SCC compared with normal epidermis (see table 1 and figure 5). In contrast, spindle cell carcinomas and basal cell carcinomas lacked membrane staining. In poorly differentiated SCCs, membrane staining was also absent, except for some faint focal staining. In tumors with mixed areas of poorly differentiated and well-differentiated tumor, membrane staining was present primarily in the areas of well-differentiated keratinized tumor.
Table 1.
Membrane scoring for tumors
| Membrane Score | Normal | AK | SCC**** | PD-SCC | Spindle**** | BCC**** |
|---|---|---|---|---|---|---|
| 3+ | 0 | 0 | 4 * | 0 | 0 | 0 |
| 2+ | 4 | 0 | 2 | 1 ** | 0 | 0 |
| 1+ | 6 | 2 | 3 | 3 ** | 0 | 1 |
| 0 | 7 | 2 | 0 | 8 | 5 *** | 7 |
Three of the four well-differentiated tumors with 3+ membrane staining were keratoacanthoma type SCC.
1 or 2+ staining was seen in four tumors with mixed poorly differentiated and well-differentiated tumors. Staining was seen only focally or in areas of well differentiated SCC.
2 Spindle cell carcinomas showed 1+ membrane staining in adjacent areas of differentiated SCC
distribution significantly different from Normal (P < 0.05; two-tailed exact trend test)
Figure 5. EP1 receptor immunolocalization in non-melanoma skin cancer.
Immunohisochemical (IHC) analyis of EP1 receptor expression was performed on formalin-fixed, paraffin-embedded archival tissue samples. In each case, 5 μm sections were deparaffinized and heat-induced epitope retrieval was done as outlined in the methods section. IHC staining was done using a monoclonal anti-human EP1 receptor antibody (clone 5F12) (A, C-F) or an isotype control (B). (A). Keratoacanthoma (400x magnification). (B). A serial section of the same keratoacanthoma stained with isotype (IgG2bκ) negative control antibody (400x). (C). Hyperplastic skin overlying the basal cell carcinoma seen in panel E. (D). Well-differentiated squamous cell carcinoma (SCC) (200x). (E). Basal cell carcinoma (400x). (F). Hematoxylin & eosin stained section corresponding to the section seen in panel C. (G). Poorly-differentiated SCC (400x). (H). Spindle cell carcinoma (400x).
In addition to the membrane staining pattern, we have previously noted in normal epidermis that the EP1 receptor is heavily localized to the granular layer, exhibiting a grainy cytoplasmic immunolocalization pattern suggestive of localization to intracellular granules [14]. In AKs and well-differentiated SCCs, this pattern was recapitulated, with intense granular-appearing cytoplasmic staining occurring only in areas of tumor exhibiting keratohyaline granules (figures 1C & F). This pattern was not observed in BCCs, poorly differentiated SCCs (PD-SCCs), or spindle cell carcinomas that lack granular layer differentiation. However, it was seen in areas of normal or hyperplastic epidermis overlying these poorly differentiated tumors (figure 1C). It should be noted that this lack of granular cytoplasmic staining with loss of keratohyaline granules was also noted in two archival samples of psoriasis, an inflammatory skin disease also known for loss of normal granular layer development (data not shown).
Finally, in addition to the cell membrane and cytoplasmic staining patterns described above, we have previously demonstrated that normal human epidermis exhibits a perinuclear/nuclear pattern of EP1 immunolocalization [14]. In figure 5 and table 2, we demonstrate that both normal human epidermis and human tumors exhibit nuclear EP1 receptor localization by IHC. Significantly, while all normal epidermal samples, AKs, and well-differentiated SCCs exhibit 1+ or greater nuclear scores, a subset of BCCs, spindle cell carcinomas and poorly differentiated SCCs exhibit a lack of nuclear staining, with a statistically significant decrease in nuclear staining noted in BCCs and PD-SCCs (see table 2). It should be noted that PGE2 receptors have been demonstrated to be localized to intracellular membranes, particularly the nuclear membrane, by multiple methodologies including electron microscopy, immunofluorescence, radioligand binding studies of cellular and intracellular membrane preparations, and immunolocalization of epitope-tagged receptors [14, 33-38]. Moreover, studies using isolated nuclei demonstrated that the receptor is coupled to nuclear calcium signaling [33].
Table 2.
Nuclear scoring for tumors
| Nuclear Score | Normal | AK | SCC | PD-SCC* | Spindle | BCC* |
|---|---|---|---|---|---|---|
| 3+ | 12 | 3 | 7 | 4 | 3 | 2 |
| 2+ | 4 | 1 | 1 | 4 | 1 | 1 |
| 1+ | 1 | 0 | 1 | 4 | 0 | 2 |
| 0 | 0 | 0 | 0 | 1 | 2 | 2 |
distribution significantly different from Normal (P < 0.05; two-tailed exact trend test)
To further validate the IHC results observed in figure 5, we next utilized mice with germ-line deletion of the EP1 receptor. In supplemental figure 1, we demonstrate that the EP1 receptor is localized in the superficial epidermis that immediately underlies the stratum corneum in normal dorsal epidermis from SKH1 mouse. Faint staining within some nuclei was also noted. However, given that mouse epidermis is much thinner than human epidermis and lacks a clearly defined granular layer, it was difficult to assess whether this staining pattern reproduced that observed in normal human epidermis. We therefore examined EP1 receptor expression in mouse skin that had been induced to undergo hyperplasia by ultraviolet B (UVB) treatment. This allowed us an opportunity to examine whether the cytoplasmic/perinuclear staining pattern seen in the granular layer of human epidermis (see figure 5C) is also observed in hyperplastic mouse epidermis. It should be noted that UVB-induced epidermal hyperplasia also results in a marked expansion of the granular layer in mice. We therefore treated SKH1 mice with 1500 J/m2 UVB and then examined EP1 receptor at 72 hours post-irradiation. This time point was chosen as it corresponds with the peak period of UVB-induced hyperplasia [20]. In supplemental figure 1B, we show that the UVB-induced hyperplastic epidermis exhibited a prominent grainy cytoplasmic staining pattern that closely mimicked the pattern seen in human epidermis. In addition, the EP1 receptor was seen to be up-regulated after 72 hours within the nuclei of epidermal keratinocytes. Finally, to verify the specificity of the monoclonal antibody, we demonstrate that mice with germ-line deletion of the EP1 receptor lacked staining for the EP1 receptor (Supplemental figure 1D). In contrast, wild-type syngeneic C57Bl/6 mice exhibit a pattern of staining in the superficial epidermis that was very similar to that observed in SKH1 mice (compare supplemental figures 1A and C).
DISCUSSION
In this study, we provide evidence that the EP1 subtype of PGE2 receptor is important in regulating normal keratinocyte differentiation in vitro. First, we show that EP1 receptor expression and the ability of EP1 agonists to induce intracellular calcium mobilization is up-regulated in a density dependent manner and that this up-regulation precedes the appearance of terminally differentiated keratinocytes. In addition, EP1 receptor antagonism inhibits density-dependent and calcium-dependent keratinocyte differentiation. Finally, we verify previous reports that EP1 receptor immunolocalization correlates with a squamous or well-differentiated phenotype in human non-melanoma skin cancer.
Our finding that the EP1 receptor is coupled to calcium mobilization is consistent with previous studies that demonstrate that the EP1 receptor is coupled to calcium mobilization in PHKs and other cell types [3, 14, 16, 33]. Inasmuch as calcium mobilization is well known to stimulate keratinocyte differentiation [8, 39], our new findings that EP1 receptor signaling is up-regulated upon reaching cellular confluence suggests a role for the EP1 receptor in regulating density-dependent keratinocyte differentiation. Thus, the next studies examined whether the EP1 receptor is involved in keratinocyte differentiation in vitro. We show that the EP1 receptor antagonist, SC51322, significantly inhibits cornified envelope formation, as well as keratin K10 and epidermal transglutaminase expression. Importantly, SC51322 is also reported to have essentially no binding activity with the EP2 receptor (Ki of >100,000 nM) and very poor binding activity with the EP4 receptor (Ki of 14,032 nM) [5]. Our studies demonstrating that SC51322 fails to block EP2 receptor-mediated cAMP production confirms the inability of SC51322 to act as an EP2 receptor antagonist. Thus, the ability of SC51322 to suppress corneocyte formation and differentiation marker expression is consistent with antagonism of the EP1 receptor, but not the EP2 receptor. However, it is possible that SC51322 may be exhibiting non-specific activity unrelated to any of the prostaglandin receptors. This appears to be unlikely, as we show that the structurally unrelated EP receptor antagonist, AH6809, also inhibits corneocyte formation. Thus, our results provide pharmacologic support for the idea that the EP1 receptor, but not the EP2 receptor, is an important regulator of keratinocyte differentiation.
While SC51322 is reported to be one of the most potent and selective antagonists of the EP1 receptor (Ki of 13.8 nM), SC51322 also exhibits considerably weaker affinity for the EP3 receptor (reported Ki of 698 nM) [5]. However, we feel that the EP1, rather than the EP3 receptor, is likely the major PGE2 receptor subtype involved in regulating keratinocyte differentiation. This conclusion is based on the following observations: First, the concentration of SC51322 used in our studies (300 nM) is well above the reported binding affinity of this antagonist for the EP1 receptor. In contrast, this concentration is less than 50% of the reported Ki of this antagonist for the EP3 receptor. Second, while SC51322 has modest ability to compete for PGE2 binding to the EP3 receptor, it is unclear whether this binding activity is associated with significant EP3 receptor antagonist activity [40]. In contrast, studies in vivo using EP1 receptor knockout and wildtype mice demonstrate the effectiveness of SC51322 in inhibiting EP1-dependent physiologic activity while studies in vitro demonstrate that SC51322 is a potent inhibitor of EP1 receptor-mediated calcium mobilization in cells over-expressing the EP1 receptor [41, 42]. Finally, our previous study has demonstrated that the EP3 receptor is not up-regulated with confluence in PHKs and is primarily expressed in the basal and lower spinous layers of intact human epidermis where it likely acts as a negative regulator of proliferation [13]. This is also consistent with our data in figure 1C, in which the EP3 receptor agonist, misoprostol, was ineffective in blocking PGE2 binding to post-confluent keratinocyte membrane preparations. However, we cannot rule out the possibility that the EP3 receptor may act cooperatively with the EP1 receptor to stimulate differentiation. This idea would be consistent with the greater effectiveness of AH6809 over SC51322 in blocking corneocyte formation. However, these differences could also be attributed to the different concentrations utilized and differences in the relative potencies of these antagonists for the EP1 receptor.
In normal epidermis, we have previously demonstrated that the EP1 receptor is localized throughout the epidermis, with perinuclear/nuclear, membrane and cytoplasmic localization [14]. However, the most prominent staining was observed in the granular layer (stratum granulosum), exhibiting a cytoplasmic granular appearance. In human tumors, we demonstrate that EP1 receptor expression exhibits increased cell membrane localization in well-differentiated keratinized tumors (well-differentiated SCCs), while membrane staining was noted to be reduced in non-keratinizing tumors (BCCs, poorly-differentiated SCCs, and spindle cell carcinomas). Interestingly, the granular cytoplasmic staining was noted only in areas where keratohyaline granules were observed histologically, suggesting that the EP1 receptor colocalizes with these keratohyalin and fillagrin-rich intracellular structures [43]. Importantly, faint nuclear staining and marked cytoplasmic/perinuclear granular layer staining is noted in normal mouse epidermis, but not in mice lacking the EP1 receptor. Moreover, in hyperplastic mouse epidermis that more closely resembles the human epidermis, the cytoplasmic/perinuclear granular layer staining is markedly enhanced, as is the nuclear staining pattern.
The above data demonstrating that the EP1 receptor is expressed in the differentiated compartment of normal and neoplastic human epidermis is in general agreement with a previous report indicating that the EP1 receptor is expressed in murine and human SCC, but not in BCC [15]. Similarly, enhanced expression of the EP1 receptor within the differentiated suprabasal compartment of mouse epidermis was also noted by a second group [44]. However, a third group demonstrated EP1 immunolocalization within the basal compartment of normal mouse epidermis [45]. The authors attribute the differences in their results to differences in methodologies or strain-specific differences. Since this study used a commercial polyclonal antibody preparation, while our current study utilized a monoclonal anti-EP1 receptor antibody developed in our laboratory, the different staining patterns could be due to the use of different reagents. However, we have previously utilized the same commercial rabbit polyclonal antibody on normal human epidermis and noted a similar staining pattern to that seen using our anti-EP1 monoclonal antibody [14]. Thus, the differences in the staining pattern are more likely due to species differences, differences in antigen retrieval methodologies or different commercial lot numbers for the polyclonal antibody. It should be noted that we have noted considerable lot-dependent differences in performance of this commercial antibody source as assessed by immunoblot analysis of HEK cell lysates over-expressing the human EP1 receptor (see supplemental figure 2). Thus, it should also be noted that the immunoblot data shown in figure 2D was performed using the same commercial lot of polyclonal anti-EP1 receptor antibody that was used in our previous study [14], and the specificity of this lot of antibody is shown in supplemental figure 2A.
In contrast to our findings with the EP1 receptor, our data also demonstrate that EP2 receptor expression and functional activity is either unchanged or suppressed in PHKs upon reaching confluence. This is consistent with previous reports demonstrating that the EP2 receptor is necessary for PGE2-induced keratinocyte proliferation and that keratinocyte proliferation is markedly suppressed upon reaching a confluent monolayer [2, 10, 12].
One of the more unusual findings of this study relate to the presence of perinuclear or nuclear EP1 receptor immunolocalization. The significance of nuclear or perinuclear EP1 receptor immunolocalization is as yet unclear, although there was a weak association with the keratinizing phenotype: While all of the normal human epidermis and well-differentiated non-melanoma skin cancers demonstrated some degree of nuclear/perinuclear staining for the EP1 receptor, a subset of basal cell carcinomas, poorly-differentiated squamous cell carcinomas and spindle cell carcinomas lacked this staining pattern. It is also possible that the perinuclear/nuclear staining pattern represents non-specific staining. However, we feel that this is unlikely. First, COX-1 and COX-2 are known to be localized to the endoplasmic reticulum and nuclear membrane [46, 47]. Moreover, nuclear membrane localization has been demonstrated for the EP1, EP3, and EP4 receptors in other cell types or tissues [33, 48, 49]. Finally, nuclear EP1 staining was increased in UVB-irradiated mouse epidermis (supplemental figure 1), while nuclear EP1 staining was not noted in EP1 knockout mice. Thus, we speculate that the EP1 receptor is responsive to PGE2 production at the nuclear envelope or endoplasmic reticulum of keratinocytes through an autocrine signaling pathway.
In summary, these studies supply evidence that the EP1 receptor is up-regulated under conditions that stimulate keratinocyte differentiation, the EP1 receptor is coupled to intracellular calcium mobilization, and EP1 receptor antagonism inhibits keratinocyte cornification and markers of keratinocyte differentiation. Taken together, these observations supply strong evidence to support a functional role for the EP1 receptor in mediating keratinocyte differentiation. Moreover, EP1 receptor expression in non-melanoma skin cancer correlates with histologic evidence of keratinization. Thus, this data suggests that the role of the EP1 receptor in regulating differentiation appears to be intact in non-melanoma skin cancer. Interestingly, the function and intracellular signaling pathway elicited by the EP1 receptor in human keratinocytes appears to differ from that observed for the other PGE2 receptor subtypes. Earlier studies demonstrated that EP2 receptor activation elicits a proliferative response in PHKs via a cAMP coupled response [10], while the EP3 receptor acts to suppress growth and induces intracellular signaling through the lipid mediators diacylglycerol and ceramide [13]. Thus, this new data indicates that the multiple PGE2 receptors, each coupled to different functional roles and intracellular signaling pathways, act to increase the diversity of cellular responses that can be elicited by PGE2.
Supplementary Material
Supplemental Figure 1: EP1 receptor immunolocalization in normal and hyperplastic mouse epidermis and absent staining in EP1 knockout mice relative to syngeneic control mice. Immunohistochemistry was performed as described in the methods secion on mouse epidermis using the mouse monoclonal anti-EP1 receptor antibody utilized in figure 5. (A). Non-irradiated control SKH1 hairless, albino mice exhibit a linear pattern of EP1 immunolocalization in the upper epidermis just superficial to the stratum corneum (long arrow). (B). UVB-induced hyperplasia results in expansion of the epidermis as well as the granular layer and results in enhanced visualization of course, grainy cytoplasmic staining within the superficial suprabasal compartment (long arrows). Nuclear staining is noted by the short arrow. (C). Wild-type C57Bl/6 mice exhibit a similar pattern of immunolocalization noted in control SKH1 mouse epidermis (panel A). (D). The epidermis from C57Bl/6 mice with germline deletion of the EP1 receptor lack significant staining by IHC.
Supplemental figure 2: Lot to lot variability in the performance of a commercial rabbit polyclonal anti-EP1 receptor antibody. Immunoblots were performed on 10 μg of membrane preparation from HEK 293 cells over-expressing the human EP1 receptor (HEK + EP1), the human EP3 receptor (HEK + EP3), or empty vector control (HEK) cells essentially as described in the methods section. (A). Immunoblot performed for EP1 receptor expression utilizing a rabbit polyclonal anti-EP1 receptor antibody (Cayman Chemical, Ann Arbor, MI). This same lot of antibody reagent was used in a previously reported study [14]. Note the specific EP1 receptor bands observed at approx 35 and 70 kDa and the non-specific band observed at approx 45 kDa. (B). Immunoblot using a subsequent lot number of the same commercial antibody source. Note that specific EP1 receptor bands are largely absent, although the non-specific band at approx 45 kDa is still observed.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Lee Ann Baldridge for her help in tissue sectioning and to Dr. Jeffrey Travers for his assistance with the calcium mobilization studies and his editorial comments. The authors also gratefully acknowledge Ms. Jennifer Snider and the surgeons within the Division of Plastic Surgery for their help in obtaining the human epidermis used in these studies.
Funding: This publication was made possible by Grant Numbers K08AR002150 and R03 AR053710 from NIAMS/NIH, a Clarian Health Values Fund Grant, a Prevent Cancer Foundation Grant, and an Indiana University-Purdue University at Indianapolis Research Support Fund Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
P.T. LaCelle is currently in the Division of Natural Science and Mathematics, Roberts Wesleyan College, Rochester, NY. N.C. Prall is currently in the Department of Dermatopathology, University of Pittsburgh Medical Center, Pittsburgh, PA. T.M. Katona is currently in the Department of Pathology, University of Arkansas for Medical Sciences, Little Rock, AR. S.D. Billings is currently in the Department of Pathology, Cleveland Clinic, Cleveland, OH.
REFERENCES
- 1.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci USA. 1999;96:7220–5. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pentland AP, Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986;77:246–51. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annual Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 4.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin Receptors: Advances in the study of EP3 receptor signaling. J. Biochem. 2002;131:781–784. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- 5.Abramovitz M, Adam M, Boie Y, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 6.Neufang G, Furstenberger G, Heidt M, Marks F, Muller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7629–34. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Research. 2002;62:3395–401. [PubMed] [Google Scholar]
- 8.Evans CB, Pillai S, Goldyne ME. Endogenous prostaglandin E2 modulates calcium-induced differentiation in human skin keratinocytes. Prostaglandins Leukotrienes & Essential Fatty Acids. 1993;49:777–81. doi: 10.1016/0952-3278(93)90025-r. [DOI] [PubMed] [Google Scholar]
- 9.Leong J, Hughes-Fulford M, Rakhlin N, Habib A, Maclouf J, Goldyne ME. Cyclooxygenases in human and mouse skin and cultured human keratinocytes: association of COX-2 expression with human keratinocyte differentiation. Experimental Cell Research. 1996;224:79–87. doi: 10.1006/excr.1996.0113. [DOI] [PubMed] [Google Scholar]
- 10.Konger RL, Malaviya R, Pentland AP. Growth Regulation of Primary Human Keratinocytes by Prostagandin E Receptor EP2 and EP3 subtypes. Biochim. Biophy. Acta. 1998;1401:221–234. doi: 10.1016/s0167-4889(97)00114-6. [DOI] [PubMed] [Google Scholar]
- 11.Konger RL, Scott GA, Landt Y, Ladenson JH, Pentland AP. Loss of the EP2 prostaglandin E2 receptor in immortalized human keratinocytes results in increased invasiveness and decreased paxillin expression. Am J Pathol. 2002;161:2065–2078. doi: 10.1016/S0002-9440(10)64485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouxhon S, Konger RL, VanBuskirk J, et al. Deletion of prostaglandin E2 EP2 receptor protects against ultraviolet-induced carcinogenesis, but increases tumor aggressiveness. Journal of Investigative Dermatology. 2007;127:439–46. doi: 10.1038/sj.jid.5700547. [DOI] [PubMed] [Google Scholar]
- 13.Konger RL, Brouxhon S, Partillo S, VanBuskirk J, Pentland AP. The EP3 receptor stimulates ceramide and diacylglycerol release and inhibits growth of primary keratinocytes. Exp Dermatol. 2005;14:914–922. doi: 10.1111/j.1600-0625.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 14.Konger RL, Billings SD, Thompson AB, et al. Immunolocalization of Low-Affinity Prostaglandin E2 Receptors, EP1 and EP2, in Adult Human Epidermis. J Invest Dermatol. 2005;124:965–970. doi: 10.1111/j.0022-202X.2005.23658.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee JL, Kim A, Kopelovich L, Bickers DR, Athar M. Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J Invest Dermatol. 2005;125:818–825. doi: 10.1111/j.0022-202X.2005.23829.x. [DOI] [PubMed] [Google Scholar]
- 16.Funk CD, Furci L, FitzGerald GA, et al. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem. 1993;268:26767–72. [PubMed] [Google Scholar]
- 17.Rys-Sikora KE, Konger RL, Schoggins JW, Malaviya R, Pentland AP. Coordinate expression of secretory phospholipase A2 and cyclooxygenase-2 in activated human keratinocytes. Am J Physiol Cell Physiol. 2000;278:C822–C833. doi: 10.1152/ajpcell.2000.278.4.C822. [DOI] [PubMed] [Google Scholar]
- 18.Konger RL, Chan TC. Epidermal growth factor induces terminal differentiation in human epidermoid carcinoma cells. Journal of Cellular Physiology. 1993;156:515–21. doi: 10.1002/jcp.1041560310. [DOI] [PubMed] [Google Scholar]
- 19.Rahal S, McVeigh LI, Zhang Y, Guan Y, Breyer MD, Kennedy CRJ. Increased severity of renal impairment in nephritic mice lacking the EP1 receptor. Canadian Journal of Physiology & Pharmacology. 2006;84:877–85. doi: 10.1139/y06-029. [DOI] [PubMed] [Google Scholar]
- 20.Tripp CS, Blomme EAG, Chinn KS, Hardy MM, LaCelle P, Pentland AP. Epidermal COX-2 Induction Following Ultraviolet Irradiation: Suggested Mechanism for the Role of COX-2 Inhibition in Photoprotection. J Invest Dermatol. 2003;121:853–861. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- 21.Kolly C, Suter MM, Muller EJ. Proliferation, Cell Cycle Exit, and Onset of Terminal Differentiation in Cultured Keratinocytes: Pre-Programmed Pathways in Control of C-Myc and Notch1 Prevail Over Extracellular Calcium Signals. J Investig Dermatol. 2005;124:1014–1025. doi: 10.1111/j.0022-202X.2005.23655.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Yuspa SH, Dlugosz AA. Differentiation of cultured human epidermal keratinocytes at high cell densities is mediated by endogenous activation of the protein kinase C signaling pathway. Journal of Investigative Dermatology. 1998;111:762–6. doi: 10.1046/j.1523-1747.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 24.Travers JB, Huff JC, Rola-Pleszczynski M, Gelfand EW, Morelli JG, Murphy RC. Identification of functional platelet-activating factor receptors on human keratinocytes. Journal of Investigative Dermatology. 1995;105:816–23. doi: 10.1111/1523-1747.ep12326581. [DOI] [PubMed] [Google Scholar]
- 25.Boer U, Neuschafer-Rube F, Moller U, Puschel GP. Requirement of N-glycosylation of the prostaglandin E2 receptor EP3βfor correct sorting to the plasma membrane but not for correct folding. Biochem J. 2000;350:839–847. [PMC free article] [PubMed] [Google Scholar]
- 26.Stillman BR, Breyer MD, Breyer RM. Importance of the extracellular domain for prostaglandin EP2 receptor function. Mol Pharmacology. 1999;56:545–551. doi: 10.1124/mol.56.3.545. [DOI] [PubMed] [Google Scholar]
- 27.Salahpour A, Bonin H, Bhalla S, Petaja-Repo U, Bouvier M. Biochemical characterization of b2-adrenergic receptor dimers and oligomers. Biol Chem. 2003;384:117–123. doi: 10.1515/BC.2003.012. [DOI] [PubMed] [Google Scholar]
- 28.Milligan G. G Protein-Coupled Receptor Dimerization: Function and Ligand Pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 29.Breitwieser GE. G Protein-Coupled Receptor Oligomerization: Implications for G Protein Activation and Cell Signaling. Circ Res. 2004;94:17–27. doi: 10.1161/01.RES.0000110420.68526.19. [DOI] [PubMed] [Google Scholar]
- 30.Ungrin MD, Carriere MC, Denis D, et al. Key Structural Features of Prostaglandin E2 and Prostanoid Analogs Involved in Binding and Activation of the Human EP1 Prostanoid Receptor. Mol Pharmacol. 2001;59:1446–1456. doi: 10.1124/mol.59.6.1446. [DOI] [PubMed] [Google Scholar]
- 31.Kaur P, Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. Journal of Investigative Dermatology. 2000;114:413–20. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 32.Polakowska RR, Graf BA, Falciano V, LaCelle P. Transcription regulatory elements of the first intron control human transglutaminase type I gene expression in epidermal keratinocytes. Journal of Cellular Biochemistry. 1999;73:355–69. [PubMed] [Google Scholar]
- 33.Bhattacharya M, Peri KG, Almazan G, et al. Nuclear localization of prostaglandin E2 receptors. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15792–7. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya M, Varma DR, Chemtob S. Nuclear prostaglandin receptors. Gene Ther. Mol. Biol. 1999;4:323–338. [Google Scholar]
- 35.Zhu T, Gobeil F, Vazquez-Tello A, et al. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Canadian Journal of Physiology & Pharmacology. 2006;84:377–91. doi: 10.1139/y05-147. [DOI] [PubMed] [Google Scholar]
- 36.Gobeil F, Jr., Vazquez-Tello A, Marrache AM, et al. Nuclear prostaglandin signaling system: biogenesis and actions via heptahelical receptors. Canadian Journal of Physiology & Pharmacology. 2003;81:196–204. doi: 10.1139/y02-163. [DOI] [PubMed] [Google Scholar]
- 37.Thorat MA, Morimiya A, Mehrotra S, Konger R, Badve SS. Prostanoid receptor EP1 expression in breast cancer. Modern Pathology. 2008;21:15–21. doi: 10.1038/modpathol.3800970. [DOI] [PubMed] [Google Scholar]
- 38.Lord JT, Ziboh VA. Specific Binding of Prostaglandin E2 to Membrane Preparations from Human Skin: Receptor Modulation by UVB-Irradiation and Chemical Agents. The Journal of Investigative Dermatology. 1979;73:373–377. doi: 10.1111/1523-1747.ep12550453. [DOI] [PubMed] [Google Scholar]
- 39.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 40.van Rodijnen WF, Korstjens IJ, Legerstee N, ter Wee PM, Tangelder G-J. Direct vasoconstrictor effect of prostaglandin E2 on renal interlobular arteries: role of the EP3 receptor. Am J Physiol Renal Physiol. 2007;292:F1094–1101. doi: 10.1152/ajprenal.00351.2005. [DOI] [PubMed] [Google Scholar]
- 41.Peti-Peterdi J, Komlosi P, Fuson AL, et al. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan Y, Zhang Y, Wu J, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tezuka T, Takahashi M. Human Hematoxylin-Stainable Protein of Keratohyalin Granules Origin. I. Extraction and Purification. J Invest Dermatol. 1987;89:400–404. doi: 10.1111/1523-1747.ep12471772. [DOI] [PubMed] [Google Scholar]
- 44.Tober KL, Thomas-Ahner JM, Kusewitt DF, Oberyszyn TM. Effects of UVB on E prostanoid receptor expression in murine skin. Journal of Investigative Dermatology. 2007;127:214–21. doi: 10.1038/sj.jid.5700502. [DOI] [PubMed] [Google Scholar]
- 45.Neumann M, Dulsner E, Furstenberger G, Muller-Decker K. The expression pattern of prostaglandin E synthase and EP receptor isoforms in normal mouse skin and preinvasive skin neoplasms. Experimental Dermatology. 2007;16:445–53. doi: 10.1111/j.1600-0625.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 46.Morita I, Schindler M, Regier MK, et al. Different intracellular locations for prostaglandin endoperoxide H synthase-1 and -2. J Biol Chem. 1995;270:10902–8. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- 47.Rangarajan A, Talora C, Mammucari C, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlotzer-Schrehardt U, Zenkel M, Nusing RM. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest. Ophthalmol. Vis. Sci. 2002;43:1475–1487. [PubMed] [Google Scholar]
- 49.Bhattacharya M, Peri K, Ribeiro-da-Silva A, et al. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. Journal of Biological Chemistry. 1999;274:15719–24. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: EP1 receptor immunolocalization in normal and hyperplastic mouse epidermis and absent staining in EP1 knockout mice relative to syngeneic control mice. Immunohistochemistry was performed as described in the methods secion on mouse epidermis using the mouse monoclonal anti-EP1 receptor antibody utilized in figure 5. (A). Non-irradiated control SKH1 hairless, albino mice exhibit a linear pattern of EP1 immunolocalization in the upper epidermis just superficial to the stratum corneum (long arrow). (B). UVB-induced hyperplasia results in expansion of the epidermis as well as the granular layer and results in enhanced visualization of course, grainy cytoplasmic staining within the superficial suprabasal compartment (long arrows). Nuclear staining is noted by the short arrow. (C). Wild-type C57Bl/6 mice exhibit a similar pattern of immunolocalization noted in control SKH1 mouse epidermis (panel A). (D). The epidermis from C57Bl/6 mice with germline deletion of the EP1 receptor lack significant staining by IHC.
Supplemental figure 2: Lot to lot variability in the performance of a commercial rabbit polyclonal anti-EP1 receptor antibody. Immunoblots were performed on 10 μg of membrane preparation from HEK 293 cells over-expressing the human EP1 receptor (HEK + EP1), the human EP3 receptor (HEK + EP3), or empty vector control (HEK) cells essentially as described in the methods section. (A). Immunoblot performed for EP1 receptor expression utilizing a rabbit polyclonal anti-EP1 receptor antibody (Cayman Chemical, Ann Arbor, MI). This same lot of antibody reagent was used in a previously reported study [14]. Note the specific EP1 receptor bands observed at approx 35 and 70 kDa and the non-specific band observed at approx 45 kDa. (B). Immunoblot using a subsequent lot number of the same commercial antibody source. Note that specific EP1 receptor bands are largely absent, although the non-specific band at approx 45 kDa is still observed.