Abstract
Background:
Mammographic density is a strong risk factor for breast cancer, usually measured by an area-based threshold method that dichotomises the breast area on a mammogram into dense and non-dense regions. Volumetric methods of breast density measurement, such as the fully-automated Standard Mammogram Form (SMF) method that estimates the volume of dense and total breast tissue, may provide a more accurate density measurement and improve risk prediction.
Methods:
In 2000-03, a case-control study was conducted of 367 newly confirmed breast cancer cases and 661 age-matched breast cancer-free controls who underwent screen-film mammography at several centres in Toronto, Canada. Conditional logistic regression was used to estimate odds ratios of breast cancer associated with categories of mammographic density, measured with both threshold and SMF (version 2.2β) methods, adjusting for breast cancer risk factors.
Results:
Median percent density was higher in cases than in controls for the threshold method (31% vs. 27%) but not for the SMF method. Higher correlations were observed between SMF and threshold measurements for breast volume/area (Spearman correlation coefficient = 0.95) than for percent density (0.68) or for absolute density (0.36). After adjustment for breast cancer risk factors, odds ratios of breast cancer in the highest compared to the lowest quintile of percent density were 2.19 (95% CI 1.28, 3.72; Pt <0.01) for the threshold method and 1.27 (95% CI 0.79, 2.04; Pt=0.32) for the SMF method.
Conclusion:
Threshold percent density is a stronger predictor of breast cancer risk than the SMF version 2.2β method in digitised images.
Introduction
Mammographic breast density corresponds to the amount of radiographically dense tissue on a mammogram, primarily representing fibroglandular tissue in the gland. Mammographic density has repeatedly been shown to be associated with risk of breast cancer, but the magnitude of this association varies depending on the method used to quantify it (1). Methods that provide continuous measurements on a quantitative scale yield larger gradients in risk than methods that simply classify density into specific categories (e.g. Wolfe patterns (2), Tabar's five-point grading system (3), Breast Imaging Reporting and Data System (BIRADS) (4)). Currently, the interactive threshold method (5), implemented with the Cumulus software, is the most widely used breast density assessment tool because it is semi-automated, provides measurements on a continuous scale, and has been shown to be a strong predictor of breast cancer risk (1, 6).
Both qualitative and currently available quantitative methods suffer from several limitations. Most are time- and labour-intensive, and hence costly when used in largescale research studies or in clinical settings. The currently favoured interactive threshold method is affected by reader subjectivity in choosing a threshold to distinguish dense from non-dense tissue and, although high reliability can be achieved with training, such subjectivity introduces measurement error leading to an attenuation of the density-breast cancer association, reduced power to detect determinants of this risk factor or to accurately measure and monitor within-woman changes in density which may be related to risk. More importantly, it is an area-based method whereby the three-dimensional structure of the breast is reduced to the projected mammogram area in which its pixels are simply assumed to represent either completely dense or completely fatty tissue, whereas in truth they represent X-ray attenuation by varying degrees of both fibroglandular and fatty tissue. Two women with similar projected areas of radiodense tissue may have different volumes of fibroglandular tissue depending on the thickness of the breast and the degree of fibroglandular tissue within the ‘dense’ area.
Volumetric measurements attempt to capture the absolute and relative volume of breast tissue that is dense and we hypothesise that, being closer to the underlying biological entity, such measures may be better predictors of breast cancer risk than area-based ones. One such implementation of the volumetric concept is the Standard Mammogram Form (SMF), developed by Highnam and Brady, which is a fully-automated method of breast density measurement that, in contrast to other volume-based methods, can be applied not only prospectively but also retrospectively to mammograms taken in the past (7). Previous evaluations of this method include assessments of its left-right breast reliability (8) as well as demonstrations of the association of its volumetric measurements with well-known density correlates (8, 9). So far, only one study has evaluated the value of SMF readings as predictors of breast cancer risk but its validity was hampered by the unavailability of data on confounders other than age at mammography (10).
In this analysis, we compare the SMF volumetric method and the interactive threshold method as predictors of breast cancer risk in a large case-control study with detailed information on imaging acquisition parameters as well as data on potential confounding variables.
Materials and Methods
Study Participants
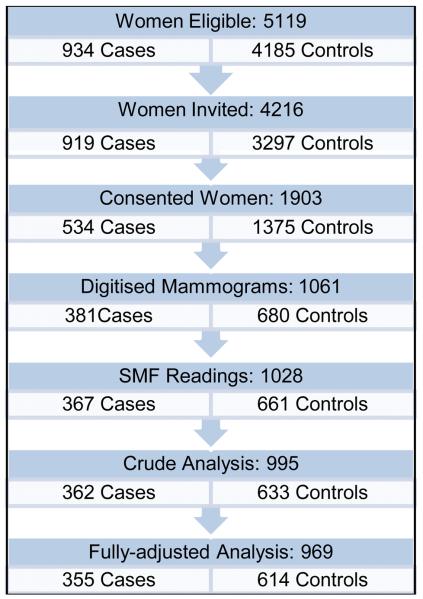
A multi-site matched case-control study was conducted and has been described in detail elsewhere (11). Briefly, recruitment of study participants took place in seven hospitals in Toronto, Canada, between March 2000 and July 2003. Cases were women with a histologically-confirmed invasive breast cancer diagnosed during the recruitment period and who had at least one screening film prior to diagnosis. Women with synchronous bilateral cancers, breast implants or reduction mammoplasty were excluded. For each case, a list of potential controls was drawn up of women who underwent mammography within one week of the case and who were the same age (in years) as the case at the time of diagnosis, but excluding those with a previous history of breast cancer or with breast implants or reductions. Two controls per case were selected randomly from this list, one matched to the same mammography machine as the case and the other counter-matched on mammography machine. As the mammography machines themselves might add extra variation in volumetric density estimations, density measurements are expected to be more comparable for films taken on the same machine. In mammography units with only one machine, a single control was selected per case. Participating women were interviewed by telephone to obtain information on demographic, anthropometric, lifestyle and reproductive characteristics and they gave consent for their mammograms to be accessed. Questionnaire data and density measurements were available for 381 (41%) cases and 680 (21%) controls (see Figure 1). The study protocol was approved by all relevant ethics committees (11).
Figure 1.
Flow Chart of Recruitment of Study Participants
Mammograms
A single cranio-caudal film was retrieved from the mammography units for each consented participant and digitised using a Lumisys 85 digitiser (Carestream Health, Rochester, NY, USA) at 260 microns and with optical density up to 4.0. If several pre-diagnostic mammograms were available the screening date closest to the date of diagnosis was chosen for analysis. To ensure blinding of the reader to the case-control status of the participant, the image of the breast contra-lateral to the cancer was selected in cases and the corresponding breast in the matched controls.
Interactive-Threshold method
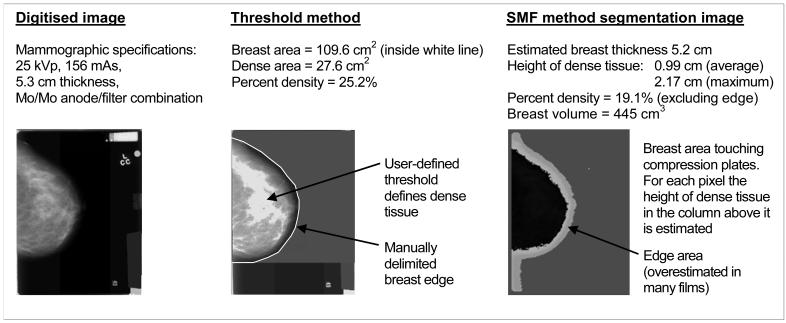
Area-based measurements of mammographic density were estimated from the digitised images using the interactive threshold method (Cumulus software, University of Toronto, Toronto, Ontario, Canada). Films were read by a single reader (NFB) in sets of approximately 120 films, comprised of pairs of randomly ordered cases and controls. Breast area was defined by delimiting the skin edge and masking the pectoral muscle (usually not visible in the cranio-caudal view) and any other non-breast tissue. The greyscale threshold subjectively distinguishing area of dense and non-dense tissue was then selected from which dense area, non-dense area and percent density were calculated (Figure 2). A random 10% sample of images within each set was read twice, as well as 10% between sets, to evaluate the reliability of the readings (intra-class correlation coefficient: 0.96).
Figure 2.
Example of cranio-caudal image mammographic density readings in the threshold and SMF methods
Standard Mammogram Form
GenerateSMF version 2.2β (Siemens Molecular Imaging Limited) is a fully automated algorithm that estimates volumes of dense and non-dense tissue, and from these, volumetric percent density. The method has been fully described elsewhere (7, 12). Briefly, it uses imaging acquisition parameters (e.g. tube voltage, film exposure time and anode-filter combination) to generate a standardised image (SMF). The process attempts to remove variations in the images that are attributable to the imaging conditions, such as breast compression, compression plate slant, anode-heel effect, varying voltages and exposures, and random scatter and glare, rather than to the breast tissue itself. Breast thickness is estimated by the algorithm in this version. After the estimation process, the film is then automatically segmented into regions. The two crucial areas are (i) area of breast tissue that touches the compression plates (sometimes referred to as ‘inside’ area) and (ii) an edge area where breast thickness is non-uniform and less than the distance between the plates and hence its volume is more difficult to estimate (Figure 2). The breast is assumed to contain only two materials (radiodense ‘interesting’ tissue and fatty tissue) and the greyscale level for each pixel in the SMF image is then computed as a function of the known X-ray attenuation coefficients of these materials to get the respective tissue thicknesses. Summing over the whole breast gives the total volume of dense tissue. SMF percent density was calculated by dividing by the breast volume excluding the edge region, because for many films in the present study the edge region was greatly overestimated (see Figure 2) due to SMFv2.2 being tuned for images from a Canon digitiser.
The SMF image quality is rated by the algorithm as excellent, poor or failed. Failed images were excluded as no estimates are provided for them, as well as SMF images of ‘poor’ quality, defined as having impossible thicknesses of dense tissue in over 5% of the breast region (for example, a dense tissue thickness greater than the breast thickness). Additionally, we visually examined all segmentation images to identify any further major errors in the segmentation process, e.g. whether the label was misidentified as the breast region.
Statistical Methods
Characteristics of the cases and controls were summarised and compared using percentages and Chi-squared (X2) tests for categorical variables, and means, standard deviations and Wilcoxon Rank Sum tests for continuous variables. Women with missing SMF density data were compared to those with such information in terms of distributions of threshold breast density measures, anthropometric and risk factor variables. A weighted kappa statistic (with weights 1, 0.75, 0.5, 0.25 and 0 for quintiles 1-5 apart) was calculated to generate the agreement index between the categories of threshold and SMF densities. Linear regression models of square-root transformed SMF and threshold densities were used to examine, in controls, mutually adjusted associations of well-known breast cancer risk factors with each density measure. Estimated linear regression coefficients were back transformed into the original scale to facilitate interpretation.
Odds ratios (OR) of breast cancer associated with SMF and threshold density measures were estimated using conditional logistic regression models for matched case-control data. Each of the density measures (SMF and threshold methods, dense, non-dense, and percent density) was included as an explanatory variable using (i) quintiles of the density distribution in controls and, for percent density, (ii) standard categories <10, 10-24, 25-49, 50-74, ≥75% for threshold percent density and, as these cut-points corresponded to the 18th, 43rd, 81st and 99th percentiles of the control distribution, SMF was also categorised at these percentiles of the control distribution. A linear test for trend across categories of these density measures was performed and P-values (Pt) reported. Three sets of odds ratio were estimated: unadjusted (i.e. associations controlled only for matching variables), adjusted for body mass index (BMI) (a known strong negative confounder at postmenopausal ages) and fully adjusted for BMI, age at mammogram, age at menarche, parity, age at first full-term pregnancy, number of full-term pregnancies, menopausal status, hormone replacement therapy (HRT) and family history of breast cancer. Stratified analyses were performed by menopausal status and whether the control was machine-matched or not. All statistical tests of significance were two-sided. The analyses were performed in Stata version 10 (College Station, Texas).
Results
Participant Characteristics
The characteristics of the 1028 women who had both threshold and SMF density readings are summarised in Table 1, stratified by case and control status. Cases and the two sets of controls were similar in terms of age at mammography, BMI, age at menarche, menopausal status, age at first full-term pregnancy, parity, number of full-term pregnancies, family history of breast cancer, and HRT use and duration. Mean age at menopause was slightly higher in cases than in controls (50.2 in cases vs. 48.7 years in each control set, P=0.03).
Table 1. Baseline and mammographic characteristics of the participants by case-control status.
| Cases n=367 |
Controls matched by machine n=361 |
Controls not matched by machine n=300 |
|
|---|---|---|---|
| Participant Characteristics | Mean (SD)1 | Mean (SD) | Mean (SD) |
|
|
|||
| Age at mammogram (years) | 59.8 (11.1) | 59.6 (11.0) | 58.2 (11.1) |
| BMI2 (kg/m2) | 26.0 (5.3) (n=365) | 25.7 (5.5) (n=360) | 25.6 (5.1) |
| Age at menarche (years) | 12.7 (1.4) (n=364) | 12.8 (1.5) | 12.7 (1.4) (n=298) |
| Post Menopausal (%) | 69.2 | 71.2 | 68.7 |
| Age at menopause (years) | 50.2 (5.5) (n=175) | 48.7 (5.7) (n=178) | 48.7 (6.2) (n=137) |
| Age at first birth (years) | 26.4 (5.0) (n=266) | 26.6 (5.4) (n=273) | 26.6 (5.5) (n=208) |
| Parity (% parous) | 72.5 | 75.4 | 69.7 |
| Number of live births | 1.7 (1.4) | 1.8 (1.4) | 1.6 (1.3) |
| Age at first birth (%) | |||
| <24 years (n=265) | 36.8 | 34.2 | 35.6 |
| 24-27 years (n=209) | 27.1 | 27.2 | 30.3 |
| 28+ years (n=272) | 36.1 | 38.6 | 34.1 |
| Breast cancer (%) in: - 1st degree relative | 21.7 | 25.9 | 23.2 |
| - 2nd degree relative | 23.6 (n=364) | 19.8 (n=359) | 21.8 (n=298) |
| HRT3 (% ever used) | 44.7 | 45.3 (n=360) | 44.7 |
| Years of HRT4 use | 4.0 (7.0) | 4.1 (7.3) (n=360) | 3.8 (7.0) |
|
| |||
| Mammographic Density Measures | Median (25th, 75th)4 | Median (25th, 75th) | Median (25th, 75th) |
|
|
|||
| Threshold | |||
| Dense area (cm2) | 36 (22, 54) | 34 (19, 48) | 34 (20, 49) |
| Non-dense area (cm2) | 91 (53.1, 139) | 95 (56, 143) | 94 (54, 149) |
| Total breast area (cm2) | 135 (99, 179) | 139 (100, 181) | 131 (97, 179) |
| Percent density | 31.1 (16.1, 46.6) | 27.1 (13.2, 45.1) | 27.2 (13.7, 45.8) |
| SMF | |||
| Dense volume (cm3) | 129 (97, 194) | 131 (93, 201) | 129 (91, 188) |
| Non-dense volume (cm3) | 372 (249, 565) | 394 (256, 577) | 376 (235, 577) |
| Total breast volume (cm3) | 524 (366, 727) | 533 (359, 755) | 513 (365, 771) |
| Percent density | 26.2 (20.2, 34.0) | 25.0 (20.8, 33.5) | 25.5 (21.0, 32.0) |
| SMF estimated thickness (cm) | 5.3 (1.0) | 5.3 (1.1) | 5.3 (1.1) |
|
| |||
| Imaging Parameters | Mean (SD) | Mean (SD) | Mean (SD) |
|
|
|||
| Voltage (kV) | 26.7 (2.0) | 26.7 (1.9) | 26.8 (2.1) |
| Exposure (mAs) | 155.0 (47.9) | 156.5 (57.7) | 146.1 (51.3) |
| Compression force (N) | 105.7 (31.4) (n=354) | 104.2 (31.3) (n=350) | 101.7 (32.5) (n=293) |
| Measured thickness5 (cm) | 5.4 (1.2) (n=366) | 5.4 (1.4) | 5.4 (1.3) |
Mean (Standard Deviation) for continuous variables and percentages for categorical variables
Body Mass Index
Hormone Replacement Therapy
Percentiles: Median (25th, 75th)
Compressed breast thickness reported by mammography machine
From the original sample of 1061 women, 33 women (3.1%) were excluded as their SMF readings were not reliable; 22 images were considered as ‘poor’ by SMF and visual examination found incorrect segmentation for a further 11 women (typically the label was identified as the breast). Women with missing SMF readings had higher threshold percent density (mean 47.9%) than women with valid SMF readings (30.8%, test of difference P<0.001) and smaller breast area (86.1cm3 vs. 146.0cm3, P<0.001) (data not shown). They had a lower mean BMI (22.5 vs. 25.8 kg/m2, P<0.001) and were less likely to be post-menopausal (51.5% vs. 69.8% post-menopausal, P=0.03). Among valid SMF readings, SMF-estimated breast thickness was highly correlated with recorded breast thickness (Spearman correlation coefficient = 0.86) and of similar magnitude although with a smaller range (mean estimated thickness = 5.30 (SD 1.07), mean recorded thickness = 5.40 (SD 1.29)), however 11% (111/1028) of differences were over 1 cm.
Overall distributions of total area/volume of the breast and of percent and absolute density are shown in Figure 3 for the whole study population (cases and controls combined). The range of SMF percent density values was much narrower, with values ranging from 11% to 65%, compared to corresponding values of 0 to 85% for the threshold method (Table 1, Figure 3). There are several women with 0% threshold densities (no dense tissue) who have positive SMF percent densities, which is not unexpected as SMF includes skin as dense tissue and so can never show 0% density. The measures of dense volume also show such differences in distribution, with no SMF dense volumes under 25cm3 whilst the threshold method has almost 3% of women with no projected dense area. High correlation (Spearman correlation coefficient = 0.95) was observed between SMF and threshold methods for breast volume/area, thus lower correlation (0.68) for percent density measures were due to lower rank correlation (0.36) of absolute density measures. Moderate agreement was found between quintiles of threshold and SMF percent densities (weighted agreement = 79.5%, kappa 0.49).
Figure 3.
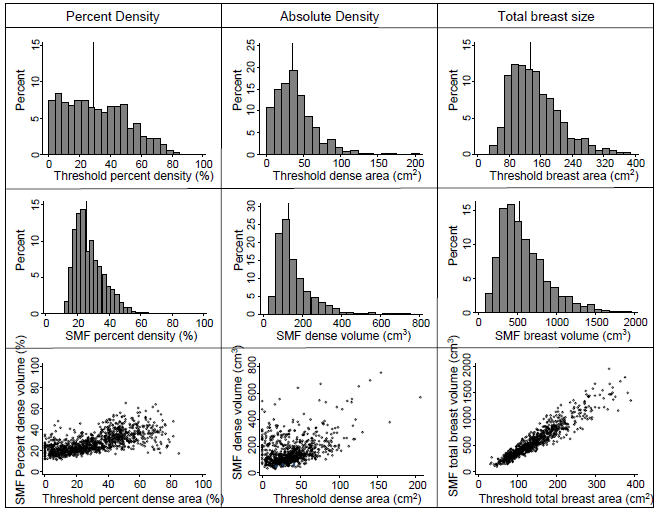
Histograms of percent and absolute density and breast size, threshold and SMF methods and their scatter plots (vertical lines locate the median value)
Associations with breast cancer risk factors in controls
Percent density was lower in the control women who were older, had higher BMI and were post-menopausal, according to both the threshold and SMF methods (Table 2). Percent density also decreased with number of full-term pregnancies and younger age at first full-term pregnancy, but these associations were only statistically significant for the threshold method. Threshold percent density was lower in women who had ever used HRT, but no association was found with SMF percent density. Absolute density decreased with age, BMI and menopausal transition when measured by the threshold method, but these associations were not apparent with SMF absolute dense volume, for which an opposite trend was observed of increasing SMF dense volume with increasing categories of BMI (Table 2). There was no evidence that age at menarche or family history of breast cancer were associated with density using either measurement method.
Table 2. Mutually adjusted associations of known determinants of mammographic density with threshold and SMF density measures in controls.
| SMF method n=650 |
Threshold method n=650 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Categories | Percent density1 | p-value2 | Absolute dense volume3 |
p-value | Percent density4 | p-value | Absolute dense area5 |
p-value |
| Age at mammogram (years) |
<50 | 0 | <0.01 | 0 | 0.41 | 0 | <0.001 | 0 | 0.01 |
| 50− | −1.5 (−3.3, 0.3) | −3.8 (−20.4, 13.9) | −5.1 (−8.6, −1.2) | −4.7 (−10.1, 1.2) | |||||
| 60− | −4.2 (−6.2, −2.0) | −15.6 (−34.7, 5.2) | −9.3 (−13.0, −5.0) | −9.8 (−15.6, −3.1) | |||||
| 70+ | −3.8 (−5.9, −1.6) | −8.8 (−28.9, 13.2) | −11.4 (−15.0, −7.4) | −10.3 (−16.3, −3.6) | |||||
|
| |||||||||
| BMIc (kg/m2) | <23 | 0 | <0.01 | 0 | <0.01 | 0 | <0.001 | 0 | <0.01 |
| 23− | −3.5 (−4.8, −2.1) | 43.9 (28.7, 59.8) | −9.9 (−12.2, −7.3) | −2.6 (−6.9, 2.1) | |||||
| 27+ | −3.9 (−5.2, −2.5) | 114.8 (96.3, 134.0) | −16.3 (−18.1, −14.3) | −7.5 (−11.6, −3.2) | |||||
|
| |||||||||
| Age at menarche (years) |
<12.5 | 0 | 0.61 | 0 | 0.76 | 0 | 0.44 | 0 | 0.24 |
| 12.5− | −0.04 (−1.4, 1.4) | −2.6 (−14.8, 10.3) | 0.3 (−2.6, 3.5) | −0.6 (−4.9, 3.9) | |||||
| 14+ | 0.67 (−0.8, 2.2) | 2.9 (−10.8, 17.2) | 2.1 (−1.2, 5.7) | 3.5 (−1.4, 8.7) | |||||
|
| |||||||||
| Post-menopausal (%) | no | 0 | <0.01 | 0 | 0.15 | 0 | 0.02 | 0 | 0.09 |
| yes | −2.7 (−4.5, −1.0) | −12.3 (−28.1, 4.6) | −4.5 (−8.0, −0.7) | −5.0 (−10.3, 0.8) | |||||
|
| |||||||||
| Age at first full-term pregnancy (years) |
<24 | 0 | 0.16 | 0 | 0.66 | 0 | 0.05 | 0 | 0.18 |
| 24− | 0.4 (−1.4, 2.2) | 3.4 (−12.5, 20.4) | 2.7 (−1.3, 6.9) | 2.6 (−3.1, 8.7) | |||||
| 28+ | 1.7 (−0.1, 3.6) | 7.9 (−8.7, 25.5) | 5.2 (1.0, 9.6) | 5.7 (−0.3, 12.1) | |||||
|
| |||||||||
| Number of full-term pregnancies |
0 | 0 | 0.23 | 0 | 0.14 | 0 | 0.04 | 0 | 0.04 |
| 1 | −0.1 (−2.4, 2.2) | 1.3 (−19.2, 23.6) | −2.0 (−6.6, 3.1) | −1.7 (−8.5, 5.9) | |||||
| 2 | −0.7 (−2.5, 1.2) | −12.5 (−28.7, 4.9) | −0.0 (−4.0, 4.4) | 1.2 (−4.8, 7.7) | |||||
| 3+ | −1.7 (−3.5, 0.1) | −14.6 (−30.3, 2.2) | −4.5 (−8.0, −0.6) | −6.0 (−11.2, −0.3) | |||||
|
| |||||||||
| Breast cancer (%) in relative |
none | 0 | 0.73 | 0 | 0.68 | 0 | 0.81 | 0 | 0.60 |
| 1st degree | 0.6 (−0.9, 2.1) | 4.5 (−8.8, 18.6) | −1.0 (−4.04,2.21) | −2.0 (−6.3, 2.8) | |||||
| 2nd degree | 0.3 (−1.2, 1.9) | 5.4 (−8.6, 20.2) | −0.0 (−3.27,3.41) | 0.8 (−4.0, 5.9) | |||||
|
| |||||||||
| HRTd (% ever used) | no | 0 | 0.29 | 0 | 0.86 | 0 | <0.01 | 0 | <0.01 |
| yes | 0.7 (−0.6, 2.0) | 1.1 (−10.7, 13.3) | 4.7 (1.73,7.89) | 6.1 (1.7, 10.7) | |||||
For a reference value of 25%.
All P values are for differences in mammographic values across categories of a given variable.
For a reference value of 130 cm3.
For a reference value of 25%.
For a reference value of 35 cm2
Breast cancer risk prediction
Cases had higher percent and absolute threshold dense areas (31.1%, 36cm2) than controls, for both machine-matched (27.1%, 34cm2) and machine counter-matched controls (27.2%, 34cm2), but breast area was similar (Table 1). These trends are not apparent in the SMF method, in which percent and absolute volumetric density seem to be very similar in cases and controls. These findings are reflected in associations with breast cancer risk, taking matched sets into account (Table 3, whose results are based on 995 women for unadjusted and BMI-adjusted models and 969 women in fully adjusted models, as some sets had either no controls or no cases after exclusion of missing SMF and risk factor SMF data). After adjusting for BMI, women in the top quintile of threshold percent density had approximately a 2.2-fold increase in breast cancer risk compared to women in the lowest quintile, which was not greatly confounded by other considered factors (OR=2.19, 95% CI: 1.28, 3.72, Pt<0.01) (Table 3). The corresponding fully-adjusted association for SMF percent density was considerably weaker, with an odds ratio of 1.27 (95% CI: 0.79, 2.04, Pt=0.32). An increased breast cancer risk was also observed with absolute threshold dense area, albeit weaker in magnitude than that for threshold percent density (OR=1.74, 95% CI 1.11, 2.74, Pt<0.01), but not with the SMF absolute volume of dense tissue (OR=0.95, 95% CI 0.58, 1.53, Pt=0.83). When looking at the odds ratios in each quintile of density (Table 3), increased risks were only seen in the 4th and 5th quintiles for SMF percent density with none being statistically significant, whereas for threshold percent density a more linear trend was observed with odds ratios significantly greater than one for quintiles 4 and 5.
Table 3. Odds ratios (95% confidence intervals) of breast cancer across categories of mammographic density measures.
| Categories of Mammographic Density Measures |
||||||
|---|---|---|---|---|---|---|
| Quintiles of Density Measures |
Q11 | Q2 | Q3 | Q4 | Q5 | Pt2 |
| SMF Percent Density | <19.6% | 19.6-23.3% | 23.4-27.9% | 28-35.1% | ≥35.2% | |
| No. Cases/controls | 79/130 | 66/134 | 57/130 | 88/135 | 77/132 | |
| OR, unadjusted2 | 1 | 0.90 (0.59, 1.37) | 0.82 (0.53, 1.28) | 1.24 (0.82, 1.87) | 1.18 (0.76, 1.84) | 0.18 |
| OR, BMI adjusted | 1 | 0.92 (0.61, 1.41) | 0.84 (0.54, 1.31) | 1.31 (0.86, 2.00) | 1.29 (0.82, 2.03) | 0.10 |
| OR, fully adjusted3 | 1 | 0.96 (0.61, 1.49) | 0.87 (0.55, 1.36) | 1.32 (0.85, 2.06) | 1.27 (0.79, 2.04) | 0.32 |
| Threshold Percent Density | <10.7% | 10.7-21.4% | 21.5-34.4% | 34.5-48.2% | ≥48.3% | |
| No. Cases/controls | 72/132 | 51/132 | 76/133 | 84/132 | 84/132 | |
| OR, unadjusted2 | 1 | 0.78 (0.50, 1.20) | 1.26 (0.83, 1.93) | 1.44 (0.94, 2.20) | 1.53 (0.98, 2.38) | 0.01 |
| OR, BMI adjusted | 1 | 0.89 (0.57, 1.39) | 1.58 (1.00, 2.48) | 1.92 (1.19, 3.08) | 2.17 (1.30, 3.63) | <0.01 |
| OR, fully adjusted3 | 1 | 0.87 (0.55, 1.38) | 1.52 (0.95, 2.44) | 1.93 (1.15, 3.26) | 2.19 (1.28, 3.72) | <0.01 |
| SMF dense volume (cm3) | <86.5 | 86.5-112.3 | 112.4-144.1 | 144.2-216.5 | ≥216.6 | |
| No. Cases/controls | 69/132 | 75/132 | 72/133 | 84/132 | 67/132 | |
| OR, unadjusted2 | 1 | 1.11 (0.73, 1.68) | 1.09 (0.72, 1.67) | 1.35 (0.90, 2.02) | 1.08 (0.71, 1.66) | 0.41 |
| OR, BMI adjusted | 1 | 1.07 (0.71, 1.63) | 1.05 (0.69, 1.62) | 1.27 (0.84, 1.93) | 0.97 (0.60, 1.55) | 0.73 |
| OR, fully adjusted3 | 1 | 1.14 (0.74, 1.78) | 1.05 (0.68, 1.63) | 1.33 (0.86, 2.04) | 0.95 (0.58, 1.53) | 0.83 |
| Threshold dense area (cm2) | <17.1 | 17.1-28.2 | 28.3-39.0 | 39.1-54.4 | ≥54.5 | |
| No. cases/controls | 73/132 | 61/132 | 63/133 | 79/133 | 91/131 | |
| OR, unadjusted3 | 1 | 0.97 (0.63, 1.49) | 0.98 (0.63, 1.51) | 1.26 (0.82, 1.92) | 1.60 (1.05, 2.45) | 0.01 |
| OR, BMI adjusted | 1 | 1.05 (0.68, 1.63) | 1.09 (0.69, 1.71) | 1.36 (0.88, 2.12) | 1.74 (1.12, 2.70) | <0.01 |
| OR, fully adjusted4 | 1 | 1.15 (0.73, 1.80) | 1.09 (0.67, 1.75) | 1.44 (0.92, 2.28) | 1.74 (1.11, 2.74) | <0.01 |
|
| ||||||
| Predetermined Categories of Density Measures |
C15 | C2 | C3 | C4 | C5 | Pt |
|
| ||||||
| SMF Percent Density | <19.1% | 19.1-24.0% | 24.1-36.1% | 36.2-52.0% | ≥52.1% | |
| No. Cases/controls | 69/119 | 88/166 | 142/257 | 59/112 | 9/7 | |
| OR, fully adjusted3 | 1 | 1.09 (0.71, 1.67) | 1.15 (0.76. 1.73) | 1.25 (0.76, 2.08) | 2.17 (0.80, 5.91) | 0.21 |
| Threshold Percent Density | <10% | 10-24% | 25-49% | 50-74% | ≥75% | |
| No. Cases/controls | 62/124 | 86/181 | 144/247 | 70/103 | 5/6 | |
| OR, fully adjusted3 | 1 | 1.34 (0.86, 2.11) | 1.99 (1.25, 3.15) | 2.65 (1.50, 4.69) | 4.24 (1.08,16.62) | <0.01 |
Quintiles of mammographic density measures
P-value for trend through categories of mammographic density measures
Unadjusted for variables other than matching factors
Adjusted for BMI, age, age at menarche, number of full-term pregnancies, age at first full-term pregnancy, menopausal status, HRT use and family history of breast cancer
Predetermined categories of mammographic density measures
A strong linear trend of increasing breast cancer risk with higher threshold percent density was also observed using the more standard cut-points of 10, 25, 50 and 75%, with approximately a 4-fold increase in breast cancer risk in the highest (>75%) compared to lowest (<10%) category (OR 4.24, 95% CI 1.08, 16.62, Pt<0.01) (Table 3). Using the equivalent percentile cut-points in SMF a linear trend was observed but with a more shallow, and statistically non-significant, gradient (for the same comparison OR=2.17, 0.80, 5.91, Pt=0.21). Inclusion of both threshold and SMF values into the same model did not affect the magnitude of the estimates shown in Table 3 (data not shown).
Stratifying on whether the control was matched on mammography machine or not revealed much stronger associations when the control was selected from a different machine, especially for the SMF method which generated a statistically significant odds ratio for the highest compared to the lowest quintile of percent density of 2.27 (95% CI 1.18, 4.38, Pt=0.01) in the unmatched controls but not in the controls matched on mammography machine (0.94 (95% CI 0.55, 1.61, Pt=0.67)) (Table 4). The associations of percent density with breast cancer risk were not affected by menopausal status for either method, but the association between absolute density and breast cancer was stronger in premenopausal women in both threshold and SMF methods. In particular, there was a marked gradient of increasing risk with increasing threshold dense area in pre-menopausal but not in post-menopausal women (Table 4).
Table 4. Odds ratios (95% confidence interval) of breast cancer across categories of mammographic density measures, by control type and menopausal status.
| Quintiles of Mammographic Density Measures |
||||||
|---|---|---|---|---|---|---|
| Q11 | Q2 | Q3 | Q4 | Q5 | Pt2 | |
| By Control Type (n=676 machine-matched, 552 machine counter-matched analyses) | ||||||
| SMF percent density | ||||||
| Matched control | 1 | 0.91 (0.56, 1.49)3 | 0.82 (0.49, 1.37) | 1.31 (0.79, 2.17) | 0.94 (0.55, 1.61) | 0.67 |
| Unmatched control | 1 | 1.20 (0.66, 2.17) | 1.09 (0.61, 1.97) | 1.68 (0.92, 3.07) | 2.27 (1.18, 4.38) | 0.01 |
| Threshold percent density | ||||||
| Matched control | 1 | 0.87 (0.52, 1.48) | 1.42 (0.83, 2.43) | 1.62 (0.92, 2.87) | 1.82 (1.00, 3.34) | 0.01 |
| Unmatched control | 1 | 1.12 (0.58, 2.14) | 2.96 (1.53, 5.73) | 4.14 (2.01, 8.52) | 4.09 (1.88, 8.91) | <0.01 |
| SMF dense volume | ||||||
| Matched control | 1 | 0.99 (0.60, 1.63) | 1.19 (0.71, 1.98) | 1.12 (0.68, 1.86) | 0.75 (0.43, 1.32) | 0.58 |
| Unmatched control | 1 | 1.32 (0.73, 2.39) | 0.97 (0.55, 1.71) | 1.78 (0.99, 3.19) | 1.43 (0.74, 2.74) | 0.15 |
| Threshold dense area | ||||||
| Matched control | 1 | 0.93 (0.54, 1.59) | 0.85 (0.49, 1.48) | 1.23 (0.73, 2.09) | 1.25 (0.74, 2.11) | 0.18 |
| Unmatched control | 1 | 1.73 (0.94, 3.17) | 1.99 (1.02, 3.87) | 2.35 (1.26, 4.39) | 3.60 (1.84, 7.05) | <0.01 |
| By Menopausal Status (n=244 premenopausal, 606 postmenopausal analyses) | ||||||
| SMF percent density | ||||||
| Premenopausal | 1 | 1.16 (0.28, 4.80) | 1.29 (0.35, 4.75) | 0.70 (0.20, 2.53) | 1.36 (0.38, 4.84) | 0.62 |
| Postmenopausal | 1 | 0.79 (0.47, 1.33) | 0.78 (0.47, 1.31) | 1.67 (0.99, 2.81) | 0.89 (0.48, 1.66) | 0.24 |
| Threshold percent density | ||||||
| Premenopausal | 1 | 3.04 (0.44, 20.91) | 2.99 (0.49, 18.22) | 7.34 (1.10, 48.95) | 6.27 (0.88, 44.90) | 0.05 |
| Postmenopausal | 1 | 0.74 (0.44, 1.24) | 1.72 (1.01, 2.93) | 1.70 (0.93, 3.11) | 1.54 (0.81, 2.94) | 0.02 |
| SMF dense volume | ||||||
| Premenopausal | 1 | 1.57 (0.54, 4.59) | 2.44 (0.83, 7.19) | 2.67 (0.91, 7.88) | 2.47 (0.78, 7.83) | 0.10 |
| Postmenopausal | 1 | 0.95 (0.56, 1.59) | 0.82 (0.48, 1.39) | 1.02 (0.60, 1.74) | 0.65 (0.36, 1.17) | 0.28 |
| Threshold dense area | ||||||
| Premenopausal | 1 | 3.37 (0.57, 19.98) | 5.44 (0.84, 35.05) | 5.41 (0.89, 32.76) | 8.45 (1.43, 49.96) | 0.01 |
| Postmenopausal | 1 | 1.01 (0.61, 1.67) | 0.86 (0.49, 1.50) | 1.24 (0.73, 2.10) | 1.20 (0.69, 2.10) | 0.38 |
Quintiles of mammographic density measures
P-value for trend through quintiles of mammographic density measures
Odds Ratio (95% CI) adjusted for BMI, age, age at menarche, number of full-term pregnancies, age at first full-term pregnancy, menopausal status, HRT use and family history of breast cancer
Discussion
The biological mechanisms underlying the association between high mammographic density and breast cancer risk are not known. Mammographic density may simply reflect the amount of fibro-glandular tissue in the breast and therefore the number of cells at risk of suffering a malignant transformation. If this is the case, the volume of fibroglandular tissue is likely to quantify risk more precisely than the projected radio-dense area seen on a mammogram and thus, theoretically, volumetric approaches to measuring breast density should, if accurate, be better predictors of breast cancer risk than area-based measures.
Three-dimensional X-ray breast imaging techniques, such as computed tomography, tomosynthesis and dual energy X-ray absorptiometry, provide direct measurements of volumetric radiological density. As none of these techniques is widely used in screening or clinical settings, attempts have been made to derive volumetric density data from images produced by the more conventional two-dimensional mammography systems (12-14). SMF is one such volumetric approach, which has the advantage of not relying on possible interference of phantoms or wedges to be placed on the X-ray plate at the time of mammography, and allowing it to be applied retrospectively to historical films. Knowledge of the thickness of the compressed breast, that is, the vertical distance between the two compression plates, is an essential element for volumetric estimations of the breast tissues. However, the thickness value recorded by the mammography equipment is not appropriate because thickness will vary across the breast as the two plates are not kept parallel. Ideally, as in the present study, data on the image acquisition parameters should be available as these are used by SMF to estimate breast thickness but, in the absence of such data, the programme is able to estimate them. But whilst the ability of the SMF to estimate breast thickness allows its retrospective use in digitised images, it may also add an extra source of error if the breast thickness estimates generated by the programme are inaccurate. Digital implementations are likely to be far more successful (15, 16).
We compared the SMF ability to predict breast cancer risk to that of the threshold method in a large case-control study with complete film acquisition data and availability of information on potential confounding variables. Contrary to our expectations, SMF percent density performed considerably worse than the threshold method, yielding weaker gradients in breast cancer risk. The risk estimates derived by the threshold method were similar to those reported in other studies (1), with percent density generating steeper gradients in breast cancer risk than total dense area. Ding et al. (10) also reported a weaker stepwise increase in the risk of breast cancer with increasing fourths of SMF percent density than with fourths of threshold percent density and, in mutually-adjusted analyses, only the latter persisted. But interpretation of their findings was limited by lack of data on potential confounding variables other than age at mammography.
The distributions of SMF percent density and dense volume values were narrower than the corresponding distributions for the threshold method, similar to previous findings (8, 9). There was only moderate rank correlation, and moderate quintile agreement, between the percent density values generated by the SMF and those generated by the threshold method, with percent differences reflecting mainly differences in their estimation of dense area/volume. However, lack of perfect agreement between the two methods would be expected as they attempt to measure different aspects of a similar underlying entity.
Consistent with the lack of a strong SMF density-breast cancer association, the SMF method produced weaker associations with known breast cancer risk factors than the threshold method. SMF percent density was associated with age at mammography, menopausal status and BMI but, in contrast to the threshold method, no relationships were found with age at first full-term pregnancy, parity or HRT use. BMI was negatively associated with threshold percent density and, to a lesser extent, threshold dense area, broadly consistent with data from other studies (17, 18). In contrast, BMI was negatively associated with SMF percent density but positively associated with SMF dense volume. Contrasting BMI associations with SMF dense volume and SMF percent density have been reported in other studies (8, 19). Women with higher BMI have lower volumetric percent densities, but as these women have larger breast volumes, their dense volume (percent density x total breast volume) was higher than in women with lower BMI whose breast volume was much smaller.
Possible reasons for the weaker breast cancer prediction ability of SMF method include the poor reliability of this method when based on a single image (8). This limitation could have been overcome if multiple images per woman were included. SMF was unable to produce density measurements for some women and, although this proportion was small (3%), these women had higher threshold density values and if SMF were to be used in a clinical setting it would be important not to overlook such images/women. Exclusion of these women from the analysis might have led to an attenuation of the density-breast cancer associations, but this should not have affected the SMF-threshold comparison as these exclusions were applied to both methods. Perhaps, more importantly, SMF relies on automatic segmentation of the breast and this may be more prone to error than the manual segmentation performed by a reader when using the interactive threshold method. SMFv2.2 was developed for films digitised using a Canon scanner and its use on Lumisys 85-digitised films could have affected the accuracy of the segmentation algorithm. Visual inspection of the SMF segmentation showed that the fatty edge was greater than it would have been if it was delimited manually. Thus, and similarly to Ding et al. (10), the breast edge was ignored in all our SMF analyses. This would have led to a slight overestimation of SMF percent density, as exclusion of the fatty edge would have reduced the total volume of the breast while affecting little the volume of dense tissue, but it is unclear how such over-estimation might have affected the SMF ability to predict breast cancer risk. The SMF version 2.2.β uses image data and the image acquisition parameters to estimate breast thickness values across the breast image. But if this estimation was affected by errors, these would have lead to inaccurate estimations of the volumes of dense and non-dense tissues in the breast and, hence, to the poor performance of SMF in this study.
Analyses stratified according to whether controls were matched on mammography machine showed that the associations with breast cancer risk were stronger when controls were selected from a different machine for both the threshold and the SMF methods. These findings are surprising as one would have expected that matching on machine would minimise potential sources of error but, as the differences were limited to one mammography site (11), they are likely to reflect differences in the practices of technologists at that location. But whatever the source of the bias affecting the set of unmatched controls, reassuringly its impact on the density – breast cancer estimates was similar for the threshold and the SMF methods (Table 4).
Other volumetric methods for breast density assessment for use with screen-film mammography, which can only be used in prospective mammographic collections, are currently being developed. One, based on a calibration step-wedge, has recently been shown, using data from the present case-control study, to be as good a predictor of breast cancer as the threshold method, but that only the latter method remained statistically significant upon mutual adjustment (11). Volumetric dense volumes in the latter study, based on the same set of films, were approximately half those estimated using SMF, revealing considerable discrepancies in defining and estimating ‘dense’ tissue such as skin, as noted earlier. Further volumetric methods have been developed for use with full-field digital mammography and attempts to improve aspects of automatic estimation are underway (15, 16, 20, 21), such as the Hologic Quantra method (21). These volumetric methods have shown good correlation with three-dimensional data from breast MRI (15), but before they can be used to aid breast cancer prediction in a clinical setting, it will be necessary to demonstrate first that they are predictive of breast cancer risk.
In short, although it is plausible to assume that volumetric methods are likely to capture more accurately the underlying biological processes leading to breast cancer, implementations of the volumetric methods for use with screen-film mammography, such as SMFv2.2 examined here, have not been found to be as good predictors of breast cancer risk as the two-dimensional interactive threshold method.
Acknowledgements
We would like to thank the technologists and other personnel of the mammography units of Mount Sinai Hospital, Women's College Hospital, Princess Margaret Hospital//The Toronto Hospital (University Health Network), Sunnybrook Health Sciences Centre, and the North York and Scarborough sites of the Ontario Breast Screening Programme, all in Toronto, Canada for their cooperation.
Financial Support: NIH RO1CA082826-01. Dr Boyd was supported by the Lau Chair in Breast Cancer Research, Dr Silva was supported by Cancer Research UK 5-year programme grant, and Z. Aitken was funded by Breast Cancer Campaign.
References
- 1.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe JN, Saftlas AF, Salane M. Mammographic parenchymal patterns and quantitative evaluation of mammographic densities: a case-control study. AJR Am J Roentgenol. 1987;148:1087–92. doi: 10.2214/ajr.148.6.1087. [DOI] [PubMed] [Google Scholar]
- 3.Tabar L, Dean PB. Mammographic parenchymal patterns. Risk indicator for breast cancer? Jama. 1982;247:185–9. [PubMed] [Google Scholar]
- 4.BI-RADS . Breast Imaging Reporting and Data System. American College of Radiology; Reston, VA: 1998. [Google Scholar]
- 5.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 6.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 7.Highnam R, Brady M. Computational Imaging and Vision. Kluwer Academic Publishers; London: 1999. Mammographic Image Analysis. [Google Scholar]
- 8.McCormack VA, Highnam R, Perry N, dos Santos Silva I. Comparison of a new and existing method of mammographic density measurement: intramethod reliability and associations with known risk factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1148–54. doi: 10.1158/1055-9965.EPI-07-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffreys M, Warren R, Highnam R, Smith GD. Initial experiences of using an automated volumetric measure of breast density: the standard mammogram form. Br J Radiol. 2006;79:378–82. doi: 10.1259/bjr/24769358. [DOI] [PubMed] [Google Scholar]
- 10.Ding J, Warren R, Warsi I, et al. Evaluating the effectiveness of using standard mammogram form to predict breast cancer risk: case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:1074–81. doi: 10.1158/1055-9965.EPI-07-2634. [DOI] [PubMed] [Google Scholar]
- 11.Boyd N, Martin L, Gunasekara A, et al. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prev. 2009;18:1754–62. doi: 10.1158/1055-9965.EPI-09-0107. [DOI] [PubMed] [Google Scholar]
- 12.Highnam R, Pan X, Warren R, Jeffreys M, Davey Smith G, Brady M. Breast composition measurements using retrospective standard mammogram form (SMF) Phys Med Biol. 2006;51:2695–713. doi: 10.1088/0031-9155/51/11/001. [DOI] [PubMed] [Google Scholar]
- 13.Pawluczyk O, Augustine BJ, Yaffe MJ, et al. A volumetric method for estimation of breast density on digitized screen-film mammograms. Med Phys. 2003;30:352–64. doi: 10.1118/1.1539038. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd JA, Herve L, Landau J, Fan B, Kerlikowske K, Cummings SR. Novel use of single X-ray absorptiometry for measuring breast density. Technol Cancer Res Treat. 2005;4:173–82. doi: 10.1177/153303460500400206. [DOI] [PubMed] [Google Scholar]
- 15.Hartman K, Highnam R, Warren R, Jackson V. Volumetric assessment of breast tissue composition from FFDM images. In: Krupinski E, editor. Digital Mammography, Lecture Notes in Computer Science 5116. Springer; Berlin/Heidelberg: 2008. pp. 33–39. [Google Scholar]
- 16.van Engeland S, Snoeren PR, Huisman H, Boetes C, Karssemeijer N. Volumetric breast density estimation from full-field digital mammograms. IEEE Trans Med Imaging. 2006;25:273–82. doi: 10.1109/TMI.2005.862741. [DOI] [PubMed] [Google Scholar]
- 17.Boyd NF, Martin LJ, Sun L, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086–92. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- 18.Haars G, van Noord PA, van Gils CH, Grobbee DE, Peeters PH. Measurements of breast density: no ratio for a ratio. Cancer Epidemiol Biomarkers Prev. 2005;14:2634–40. doi: 10.1158/1055-9965.EPI-05-0824. [DOI] [PubMed] [Google Scholar]
- 19.Jeffreys M, Warren R, Highnam R, Davey Smith G. Breast cancer risk factors and a novel measure of volumetric breast density: cross-sectional study. Br J Cancer. 2008;98:210–6. doi: 10.1038/sj.bjc.6604122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufhold J, Thomas JA, Eberhard JW, Galbo CE, Trotter DE. A calibration approach to glandular tissue composition estimation in digital mammography. Med Phys. 2002;29:1867–80. doi: 10.1118/1.1493215. [DOI] [PubMed] [Google Scholar]
- 21. http://www.hologic.com/breast-screening/volumetric-assessment/