Abstract
Anti-cancer drugs targeting protein kinases include small molecule inhibitors and monoclonal antibodies. Feedback loops and cross talk between signalling pathways impact significantly on the efficacy of cancer therapeutics and resistance to targeted agents is a major barrier to effective treatments. Increasingly, therapies are being designed to target multiple kinase pathways. This can be achieved using a single agent which inhibits multiple signalling pathways or a combination of highly selective agents. In this review we discuss the principles of specifically targeting multiple kinase pathways with particular reference to angiogenic signalling pathways.
Background
Introduction
Dysregulation of protein kinases in cancer cells is extremely common. Consequently protein kinases are attractive targets for anti-cancer drugs, including small molecule inhibitors which usually act to block binding of ATP or substrate to the catalytic domain of the tyrosine kinase (TK), and monoclonal antibodies which specifically target receptor tyrosine kinases (RTKs) and their ligands. The most exquisite example of successful targeted therapy is perhaps that of imatinib, designed specifically to target an abnormal, constitutively active BCR-ABL tyrosine kinase found in >90% of cases of chronic myeloid leukaemia (1). In solid malignancies, it is unusual for a single kinase abnormality to be the sole cause of disease and it is unlikely that tumours are dependent on only one abnormally activated signalling pathway. Instead multiple signalling pathways are dysregulated. Furthermore, even single molecular abnormalities may have multiple downstream effects. Thus, unless it is possible to target a single key underlying defect, it is likely that therapies will be more effective by inhibiting a number of downstream targets.
Advantages of such a ‘multi-targeted’ approach include the potential for increased efficacy and reduced resistance by simultaneous inhibition of multiple pathways and common escape pathways. Disadvantages include possible increased cost and toxicity. Another important question is whether simultaneous or sequential administration of targeted drugs produces superior efficacy. The theoretical background for simultaneously targeting multiple targets is not the same as simultaneously using multiple agents. Employing sequential use of non-cross resistant therapies may in some cases result in improved outcomes. Importantly, even agents with similar modes of actions, such as sunitinib and sorafenib, appear to demonstrate a rather low level of cross-resistance as demonstrated by two clinical trials comparing the sequential use of sunitinib and sorafenib and vice versa(2, 3). Sequential therapy may also be associated with a more favourable toxicity profile but ultimately this is a question which will need to be resolved in clinical trials.
Multiple pathways can be targeted either by using a single agent which inhibits multiple signalling pathways or by using a combination of highly selective agents. While use of a single multi-targeted agent offers convenience, potential limitations include difficulties in obtaining sufficient potencies against multiple targets in tumour cells without excessive toxicity from cross-reactivity with normal tissues. Differing affinities for the receptors may result in relatively greater inhibition of one target to achieve adequate inhibition of another resulting in toxicity. In contrast, combining selective agents with the aim of achieving additive or synergistic effects may allow high target selectivity with reduced systemic effects, though this is at the risk of potential pharmacodynamic and pharmacokinetic interactions between the drugs. Ideally, combination therapies should use effective agents with differing mechanisms of action and adverse effect profiles.
In this review we discuss the principles of specifically targeting multiple kinase pathways.
Angiogenic Signalling Pathways
Angiogenesis is crucial for tumour progression and metastasis and is increasingly a target for cancer therapies. The vascular endothelial growth factor (VEGF) family of proteins consist of numerous subtypes, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and placenta-growth factor-1(reviewed within(4)), most of which bind to cell membrane-associated RTKs, the VEGF-receptors (VEGFRs). The binding of VEGF ligand to its receptor initiates activation of downstream signalling pathways, including the RAF-MEK-ERK and PI3K pathways, which ultimately lead to endothelial cell activation, proliferation, migration and survival (Figure 1a). Increased VEGF expression is found in a variety of human tumours including colorectal cancer (CRC), non-small cell lung cancer (NSCLC), breast and ovarian cancers and is correlated directly with increased neovascularisation within the tumour (reviewed in (5)). Drugs targeting the VEGF pathways include the monoclonal antibody bevacizumab and the small molecule inhibitors sunitinib, sorafenib and valatinib. Additional positive regulators of angiogenesis and their receptors include fibroblast growth factor (FGF, FGF-receptor), platelet derived growth factor (PDGF, PDGF-receptor), angiopoietin 1 & 2 (Tie2 receptor) and transforming growth factor-β (TGF- β, TGF-β-R). Moreover, increasing evidence suggests a link between the EGFR and HER2 pathways and VEGF-dependent angiogenesis and preclinical studies have demonstrated both direct and indirect angiogenic effects of EGFR signalling (reviewed in (6)). Upregulation of tumour proangiogenic factors and EGFR-independent tumour induced angiogenesis has been suggested as a potential mechanism by which tumour cells might overcome EGFR inhibition.
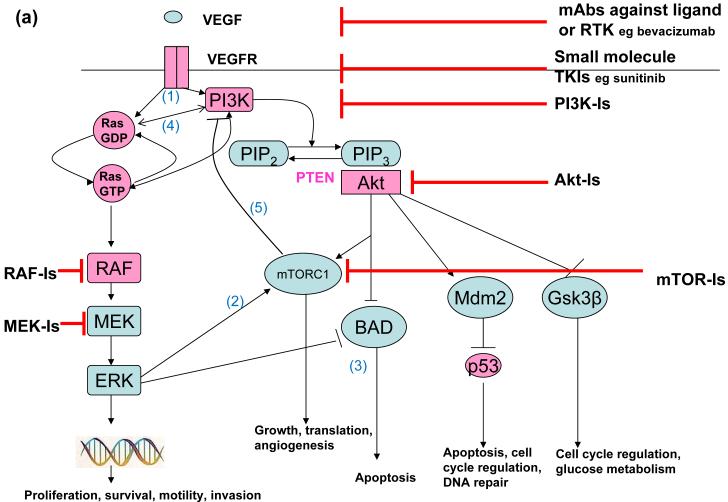
Figure 1a. VEGF signalling and potential therapeutic targets.
The binding of VEGF to its receptor initiates activation of both the PI3K-Akt and RAF-MEK-ERK pathways (1). Each pathway has its own distinct downstream effects. However, they also converge on at least two important downstream targets, mTORC1 (2) and BAD (3), which plays a key role in apoptosis. Furthermore, Ras binds directly to PI3K and each influences activation of the other pathway(4). mTORC1 inhibition leads to activation of both PI3K and ERK signalling by abrogating feedback inhibition (5). Potential points of therapeutic inhibition are highlighted. Components in the signalling pathway that are mutated in cancers are shown in pink.
Targeting the phosphoinositide 3-kinase (PI3K) and RAF-MEK-ERK pathways
The PI3K-Akt signalling pathway is inappropriately activated in many cancers (reviewed in (7), Figure 1a). This may result from RTK induced activation of PI3K; tumours in which PI3K is activated by multiple RTKs are invariably resistant to a single specific RTKI (reviewed in (7)). Alternatively, several genetic abnormalities are known to activate PI3K-Akt signalling. These include loss of the PTEN tumour suppressor (reviewed in (8)), somatic activating mutations in the class Ia PI3K catalytic subunit (p110-α) (9) or regulatory subunit (10) and genetic alterations of all three Akt isoforms (reviewed in (7)). PI3K also binds directly to ras (11) and there is increasing evidence that Ras activation modulates PI3K function, and vice-versa, though the importance of this interaction is as yet unquantified. Several small molecules that inhibit the PI3K-Akt signalling pathway are in clinical development, including mammalian target of rapamycin (mTOR) inhibitors, PI3K inhibitors, dual PI3K-mTOR inhibitors and Akt inhibitors (reviewed in (7, 12). Interestingly, the p110 subunits of PI3K and mTOR share similar structures, and small molecule inhibitors of p110 often also inhibit mTOR.
Targeting multiple pathways is likely to be the optimum therapeutic strategy
Multi-targeted therapies may target several points within a single pathway, or multiple distinct pathways. It is becoming increasingly clear that feedback loops and crosstalk between signalling pathways, including the PI3K/Akt and RAF-MEK-ERK pathways impact significantly on the efficacy of cancer therapeutics (Figure 1a). For example mTOR-complex 1 (mTORC-1) inhibition leads to activation of both PI3K signalling by abrogating feedback inhibition (13) and ERK signalling through a feedback loop which depends on an S6 kinase-PI3K-Ras pathway (14). Such preclinical studies emphasise the potential of a combined therapeutic approach with mTORC1 and MEK inhibitors (Figure 1b).
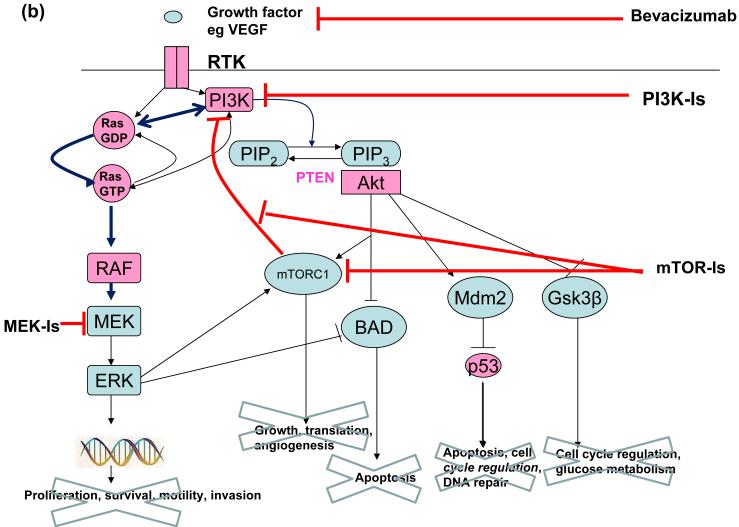
Figure 1b. Combination therapies including mTOR Inhibitors.
Combining mTOR-Is with VEGF antagonists such as bevacizumab may result in additive or synergistic efficacy. Alternatively, preclinical evidence suggests that combining mTOR-Is with MEK-Is and/or PI3K-Is may improve clinical efficacy by abrogating downstream effects induced by mTORC-I induced activation of the RAF-MEK-ERK and PI3K pathways.
Likewise, in cancers with mutant RTKs or oncogenes such as RAS that activate both the RAF-MEK-ERK and PI3K pathway, blocking the PI3K pathway can actually upregulate signalling of the RAF-MAPK pathway because the two pathways have cross-inhibitory effects (15). It is therefore likely that the most useful role for drugs targeting molecules downstream to the RTKs may be as combination therapies rather than as single agents. Administration of mTORC1 inhibitors concurrently with PI3K and MEK inhibitors could theoretically circumvent resistance due to the feedback loops described above, and combining PI3K and MEK inhibitors may alleviate problems associated with cross-inhibitory effects between the two pathways (16)(Figure 1b).
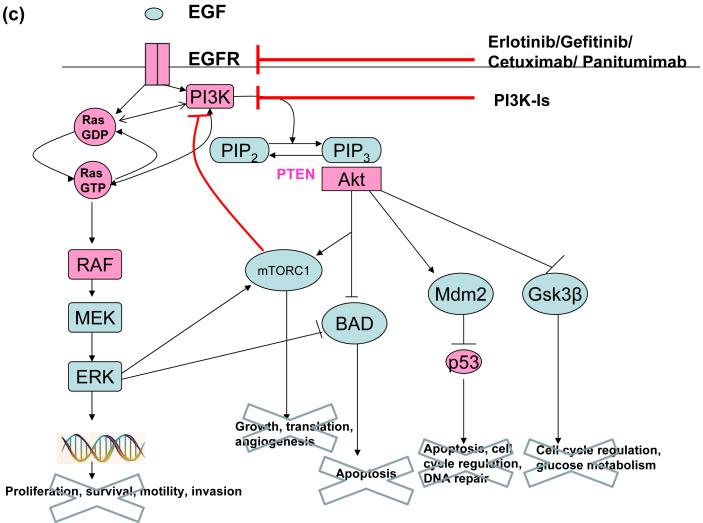
Lung cancers which are sensitive to EGFR inhibitors (EGFR-Is) have PI3K and ERK activation that is under sole control of the EGFR (EGFR oncogene addiction). Such tumours can be rendered resistant to EGFR-Is simply by maintaining PI3K signalling, and reactivation of PI3K signalling is almost invariably seen in cancers which naturally develop resistance to EGFR-Is (reviewed in (7)). Combining PI3K inhibitors with EGFR-Is is a sensible strategy to increase the proportion of cancers that benefit from EGFR-Is, and delay the development of resistance in those tumours which are initially responsive (Figure 1c). Similarly, there is evolving evidence that inhibition of both the PI3K-Akt and RAF-MEK-ERK pathways might be substantially more effective than inhibition of either pathway alone. For example in a mouse model of lung cancer that expressed mutant k-ras, the combination of PI3K inhibitors and MEK inhibitors was highly effective, while the use of either agent alone wasn’t (16).
Figure 1c. Combinations of EGFR-Is and PI3K-Is may improve clinical efficacy.
Reactivation of PI3K signalling is a common mechanism of resistance to therapies targeting the EGFR. Combining these agents with PI3K-Is may be one way to overcome this resistance mechanism.
Clinical Translational Advances
Combining Anti-angiogenic Agents in the Clinic
Combination therapies with mTOR-Is (Figure 1b)
Although mTOR was only recently defined as a member of the PI3K pathway, it is the first node of the pathway to be targeted in the clinic. mTOR exists in two multiprotein complexes: mTORC1 and mTORC2. The rapamycin analogues temsirolimus (17) and everolimus(18) both inhibit mTORC1 and are approved as treatments for advanced clear cell renal carcinoma (ccRCC). Rapalogs have also shown single-agent activity in lymphoma but have failed to show any appreciable single agent activity in many other tumour types (reviewed in (19)). Selective ATP-competitive inhibitors of mTOR which are currently in clinical trials inhibit both mTORC1 and mTORC2 and impair cell growth and proliferation more effectively than rapamycin, suggesting the potential for increased clinical efficacy (20).
Combinations of mTOR-Is and VEGF antagonists have already reached clinical trials. Preliminary results of a phase I study of temsirolimus and bevacizumab in metastatic ccRCC were encouraging with 8 partial responses in 14 patients (21), and phase II data also suggests the combination of bevacizumab and everolimus is tolerable at full doses of both agents (22). Unfortunately both sorafenib (23) and sunitinib (24) in combination with temsirolimus have shown significant toxicity. The ECOG E2804 randomised phase II study of temsirolimus/bevacizumab, temsirolimus/sorafenib and single agent bevacizumab is evaluating temsirolimus combinations in more detail.
Numerous other rational combinations can be conceived and are being tested, including mTOR-Is with inhibitors of the insulin-like growth factor 1 receptor (IGF1R, in preclinical models IGF1R inhibition prevents rapamycin-induced Akt activation and sensitizes tumour cells to mTOR-inhibition (25)), mTOR-Is with trastuzumab (in HER-2 positive breast cancer cell lines trastuzumab has been shown to inhibit feedback activation of Akt(26)), and other RTKIs in combination with inhibitors of the PI3K/ RAF-MEK-ERK/mTOR pathways.
Combining anti-angiogenic agents with EGFR-Is
Both bevacizumab and cetuximab are independently approved in combination with chemotherapy to treat metastatic colorectal cancer (mCRC) and several studies have assessed their combined use (ref -6-9)). Unfortunately their results have not been encouraging and it seems that addition of cetuximab to bevacizumab in mCRC, with or without chemotherapy, does not improve outcome and in some patients is detrimental.
An alternative approach is to combine bevacizumab with EGFR TKIs, such as erlotinib. Phase II studies in NSCLC have produced encouraging results (27, 28) supporting further evaluation of this approach in phase III clinical trials. Studies in pancreatic(29), breast(30), mesothelioma(31), gynaecological tumours(32) and clear cell renal cell carcinoma (ccRCC)(33) have not shown sustained efficacy, though encouraging reports have been described in early hepatocellular cancer(34) and head and neck squamous cell carcinoma(35). Studies which combined erlotinib and bevacizumab plus a chemotherapy regime in CRC have in general shown an unacceptable level of toxicity (36).
In conclusion, it seems that combining targeted agents does have the potential to increase efficacy, though whether this effect is additive or synergistic is as yet unclear. Toxicity is a considerable limiting factor and cost may be prohibitive.
Using a single agent to target more than one pathway
Increasingly “multi-targeted” agents are being developed with the goal of inhibiting more than one pathway simultaneously. Multi-targeted agents currently licensed for use include sorafenib, sunitinib, lapatinib and pazopanib. Additional agents under investigation include Vandetanib, an oral inhibitor of VEGFR and EGFR signalling which also has activity versus Ret TK. A phase II study in advanced NSCLC demonstrated a significant prolongation of PFS versus gefitinib, and vandetanib is currently being evaluated as a monotherapy in two randomized phase III studies in advanced NSCLC(37). Further studies are evaluating its use in colorectal cancer. Regorafinib is an orally active, potent multi-kinase inhibitor with a kinase inhibition profile targeting VEGFR, PDGFR, TIE2, KIT, RET and FGFR. Preliminary data from an open label phase II study of previously untreated patients with metastatic ccRCC showed promising anti-tumour activity with disease control in 81% of patients (38). Vatalanib is another agent which inhibits all known VEGFRs, as well as PDGF-β-R and c-kit, but is most selective for VEGFR-2. Addition of vatalanib to chemotherapy in mCRC did not improve overall survival in two large randomised controlled phase III studies(39, 40) though a meta-analysis of these trials suggested that vatalanib did significantly improve progression free survival in patients with high levels of LDH (41).
Development of resistance
Resistance to targeted agents is eventually inevitable. The types of resistance may be classified as intrinsic resistance, which is present prior to starting treatment and acquired resistance which evolves during the course of therapy. Mechanisms of resistance include (a) upregulation of alternative RTKs which activate the same downstream targets. For example, amplification of the Met oncogene may lead to resistance to EGFR-Is by activating erbB-3 signalling (42); (b) constitutive activation of downstream mediators. For instance activating mutations in k-ras have been significantly associated with lack of response to EGFR-Is (reviewed in (42)) and four separate studies of mCRC demonstrated that adding an EGFR-I (cetuximab or panitumumab) to standard chemotherapy only benefits patients with wild-type k-ras (43-46); (c) upregulation of the ligand for the targeted receptor. For example hypoxia-triggered upregulation of proangiogenic factors such as FGF and PDGF-β in the presence of anti-VEGF agents can restimulate tumour angiogenesis in a VEGF-independent manner (42); (d) the existence of specific mutations within the targeted RTK. For example a T790M mutation in the EGFR confers resistance to ATP-competitive kinase inhibitors such as gefitinib and erlotinib by increasing the ATP affinity of the EGFR by more than an order of magnitude (47) .
As we learn more about mechanisms of resistance it will become easier to devise intelligent strategies to overcome them. Targeting multiple pathways at multiple levels may be one strategy to surmount resistance.
Conclusions
To develop effective therapeutic strategies which overcome or prevent drug resistance we must continuously anticipate the potential mechanisms underlying resistance and devise methods to circumvent them. To achieve this, it is critical to design genotype directed clinical trials, matching the therapy to the patient, rather than testing random combinations in a broad selection of cancers. Assimilation of the most convincing pre-clinical data will assist in patient selection, and such a strategy is more likely to prioritize the most active therapies. In turn, preclinical models must be re-evaluated and adjusted in view of the results from clinical trials.
Our ways of assessing tumour response need reviewing. Kinase-inhibition often has anti-proliferative rather than anti-apoptotic effects and anti-tumour activity may not therefore necessarily lead to tumour regression. Instead long lasting disease stabilization may be seen, implying a need to modify the traditionally used RECIST criteria to account for this phenomenon. It might be expected that combining therapies will increase toxicity. However this risk may be overcome with intelligent dosing strategies. In chronic myeloid leukaemia, cytotoxicity with transient potent target inhibition using dasatinib is equivalent to prolonged target inhibition (48), and in metastatic ccRCC sunitinib shows activity either when administered within an intermittent 4 weeks on, 2 weeks off dosing schedule (50mg/day) or with a continuous once daily administration of 37.5mg, suggesting the potential for flexible dosing (49). Intermittent potent kinase inhibitor therapy may be a tool to minimise the toxicity of targeted therapy.
It is important to understand why therapies fail when they do. Is it because they don’t potently inhibit their target in vivo, or because effective target inhibition does not produce the desired results? Pharmacodynamic assessments of target inhibition are necessary to answer this; at present this requires evaluation of cancer specimens after drug treatment, and developing less invasive ways of measuring target inhibition, potentially for example by assessing circulating tumour cells(50), would be invaluable. Equally, identification of robust biomarkers that can be used with confidence to select patients most likely to benefit from treatment is a crucially important part of the development of new agents. The holy-grail for targeted therapies is unlikely to be a highly specific agent targeting a single molecule. Instead, approaches targeting more than one molecule in more than one pathway are likely to be the most successful. Informed combinations, directed at bypassing feedback loops and interrupting cross talk between signaling pathways may improve therapeutic outcomes.
Abbreviations
- BAD
Bcl2-associated agonist of cell death
- EGF
epidermal growth factor
- EGFR
EGF receptor
- ERK
extracellular regulated kinase
- GSK-3β
glycogen synthase kinase-3 β
- mAb
monoclonal antibody
- MEK
mitogen activated protein kinase kinase
- PI3K
phosphoinositide-3-kinase
- PIP2
phosphatidylinositol bisphosphate
- PIP3
phosphatidylinositol trisphosphate
- mTORC1
mammalian target of rapamycin complex 1
- RTK
receptor tyrosine kinase
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002 Feb 28;346(9):645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 2.Dudek AZ, Zolnierek J, Dham A, Lindgren BR, Szczylik C. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009 Jan 1;115(1):61–7. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 3.Sablin MP, Negrier S, Ravaud A, Oudard S, Balleyguier C, Gautier J, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009 Jul;182(1):29–34. doi: 10.1016/j.juro.2009.02.119. discussion. [DOI] [PubMed] [Google Scholar]
- 4.Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006 Mar;63(5):601–15. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox SB, Gasparini G, Harris AL. Angiogenesis: pathological, prognostic, and growth-factor pathways and their link to trial design and anticancer drugs. Lancet Oncol. 2001 May;2(5):278–89. doi: 10.1016/S1470-2045(00)00323-5. [DOI] [PubMed] [Google Scholar]
- 6.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008 Sep;5(9):521–30. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009 Aug;9(8):550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 8.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004 Jul 15;22(14):2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 9.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 10.Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, et al. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001 Oct 15;61(20):7426–9. [PubMed] [Google Scholar]
- 11.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994 Aug 18;370(6490):527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 12.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009 Aug;8(8):627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006 Feb 1;66(3):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008 Sep;118(9):3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun T, Gjoerup O, Roberts TM. Tangled webs: evidence of cross-talk between c-Raf-1 and Akt. Sci STKE. 1999 Dec;21(13):PE1. doi: 10.1126/stke.1999.13.pe1. 1999. [DOI] [PubMed] [Google Scholar]
- 16.Yu K, Toral-Barza L, Shi C, Zhang WG, Zask A. Response and determinants of cancer cell susceptibility to PI3K inhibitors: combined targeting of PI3K and Mek1 as an effective anticancer strategy. Cancer Biol Ther. 2008 Feb;7(2):307–15. doi: 10.4161/cbt.7.2.5334. [DOI] [PubMed] [Google Scholar]
- 17.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007 May 31;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 9;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 19.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009 May 1;27(13):2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Activesite inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009 Feb 10;7(2):e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merchan J, Liu G, Fitch T, et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma:phase I safety and activity results [abstract] Journal of Clinical Oncology. 2007;25 Abstract 5034. [Google Scholar]
- 22.Whorf RC, Spigel DR, Yardley DA, Burris HA, III, Waterhouse DM, Vazquez ER, Greco FA. Phase II study of bevacizumab and everolimus (RAD001) in the treatment of advanced renal cell carcinoma (RCC) Journal of Clinical Oncology. 2008;26(suppl) JDH. Abstr 5010. [Google Scholar]
- 23.Patnaik A, Cooper J, Papadopoulos K, Beeram M, Mita C, Mita MM, Hufnagel D, Izbicka E. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib, a multi-targeted kinase inhibitor in combination with temsirolimus, an mTOR inhibitor in patients with advanced solid malignancies. Journal of Clinical Oncology. 2007;25S:3512. AR. [Google Scholar]
- 24.Fischer P, Carducci MA, McDermott DF, Hudes GR, Lubiniecki GM, Gelder MS, Senico P, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Journal of Clinical Oncology. 2008;26(Suppl) doi: 10.3816/CGC.2009.n.004. PP. Abstr 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007 Mar 22;26(13):1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 26.Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007 Oct 1;13(19):5883–8. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 27.Groen HJ. A phase II study of erlotinib and bevacizumab in patients with previously untreated stage IIIB/IV non-small cell lung cancer. Journal of Clinical Oncology. 2007;25(18S):7625. EFSaAD. [Google Scholar]
- 28.Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007 Oct 20;25(30):4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 29.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009 May 1;27(13):2231–7. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 30.Dickler MN, Rugo HS, Eberle CA, Brogi E, Caravelli JF, Panageas KS, et al. A phase II trial of erlotinib in combination with bevacizumab in patients with metastatic breast cancer. Clin Cancer Res. 2008 Dec 1;14(23):7878–83. doi: 10.1158/1078-0432.CCR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackman DM, Kindler HL, Yeap BY, Fidias P, Salgia R, Lucca J, et al. Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer. 2008 Aug 15;113(4):808–14. doi: 10.1002/cncr.23617. [DOI] [PubMed] [Google Scholar]
- 32.Nimeiri HS, Oza AM, Morgan RJ, Friberg G, Kasza K, Faoro L, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol. 2008 Jul;110(1):49–55. doi: 10.1016/j.ygyno.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bukowski RM, Kabbinavar FF, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007 Oct 10;25(29):4536–41. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009 Feb 20;27(6):843–50. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 35.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10(3):247–57. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyerhardt JA, Stuart K, Fuchs CS, Zhu AX, Earle CC, Bhargava P, et al. Phase II study of FOLFOX, bevacizumab and erlotinib as first-line therapy for patients with metastatic colorectal cancer. Ann Oncol. 2007 Jul;18(7):1185–9. doi: 10.1093/annonc/mdm124. [DOI] [PubMed] [Google Scholar]
- 37.Natale RB, Bodkin D, Govindan R, Sleckman BG, Rizvi NA, Capo A, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase ii study. J Clin Oncol. 2009 May 20;27(15):2523–9. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 38.Eisen T, Nathan P, Harper P, Wojtukiewicz M, Nicholson S, Bahl A, Tomczak P, Wagner A, Quinn D. Phase II trial of the oral multikinase inhibitor BAY 73–4506 as 1st-line therapy in patients with metastatic or unresectable renal cell cancer (RCC) European Journal of Cancer Supplements. 2009;7:424. HJ. [Google Scholar]
- 39.Tyagi P. Vatalanib (PTK787/ZK 222584) in combination with FOLFOX4 versus FOLFOX4 alone as first-line treatment for colorectal cancer: preliminary results from the CONFIRM-1 trial. Clin Colorectal Cancer. 2005 May;5(1):24–6. doi: 10.1016/s1533-0028(11)70162-1. [DOI] [PubMed] [Google Scholar]
- 40.Koehne C, Lin E, et al. Results of an interim analysis of a multinational randomized, double-blind, phase III study in patients (pts) with previously treated metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo (CONFIRM 2) J Clin Oncol. 2006:24. BE. [Google Scholar]
- 41.Major P, Lenz H, et al. Major P, Trarbach T, Lenz H, et al., editors. A meta-analysis of two randomized, double-blind, placebo-controlled, phase III studies in patients (pts) with metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK/ZK to determine clinical benefit on progression-free survival (PFS) in high LDH pts. J Clin Oncol. 2006;24:153S. TT. [Google Scholar]
- 42.Dempke WC, Heinemann V. Resistance to EGF-R (erbB-1) and VEGF-R modulating agents. Eur J Cancer. 2009 May;45(7):1117–28. doi: 10.1016/j.ejca.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 43.Punt CJ, Rodenburg CJ, et al. Randomized phase III study of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab in advanced colorectal cancer (ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG) Journal of Clinical Oncology. 2008;26(15S):LBA4011. doi: 10.1093/annonc/mdm607. TJ. [DOI] [PubMed] [Google Scholar]
- 44.Randolph J, Hecht EM, Tarek Chidiac, Carroll Scroggin, Christopher Hagenstad, David Spigel, John Marshall, Allen Cohn, David McCollum, Philip Stella, Robert Deeter, Seta Shahin, Rafael G. Amado A Randomized Phase IIIB Trial of Chemotherapy, Bevacizumab, and Panitumumab Compared With Chemotherapy and Bevacizumab Alone for Metastatic Colorectal Cancer. Journal of Clinical Oncology. 2009;27(5):672. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 45.Cutsem EV. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. Journal of Clinical Oncology. 2008;26(Suppl) Abstract 2. [Google Scholar]
- 46.Bokemeyer C, Hartmann JT, De Braud FG, Volovat C, Nippgen J, Stroh C, Celik I, Koralewski P. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer (mCRC) with FOLFOX with or without cetuximab. Journal of Clinical Oncology. 2008;26 IB. Abstract 4000. [Google Scholar]
- 47.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008 Jul 1;26(19):3204–12. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 49.Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009 Sep 1;27(25):4068–75. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 50.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008 Jul 24;359(4):366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]