Abstract
Here we show a unique example of male infertility conferred by a gene knock-out of the sperm-specific, pH-dependent SLO3 potassium channel. In striking contrast to wild-type sperm which undergo membrane hyperpolarization during capacitation, we found that SLO3 mutant sperm undergo membrane depolarization. Several defects in SLO3 mutant sperm are evident under capacitating conditions, including impaired motility, a bent “hairpin” shape, and failure to undergo the acrosome reaction (AR). The failure of AR is rescued by valinomycin which hyperpolarizes mutant sperm. Thus SLO3 is the principal potassium channel responsible for capacitation-induced hyperpolarization, and membrane hyperpolarization is crucial to the AR.
Keywords: SLO3, capacitation, sperm, acrosome reaction
Introduction
The SLO3 potassium channel is a closely related paralogue of the high conductance calcium-activated potassium channel, SLO1. Notably, however, SLO3 is expressed only in mammalian testes [1, 2] while SLO1 is widely expressed in many tissues and in many phyla, including invertebrates [3]. Furthermore, SLO3 channels are activated by alkalinization and lack the calcium sensors present in SLO1 channels [1, 3]. Mammalian sperm are unable to fertilize eggs immediately after ejaculation. They acquire fertilization capacity after residing for a certain time in the female genital tract through a process called capacitation. Capacitation is associated with an increase in intracellular pH (pHi)[4, 5] and hyperpolarization of the sperm plasma membrane [6; 7]. SLO3 channels are only found in mammals, the only class of vertebrate animals requiring capacitation prior to fertilization. This, in addition to its localization in spermatocytes and its activation by intracellular alkalinization, make the SLO3 potassium channel a likely candidate to account for the hyperpolarization that occurs during capacitation. NH4Cl induced increases in sperm pHi in patch clamp experiments have been shown to activate two major conductances, an outward K+ conductance postulated to be carried by SLO3 channels [8, 9] (also called Ksper [9]), and an inward conductance permitting Ca2+ entry carried by CatSper cation selective channels [10]. Here we show that SLO3 mutant sperm subjected to conditions normally used to capacitate sperm, undergo membrane depolarization, in contrast to wild-type (wt) sperm which undergo membrane hyperpolarization when subjected to the same conditions. The membrane depolarization seen in SLO3 mutant sperm could reflect a slight increase in the activity of the pH-dependent CatSper channel [10, 11] which is not offset by the increase in SLO3 K+ channel activity. Surprisingly, sperm from the SLO3 homozygous knock-out are deficient in the acrosome reaction (AR) even when exposed to A23187, an ionophore which directly permits calcium entry obviating the need to activate other Ca2+ channels. However, we observe that the mutant deficiency in the AR is largely rescued by the application of the K+ ionophore valinomycin in the presence of A23187. This suggests that, in addition to a rise in [Ca2+] in the cytosol, the AR may require an additional voltage-dependent process. These results clearly demonstrate that the SLO3 channel is a key player in the fertilization process and no other channel seems to be able to undertake its function.
Material and Methods
SLO3 knockout mouse
The SLO3 (Kcnu1) null mutation was synthesized by removal of the first two coding exons of the Kcnu1 gene (TG0050 TIGM). This removed the initiation codon and DNA sequence encoding the first and partial second membrane spanning domains. The next possible downstream site of translational initiation would, if used, result in the synthesis of a short out-of-frame polypeptide.
Animals
All procedures described herein were reviewed and approved by the Animals studies Committee of Washington University (St Louis) and were performed in accord with the NIH Guiding Principles of the care and use of laboratory animals.
Testis total membrane preparation and Western blot analysis
Testis from knockout (−/−), heterozygous (+/−) and wt (+/+) mice were processed in a Teflon/glass homogenizer in 250 mM sucrose, 10 mM HEPES, pH 7.40 plus protease inhibitor cocktail (0.01 U/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml benzamidine, 1 μg/ml antipain, 5 μg/ml trypsin inhibitor, 1 μg/ml chymostatin, 1 μg/ml pepstatin A, 1 mM PMSF). Suspensions were spun down at 10,000 g at 4 °C for 1 min; the supernatants were spun down 200,000 g at 4 °C for 45 min. Pellets were then dispersed by 1 % SDS and protein measured using BCA protein assay kit (Pierce, Rockfold, IL). 100 micrograms of total membrane proteins were treated 15 min at 60 °C in loading buffer plus 50 mM DTT and then loaded on a 7.5 % PAGE-SDS gel and subsequently transferred to a nitrocellulose membrane. The Western blot was probed with anti- monoclonal antibody (NeuroMab Anti-SLO3, clone N2/16) (2.8 ug/ml in 5 % low fat milk, PBS, 0.5 % Tween 20) and IRDye 680 donkey anti-mouse IgG (LI-COR Biosciences, Lincoln, NE) as secondary antibody (1 in 5000 dilution). Band intensities were measured using an Odyssey Infrared Imaging System (LI-COR Bioscience, Lincoln, NE).
Assay for Acrosome reaction
Caudal epididymal sperm were collected in HS medium (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 20 HEPES, 5 glucose, 10 lactic acid, 1 Na-pyruvate, supplemented with 15 mM of NaHCO3 and 5 mg/ml of bovine serum albumin at 37 °C. The swim up method [12] was used to separate sperm with > 90% motility. The sperm suspension was incubated for 10 min and the top 500 μl, was separated and capacitated by incubating at 37 °C for 40 min using the technique of Visconti et al [13]. AR was induced after capacitation in a 50 μl aliquot by 15 μM Ca2+ ionosphore A23187 and incubating at 37°C. The percentage of AR was determined 30 min later using Coomassie Blue staining [14]. At least 200 sperm were assayed for each experimental condition to calculate the percentage of AR.
Measurement of membrane potential
Mature sperm from caudal epididymides were capacitated in vitro as described above. After 40 min of incubation, the potential-sensitive dye 3,3′-dipropylthiocabocyanine iodine (DiSC3) was added to a final concentration of 1 μM. Fluorescence was monitored with a Varian Cary Eclipse Fluorescence Spectrophotometer at 620/670 nm excitation/emission wavelength pair [15]. Mithocondrial membrane potential was dissipated with 500 nM carbonyl cyanide m-chorophenylhydrazone (CCCP). Cell hyperpolarization decreases the dye fluorescence. Recordings were initiated after reaching steady-state fluorescence (1–3 min) and were converted to membrane potential as described previously [14].
Sperm motility analysis
Computer assisted sperm analysis (CASA) was performed to determine sperm motility characteristics. Sperm from three pairs of mature wt and SLO3 mutant mice of approximately four months of age were incubated in HS medium: and motility parameters were assessed using the HTM-IVOS Vs12 Integrated Visual Optical System (Hamilton-Thorne Research Danvers, MA, USA).
Electrophysiology
Whole cell currents recordings from testicular sperm were performed essentially as described [8]. The internal pipette solution contained in mM: 130 K-methansulphonate, 8 KCl, 20 KF, 2.5 CaCl2, 1 MgCl2, 5 EGTA 2 HEPES, pH 6 with NaOH. The external solution contained: 118 mM Na-methanesulfonate, 8 NaCl, 2.5 CaCl2, 2 KSO4 1 MgCl2 10 HEPES, and supplemented with glucose to achieve an osmolarity of 290 mOsm. Currents recorded with an Axopatch 200A amplifier, filtered at 2–5 kHz (4-pole Bessel filter), were digitized at 5–10 kHz using a PC equipped with a DigiData 1200 (Axon). Data capture and analysis were performed with pCLAMP software (Axon, Molecular Devices, Palo Alto, CA) and Origin 6 (Microcal Software, Northampton MA).
In vitro fertilization
Oocytes were recovered from superovulated female mice 13 hs after 10 Units of hCG (human chorionic gonadotropin) injection. Sperm were collected from cauda epididymides of wt and SLO3 mutant mice and capacitated in vitro at 37 °C for 1 hr. Capacitated sperm at a concentration of approximately 105 sperm/ml were co-incubated with eggs for 6 hrs and unbound sperm were subsequently washed away. After 24 hrs incubation the embryos were observed under light microscopy. The development of two-cell stage was considered successful fertilization. Some sperm were incubated with zona pellucida (ZP)-free eggs. The ZP-free eggs were prepared as follows. Oocytes were treated with hyaluronidase (10 mg/ml) for 10 min and then washed with media. Cumulus-free oocytes were transferred into acidic Tyrode’s solution (Sigma) for 30 s to dissolve the ZP. The oocytes were then washed twice and incubated with sperm.
Results
The SLO3 knockout mouse is infertile
We confirmed the absence of the SLO3 protein by Western blot analysis of testes from homozygous null SLO3− mutants which showed no detectable SLO3 protein (Fig. 1). Western blots of heterozygous SLO3+/SLO3− animals showed approximately half the level of protein. Homozygous null SLO3− mutant males were infertile. Matings of 8 homozygous SLO3− males with multiple wt females (2 females per male) produced no offspring in a period of approximately four months, while 9 out of 10 homozygous mutant females mated to wt males produced offspring during the same period. Heterozygous SLO3+/SLO3− animals of both sexes were fertile; 17/18 heterozygous males produced offspring; 24/25 heterozygous females produced offspring. The genotypes of offspring produced by intercrossing heterozygous (+/−) males and females exhibited roughly Mendelian proportions (46 +/+; 102 +/−; 41 −/−) which suggest that the mutation did not affect embryonic development. The presence of vaginal plugs noted in females mated to SLO3 homozygous mutant males suggested normal mating behavior in mutant males. Body and testes weight were not significantly different between wt and mutant SLO3 males. Neither SLO3 homozygous mutant adult mice nor sperm showed any morphological abnormalities, except for the fact that, after the capacitation incubating period, 70% of mutant sperm showed flagellar angularity between the midpiece and the principal piece, compared to only 30% of the wt sperm (n = 4 animals; and > 200 sperm/animal). Indeed, many of the mutant sperm had a “hairpin” configuration in which the midpiece was folded back 180° and aligned along the principal piece (see online supplementary videos). Such abnormalities are known to be associated with sperm having deficient osmoregulation and volume control [16], a condition not unexpected to result from the mutant ablation of a major ion channel.
Fig 1. SLO3 channel is absent in mutant mice as illustrated by western blot analysis.
Equal amounts of membrane protein from testis of wild type (wt)(+/+), heterozygous mutant (+/−) and homozygous mutant (−/−) mice were stained with anti-SLO3 antibody (methods). The SLO3 protein was undetectable in homozygous mutants (−/−) and present in approximately half the wt amount in heterozygous (+/−) mice.
SLO3 mutant sperm depolarize during capacitation
Intrinsic to the maturation of spermatozoa is the process of capacitation which includes a pHi raise [4, 5] and an accompanying membrane hyperpolarization [6]. In our initial characterization of the SLO3 potassium channel [1, 2] we noted that because of its pH sensitivity, the SLO3 channel would be a good candidate to alter sperm membrane potential during capacitation. More recent studies showing that there is a pH-sensitive potassium current in sperm [8, 9] that hyperpolarizes these cells upon exposure to external NH4Cl [9] have supported this hypothesis. However, none of these studies provide conclusive evidence that the SLO3 potassium channel is responsible for the pH-dependent potassium current. More importantly, since these experiments were not done under conditions normally used to capacitate sperm, (these experiments were conducted with more immature stages of the sperm: corpus epididymes and testicular sperm where the patch clamp experiments are accessible) there is no evidence that the SLO3 potassium channel plays a critical role during capacitation. A rigorous test of the hypothesis that the SLO3 potassium channel is responsible for membrane hyperpolarization during capacitation would be the observation that caudal epididymal sperm from SLO3 null mutant fail to undergo membrane hyperpolarization when exposed to physiological conditions that are well known to capacitate sperm of wt animals in vitro. Thus, we measured the membrane potentials of both wt and SLO3 null mutant sperm, before and after capacitation. In vitro capacitation was undertaken by incubating caudal epididymal sperm at 37°C for 1hr in HS medium (methods) supplemented with 15 mM NaHCO3, and 5mg/ml of bovine serum albumin. Membrane potential was measured using the potential-sensitive dye 3,3′-dipropylthiocabocyanine iodine as described ( see methods). The results of these measurements were striking; unlike wt sperm, SLO3 null mutant sperm failed to hyperpolarize their membrane potential after capacitation. Indeed, they showed a depolarization (Fig. 2). Notably, before capacitation no significant difference was evident between the membrane potential of the mutant and wt sperm. Only after the in vitro capacitation process occurred did differences become evident. This suggests that capacitation conditions trigger a membrane conductance that hyperpolarizes the sperm. Although different ion channels have been claimed as candidates to produce such hyperpolarization [14, 17, 18], we found that the removal of SLO3 channels alone is sufficient to remove the hyperpolarization. The membrane depolarization seen in SLO3 mutant sperm after capacitation may be the consequence of a slight increase in the activity of the pH-dependent CatSper channel [10, 11]
Fig 2. SLO3 mutant sperm lack capacitation induced hyperpolarization and, in contrast to wt, depolarize after capacitation.
Membrane potentials before and after in vitro capacitation are illustrated for wt and SLO3 homozygous mutant (−/−) sperm (error bars show SEM). Sperm membrane potential was measured using the fluorescence dye DiSC3 as described in methods. Uncapacitated wt sperm have a membrane potential value of −47 ± 3.3 mV and hyperpolarize to −62.2 ± 2.9 mV (n =4) after capacitation. Uncapacitated SLO3 mutant sperm have a membrane potential of −48 mV ± 3.9 mV and depolarize during capacitation to −38.8 ± 2.8 mV (n = 6). Membrane potential differences between wt and mutant sperm are significant for all pairwise comparisons at the 0.05 % level (paired t-test).
SLO3 mutant sperm do not have pH-sensitive potassium currents
By whole cell voltage clamp experiments of mutant and wt sperm we showed that the SLO3 gene encodes the potassium channels responsible for the pH-sensitive current present in testicular sperm. To demonstrate this we used the technique of Kirichock et al, 2006 [11]. In these experiments we used testicular mouse sperm, which are less mature than epididymal sperm and have a larger cytoplasmic droplet. As undertaken previously [8, 9], internal alkalinization was achieved by the addition of NH4Cl. The results of these experiments show that, in contrast to wt sperm, SLO3 null mutant sperm lack the alkalinization-induced increase in outward current (Fig. 3). In addition there was a qualitative difference noted between wt and mutant; outward currents in wt sperm had a higher noise level suggestive of the presence of high conductance channels, that was not observed in the outward currents recorded from SLO3 null mutant sperm.
Fig 3. Mutant SLO3 sperm lack pH sensitive K+ currents.
Representative whole cell voltage clamp recordings and current/voltage relationships from testicular sperm are shown for wt (A) and SLO3 mutant sperm (B). Whole cell currents were evoked by 10 mV voltage steps from −80 to +60 mV at a holding potential of −50 mV. Currents are shown in control conditions and after bath application of 40 mM NH4Cl. In control experiments with wt sperm we noted that the relative increase in outward current amplitude after alkalization with NH4Cl was somewhat smaller than that reported in a previous paper (Navarro et al, 2007). However this may be due to the different stages of sperm that were analyzed; in our study testicular sperm were used, while in the previous study, more mature epididymal sperm were used. The bar graphs in (C) show a comparison of outward current amplitudes evoked at +60 mV for wt (n=4) and mutant SLO3 sperm (n=4), before (control) and after (NH4Cl) exposure to 40 mM NH4Cl.
SLO3 mutant sperm have impaired progressive motility and acrosome reaction (AR)
As expected, SLO3 null mutant sperm, which undergo abnormal depolarization after capacitation, were markedly deficient in progressive motility relative to sperm from wt animals (Fig. 4). Perhaps the most unexpected result was the observation that sperm from the SLO3 homozygous knockout are deficient in the AR when treated with the calcium ionophore A23187 which should bypass the need to activate other Ca2+ channels. A23187 potently elicits the AR in wt sperm (Fig. 5). These results are consistent with our in vitro fertilization results which show that even after removal of the ZP, the mutant sperm are unable to fertilize the eggs (see fig 6). However, we have noted that the mutant AR phenotype is substantially rescued by the inclusion of the K+ ionophore valinomycin which is able to substitute for the SLO3 mutant deficit in potassium ion conductance and result in membrane hyperpolarization in SLO3 mutant sperm.
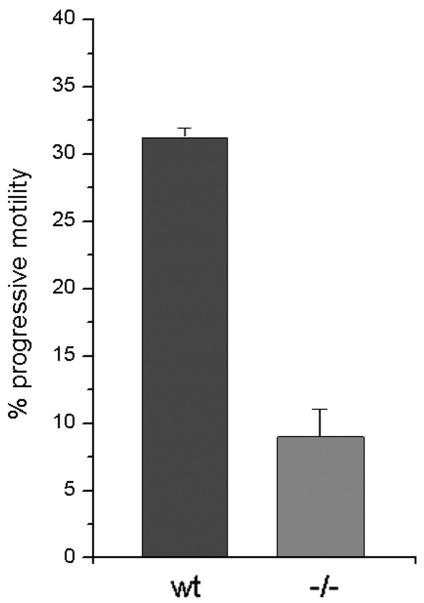
Fig 4. Mutant SLO3 sperm display less progressive motility.
Percentage of progressive motility is shown for wt and mutant SLO3 −/− sperm. Sperm showing progressive motility were counted as a percentage of total sperm using computer assisted sperm analysis (CASA). While 31.3 ± 0.7 % of wt sperm showed progressive motility, only 9 ± 2.1 % of mutant sperm showed progressive motility for both wt (n=3) and mutant (n=3) animals; approximately 1000 sperm were counted for each animal). Differences were significant (p < 0.05).
Fig 5. Acrosome reaction (AR) induced by A23187 is impaired in SLO3 mutant sperm, & rescued by valinomycin.
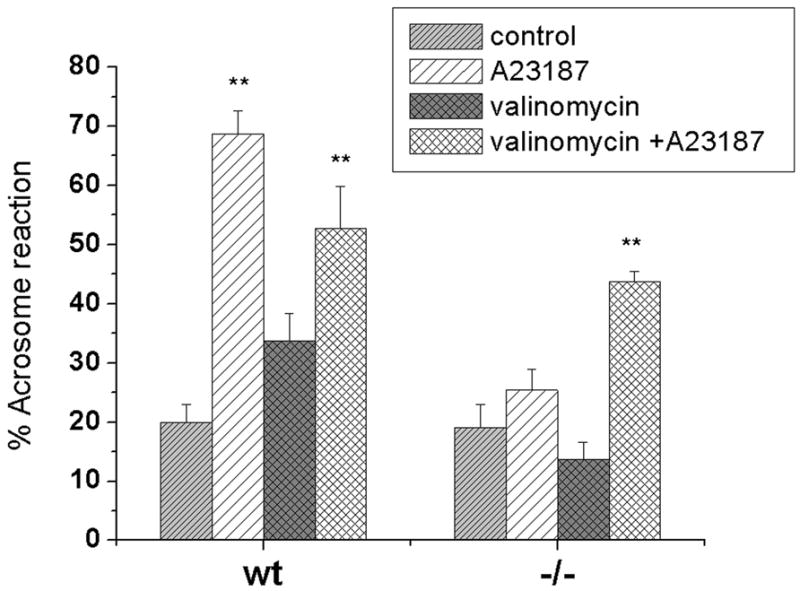
The percentage of spontaneous AR is similar in wild-type (WT) and SLO3 mutant sperm after exposure to capacitating conditions. However, the addition of A23187 greatly increases AR in WT (p≪.001) but has no significant effect on SLO3 mutant sperm (p=0.09). The addition of valinomycin, however, to A23187 greatly increases the AR reaction in SLO3 mutant sperm (p<0.02). 200 sperm for each condition were counted as acrosome reacted (no staining in the acrosomal region) or as acrosome intact (dark blue staining over the acrosomal region). [Numbers of animals for each condition: WT spontaneous=13; WT A23187=9; WT valinomycin=3; WT A23187+valinomycin=3; SLO3 mutant (SLO3): SLO3 spontaneous=11; SLO3 A23187=8; SLO3 valinomycin=3; SLO3 valinomycin=A23187=3]
Fig 6. Sperm from SLO3 mutants are not capable of fertilization under in vitro conditions.
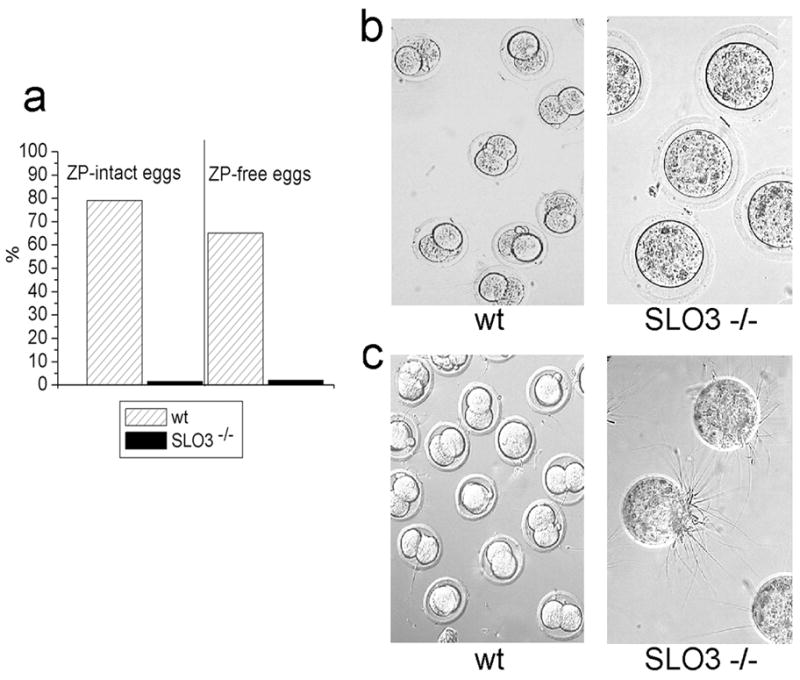
Results of in vitro fertilization (IVF) experiments performed with wt(n = 2 animals) and SLO3 mutant sperm from 2 animals a. % of two stage cell embryos observed after IVF using wt or SLO3 mutant sperm. Capacitated wt and mutant SLO3 mutant sperm were incubated with superovulated oocytes with ZP intact from C57BL/6 wt mice (145 and 134 eggs respectively), and with the ZP removed (94 and 88 eggs respectively). The percentage (respectively for wt and SLO3 mutant sperm) of two-stage embryos was: 79% (114/145) and 1.5% (2/134) respectively in intact-ZP eggs, and 65% (61/94) and 2% (2/88) in ZP-free eggs. b. representative micrographs of intact-ZP eggs after 24 hs of fertilization with wt or SLO3 mutant sperm. c. representative micrographs of eggs with ZP removed after 24 hrs of fertilization with wt or mutant SLO3 sperm.
Discussion
In our initial characterization of the SLO3 potassium channel [1, 2] we noted that the pH sensitivity of the SLO3 channel made it a good candidate to alter sperm membrane potential during capacitation. More recent studies have supported this hypothesis [8, 9]. However, those experiments were not done under conditions normally used to capacitate sperm and several ion channels have been claimed as candidates to produce such hyperpolarization [14, 17, 18]. However, we found that the genetic removal of the SLO3 gene alone is sufficient to completely remove hyperpolarization in capacitating conditions, and indeed, produce a slight depolarization instead which might be the consequence of an increase in the activity of the pH-dependent CatSper channel [10, 11, 19] or the activation of high-V-dependent Ca2+ channels present in sperm [20, 21]. As a consequence of the depolarization seen in SLO3 mutant mouse sperm, the Ca2+ driving force would be reduced and consequently calcium influx into the flagellum will be reduced impairing flagellar motility, thus explaining the abnormalities of motility we see in these mutants [10, 19, 22, 23].
The most unanticipated defect, however, is the deficiency in the AR when SLO3 mutant sperm are exposed to the Ca2+ ionophore A23187. Although control of membrane potential has been hypothesized to be a key step in calcium entry [24, 25], the addition of A23187 should bypass the need for calcium entry through any native calcium plasma membrane channel. Yet SLO3 mutant sperm still fail to achieve exocytosis under these conditions. The fact that valinomycine rescue the AR phenotype seems to suggest the presence of an additional membrane potential dependent process in addition to calcium ion entry. One possible candidate is the novel mammalian sperm specific Na+/H+ exchanger, sNHE, which contains a putative voltage sensor [26]. Overall our results highlight the involvement of SLO3 channels in multiple mechanisms of male fertility and may have revealed the presence of an essential voltage-sensitive process not previously known. These results also suggest several compelling reasons to consider the SLO3 channel an attractive pharmacological target for male contraception.
Legend to SI video files: File designation mutant HR.avi indicates sperm of SLO3 homozygous knockout mutant; wt HR.avi indicates wild-type sperm. Sperm were collected from cauda epididymides of wild-type and SLO3 −/− mutant mice and capacitated in vitro at 37 °C for 1 hr as indicated in Materials and Methods. Mutant sperm show a high percentage of flagellar angularity between the midpiece and the principal piece (see text). In addition, hyperactivation was not evident in mutant sperm. Each of the video files contains 30 frames (15 FPS).
Supplementary Material
Acknowledgments
NIH Grants: 1R21HD056444-01A1 (to CS) and R01 NS0661871-01(to LS) and CONACyT-Mexico, 49113 (to AD), DGAPA/UNAM IN211809 (to AD), and the Wellcome Trust (to AD). We thank Dr. Kelle Moley, Erica Schoeller, Julie Schmitt, Janet Willard, Mia Wallace, Harry Fraser, Stephanie Higgins and Dr. Paul Schlesinger for technical assistance and Travis Hage for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–3516. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 2.Santi CM, Butler A, Kuhn J, Wei A, Salkoff L. Bovine and mouse SLO3 K+ channels: Evolutionary divergence points to a RCK1 region of critical function. J Biol Chem. 2009;284(32):21589–98. doi: 10.1074/jbc.M109.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salkoff L, Butler A, Ferreira G, Santi CM, Wei A. High conductance Potassium Channels of the Slo Family. Nature Reviews Neuroscience. 2006;5:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 4.Vredenburgh-Wilberg WL, Parrish JJ. Intracellular pH of bovine sperm increases during capacitation. Mol Reprod Biol. 1995;40 (4):490–502. doi: 10.1002/mrd.1080400413. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na+, Cl−, and HCO3− dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996;173:510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Y, Clark EN, Florman HM. Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol. 1995;71(2):554–63. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- 7.Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM. Control of the low voltage activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci U S A. 1999;96(12):6757–62. doi: 10.1073/pnas.96.12.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-López P, Santi CM, Treviño CL, Ocampo-Gutiérrez AY, Acevedo JJ, Alisio A, Salkoff LB, Darszon A. Mouse sperm K+ currents stimulated by pH and cAMP possibly encoded by Slo3 channels. BBRC. 2009;381:204–209. doi: 10.1016/j.bbrc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm. Proc Natl Acad Sci U S A. 2007;104(18):7688–92. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439(7077):737–40. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 12.Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003 Nov 14;1:108. doi: 10.1186/1477-7827-1-108. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf GS, Tezón JG. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod. 1999;61(1):76–84. doi: 10.1095/biolreprod61.1.76. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz-Garay C, De la Vega-Beltrán JL, Delgado R, Labarca P, Felix R, Darszon A. Inwardly rectifying K(+) channels in spermatogenic cells: functional expression and implication in sperm capacitation. Dev Biol. 2001;234(1):261–74. doi: 10.1006/dbio.2001.0196. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa F, Darszon A. Mouse sperm membrane potential: changes induced by Ca2+ FEBS Lett. 1995;372(1):119–25. doi: 10.1016/0014-5793(95)00962-9. [DOI] [PubMed] [Google Scholar]
- 16.Cooper TG, Yeung C, Wagenfeld A, Nieschlag E, Poutanen M, Huhtaniemian I, d Sipilä P. Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol. 2004;216 (1–2):55–63. doi: 10.1016/j.mce.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo JJ, Mendoza-Lujambio I, de la Vega-Beltrán JL, Treviño CL, Felix R, Darszon A. KATP channels in mouse spermatogenic cells and sperm, and their role in capacitation. Dev Biol. 2006 Jan 15;289(2):395–405. doi: 10.1016/j.ydbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-González EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, López-González I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem. 2006;281(9):5623–33. doi: 10.1074/jbc.M508172200. [DOI] [PubMed] [Google Scholar]
- 19.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wennemuth G, Westenbroek RE, Xu T, Hille B, Babcock DF. Cav2.2 and Cav2.3 (N-and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J Biol Chem. 2000;275:21210–21217. doi: 10.1074/jbc.M002068200. [DOI] [PubMed] [Google Scholar]
- 21.Babcock DF, Pfeiffer DR. Independent elevations of cytosolic Ca2+ and pH of mammalian sperm by voltage dependent and pH-sensitive mechanisms. J Biol Chem. 1987;262:15041–15047. [PubMed] [Google Scholar]
- 22.Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A. 2003;100(25):14864–8. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marquez, Suarez Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca influx. Biol Reprod. 2007;76:660–665. doi: 10.1095/biolreprod.106.055038. [DOI] [PubMed] [Google Scholar]
- 24.Florman HM, Corron ME, Kim TDH, Babcock DF. Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol. 1992;152:304–314. doi: 10.1016/0012-1606(92)90137-6. [DOI] [PubMed] [Google Scholar]
- 25.Florman HM. Sequential focal and global elevations of sperm intracellular Ca2+ are initiated by the zona pellucida during acrosomal exocytosis. Dev Biol. 1994;165:152–164. doi: 10.1006/dbio.1994.1242. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, King SM, Quill TA, Doolitle LK, Garbers DL. A new sperm-specific N+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.