Abstract
Objective
To track changes in the proportion of persons ages 18–64 with systemic lupus erythematosus (SLE) who were employed from diagnosis through 2004, to estimate changes in annual work hours during this time, and to describe risk factors for work loss among those employed at diagnosis.
Methods
A structured telephone survey was administered to a cohort of 982 persons with SLE, which was assembled between 2002 and 2004. Of the 900 enrolled in 2002–2003, 832 (92%) were re-interviewed in 2004. We tabulated the proportion employed at diagnosis, at baseline interview, and at followup in 2004. Among individuals employed at each time frame, we estimated the hours of work per year. We then used the Kaplan-Meier method to estimate time until work loss among individuals employed at diagnosis and Cox proportional hazards regression to describe the risk factors for such work loss.
Results
Between diagnosis and followup interview, the proportion employed declined from 74% to 54%. Over the same period, hours of work per year declined by 32.2% among all individuals with a work history, but by only 1% among those continuously employed. Among individuals working at diagnosis, the proportion employed declined by 15% and 63% after 5 and 20 years, respectively. Demographics (age, sex, and education) and work characteristics (physical and psychological demands of jobs and level of control) were the principal determinants of work loss.
Conclusion
Total cessation of employment, rather than reduced hours among employed persons, accounts for most of the decline in annual work hours among persons with SLE.
Keywords: Systemic lupus erythematosus, Employmentm, Productivity
INTRODUCTION
Work disability, particularly in chronic diseases with onset in the early part of a person’s work life, has profound repercussions for the individual and family, including loss of self-esteem, opportunity to socialize with one’s peer group, and both current earnings and the ability to accumulate assets for retirement. In the rheumatic diseases, work loss accounts for the majority of the economic costs of illness (1). Most of the research on work disability in the rheumatic diseases has focused on rheumatoid arthritis (2–8). Despite onset at a young age and the potential severity of systemic lupus erythematosus (SLE), there have been relatively few studies of employment among persons with SLE. These few studies indicate that work limitation and work loss are common (9–16). However, these studies have either had small sample sizes or respondents exclusively sampled from tertiary care centers or both.
The present report describes the work dynamics of persons with SLE from the year of diagnosis through 2004. Data for the study were derived from a large and heterogeneous US cohort of persons with SLE. The specific goals were to track the change in the proportion of individuals who worked from diagnosis through the first and second annual interviews, to estimate the change in annual work hours over these time frames, to describe the pattern of retrenchment from employment among those working at diagnosis, and to estimate the risk factors for work loss.
MATERIALS AND METHODS
Enrollment and followup
The data source for the present study was the University of California, San Francisco (UCSF) Lupus Outcomes Study (LOS). Participants in the LOS had formerly participated in a study of genetic risk factors for SLE outcomes (17,18). These participants became eligible for the LOS upon completion of their involvement in the genetics study. Between September 2002 and August 2003, we sought to enroll the 1,075 persons with SLE who had participated in the genetics study into the LOS. We were able to contact 897 individuals and conduct initial interviews with 711 (66% of the 1,075 and 79% of the 897). Between August 2003 and December 2004, another 440 participants in the lupus genetics study became eligible for the LOS, of whom we contacted 360 (82%) and interviewed 271 (61% of the eligible group and 75% of those contacted). Overall, 1,519 persons with SLE who had been in the genetics study were eligible for the LOS, 1,265 (83%) were successfully contacted, and 982 had at least an initial interview by the end of 2004 (65% of all persons eligible and 78% of those successfully contacted).
The 982 LOS participants were recruited from both clinical and community-based sources, including UCSF-affiliated clinics (22%); non-UCSF rheumatology offices (11%); lupus support groups and conferences (26%); and newsletters, Web sites, and other forms of publicity (41%). Therefore, 66% of the LOS participants were derived from nonclinical sources.
In 2004, we conducted a second annual interview with 832 (92%) of the 900 LOS participants initially enrolled prior to the end of 2003. Of the 68 participants we did not re-interview, 11 (1%) had died, 11 (1%) were medically unable to participate in an interview, 22 (2%) declined further participation, and 24 (3%) were lost to followup. Re-interview rates were significantly higher among women than men (93% versus 83%), whites than nonwhites (94% versus 88%), and persons with higher rates of formal education, but there was no difference in followup rates by poverty status or age. Individuals reporting end stage renal disease, pulmonary manifestations, and longer durations of disease were significantly less likely to be re-interviewed, but there was no difference in followup rates by other health status measures.
The 982 LOS participants were derived from 955 distinct families, or ~1.03 per family stratum. This small nesting effect reduced the effective sample size to 962 observations. Because the design effect is so small, we did not incorporate the design effect into the analyses described below.
Data
The LOS database includes information abstracted from the medical records of participants as part of the prior genetics study. The medical record abstraction was conducted by trained chart reviewers who were either rheumatologists or registered nurses working under a rheumatologist’s supervision. The information in the medical records was used to assess whether individuals met the American College of Rheumatology criteria for SLE (19,20).
The principal source of data for the LOS was a structured 1-hour telephone interview conducted by trained survey workers. The content of the LOS survey is summarized in Table 1. As can be seen from the table, the survey used well-validated items covering the following major domains: demographics and socioeconomic status, SLE status, general health and functional status, mental and cognitive status, health care utilization, and employment. In the baseline survey, identical employment items were administered for the year in which SLE was first diagnosed and for the year prior to the interview. In the followup interview, employment items were only administered for the year prior to that interview.
Table 1.
Content of Lupus Outcomes Study annual telephone survey*
| Type of variable | Examples and source |
|---|---|
| Demographics/socioeconomic characteristics | Age, sex, race/ethnicity, marital status, education, family income, and household size, modified from the National Health Interview Survey (31) |
| Status of SLE | Systemic Lupus Activity Questionnaire (32), recent disease manifestations, checklist of medications |
| General health and functional status | Checklist of chronic conditions not related to SLE, current height and weight, pregnancy history, smoking status (31); SF-12 (33), SF-36 physical function subscale (34) |
| Mental health and cognitive status | CES-D (35), Medical Outcomes Study Cognitive Function battery (36), Hopkins Verbal Learning Test (37), Perceived Stress (38), Cognitive Symptoms Inventory (39) |
| Health insurance coverage | Type and source of coverage; specific aspects of coverage such as copayments, availability of specialist care, and drug coverage, developed for the Medical Expenditures Panel Survey (40,41) |
| Health care utilization | Name and location of primary SLE physician; doctor visits in the past 12 months, drugs and other treatments, emergency department use, hospitalization, and surgery over the preceding 12 months (42,43) |
| Employment | Employment status, hours and weeks of work, and occupations and industries (27); work schedules (44); physical and cognitive demands of jobs (45); psychological demands of jobs and control over work (46) |
SLE = systemic lupus erythematosus; SF-12 = Short Form 12; SF-36 = Short Form 36; CES-D = Center for Epidemiologic Studies Depression Scale.
Human subjects review
The enrollment and data collection protocol was approved by the UCSF Committee on Human Research.
Analysis
Inclusion and exclusion criteria
We report the results of 2 types of analyses below. In the first analysis, we examined change in employment status over time of the 878 persons between ages 18 and 64 with a history of employment for pay or profit at some point in their lives. In the second analysis, we evaluated the time until work cessation among the 673 participants employed at diagnosis of SLE. In this analysis, persons currently age ≥65 years were included; they become censored observations if they reached age 65 before leaving work.
Assessing impact of attrition
Of the 878 study participants with a work history who were between 18 and 64 years of age, 804 had completed their initial LOS interviews by the end of 2003 and were therefore eligible for re-interviews in 2004. Of these 804, re-interviews were conducted with 748 (93%). Because of the potential impact of differential rates of followup, we developed attrition weights (21). We used the attrition weights only for those analyses that specifically looked at change between the 2003 and 2004 interviews. The analyses of time until cessation of employment did not incorporate attrition weights because the statistical techniques used for these analyses (described below) are designed to account for loss to followup or death.
Specific analyses
Description of employment changes
For the description of the study population, we determined frequency distributions (for categorical variables) and means and SDs (for continuous variables). We then used contingency table analysis to show transitions in employment status between the year of diagnosis and the years of baseline and followup interviews.
Three measures of productivity were used: hours of work per week; weeks of work per year; and the product of these 2 measures, hours of work per year. We also determined the percentage change in these measures from the year of diagnosis to the followup year. The foregoing measures were assessed for 3 strata: all individuals who have ever worked; those working at diagnosis (n = 627), first interview (n = 477), and followup interview (n = 405); and those working at all 3 time frames (n = 314). The first strata demonstrated the loss of productivity due to cessation and reduction of work, and the second and third demonstrated productivity losses among all employed individuals at each time frame and among those continuously employed, respectively.
Estimate of duration between diagnosis and work loss
We first used the Kaplan-Meier method (22) to estimate the duration of time until work loss occurred among individuals working at the time of SLE diagnosis. We then used Cox proportional hazards regression (23) to estimate risk factors for work loss among the 673 persons with SLE employed in the year of diagnosis. In this analysis, right censorship occurred if an individual who was interviewed in the baseline year was not re-interviewed in the followup year, if an individual reached age 65 without having experienced work loss, or if an individual was still working as of the followup year.
In the LOS questionnaire, respondents report the approximate interval during which they stopped working. Accordingly, the exact date of work cessation is not available. To account for this in both the Kaplan-Meier and Cox models, we used the method outlined by Lindsey and Ryan for interval censored data (24). Based on their method, we calculated the interval during which work cessation would have occurred based on the number of years following diagnosis. We then created 3 variables to capture the beginning, middle, and end point of that interval, and we performed survival analyses with each of these variables in turn. Because the results did not differ appreciably among the 3 end points, we report the results from the principal analysis using the midpoint of the interval.
For the survival analyses, from the original 982 participants, we excluded 288 (29%) who were not employed at diagnosis. An additional 21 (2%) participants were excluded because they were either ≥65 years of age at the time of diagnosis, turned 65 during the work loss interval described above, or did not provide information as to when they last worked, leaving 673 for these analyses.
In the proportional hazards regressions, we included disease duration, demographic characteristics, and characteristics of the job held at diagnosis as independent variables. The demographic characteristics included age by category, female sex, white versus nonwhite race, education by category, and marital status. The work characteristics included the number of physical demands on the job (walking; using stairs or inclines; sitting for long periods; stooping, crouching, or kneeling; lifting and carrying weights of 10 and/or 50 pounds; repeating the same hand motion at least 30 times per hour; bending over or twisting around; and using hand tools); the number of cognitive demands on the job (concentrating for long periods, interacting with other individuals, using computers); the conjoint presence of high levels of psychosocial job demands and low levels of control, with other combinations of demands and control as references; occupations (indicator variables for administrative support and clerical occupations; crafts and operatives; and sales, technical, and services occupations, with professional occupations as the reference); and industries (indicator variables for extractive, construction, and manufacturing industries; personal, business, and repair services; and a miscellaneous category including government, transportation/utilities, wholesale/retail trade, finance, insurance, and real estate; with professional services as the reference).
In the proportional hazards regressions, we were unable to include medical characteristics, except duration of disease, as independent variables because such characteristics may not have been present at the time of diagnosis, may not have preceded the loss of work, and/or the time at which the characteristics were manifest may not have been accurately reported. For example, significant symptoms may have developed after work cessation had occurred.
RESULTS
The demographic characteristics of all LOS participants and of those between ages 18 and 64 years who had ever been employed and who were employed in the year of diagnosis are shown in Table 2. Except for a higher proportion of individuals who were ever employed being below the poverty level compared with those employed at diagnosis (because the former group presumably includes those rendered poor by work cessation), there were no substantial differences in the demographic characteristics of the 2 groups. On average, LOS participants were in their mid-forties. More than 90% were women, ~60% were married, and >60% had completed at least some college, with approximately 1 in 7 having some postbaccalaureate education.
Table 2.
Demographic and medical characteristics of Lupus Outcomes Study participants*
| Age 18–64 at baseline |
|||
|---|---|---|---|
| Characteristic | All study participants (n = 982) | Ever employed (n = 878) | Employed at diagnosis (n = 646) |
| Demographic characteristics | |||
| Age, mean ± SD years | 47.0 ± 13.2 | 44.8 ± 11.0 | 45.3 ± 10.6 |
| Female sex | 90.9 | 91.7 | 91.8 |
| White | 66.3 | 65.6 | 66.3 |
| Married | 60.8 | 61.1 | 63.6 |
| Education | |||
| High school or less | 21.1 | 19.7 | 18.6 |
| Some college | 41.8 | 41.2 | 42.0 |
| College | 23.5 | 25.2 | 24.8 |
| Postgraduate | 13.7 | 13.9 | 14.7 |
| Below poverty level† | 11.9 | 11.8 | 8.7 |
| Medical characteristics | |||
| Fair or poor health | 41.3 | 41.2 | 41.0 |
| Duration of SLE, mean ± SD | 12.8 ± 8.6 | 12.5 ± 8.4 | 12.3 ± 8.3 |
| Symptoms | |||
| Weight loss | 18.1 | 18.7 | 17.3 |
| Fatigue | 76.2 | 76.5 | 76.9 |
| Fevers | 17.9 | 18.6 | 18.6 |
| Muscle pain | 68.2 | 68.6 | 69.0 |
| Muscle weakness | 53.6 | 52.9 | 52.6 |
| Joint stiffness | 80.4 | 80.3 | 80.0 |
| Joint swelling | 53.9 | 54.5 | 55.1 |
| Organ manifestations | |||
| Severe kidney problems | 8.2 | 8.5 | 7.7 |
| Lung problems | 39.8 | 39.8 | 38.9 |
| Clotting disorders | 30.9 | 30.2 | 30.5 |
| Vision loss | 10.3 | 11.1 | 11.6 |
| Seizures | 17.2 | 17.5 | 15.8 |
Values are the percentage unless otherwise indicated. SLE = systemic lupus erythematosus.
Missing for 64 subjects.
There were no substantial differences in the current medical characteristics of participants who had ever been employed and those employed at diagnosis (Table 2). The LOS participants had had SLE for an average of >12 years. More than 40% of the LOS participants reported being in fair or poor health. More than three-quarters reported joint stiffness and fatigue due to SLE in the past 3 months, whereas more than half reported muscle pain and weakness and joint swelling. The proportion of participants who reported weight loss and fever was much smaller. With respect to specific organ manifestations, slightly less than 1 in 10 reported end stage renal disease (defined by receipt of dialysis or transplantation); approximately 1 in 9 reported vision loss, 1 in 6 a history of seizures, 1 in 3 a clotting disorder, and 4 in 10 a pulmonary manifestation.
The transitions in employment status between the year of diagnosis (~12 years ago on average), the baseline interview, and the followup interview are displayed in Figure 1. Overall, the proportion employed declined from 74% in the diagnosis year to 55% at the time of the baseline interview and 54% a year later. At each time interval, the proportion of individuals who entered work was approximately equal to the proportion who left work. Therefore, whereas 40% of participants working in the diagnosis year had stopped working by the baseline interview, 41% of those not working at diagnosis had started by the baseline interview; similarly, 8% of those working at the baseline interview had stopped working by the following year, and the same proportion started working in the latter year.
Figure 1.
Employment status in diagnosis year, baseline, and followup among 748 respondents under age 65, with employment history, who were followed through wave 2. Adjusted for attrition in wave 2.
The productivity losses associated with SLE among LOS participants are shown in Table 3. Among individuals ever employed, hours of work per week declined by 35.4%, from 29.1 to 18.8, between the year of diagnosis and 2004; weeks per year declined by 23.7%, from 31.6 to 24.1; and hours per year declined by 32.2%, from 1,287 to 873.
Table 3.
Productivity losses from diagnosis year to followup interview among Lupus Outcomes Study participants with work history, age 18–64 at baseline interview*
| No. | Hours/week | Weeks/year | Hours/year | |
|---|---|---|---|---|
| All participants ever employed | ||||
| Diagnosis year | 859 | 29.1 (27.6–30.5) | 31.6 (30.1–33.1) | 1,287 (1,215–1,358) |
| Baseline | 864 | 19.0 (17.6–20.3) | 24.1 (22.5–25.7) | 864 (798–931) |
| Followup | 742 | 18.8 (17.3–20.3) | 24.1 (22.3–25.8) | 873 (799–948) |
| % change, diagnosis year to followup | −35.4 | −23.7 | −32.2 | |
| Participants employed at specific time period | ||||
| Diagnosis year | 627 | 39.8 (38.7–40.9) | 43.3 (42.3–44.4) | 1,763 (1,695–1,830) |
| Baseline | 477 | 34.4 (33.1–35.7) | 43.6 (42.3–44.9) | 1,566 (1,490–1,641) |
| Followup | 405 | 34.8 (33.1–35.7) | 44.6 (43.2–45.9) | 1,616 (1,530–1,701) |
| % change, diagnosis year to followup | −12.6 | 3.0 | −8.3 | |
| Participants employed at all time periods (n = 314)† | ||||
| Diagnosis year | 314 | 39.2 (37.7–40.8) | 44.3 (42.9–45.7) | 1,775 (1,676–1,872) |
| Baseline | 314 | 35.4 (33.9–36.9) | 45.8 (44.4–47.2) | 1,671 (1,582–1,760) |
| Followup | 314 | 37.1 (35.4–38.7) | 46.5 (45.2–47.9) | 1,758 (1,665–1,851) |
| % change, diagnosis year to followup | −5.4 | 5.0 | −1.0 | |
Values are the mean (95% confidence interval) unless otherwise indicated. Followup interview results adjusted for attrition. Nineteen respondents were missing data for hours and/or weeks of work during diagnosis year, 14 respondents were missing data for hours and/or weeks of work for wave 1, 6 respondents were missing data for hours and/or weeks of work for wave 2.
Excludes 30 respondents with missing data in any time period.
Among persons employed at each time period (Table 3), hours per week declined between the year of diagnosis and followup interview by 12.6%, weeks per year actually increased by 3.0%, and hours per year declined by 8.3%. After multiplying the hours per year by the number of persons working in each time frame, there were 1,105,401 total hours worked among those employed in the diagnosis year, 746,982 hours at the baseline interview, and 654,480 a year later (data on annual work hours not shown). Among the 314 persons continuously employed from the year of diagnosis through followup interview (Table 3), hours per week declined by only 5.4%, weeks per year increased by 5.0%, and hours per year decreased slightly by 1.0%.
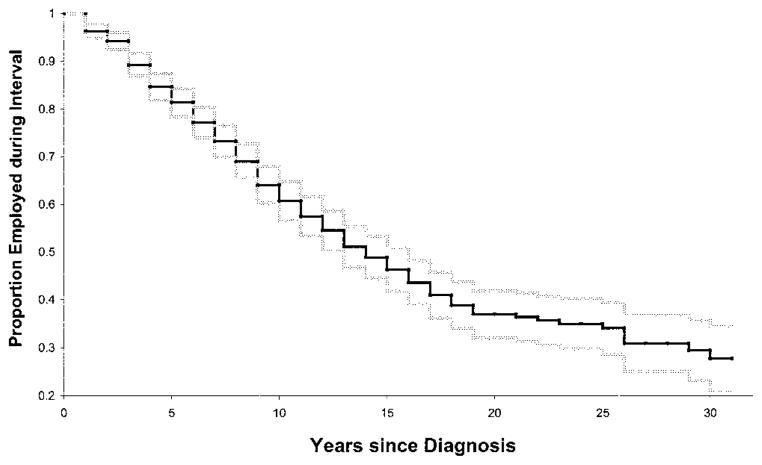
An estimate from the Kaplan-Meier analysis of the duration of time in years until cessation of work among participants employed at diagnosis is provided in Figure 2. By 5 years after diagnosis, 15% (95% confidence interval [95% CI] 13–18%) had stopped working; by 10, 15, and 20 years, ~36% (95% CI 32–40%), 51% (95% CI 47–56%), and 63% (95% CI 58–68%), respectively, had stopped working. Almost three-quarters had stopped working by 30 years after diagnosis. Note that, because of the large sample size, the estimates of the proportion working at each interval were estimated with great precision up to 25 years after diagnosis.
Figure 2.
Proportion employed (95% confidence interval), by year since diagnosis, among persons with systemic lupus erythematosus under age 65 who were employed at diagnosis.
The hazard ratios and 95% CIs for the variables associated with work loss from the Cox regression analysis are shown in Table 4. The results of 3 models are presented: 1 in which only duration of disease and demographics were included as independent variables; 1 in which only work characteristics were included; and 1, the full model, in which both were included. As indicated by the model chi-square values, both of the first 2 models were significantly related to the hazard of work loss. A comparison of the full model chi-square value with those of the smaller models indicates that both sets of variables contributed significantly to the explanation of the risk of work loss. The individual parameter estimates, however, did not change very much between the smaller models and the full model; therefore in the text we report the results from the latter model only. Longer duration of disease was associated with a reduced risk of work loss. Consistent with expectations, younger workers experienced a substantially reduced risk of work loss. Women had a substantially elevated risk of work loss. Education was strongly and inversely related to the risk of work loss, with individuals with a high school education or less experiencing more than a 3-fold risk of work loss relative to those with postgraduate education, and even those with some college experiencing more than a 2-fold risk. Among the job characteristics, the risk of work loss increased with each increment of physical job demands. In addition, participants reporting the simultaneous occurrence of high levels of psychosocial job demands and low levels of job control had a highly elevated risk of work loss. No one occupation or industry category was significantly associated with the risk of work loss.
Table 4.
Risk factors for work loss among 673 participants employed in diagnosis year (from Cox proportional hazards analysis)*
| Variable | Model |
||
|---|---|---|---|
| Disease duration and demographics | Work characteristics | Full model† | |
| Disease duration, years | 0.92 (0.91–0.94) | 0.93 (0.91–0.94) | |
| Ages 18–34‡ | 0.44 (0.30–0.64) | 0.46 (0.30–0.69) | |
| Ages 35–54 | 0.54 (0.42–0.70) | 0.49 (0.37–0.63) | |
| Female sex | 1.54 (0.99–2.38) | 1.87 (1.17–3.01) | |
| White | 0.94 (0.75–1.19) | 0.92 (0.73–1.17) | |
| High school education or less§ | 3.87 (2.49–5.99) | 3.84 (2.40–6.14) | |
| Some college | 2.58 (1.71–3.90) | 2.51 (1.62–3.87) | |
| College graduate | 1.66 (1.06–2.62) | 1.67 (1.05–2.65) | |
| Currently married¶ | 0.93 (0.68–1.26) | 0.90 (0.65–1.23) | |
| Widowed, separated, or divorced | 0.98 (0.66–1.45) | 0.98 (0.66–1.46) | |
| Sum of physical job demands | 1.07 (1.03–1.11) | 1.06 (1.02–1.11) | |
| Sum of cognitive job demands | 1.11 (1.02–1.21) | 1.02 (0.93–1.12) | |
| High job demands, low job control | 1.32 (1.04–1.67) | 1.35 (1.06–1.72) | |
| Administrative support/clerical occupations | 0.90 (0.67–1.21) | 0.76 (0.55–1.04) | |
| Crafts/operative occupations | 1.02 (0.65–1.60) | 1.13 (0.70–1.83) | |
| Sales/technical/services occupations | 1.07 (0.79–1.45) | 0.87 (0.63–1.19) | |
| Extractive/construction/manufacturing industries | 1.23 (0.82–1.84) | 1.20 (0.79–1.81) | |
| Personal/business/repair services | 1.02 (0.78–1.33) | 0.99 (0.75–1.31) | |
| Miscellaneous industries# | 1.19 (0.82–1.73) | 1.25 (0.85–1.83) | |
| Model chi-square | 135.3, 10 df** | 33.0, 9 df** | 162.2, 19 df** |
Values are the hazard ratio (95% confidence interval) unless otherwise indicated. Excludes 17 individuals who were 65 or over as of diagnosis, or who turned 65 within the work loss interval, and 3 who did not report when they last worked.
Full model chi-square compared with prior models (P < 0.001).
Ages 55–64 as reference.
More than college graduate as reference.
Never married as reference.
Includes government, transportation/utilities, wholesale/retail trade, and finance/insurance/real estate.
P < 0.001.
DISCUSSION
In rheumatoid arthritis, several analysts have observed reductions in work disability rates over time (4,25), with some evidence that this may be associated with emerging treatments (26), although it also may be an artifact of methods of analysis or of differences among nations in employment (4). The amount of literature on work disability in persons with SLE is much smaller and, with the exception of 2 of the studies (14,16), the sample sizes of these studies were relatively small and the populations studied were relatively homogeneous. The present study was designed to provide statistically reliable estimates of the employment outcomes of individuals with SLE in a heterogeneous sample in the US. Indeed, approximately two-thirds of the respondents were enrolled independent of clinical environments, with ~25% being derived from lupus support groups and patient conferences and another 41% as a result of publicity in newsletters, Web sites, and public service announcements. Of note, more than one-third of the study participants in the LOS were members of racial and ethnic minorities, more than 1 in 8 reported less than a poverty-level income, and almost 10% reported a history of dialysis and/or a kidney transplant.
Consistent with prior studies of work disability in persons with SLE, we found relatively high rates of work cessation. However, the overall decline in employment masks a high rate of transitions into as well as out of employment. The rate of transitions into employment may reflect the relatively young age at onset, the fluctuating nature of SLE, or the educational attainment of many of the study participants.
In the years since diagnosis, the productivity of the persons with SLE, defined as the number of hours worked per year by all those with some work history, declined by nearly one-third. In contrast, among those working continuously from diagnosis to followup interview, such hours declined by only ~1%. Therefore, consistent with findings for rheumatoid arthritis, overall productivity among persons with SLE is much more a function of whether or not such persons continue to work than of how much they work if they continue to be employed. At diagnosis, the employment rate and hours and weeks of work of the persons with SLE were remarkably similar to those among all US working-age persons of comparable demographic characteristics, suggesting that it was the development of SLE, rather than other characteristics, that reduced their labor force participation (27).
Because of the large sample size of the present study, we were able to estimate the proportion of participants remaining employed with relatively great precision up to 25 years after diagnosis. By a decade after diagnosis, ~36% of those employed at diagnosis had stopped working; by 15 years, slightly more than half had stopped working; and by 25 years, just under three-quarters had stopped working.
Why do persons with SLE stop working? In the large literature on rheumatoid arthritis, most studies demonstrate that demographic factors and the nature of premorbid jobs largely determine work outcomes (5,7). Although work factors, including both the physical and psychosocial demands of jobs, affected work outcomes in this cohort, demographic and disease variables appear to play a larger role. In particular, women with SLE had much higher rates of work loss, as did persons with low levels of education, whereas younger workers experienced lower rates. The impact of education was particularly strong, with a 3-fold risk of work loss occurring among those without a high school education relative to those with postbaccalaureate training. Of course, for those with early SLE onset, the time to reach the critical threshold of education for protection against work loss may have already passed; for many others, the economic means to attain an advanced degree may never have been present. Contrary to expectations, once age was taken into account, individuals with longer disease duration had slightly lower rates of work loss. This finding may be an artifact of a healthy worker effect (28) in which those with an especially high a priori risk of work loss died prior to study enrollment. The study by Mau and colleagues (16), based on a population registry, found that longer durations were associated with higher rates of work loss, consistent with the view that a healthy worker effect did affect the results of the present study.
The finding that high levels of physical demands of jobs and the combination of high psychological demands and low levels of autonomy place persons with SLE at risk for work loss is consistent with the results of studies in rheumatoid arthritis (5,7) and nonrheumatic conditions (29,30). However, the risks of high demands and low autonomy may be particularly acute in persons with SLE given the high rate of cognitive impairment and psychological disturbance associated with this condition. Moreover, high rates of transition into and out of employment may preclude persons with SLE from establishing a long-term relationship with an employer with which to negotiate flexible working conditions.
The generalizability of the present study is limited by the absence of a true population-based sample and by the lack of measurement of disease factors prior to changes in employment. As to the former, the effect is mitigated by the fact that almost two-thirds of persons with SLE were enrolled outside of clinical environments. Nevertheless, it is possible that the prevalence of work loss in the study sample differs from what might be observed in a population-based national sampling frame if that were feasible in such a rare condition. As to the latter, persons with SLE will be observed on a prospective basis. Given that many of these individuals still work, we will be able to ascertain the role of SLE-related manifestations and symptoms on subsequent changes in employment.
In this sample of persons with SLE, diagnosis occurred when individuals were in their mid-thirties on average. Given that half of those employed at diagnosis stopped working within 15 years, such individuals are losing almost 2 decades of their work lives. Because much of the accrual of retirement savings occurs in these last 2 decades of a career, after one’s responsibilities to one’s children have passed, persons with SLE will have to face retirement with a much greater risk of poverty. Indeed, it appears that almost no one in this cohort sustains employment to the normal age of retirement.
Acknowledgments
The authors gratefully acknowledge the assistance of Janet Stein, Jessica Spry, and Rosemary Prem.
Dr. Yelin’s work was supported by the Agency for Health-care Research and Quality/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1-R01-HS-013893, the State of California Lupus Fund, and the Arthritis Foundation. Dr. Yazdany’s work was supported by the American College of Rheumatology/Research and Education Foundation Physician Scientist Development award. Dr. Criswell’s work was supported by NIH grants K24-AR-02175 and R01-AR-44804.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Yelin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Drs. Yelin, Katz, and Criswell. Acquisition of data. Drs. Yelin and Criswell.
Analysis and interpretation of data. Drs. Yelin, Criswell, Yazdany, Gillis, and Panopalis, and Ms Trupin.
Manuscript preparation. Drs. Yelin, Katz, Criswell, Yazdany, Gillis, and Panopalis.
Statistical analysis. Dr. Yelin and Ms Trupin.
Creation of original SLE cohort. Dr. Criswell.
References
- 1.Yelin E, Cisternas MG, Pasta DJ, Trupin L, Murphy L, Helmick CG. Medical care expenditures and earnings losses of persons with arthritis and other rheumatic conditions in the United States in 1997: total and incremental estimates. Arthritis Rheum. 2004;50:2317–26. doi: 10.1002/art.20298. [DOI] [PubMed] [Google Scholar]
- 2.Yelin E, Meenan R, Nevitt M, Epstein W. Work disability in rheumatoid arthritis: effects of disease, social, and work factors. Ann Intern Med. 1980;93:551–6. doi: 10.7326/0003-4819-93-4-551. [DOI] [PubMed] [Google Scholar]
- 3.Yelin E, Henke C, Epstein W. The work dynamics of the person with rheumatoid arthritis. Arthritis Rheum. 1987;30:507–12. doi: 10.1002/art.1780300504. [DOI] [PubMed] [Google Scholar]
- 4.Allaire SH. Update on work disability in rheumatic diseases. Curr Opin Rheum. 2001;13:93–8. doi: 10.1097/00002281-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Sokka T, Pincus T. Markers for work disability in rheumatoid arthritis [review] J Rheumatol. 2001;28:1718–22. [PubMed] [Google Scholar]
- 6.Callahan L, Yelin E. The social and economic consequences of rheumatic disease. In: Klippel J, editor. Primer on the rheumatic diseases. Atlanta: Arthritis Foundation; 2001. [Google Scholar]
- 7.Verstappen SM, Bijlsma JW, Verkleij H, Buskens E, Blaauw AA, ter Borg EJ, et al. the Utrecht Rheumatoid Arthritis Cohort Study Group. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheum. 2004;51:488–97. doi: 10.1002/art.20419. [DOI] [PubMed] [Google Scholar]
- 8.De Croon EM, Sluiter JK, Nijssen TF, Dijkmans BA, Lankhorst GJ, Frings-Dresen MH. Predictive factors of work disability in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2004;63:1362–7. doi: 10.1136/ard.2003.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturfelt G, Nived O. Clinical inconsistency, benign course and normal employment rates in unselected systemic lupus erythematosus. Clin Exp Rheumatol. 1985;3:303–10. [PubMed] [Google Scholar]
- 10.Stein H, Walters K, Dilon A, Schulzer M. Systemic lupus erythematosus: a medical and social profile. J Rheumatol. 1986;13:570–6. [PubMed] [Google Scholar]
- 11.Karlson EW, Daltroy LH, Lew RA, Wright EA, Partridge AJ, Roberts WN, et al. The independence and stability of socioeconomic predictors of morbidity in systemic lupus erythematosus. Arthritis Rheum. 1995;38:267–73. doi: 10.1002/art.1780380217. [DOI] [PubMed] [Google Scholar]
- 12.Partridge AJ, Karlson EW, Daltroy LH, Lew RA, Wright EA, Fossel AH, et al. Risk factors for early work disability in systemic lupus erythematosus: results from a multicenter study [review] Arthritis Rheum. 1997;40:2199–206. doi: 10.1002/art.1780401214. [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe N, Clarke AE, Gordon C, Farewell V, Isenberg DA. The association of socio-economic status, race, psychosocial factors and outcome in patients with systemic lupus erythematosus. Rheumatology (Oxford) 1999;38:1130–7. doi: 10.1093/rheumatology/38.11.1130. [DOI] [PubMed] [Google Scholar]
- 14.Clarke AE, Penrod J, St Pierre Y, Petri MA, Manzi S, Isenberg DA, et al. the Tri-Nation Study Group. Underestimating the value of women: assessing the indirect costs of women with systemic lupus erythematosus. J Rheumatol. 2000;27:2597–604. [PubMed] [Google Scholar]
- 15.Boomsma MM, Bijl M, Stegeman CA, Kallenberg CG, Hoffman GS, Tervaert J. Patients’ perceptions of the effects of systemic lupus erythematosus on health, function, income, and interpersonal relationships: a comparison with Wegener’s granulomatosis. Arthritis Rheum. 2002;47:196–201. doi: 10.1002/art.10341. [DOI] [PubMed] [Google Scholar]
- 16.Mau W, Listing J, Huscher D, Zeidler H, Zink A. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol. 2005;32:721–8. [PubMed] [Google Scholar]
- 17.Freemer M, King T, Jr, Criswell L Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis [article online] 2005 doi: 10.1136/ard.2005.039438. URL: http://ard.bmjjournals.com/cgi/rapidpdf/ard.2005.039438v1. [DOI] [PMC free article] [PubMed]
- 18.Parsa A, Lovett DH, Peden EA, Zhu L, Seldin MF, Criswell LA. Renin-angiotensin system gene polymorphisms predict the progression to renal insufficiency among Asians with lupus nephritis. Genes Immun. 2005;6:217–24. doi: 10.1038/sj.gene.6364179. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Yelin E, Trupin L, Katz P. The prevalence and impact of managed care for persons with rheumatoid arthritis in 1994 and 1999. Arthritis Rheum. 2002;47:172–80. doi: 10.1002/art.10340. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Soc. 1958;53:457–81. [Google Scholar]
- 23.Cox D. Regression models and life tables (with discussion) J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 24.Lindsey JC, Ryan LM. Tutorial in biostatistics: methods for interval-censored data [published erratum appears in Stat Med 1999;18:890] Stat Med. 1998;17:219–38. doi: 10.1002/(sici)1097-0258(19980130)17:2<219::aid-sim735>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis. Work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–17. [PubMed] [Google Scholar]
- 26.Yelin E, Trupin L, Katz P, Lubeck D, Rush S, Wanke L. Association between Etanercept use and employment outcomes among patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:3046–54. doi: 10.1002/art.11285. [DOI] [PubMed] [Google Scholar]
- 27.US Bureau of the Census. Current population survey technical documentation. Washington, DC: US Department of Commerce; 1993. [Google Scholar]
- 28.Wen CP, Tsai SP, Gibson RL. Anatomy of the healthy worker effect: a critical review. J Occup Med. 1983;25:283–9. [PubMed] [Google Scholar]
- 29.Yelin EH, Greenblatt RM, Hollander H, McMaster JR. The impact of HIV-related illness on employment. Am J Public Health. 1991;81:79–84. doi: 10.2105/ajph.81.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yelin E, Henke J, Katz PP, Eisner MD, Blanc PD. Work dynamics of adults with asthma. Amer J Ind Med. 1999;35:472–80. doi: 10.1002/(sici)1097-0274(199905)35:5<472::aid-ajim4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics. National Health Interview Survey (NHIS), Public Use Data Release, NHIS Survey Description, 2003. 2002 URL: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2002/srvydesc.pdf.
- 32.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 33.Ware J, Jr, Kosinski M, Keller SD. A 12-item short form health survey: construction of scales and preliminary tests. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Ware J, Snow K, Kosinski M, Gandek B. SF-36 Health Survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 35.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 36.Stewart A, Ware JJ. Measuring functioning and well-being: the Medical Outcomes Study approach. Durham (NC): Duke University Press; 1992. [Google Scholar]
- 37.Brandt J, Benedict R. Hopkins verbal learning test: revised. Lutz (FL): Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 38.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 39.Pincus T, Swearingen C, Callahan L. A self-report cognitive symptoms inventory to assess patients with rheumatic diseases: results in eosinophiliamyalgia syndrome (EMS), fibromyalgia, rheumatoid arthritis (RA), and other rheumatic diseases [abstract] Arthritis Rheum. 1996;39 (Suppl 9):S261. [Google Scholar]
- 40.Cohen JW, Monheit AC, Beauregard KM, Cohen SB, Lefkowitz DC, Potter DE, et al. The Medical Expenditure Panel Survey: a national information resource. Inquiry. 1996–1997;33:373–89. [PubMed] [Google Scholar]
- 41.Kerwin J, Cantor D, Sheridan S. Results of rounds 3 and 4 of managed care cognitive interviews for the household portion of the MEPS: report to AHCPR. Westat, Inc; Rockville, MD: 1995. [Google Scholar]
- 42.Clarke AE, Esdaile JM, Bloch DA, Lacaille D, Danoff DS, Fries JF. A Canadian study of the total medical care costs for patients with systemic lupus erythematosus and the predictors of costs. Arthritis Rheum. 1993;36:1548–59. doi: 10.1002/art.1780361109. [DOI] [PubMed] [Google Scholar]
- 43.Gironimi G, Clarke AE, Hamilton VH, Danoff DS, Bloch DA, Fries JF, et al. Why health care costs more in the US: comparing health care expenditures between systemic lupus erythematosus patients in Stanford and Montreal. Arthritis Rheum. 1996;39:979–87. doi: 10.1002/art.1780390615. [DOI] [PubMed] [Google Scholar]
- 44.Bureau of Labor Statistics. Workers on flexible and shift schedules in May 2004. US Department of Labor; 2005. URL: http://www.bls.gov/cps/ [Google Scholar]
- 45.The health and retirement study: a longitudinal study of health, retirement, and aging. Survey Research Center, Institute for Social Research, University of Michigan. 2002 URL: http://hrsonline.isr.umich.edu/intro/sho_uninfo.php?hfyle=sample&xtyp=2.
- 46.Karasek R, Schwartz J, Theorell T. Final report to national institute of occupational safety and health. New York: Columbia University; 1982. Job characteristics, occupation, and coronary heart disease. [Google Scholar]