Abstract
Arsenic is a well known human skin carcinogen whose mechanism of action remains to be elucidated. In this work using cultured human epidermal cells, arsenite suppressed accumulation of the transcriptionally active intracellular domain of Notch1. The cells responded to an active peptide from the Notch1 ligand, Jagged1, with increased levels of differentiation marker mRNAs and decreased colony forming ability. Arsenite suppressed Jagged1 effects and expression of Jagged1 mRNA as well. Moreover, exposure of the cells to a γ-secretase inhibitor prevented Notch1 processing, decreased cell size and differentiation marker expression and increased proliferative potential, all effects that occur with arsenite treatment. Thus, arsenite action in suppressing keratinocyte differentiation while maintaining germinative capability could be due to inhibition of Notch1 signaling subsequent to ligand binding. This work also revealed that such arsenite action depends upon epidermal growth factor receptor kinase activity. These findings may help explain how arsenite, by decreasing generation of the tumor suppressor Notch1, contributes to skin carcinogenesis.
INTRODUCTION
The Notch signaling pathway regulates a broad spectrum of developmental processes as a result of contact between a membrane receptor of the Notch family with a transmembrane ligand of the Delta or Jagged (Serrate) families on an adjacent cell (Artavanis-Tsakonas et al., 1999). The Notch1 membrane receptor is synthesized as a 300 kDa precursor that undergoes three distinct cleavages (Fortini, 2002). First, cleavage in the trans-Golgi network by a furin-like convertase produces a noncovalently associated heterodimer at the cell surface. Upon binding a ligand from an adjoining cell, the receptor is then cleaved extracellularly close to the transmembrane domain by a disintegrin/metalloprotease family member, tumor necrosis factor-α-converting enzyme. The membrane-anchored carboxy-terminal product of this cleavage is subsequently processed by the γ-secretase complex, leading to release of the Notch intracellular domain (NICD), which translocates into the nucleus and binds the transcription factor RBP-Jk, resulting in transcriptional repression or activation of Notch target genes. The best characterized targets of Notch regulated transcriptional activation are members of the HES and HERP families of basic helix-loop-helix transcriptional repressors (Iso et al., 2003).
The Notch signaling pathway is important in determining keratinocyte fate. Notch1 is expressed throughout the living layers of human interfollicular epidermis with highest levels observed in the spinous layer (Lowell et al., 2000; Nickoloff et al., 2002). Overexpression of the ligand Delta1 in adjoining epidermal cells in culture promotes differentiation of putative stem cells (Lowell et al., 2000), and treatment of cultures with an active peptide from the ligand Jagged1 increases expression of differentiation markers (Nickoloff et al., 2002). Similar findings in mouse keratinocyte cultures (Rangarajan et al., 2001) led to the demonstration of Notch1 as a tumor suppressor in mouse skin, where its ablation led to increased incidence of spontaneous tumors and to promotion of dimethylbenzanthracene carcinogenicity (Nicolas et al., 2003). Notch1 expression is frequently depressed in human squamous cell carcinomas and cell lines derived from them, while suppression of Notch1 signaling in culture increases clonal growth capability of the normal epidermal cells and, in cells transplanted to mouse skin, promotes tumors induced by oncogenic ras (Lefort et al., 2007). Interference with Notch1 signaling by expressing a dominant negative mastermind-like protein, a transcriptional coactivator associated with the NICD, has now produced a mouse model for human squamous cell carcinoma (Proweller et al., 2006).
Arsenic, a well known human carcinogen, produces cancer of the skin and other organs upon chronic exposure to high levels in drinking water. Elucidating a mechanistic basis for such action in human target cells remains a priority in view of continuing concern about worldwide exposures and the risks they pose (Hughes et al., 2007). To this end, the roles of arsenic as a co-promoter in mouse epidermis expressing a v-ras oncogene (Germolec et al., 1997) and as a co-carcinogen with UV light (Burns et al., 2004) have been demonstrated, and a mouse model for studying arsenic carcinogenesis in other organs by transplacental exposure has recently become available (Waalkes et al., 2004).
For investigative purposes, keratinocyte cultures provide a good model for studying actions of toxic agents that adversely affect the skin. Epidermal cells, propagated with 3T3 feeder layer support, exhibit many characteristics of epidermis in vivo including stratification, expression of specific differentiation markers and production of enlarged superficial squames (Green, 1979), and they readily adopt normal histology after grafting onto human subjects (Compton, 1996). Treatment of cultured human epidermal cells with arsenite suppresses differentiation marker expression (Kachinskas et al., 1997) while maintaining proliferative potential, suggesting it interferes with important signal transduction pathways (Jessen et al., 2001). Identifying these pathways, particularly if they affect the fate of germinative cells, could help elucidate the influence of arsenic on the neoplastic process in vivo. The plausibility of a connection to carcinogenesis is supported by observations that arsenite maintains elevated β-catenin and β1-integrin levels in the cultures as well as rapid adherence of keratinocytes to type IV collagen upon passage (Patterson et al., 2005), characteristics of populations enriched in putative stem cells (Jones and Watt, 1993).
The above effects of arsenite all require active EGFR as they are prevented by co-treatment with EGFR inhibitors (Patterson and Rice, 2007). In contrast to EGFR signaling, insulin/IGF-I signaling in post-confluent keratinocyte cultures decreases proliferative potential (Patterson and Rice, 2007) and increases differentiation marker expression (unpublished), effects that are largely prevented by arsenite exposure. The observation that arsenite and Notch signaling have opposite effects in keratinocytes on proliferative potential and differentiation prompted the present investigation to determine whether Notch signaling is affected by arsenite exposure.
RESULTS
Arsenite suppresses NICD in parallel to differentiation
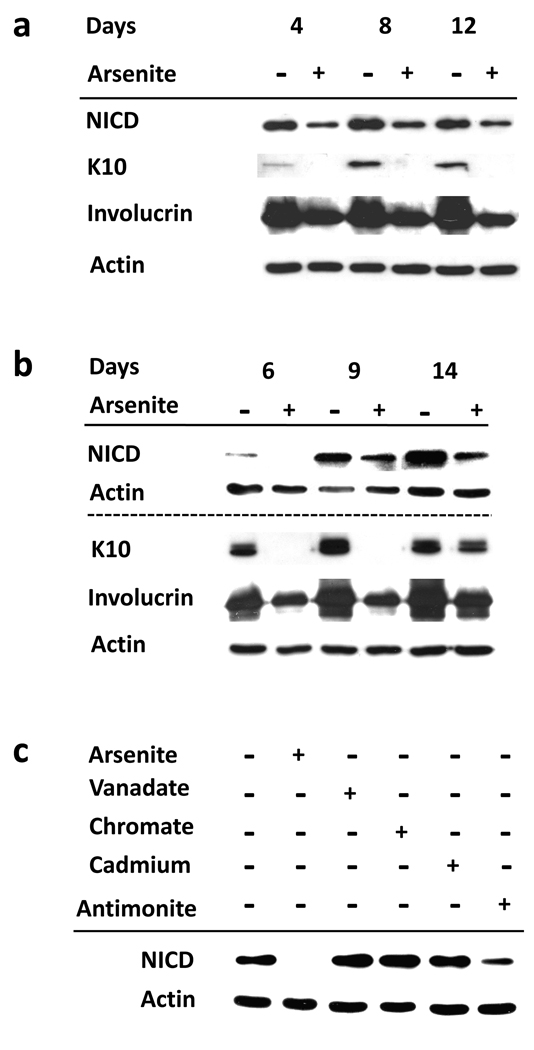
When keratinocyte cultures reach confluence, their differentiated state increases during the next 10 days while their proliferative potential declines, phenomena that arsenite treatment largely prevents (Patterson et al., 2005). Immunoblotting of extracts from normal human epidermal cells (Figure 1a) or from the SIK spontaneously immortalized epidermal keratinocyte line (Figure 1b) with antiserum specific for NICD, the activated form of Notch1, showed that this γ-secretase cleavage product increased after confluence in parallel to the differentiation marker proteins keratin 10 (K10) and involucrin. All of these changes were suppressed by arsenite treatment (Figure 1a,b). While arsenite, antimonite, vanadate, chromate and cadmium all suppress differentiation marker expression (Rea et al., 2003), only arsenite and antimonite are able to preserve colony forming ability (Patterson et al., 2005) and suppress NICD accumulation (Figure 1c).
Figure 1. Suppression of NICD and differentiation in keratinocytes by arsenite.
Starting at 90% confluence, normal epidermal (a) or SIK (b) cultures were treated with 2 µM arsenite for the indicated number of days. (c) SIK cultures were treated with 2 µM arsenite, 3 µM vanadate, 5 µM chromate, 10 µM cadmium or 5 µM antimonite as indicated for 11 days. Immunoblot analysis of NICD was performed using cleaved Notch1 (Val1744) antibody, which detects endogenous levels of the cytosolic domain of Notch1 only when cleaved between Gly1743 and Val1744. β-Actin was used as loading control. The dotted line in (b) separates 2 different blots.
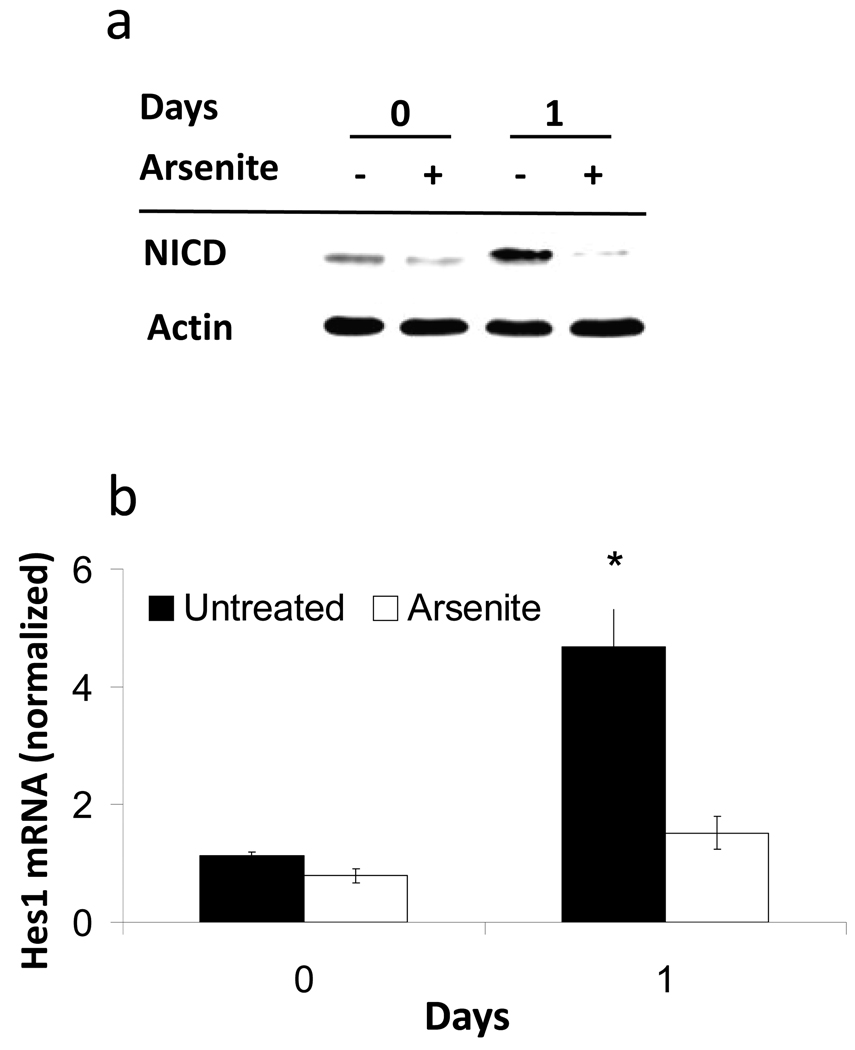
When keratinocytes are placed in suspension, they display a rapid reduction in colony forming ability as they become terminally differentiated, a process that arsenite treatment impedes (Patterson et al., 2005). In suspension culture, NICD levels increased after 1 day, while arsenite prevented this effect (Figure 2a). In addition, after 1 day in suspension Hes1 mRNA, a transcriptional Notch1 target, was induced in the absence but not in the presence of arsenite (Figure 2b).
Figure 2. Arsenite suppression of NICD protein and Hes1 mRNA in suspension culture.
(a) Immunoblots of NICD and β-actin (loading control) performed on extracts of SIK before (0) and after one day (1) in suspension. (b) Values for Hes1 mRNA expression (mean ± SD), measured using real-time PCR, are given relative to mRNA in untreated SIK cultures at zero days and normalized to 18S RNA. The difference between treated and untreated samples after one day suspension was significant (*, p <0.05).
γ-Secretase inhibition mimics arsenite in altering keratinocyte differentiation and proliferative potential
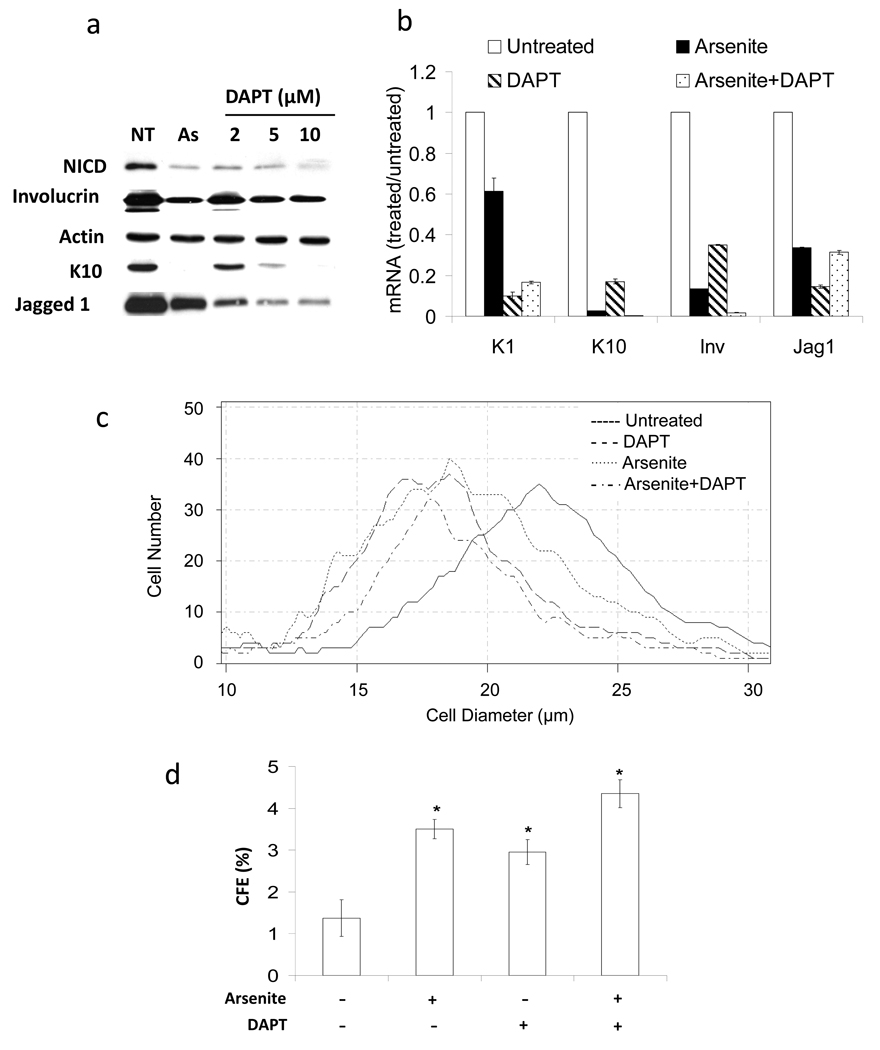
To investigate whether interruption of Notch signaling alone would replicate the consequences of arsenite exposure, cells were treated in parallel with arsenite or with the specific γ-secretase inhibitor, DAPT (N-(N-(3,5-difluorophenacetyl)-L-alanyl)-S-phenylglycine t-butyl ester). DAPT potently suppressed NICD accumulation with effects similar to those achieved by arsenite treatment (Figure 3a). Furthermore, γ-secretase inhibition by arsenite or DAPT treatment led to suppression of Jagged1 protein and mRNA as well as differentiation marker proteins and mRNAs (Figures 3a,b), although the amount of suppression by arsenite and DAPT was not identical, likely due to additional targets of each agent.
Figure 3. Inhibition of γ-secretase activity preserves proliferative potential of keratinocytes and suppresses differentiation.
SIK cultures were treated one day before confluence with 2 µM arsenite and/or with the indicated concentrations of DAPT (a) or 10 µM (b–d). After 7 days of treatment, cultures were harvested and assayed. (a) Immunoblot analysis was performed using antibodies to the indicated proteins; β-actin was used as a loading control. (b) mRNA levels of K1 and K10, involucrin (Inv) and Jagged1 (Jag1) were measured using real-time PCR. mRNA expression, normalized to endogenous 18S RNA, is presented relative to untreated cultures (value of 1). (c) Distribution of cell sizes from treated and untreated cultures. (d) Significant differences in CFE between untreated and each treatment (*p<0.01). Each panel is representative of 2 independent experiments. Values in b and d are mean ± SD.
DAPT and arsenite treatment individually decreased cell size (Figure 3c) and increased colony forming efficiency (CFE) compared to untreated cultures (Figure 3d). In general, co-treatment with DAPT and arsenite had little additive effect on differentiation marker or Jagged1 mRNA expression (Figure 3b), cell size (Figure 3c) or CFE (Figure 3d).
Arsenite suppresses Notch1 processing at a step after ligand binding
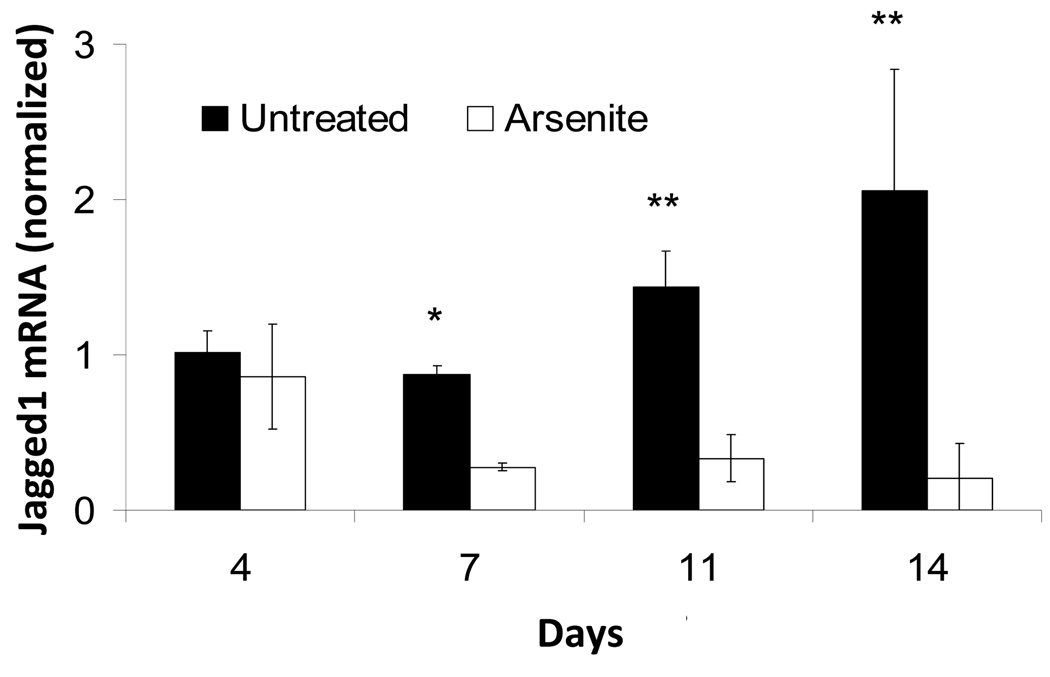
To find whether arsenite suppression of Jagged1 protein reflected a transcriptional effect, mRNA levels were measured as a function of time after confluence. As shown in Figure 4, expression approximately doubled after 2 weeks. By contrast, treatment of the cultures with arsenite greatly suppressed the mRNA levels so that after that time the levels differed by an order of magnitude. This observation raised the possibility that arsenite could produce its effects downstream of NICD in this fashion.
Figure 4. Time course of Jagged1 mRNA accumulation in SIK cultures with and without 2 µM arsenite treatment.
Values (mean ± SD) are given relative to untreated cultures harvested after 4 days and normalized to 18S RNA. Significant differences between untreated and arsenite treated samples (*p<0.05, **p<0.01) are representative of 5 independent experiments.
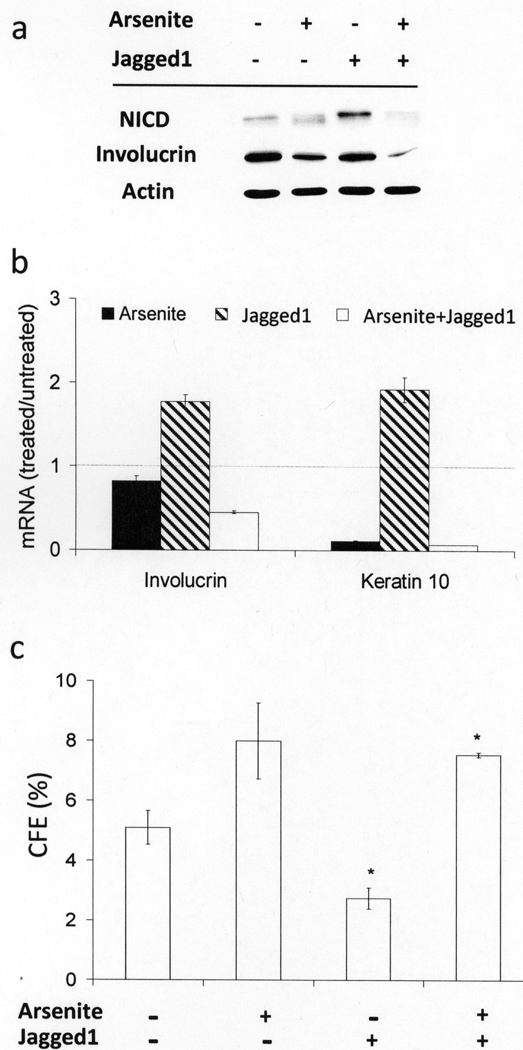
When cultures were treated with a synthetic peptide derived from Jagged1 that has Notch agonist activity in vitro and stimulates terminal differentiation and cornification of human keratinocytes (Nickoloff et al., 2002), NICD levels increased (Figure 5a) and involucrin and K10 mRNAs were induced (Figure 5b). Furthermore, colony forming efficiency (CFE) was reduced by half with Jagged1 treatment (Figure 5c). In the presence of the Jagged1 peptide, however, arsenite treatment still suppressed NICD level and differentiation marker expression, while preserving proliferative potential (Figure 5), indicating that arsenite also targets Notch1 signaling regardless of ligand availability.
Figure 5. Jagged1 peptide effects on arsenite-treated keratinocytes.
90% confluent SIK cultures were pre-treated for 24 hr with 2 µM arsenite prior to addition of synthetic Jagged1 peptide into the medium at a final concentration of 40 µM. Cultures were treated for 3 days and then harvested and assayed. (a) Protein levels of NICD were detected by immunoblotting; β-actin was used as loading control. (b) mRNA levels of involucrin and K10 were measured by real-time PCR. Values are given relative to mRNA in untreated cultures, indicated by the horizontal dotted line. (c) CFE after Jagged1 or arsenite treatment. The results (mean ± SD) are representative of 2 independent experiments, where * indicates the values for cultures treated with Jagged1 ± arsenite were significantly different (p<0.02).
Regulation of Notch signaling by EGF and insulin/IGF-I receptors
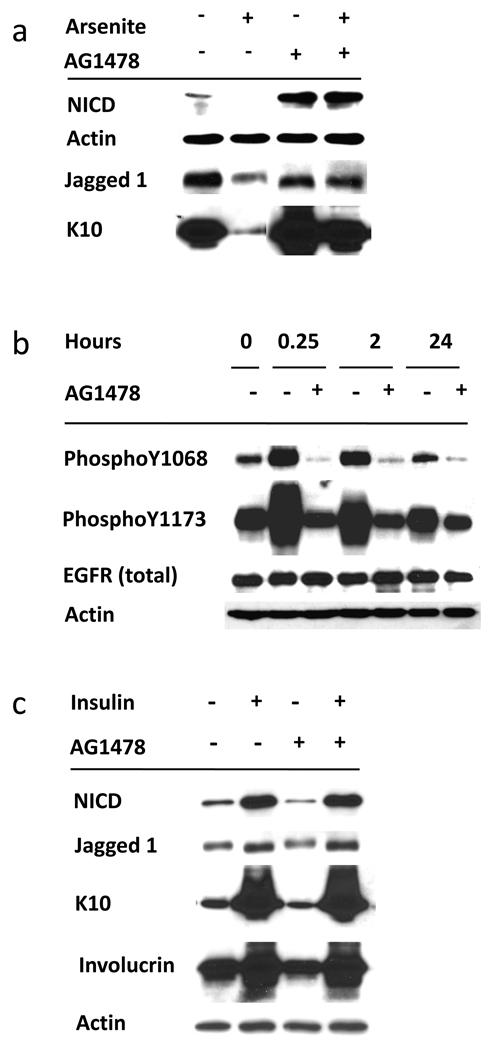
Treatment of cultures with the EGFR tyrosine kinase inhibitor AG1478 alone enhanced NICD accumulation and, in the presence of arsenite, it completely reversed arsenite suppression of NICD, Jagged 1 and K10 (Figure 6a). In these experiments the inhibitor suppressed EGF-mediated phosphorylation of tyrosines 1068 and 1173 without altering EGFR protein levels (Figure 6b) in line with known effects of EGFR inhibition (Peus et al., 1997).
Figure 6. EGFR inhibition induces Notch1 activation and reverses the arsenite effect on Notch1.
(a) SIK cultures (90% confluent) were pre-treated for 1 hr with 1 µM AG1478 followed by treatment with 2 µM arsenite for 4 days. Cultures were harvested and subjected to immunoblot analysis using antibodies as indicated; β-actin was used as a loading control. Results are representative of 3 independent experiments. (b) Demonstration of AG1478 efficacy in EGFR inhibition using antibodies to autophosphorylation sites. Confluent SIK cultures were treated with AG1478 (or solvent control) for 1 hr before EGF addition and harvested at the indicated times. (c) SIK cultures were grown to confluence in the presence of insulin, which was then removed from those cultures indicated by (-). Cultures were treated with AG1478 as indicated (added at each medium change) starting at confluence and harvested 2 weeks later.
In 3 independent experiments, 9 day treatment with arsenite suppressed expression not only of involucrin and K10 mRNAs (to 2 ± 1% and 2 ± 2%, respectively), but also of Jagged1 and Hes1 (to 42 ± 12% and 55 ± 6%, respectively, of untreated control values). This action also was prevented by treatment with AG1478 as shown by the representative experiment in Table 1. In these experiments, inhibition of EGFR activity by treatment with AG1478 alone stimulated levels of each mRNA, and co-treatment with arsenite resulted in levels close to those of untreated control cultures.
Table 1.
Relative mRNA levels in SIK cultures treated with arsenite and AG1478
| Marker | % of Untreated1 | |||
|---|---|---|---|---|
| Arsenite | AG1478 | Arsenite+AG1478 | ||
| Hes1 | 57±4 | 158±3 | 107±2 | |
| Jagged1 | 38±0.4 | 171±2 | 119±4 | |
| Keratin 10 | 0.4±0.01 | 366±9 | 138±1 | |
| Involucrin | 2.3±0.2 | 230±12 | 86±2 | |
mRNA values, measured by real time PCR and expressed relative to those of parallel untreated samples (all normalized to GUSB), are means and standard deviations of duplicate samples. The experiment was repeated 4 times with similar results.
Standard keratinocyte culture medium contains a high concentration of insulin to stimulate IGF-I signaling. In post-confluent cells, removal of insulin increased proliferative potential (Patterson and Rice, 2007) and decreased NICD, Jagged1, involucrin and K10 (Figure 6c). These effects were independent of EGFR signaling as they were unchanged in the presence of the inhibitor AG1478.
DISCUSSION
Present results show clear inhibition by arsenite of Notch1 signaling components (NICD, Jagged1, Hes1) that likely contribute to expression of differentiation features (K1, K10, involucrin, cell size) and germinative capability after confluence. This finding contributes to our understanding of the actions of arsenite inasmuch as Notch1 signaling is known to affect the balance of germinative cell persistence versus their loss through differentiation. By contrast, and showing the specificity of arsenite, the actions of cadmium, vandate and chromate in suppressing differentiation (Rea et al., 2003) appear not to be mediated through the Notch1 pathway since neither proliferative potential (Patterson et al., 2005) nor NICD level were altered. That antimonite has an effect like arsenite is consistent with our previous finding that these agents preserve proliferative potential similarly (Patterson and Rice, 2007).
Insulin, probably acting through the IGF-I receptor, has been shown to be responsible for the loss of proliferative potential and decline of EGFR levels after confluence. These effects are largely prevented by arsenite and require the activity of the EGFR (Patterson and Rice, 2007). Present results extend our knowledge by showing that NICD levels and differentiation markers are stimulated by insulin in the medium and are overcome by the combined action of arsenite and EGFR. These results lead to the following model. In confluent keratinocytes, IGF-I receptor signaling generates a pro-differentiation signal, leading to loss of EGFR, followed by loss of proliferative potential and accumulation of NICD and differentiation markers. Arsenite, by preventing loss of EGFR, suppresses this process. In the absence of insulin, addition of an EGFR inhibitor has no effect on NICD or differentiation markers, signifying that the role of EGFR signaling is to oppose a pro-differentiation signal generated by insulin.
Notch1 acts as a tumor suppressor in mouse skin where its ablation increases both spontaneous and chemical carcinogen-induced basal cell carcinomas (Nicolas et al., 2003). In addition, suppression of Notch signaling by decreased γ-secretase activity due to decreased presenilin 1 (Xia et al., 2001) or nicastrin (Li et al., 2007) or due to expression of a dominant negative Notch inhibitor (Proweller et al., 2006) also increases skin tumors, mostly squamous cell carcinomas. In all of these cases, nuclear β-catenin was increased in neoplastic lesions, reminiscent of the increase observed after chronic exposure of cultured keratinocytes to arsenite (Patterson et al., 2005). In epidermis, Notch expression is upregulated in suprabasal layers and, in cultured keratinocytes, activation results in increased differentiation (reviewed by Watt et al., 2008). Present findings, consistent with premature or enhanced differentiation upon EGFR inhibition (Peus et al., 1997), suggest that increased EGFR signaling in epidermis leads to decreased Notch signaling. Consistent with this expectation, increased EGFR signaling is often observed in human and mouse squamous cell carcinomas and its disruption results in decreased growth of v-rasHa papillomas, interpreted as a requirement for EGFR in maintaining the proliferative population (Hansen et al., 2000). These observations suggest that arsenite increases skin cancer because it increases or prolongs EGFR signaling, resulting in maintenance of the proliferative population (carcinogen target cells) and decreased Notch activation, thereby preventing loss of initiated cells due to differentiation.
The molecular target of arsenite that reduces NICD production remains to be identified. Arsenite prevented cell responses to Jagged1, indicating that it is acting downstream of ligand production. Exposure to arsenite could alter interaction of Notch1 with activating ligands, release of NICD by proteolysis or half life of NICD. Notch1 mRNA, quantified by real time PCR analysis, or precursor protein levels, detected with a C- terminal antibody (unpublished) were not altered by arsenite. If NICD suppression were due to direct inhibition of γ-secretase activity, then proteolysis of other γ-secretase substrates, such as E-cadherin, should be altered by arsenite treatment. Although DAPT altered the processing of both E-cadherin and NICD, the processed forms of E-cadherin in arsenite-treated cells were identical to those in untreated cells (unpublished), suggesting that arsenite reduces NICD by another mechanism.
On the other hand, since elements of membrane dynamics influence Notch signaling, arsenite could interfere with a processing step such as ubiquitination or endocytosis (Gupta-Rossi et al., 2004; Le Borgne, 2006). Several negative regulators of Notch signaling have been identified; Numb and Sel10 promote ubiquitination and degradation of Notch (McGill and McGlade, 2003; Welcker and Clurman, 2008), while LRF is a transcriptional repressor that prevents activation of Notch (Maeda et al., 2007). In preliminary experiments, arsenite treatment had little effect (< 2 fold) on levels of mRNAs encoding these proteins, although protein levels or function have not yet been examined. Since arsenite also alters EGF receptor levels in keratinocytes (Patterson and Rice, 2007), the possibility of more global effects on trafficking of membrane proteins merits further investigation.
Elucidating the details of Notch signaling is important to understand development and differentiation in a variety of cell types. In contrast to its tumor suppressive action in the skin, Notch signaling has attracted attention because activating mutations can promote growth of hematopoietic and certain other neoplasias (Miele et al., 2006). Use of arsenic to induce remission of acute promyelocytic leukemia appears to be more effective than all-trans retinoic acid in removing leukemic stem cells (Zheng et al., 2007). If arsenite inhibits NICD generation in these cells as it does in keratinocytes, this property could contribute to its effectiveness. Understanding the mechanism of arsenite action could lead to other potential clinical uses as well as alert clinicians to possible side effects of its use as a therapeutic.
MATERIALS AND METHODS
Cell culture
Cultures of SIK spontaneously immortalized (Rice et al., 1993 ) or normal human epidermal keratinocytes were propagated in DMEM/F12 (2:1) medium supplemented with fetal bovine serum (5%), hydrocortisone (0.4 µg/ml), EGF (10 ng/ml), adenine (0.18 mM), insulin (5 µg/ml), transferrin (5 µg/ml) and triiodothyronine (20 pM) using a feeder layer of lethally irradiated 3T3 cells (Allen-Hoffmann and Rheinwald, 1984). Medium was further supplemented with cholera toxin (10 ng/ml) at inoculation, then with EGF (10 ng/ml) starting at the first medium change. Cells were grown until just before confluence with medium changes at 3 to 4 day intervals, at which time they were treated with 2 µM sodium arsenite, 5 µM potassium antimony tartrate, 3 µM sodium vanadate, 5 µM potassium chromate, 10 µM cadmium chloride (all from Fisher Scientific, Pittsburgh, PA) as indicated, or designated concentrations of DAPT from EMD Bioscience (La Jolla, CA). In certain instances, cells were first pre-treated for 1 hr with 1 µM AG1478 (LC Laboratories, Woburn, MA).
Suspension induced terminal differentiation
Keratinocyte cultures were treated a day before confluence with 2 µM sodium arsenite for 24 hr. At confluence, any remaining 3T3 cells were removed with EDTA, and the keratinocytes were dispersed by trypsinization. Two million cells were suspended in 10 ml of culture medium containing 1.4% methylcellulose (Green, 1977) with continuing arsenite treatment in suspension for 1 day. At indicated time points, cells were diluted 10-fold with phosphate buffered saline and recovered by low speed centrifugation. After 3 washes with phosphate buffered saline, the cells were used for immunoblotting or real-time RT-PCR as indicated.
Colony forming efficiency assay
Cultures were treated for indicated times, trypsinized, and 1000 or 10,000 cells were inoculated in 6 cm dishes. Cells were allowed to grow with feeder layer support until most were 2–5 mm in diameter, typically 1 to 2 weeks. The colonies were fixed and visualized by staining with Rhodanile blue (Rheinwald and Green, 1975). CFE values (%) are presented as the number of macroscopic colonies (>50 cells) divided by the number of cells inoculated times 100.
Cell size determination
Cultures were treated as indicated, trypsinized and the size distribution was analyzed with a Beckman Coulter Multisizer3. Representative graphs were obtained using Multisizer 3.51 software.
Jagged1 peptide treatment
A synthetic peptide corresponding to Jagged1 residues 188–204 (CDDYYYGFGCNKFCRPR) (Nickoloff et al., 2002) was purchased from GeneMed Synthesis (San Francisco, CA). Peptide stock solutions (10 mM) were prepared in sterile distilled water, aliquoted and stored frozen. Cultures were treated with 40 µM Jagged1 peptide diluted in medium immediately before use.
Immunoblot analysis
Cells were scraped directly into buffer containing 2% SDS, 62.5 mM Tris (pH 6.8) and 10% glycerol. Protein was measured with bicinchoninic acid (Pierce Chem Co, Rockford IL) before addition of dithiothreitol to 50 mM. Equal amounts (20 µg) of protein were submitted to sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinyldifluoride membranes, blocked with 5% dry milk in TBST, incubated with the indicated antibodies and detected using ECL Plus chemiluminescence detection reagent (Amersham Biosciences, Piscataway, NJ). Antibodies were obtained from the following sources: cleaved Notch1 (Val1744) and Jagged1 rabbit monoclonal (Cell Signaling, Danvers MA), K10 mouse monoclonal (NeoMarkers, Fremont, CA), β-actin mouse monoclonal from Sigma (St. Louis, MO) and involucrin (Rice and Green, 1979).
Real-time reverse transcription PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). To avoid amplification of genomic DNA, RNA was pretreated with DNase (DNA-free Ambion kit, Applied Bioststems, Foster City, CA). c-DNA synthesis was performed using High Capacity cDNA Archive Kit (Applied Biosystems). The cDNA served as a template in quantitative real-time PCR utilizing TaqMan Fast Universal PCR Master Mix and TaqMan Gene Expression inventoried assay probes that amplified sequences across intron/exon boundaries (Applied Biosystems). The assays were performed on an ABI 7500 Fast Sequence Detection System. mRNA expression was normalized to 18S or GUSB RNA, neither of which appeared to change with the treatments.
Statistics
Student’s 2-tailed t-tests were conducted in Excel for estimation of significance.
ACKNOWLEDGMENTS
We thank Ms. Qin Qin for expert technical assistance Dr. Timothy J. Patterson for valuable discussions. These experiments were supported by USPHS Grants 2 P42 ES 04699, R01 AR27130 and 5 T32 ES07059.
Abbreviations
- CFE
colony forming efficiency
- DAPT
N-(N-(3,5-difluorophenacetyl)-L-alanyl)-S-phenylglycine t-butyl ester
- EGFR
epidermal growth factor receptor
- IGF-I
insulin-like growth factor
- K1
keratin 1
- K10
keratin 10
- NICD
Notch1 intracellular domain
- SIK
spontaneously immortalized keratinocytes
- TACE
tumor necrosis factor-α converting enzyme
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc Natl Acad Sci USA. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: A dose-response study. Environ Hlth Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton CC. Cultured epithelial autografts for burn wound resurfacing: Review of observations from an 11-year biopsy study. Wounds. 1996;8:125–133. [Google Scholar]
- Fortini ME. γ-Secretase-mediated proteolysis in cell surface receptor signaling. Nat Rev Molec Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Boorman GA, Wilmer JL, Yoshida T, Simeonova PP, et al. Arsenic can mediate skin neoplasia by chronic stimulation of keratinocyte-derived growth factors. Mutation Res. 1997;386:209–218. doi: 10.1016/s1383-5742(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of human epidermal cells. Cell. 1977;11:405–415. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1979;74:101–138. [PubMed] [Google Scholar]
- Gupta-Rossi N, Six E, LeBail O, Logeat F, Chastagner P, Olry A, et al. Monoubiquitination and endocytosis direct γ-secretase cleavage of activated Notch receptor. J Cell Biol. 2004;166:73–83. doi: 10.1083/jcb.200310098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LA, Woodson RL, Holbus S, Strain K, Lo Y-C, Yuspa SH. The epidermal growth factor receptor is required to maintain the proliferative population in the basal compartment of epidermal tumors. Cancer Res. 2000;60:3328–3332. [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM, Kitchin KT. Research approaches to address uncertainties in the risk assessment of arsenic in drinking water. Toxicol Appl Pharmacol. 2007;222:399–404. doi: 10.1016/j.taap.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Jessen BA, Qin Q, Phillips MA, Phillips DL, Rice RH. Keratinocyte differentiation marker suppression by arsenic: Mediation by AP1 response elements and antagonism by tetradecanoylphorbol acetate. Toxicol Appl Pharmacol. 2001;174:302–311. doi: 10.1006/taap.2001.9227. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kachinskas DJ, Qin Q, Phillips MA, Rice RH. Arsenate suppression of human keratinocyte programming. Mutation Res. 1997;386:253–261. doi: 10.1016/s1383-5742(97)00015-x. [DOI] [PubMed] [Google Scholar]
- Le Borgne R. Regulation of Notch signaling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKα kinases. Genes Develop. 2007;21:562–577. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Das P, Smithson L, Fauq A, et al. EGFR and notch pathways participate in the tumor suppressor function of γ-secretase. J Biol Chem. 2007;282:32264–32273. doi: 10.1074/jbc.M703649200. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones PH, LeRoux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by Delta-Notch signaling at the boundaries of stem cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, et al. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278:23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Molec Med. 2006;6:905–918. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin J-Z, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kB and PPARγ. Cell Death Differen. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Reznikova TV, Phillips MA, Rice RH. Arsenite maintains germinative state in cultured human epidermal cells. Toxicol Appl Pharmacol. 2005;207:69–77. doi: 10.1016/j.taap.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Rice RH. Arsenite and insulin exhibit opposing effects on epidermal growth factor receptor and keratinocyte proliferative potential. Toxicol Appl Pharmacol. 2007;221:119–128. doi: 10.1016/j.taap.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997;109:751–756. doi: 10.1111/1523-1747.ep12340759. [DOI] [PubMed] [Google Scholar]
- Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, et al. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006;66:7438–7444. doi: 10.1158/0008-5472.CAN-06-0793. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH. Global alteration of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis. 2003;24:747–756. doi: 10.1093/carcin/bgg010. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: Activation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice RH, Steinmann KE, deGraffenried LA, Qin Q, Taylor N, Schlegel R. Elevation of cell cycle control proteins during spontaneous immortalization of human keratinocytes. Molec Biol Cell. 1993;4:185–194. doi: 10.1091/mbc.4.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: Inorganic arsenic is a transplacental carcinogen in mice. Toxicol Appl Pharmacol. 2004;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- Xia X, Qian S, Soriano S, Wu Y, Fletcher AM, Wang X-J, et al. Loss of presenilin 1 is associated with enhanced β-catenin signaling and skin tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10863–10868. doi: 10.1073/pnas.191284198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Seshire A, Ruster B, Bug G, Beissert T, Puccetti E, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARα-positive leukemic stem cells. Haematologica. 2007;92:323–331. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]