Abstract
Objective
To estimate health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus (SLE) in the US.
Methods
Data were derived from the University of California, San Francisco Lupus Outcomes Study. Participants provided information on their health care resource use and employment. Cost estimates were derived for both direct health care costs and costs related to changes in work productivity. Direct health care costs included costs for hospitalizations, emergency department services, physician visits, outpatient surgical procedures, dialysis, and medications. Productivity costs were estimated by measuring changes in hours of work productivity since diagnosis of SLE; these estimates were also compared with normal US population data.
Results
For the total population of participants, the mean annual direct cost was $12,643 (2004 US dollars). The mean annual productivity cost for subjects of employment age (≥18 and <65 years) was $8,659. The mean annual total cost (direct and productivity) for subjects of employment age was $20,924. Regression results showed that greater disease activity, longer disease duration, and worse physical and mental health were significant predictors of higher direct costs; older age predicted lower direct costs. Older age, greater disease activity, and worse physical and mental health status were significant predictors of higher costs due to changes in work productivity.
Conclusion
Both direct health care costs and costs associated with changes in work productivity are substantial and both represent important contributors to the total costs associated with SLE.
INTRODUCTION
Rheumatic diseases are costly to society and are a heavy burden on patients (1–3). A number of studies have already demonstrated that conditions such as rheumatoid arthritis (RA) and osteoarthritis impose a considerable financial burden (4–9). Fewer studies have assessed the economic impact of systemic lupus erythematosus (SLE), a chronic and complex systemic disease that affects a young population and produces a myriad of disease manifestations. Although less prevalent than osteoarthritis and RA, the complexity of SLE often entails heavy resource use, whereas the younger age at onset may result in disability during a person’s most productive years. Understanding the economic consequences of SLE will help define the magnitude of this disease and assist policymakers in the allocation of health care resources and research dollars.
Costs associated with a given illness include both direct and indirect costs. Direct costs are expenditures for resources used in the prevention, diagnosis, and treatment of an illness; indirect or productivity costs represent the value of labor earnings lost because of disease-related disability. There are currently few studies that have assessed both the direct and indirect costs of SLE. One study evaluated costs among SLE patients attending tertiary care clinics in each of 3 countries: the US, Canada, and the UK (10–13). However, in order to facilitate comparisons, cost estimates were made using Canadian prices and wages, which may have resulted in an overestimation or underestimation of costs in the US and the UK. Only 2 other studies have evaluated the direct and indirect costs of SLE, both in European countries (14,15). Therefore, there appears to be a paucity of SLE cost data in the US. Moreover, the data in the current literature are largely derived from subjects attending tertiary care clinics, which may not represent the full spectrum of persons living with SLE.
The objective of the current study was to estimate, from a societal perspective, the direct health care costs incurred by persons with SLE living in the US, as well as costs related to changes in work productivity. To our knowledge, this is the first large-scale study assessing the costs associated with SLE, using American prices, in a large US SLE population.
SUBJECTS AND METHODS
Subjects
Data for this study were derived from a cohort of 815 subjects participating in the second annual interview of the University of California, San Francisco (UCSF) Lupus Outcomes Study (LOS), a large cohort of persons with SLE (age ≥ 18 years) living in the US enrolled in a longitudinal survey (16). All participants met at least 4 of the 11 American College of Rheumatology revised criteria for the classification of SLE (17) as updated in 1997 (18) after chart review by a rheumatologist or a nurse working under the supervision of a rheumatologist. Enrollment for the LOS began in September 2002 and the full cohort, consisting of 957 participants, was recruited from a variety of clinical and community-based sources. Twenty-three percent of participants were recruited from UCSF-associated clinics and 11% from non-UCSF rheumatology offices. The remaining 66% were recruited from various community-based sources, including outreach at national support group meetings and patient conferences, and through advertisements in patient newsletters and magazines. The latter recruitment sources allowed us to capture individuals from a large number of states. Seventy-five of the 957 participants were enrolled in 2004–2005. Therefore, our sample of 815 derives from the 882 LOS participants, enrolled prior to the end of 2003, who were available to complete their second wave of interviews between January 2004 and April 2005. Data were derived from the second wave of interviews because they included information regarding disease activity that was absent from the first wave of interviews. Seventy-two percent of subjects participating in the second wave of interviews were residing in California. The study protocol was approved by the UCSF Committee on Human Research.
Data
All participants completed structured, 1-hour telephone interviews conducted by trained interviewers. The survey included validated items pertaining to demographic and socioeconomic characteristics, SLE disease activity and manifestations, general health, mental health, employment, health resource use, medications, and health insurance coverage.
Measures
Health care resource use
Participants were queried about their health care resource use over the preceding 12 months, including visits to physicians by specialty and to other health care professionals, acute and long-term care hospitalizations, use of emergency department services, outpatient surgical procedures, dialysis, and medications.
Employment
Participants were queried about both their current work situation and their work situation in the year their SLE was diagnosed, using a series of questions employed by the Bureau of Labor Statistics to derive data from the Current Population Survey (19). Participants were considered employed if they reported that they had a job or that they had done any work for pay or profit. Additionally, participants provided information regarding the number of hours per week and the number of weeks per year they typically worked, both currently and in the year of SLE diagnosis.
Demographic variables
Demographic information collected included age, sex, race/ethnicity (dichotomized into white or nonwhite), marital status (categorized as being currently married or living with a partner versus other), and education (reported as less than high school, high school graduate, some college, trade or vocational school, college graduate, or postgraduate degree).
Disease and general health status
SLE disease activity was assessed using the Systemic Lupus Activity Questionnaire (SLAQ) (20,21), a validated, patient-reported assessment that has been found to correlate strongly with the Systemic Lupus Activity Measure Revised (20). Disease duration was calculated as the number of years since the year of SLE diagnosis, which was determined by review of the medical record. Health status was assessed by the Medical Outcomes Study Short Form 12 (SF-12), which includes a physical component summary (PCS) score and a mental component summary (MCS) score (22).
Overview of cost estimates
Cost estimates were derived for both direct health care costs and indirect costs related to changes in work productivity. For the most part, Medicare reimbursement rates were used to value direct costs, because they represented a reasonable proxy for the long-run opportunity costs of medical care (4). Medications were valued at the median average wholesale price. For this study, we chose to include all health care costs, not just costs related to SLE. Given the complexity of the disease, it is difficult to distinguish health resource use that results from SLE versus other conditions. Therefore, estimates reflect all costs for health care incurred by participants, and not only the additional costs that result as a consequence of SLE.
Estimates of direct health care costs
Inpatient acute care costs
Inpatient acute care hospital costs for both surgical and medical admissions were estimated using methods that approximated those used by Medicare in deriving reimbursement amounts. Two major cost components were considered: hospital charges and professional fees. Because hospital charges are based on diagnostic-related group (DRG) codes, a DRG code was first assigned to each hospital admission based on the subject’s description of the primary procedure or diagnosis. Based on this DRG code, a national average cost for the year 2004 was obtained using information derived from the Healthcare Cost and Utilization Project (23). Inpatient professional fees were estimated using the 2004 National Physician Fee Schedule (online at http://www.cms.hhs.gov/PhysicianFeeSched/) as follows: a Current Procedural Terminology (CPT) code was first assigned to each hospital admission based on the primary procedure or diagnosis. Then, based on the CPT code, we determined the corresponding relative value unit (RVU). Medicare calculates payments in terms of RVUs and issues a conversion factor that converts the RVUs to dollars. The professional fee is the product of the RVU and the 2004 conversion factor of 37.3374. The survey did not include sufficient information to estimate fees for anesthesiologists, pathologists, radiologists, and other specialists. Therefore, the professional fee was multiplied by 1.5 in order to account for these potential additional fees. Sensitivity analyses were also performed using other multipliers (× 1.0 and × 2.0).
Inpatient long-term care costs
Inpatient long-term care hospital costs (that include stays in a rehabilitation facility or a nursing home) were also estimated using methods that approximate Medicare reimbursement. A standard 2004 federal rate of $35,726.18, which is used by Medicare to calculate long-term care costs, was multiplied by a diagnosis-specific RVU to arrive at the full payment amount. A long-term stay admission that is shorter than the expected number of days for a particular diagnosis (as estimated by Medicare) results in a lesser payment.
Outpatient visits to health professionals
Costs for outpatient visits to physicians (general practitioners, rheumatologists, and other specialists) and other health professionals were estimated using the 2004 National Physician Fee Schedule.
Emergency department visits
Costs for emergency department services were estimated by multiplying the number of emergency department visits by an average cost per visit, which included 2 components: professional fees and hospital charges. Professional fees were estimated using the 2004 National Physician Fee Schedule and were estimated based on a moderate level of emergency department care (CPT code 99283). Hospital charges used were derived by estimates made by Bamezai et al (24).
Outpatient surgical procedures
Costs for outpatient surgical procedures were estimated by a method similar to that used for determining inpatient hospital costs. Two cost components were considered: institutional charges and professional fees. Institutional charges were estimated as follows: an ambulatory payment classification (APC) code was assigned to each case based on the procedure being performed. The RVU for the corresponding APC code was then multiplied by the 2004 conversion factor to arrive at the institutional charges. Professional fees were determined in a manner similar to that for inpatient hospitalizations.
Medications
Information regarding medication use was limited to whether the subject was currently taking a particular medication and whether or not they had been taking that medication continuously since the time of the previous interview (1 year). For those subjects taking a particular medication continuously for 1 year, the average annual cost per year (for an average dose of medication) was determined using the Red Book, 2005 edition (25). When subjects had not been taking a particular medication continuously for 1 year, an average treatment duration of 6 months was assumed. We performed sensitivity analyses in order to estimate the differences in costs assuming a shorter (3 months) or longer (9 months) duration.
Renal dialysis
For participants reporting that they had been on dialysis continuously, 12 months of continuous dialysis was assumed. For a small number of participants in whom a kidney transplant obviated the need for dialysis, the number of months of dialysis was estimated based on the date of their surgery. Monthly costs for dialysis, totaling $1,596.60 per month, were derived from estimations made by the US Renal Disease Data System (26).
Changes in work productivity
Productivity costs were determined only for participants of traditional working age (i.e., ≥ 18 years old at age of diagnosis and <65 years old at the time of the study). We first determined work force participation by estimating annual hours engaged in paid employment, both at the year of diagnosis and at the time of the study. The variable annual hours of work was calculated by taking the product of hours of work per week and weeks of work per year. There was incomplete employment data for 14 cases, and these were calculated by substituting mean values. To estimate the mean annual income at the year of diagnosis and at the time of the study (both in 2004 US dollars), we multiplied the number of annual hours of work per year by the average national hourly wage for 2004, $18.09 (27). Changes in work productivity were determined by subtracting annual income at the time of the study from annual income at the time of diagnosis. We also compared annual income at the time of the study with the mean income (in 2004 US dollars) of a normal age- and sex-matched US population derived from the Current Population Survey.
Statistical analysis
Demographic, disease, cost, and employment characteristics were expressed using means, SDs, and proportions, as appropriate. We used univariate and multivariate multiple linear regression to determine predictors of increased costs for both direct and indirect costs. Due to skewness of the direct cost data, the results were log-transformed for the regression model. Covariates in the regression models included age, sex, ethnicity, marital status, education (college degree or higher versus no college degree), disease activity, disease duration, and the SF-12 PCS and MCS scores. All statistical analyses were performed using SPSS statistical software, version 15.0 (SPSS, Chicago, IL).
RESULTS
A total of 812 subjects were included in the analysis. Three subjects were excluded because they did not provide complete health resource use data. Baseline demographic and disease characteristics are shown in Table 1. The majority of subjects were women (92.6%), white (74.0%), and married or living with a partner (58.4%). The mean age at the time of the interview was 48.2 years and the mean age at the time of diagnosis was 34.5 years, corresponding to a mean disease duration of 13.7 years.
Table 1.
Demographic and disease characteristics of study participants*
| Value | |
|---|---|
| Demographic characteristics | |
| Age, mean ± SD years | 48.2 ± 12.8 |
| Female sex | 92.6 |
| White | 74.0 |
| Married or living with a partner | 58.4 |
| Education | |
| Less than high school | 2.6 |
| High school | 11.7 |
| Some college | 26.3 |
| Associate’s degree/trade or vocational school | 20.7 |
| College graduate | 22.7 |
| Postgraduate | 16.0 |
| Employed† | 44.5 |
| Disease characteristics | |
| Age at diagnosis, mean ± SD years | 34.5 ± 13.3 |
| Disease duration, mean ± SD years | 13.7 ± 8.5 |
| Disease activity, mean ± SD SLAQ score‡ | 12.4 ± 8.0 |
| Disease activity, SLAQ score quartiles‡ | |
| <6 | 22.5 |
| 6–11 | 27.5 |
| 12–16 | 21.7 |
| ≥17 | 28.3 |
| SLE flare in past 3 months | 47.4 |
| Selected organ manifestations | |
| Renal | 21.0 |
| On dialysis | 2.6 |
| Pulmonary | 20.7 |
| Pericarditis | 5.1 |
| Seizures | 3.5 |
| SF-12, mean ± SD score | |
| MCS | 47.5 ± 12.9 |
| PCS | 37.3 ± 6.5 |
Values are the percentage unless otherwise indicated. SLAQ = Systemic Lupus Activity Questionnaire; SLE = systemic lupus erythematosus; SF-12 = Medical Outcomes Study Short Form 12; MCS = mental component summary; PCS = physical component summary.
Had a job or did any work for pay or profit.
Range 0–40.
Data pertaining to health care use and average direct health care costs are shown in Table 2. Twenty-one percent of participants had at least 1 acute care hospitalization over the preceding year, and the overwhelming majority (99.9%) had seen a physician in the outpatient setting at least once. Thirty-nine percent had used emergency department services at least once in the previous year, and 2.6% had received dialysis during the previous year. Acute care hospital admissions accounted for almost half of all direct health care costs (48.7%), resulting in a mean annual cost of $6,153 per participant. A quarter of costs were due to medications and 11.6% of costs resulted from outpatient visits to physicians. The estimated total direct health care cost was $12,643. Sensitivity analyses in areas of uncertainty (i.e., professional fees, medications) resulted in a lower estimate of $10,359 and a higher estimate of $13,846 annual direct cost per patient (Table 2).
Table 2.
Health care use and estimated direct health care costs for all study participants (n = 812)
| Resource | Participants consuming resource, % |
Usage among participants consuming resource |
Overall usage, all participants |
Annual cost per person, mean ± SD* |
Share of total direct cost, % |
|---|---|---|---|---|---|
| Direct health care costs | |||||
| Inpatient | |||||
| Acute care hospitalizations | 21.1 | 1.5 | 0.3 | 6,153 ± 19,977 | 48.7 |
| Long-term care hospitalizations | 0.9 | 1 | 0.01 | 177 ± 2,213 | 1.4 |
| Outpatient | |||||
| Total physician visits | 99.9 | 17.8 | 17.8 | 1,465 ± 1,212 | 11.6 |
| Other health professional visits | 41.3 | 22.6 | 9.3 | 186 ± 554 | 1.5 |
| Physiotherapy visits | 21.0 | 16.8 | 3.5 | 201 ± 697 | 1.6 |
| Emergency department visits | 39.1 | 2.3 | 0.9 | 369 ± 816 | 2.9 |
| Outpatient surgical procedures | 21.8 | 1.6 | 0.3 | 408 ± 1,159 | 3.2 |
| Dialysis, months/year | 2.6 | 10.7 | 0.3 | 440 ± 2,828 | 3.5 |
| Medications | 99.0 | 6.1 | 6.0 | 3,244 ± 3,799 | 25.7 |
| Total direct cost | 12,643 ± 23,741 | 100.0 | |||
| Total direct cost (low estimate)† | 10,359 ± 22,727 | ||||
| Total direct cost (high estimate) | 13,846 ± 24,426 |
In 2004 US dollars.
Where uncertainty existed, low and high estimates were made based on varying levels of resource use. For example, the low estimate assumes less intense use of inpatient physician services (1 physician involved in care) and a shorter duration of medication use (3 months).
Employment characteristics and estimated productivity costs are shown in Table 3. In the year of SLE diagnosis, 76.8% of participants had been employed, whereas only 48.7% were employed at the time of the study. As expected, annual hours of employment also decreased since the year of diagnosis, from 1,378.2 hours per year to 899.5 hours per year. Applying the 2004 national average hourly wage of $18.09, the mean income of working-age participants decreased from $24,931 in the year of diagnosis to $16,272 at the time of the study, representing a productivity cost of $8,659. Given that employment characteristics of participants in the year of diagnosis aligned so closely with national averages (Table 3), we can assume that this decrease resulted largely as a consequence of SLE.
Table 3.
Employment status and work productivity at year of diagnosis and current year, and estimated productivity costs among working-age individuals (n = 651)*
| National average† |
Lupus Outcomes Study participants |
Change (current year to diagnosis year) |
||
|---|---|---|---|---|
| Diagnosis year | Current year | |||
| Employed, %‡ | 75.3 | 76.8 | 48.7 | −28.1 |
| Labor force activity, mean ± SD | ||||
| Hours per week | 27.0 | 30.8 ± 20.6 | 19.1 ± 20.8 | −11.7 ± 25.4 |
| Weeks per year | 33.8 | 33.7 ± 21.9 | 24.6 ± 24.6 | −9.1 ± 28.8 |
| Hours per year§ | 1,270.2 | 1,378.2 ± 1,045.4 | 899.5 ± 1,047.6 | −478.6 ± 1,264.8 |
| Estimated annual income, mean ± SD 2004 US dollars¶ | 22,978.68 | 24,931 ± 18,911 | 16,273 ± 18,951 | −8,659 ± 22,880 |
Employment age is ≥18 and <65 years at current year.
Based on data derived from the Current Population Survey for women ages 25–54 years.
Had a job or did any work for pay or profit.
Hours per year is a calculated variable; it represents the mean hours per year per subject (calculated for each subject by taking the product of hours per week and weeks per year).
Estimates based on average national hourly earnings in 2004 US dollars ($18.09).
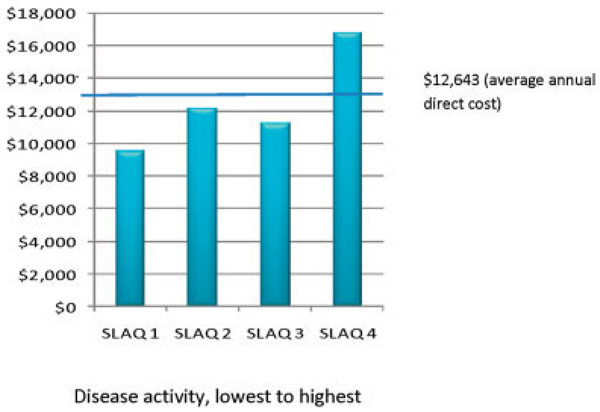
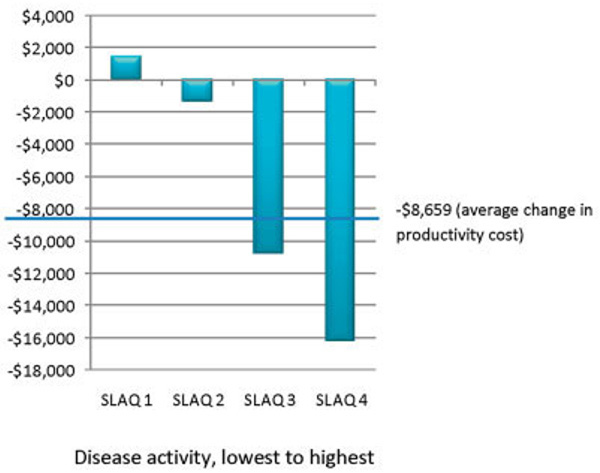
Figures 1 and 2 show the average direct health care and productivity costs for participants with various levels of disease activity, by SLAQ score quartiles. The group of participants with the lowest level of disease activity incurred direct costs of $9,501 (95% confidence interval [95% CI] $6,587, $12,416), whereas the group with the highest level of disease activity incurred direct costs of $16,761 (95% CI $12,702, $20,819) (Figure 1). Productivity costs by disease activity are shown in Figure 2. Participants in the group with the lowest level of disease activity actually experienced an increase in productivity of $−1,476 (95% CI $−4,845, $1,893), whereas the group with the highest level of disease activity incurred considerable productivity losses of $16,163 (95% CI $13,029, $19,297).
Figure 1.
Direct health care costs (in 2004 US dollars) by disease activity (Systemic Lupus Activity Questionnaire [SLAQ] score quartile).
Figure 2.
Change in productivity (in 2004 US dollars) since diagnosis by disease activity (Systemic Lupus Activity Questionnaire [SLAQ] score quartile).
Table 4 shows results of multivariate regression analyses of predictors of direct health care costs and productivity costs. Due to skewness of direct cost data, the regression analysis was performed on log-transformed direct costs. Older age predicted lower costs, whereas greater disease activity (as expressed by the SLAQ score), greater disease duration, and worse physical and mental health status (i.e., lower scores on the SF-12 PCS and MCS) predicted higher costs. Productivity costs were not skewed, and so results were not log-transformed for the regression analysis. Predictors of higher productivity costs included older age, greater disease activity, and worse physical and mental health status.
Table 4.
Predictors of direct health care and productivity costs*
| Direct health care costs, all participants† |
Productivity costs, participants age <65 years |
|||
|---|---|---|---|---|
| Regression-adjusted coefficient (95% CI) |
P | Regression-adjusted coefficient (95% CI) |
P | |
| Age, years | −0.007 (−0.013, −0.001) | 0.032 | 342.6 (151.8, 533.4) | < 0.0001 |
| Female sex | −0.158 (−0.458, 0.142) | 0.302 | −202.1 (−7,692.4, 7,288.3) | 0.627 |
| White‡ | −0.153 (−0.331, 0.025) | 0.091 | −1,670.7 (−5,211.9, 1,870.4) | 0.355 |
| Marital status§ | −0.105 (−0.260, 0.048) | 0.178 | 1,248.2 (−1,817.6, 4,313.9) | 0.424 |
| Education¶ | −0.033 (−0.194, 0.128) | 0.691 | −2,893.3 (−7,212.7, 1,432.1) | 0.190 |
| Disease activity, SLAQ score | 0.019 (0.005, 0.033) | 0.007 | 263.5 (16.1, 510.9) | 0.037 |
| Disease duration, years | 0.017 (0.008, 0.026) | 0.000 | −53.8 (−238.6, 131.1) | 0.568 |
| SF-12 | ||||
| MCS | −0.039 (−0.052, −0.025) | 0.000 | −345.4 (−489.0, −201.7) | < 0.0001 |
| PCS | −0.019 (−0.027, −0.012) | 0.000 | −277.5 (−530.9, −24.1) | 0.032 |
95% CI = 95% confidence interval; SLAQ = Systemic Lupus Activity Questionnaire; SF-12 = Medical Outcomes Study Short Form 12; MCS = mental component summary; PCS = physical component summary.
Due to skewness of direct cost data, a logarithmic transformation was performed prior to regression analysis.
White race versus other.
Currently married or living with a partner.
College graduate.
DISCUSSION
We have estimated the annual costs incurred by persons with SLE in a large US cohort, including the direct costs associated with the provision of health care and the costs associated with changes in work productivity. This analysis indicates that persons with SLE incur a mean annual direct cost of $12,643 in 2004 US dollars. Participants of working age incurred mean annual productivity costs of $8,659, assuming an average national hourly wage of $18.09. Our regression analyses showed that the strongest predictors of increased direct costs were greater disease activity, longer disease duration, and worse mental and physical health status (as assessed by the SF-12 health status questionnaire), whereas older age predicted lower direct costs. Older age has been shown to be a predictor for decreased costs in one other study of SLE patients (15), whereas disease activity and worse physical health status have been shown to predict higher costs (14,15). With respect to productivity costs, older age, greater disease activity, and worse physical and mental health status predicted higher costs. These predictors are similar to those seen in previous studies, which also showed disease activity and physical health status to be significant predictors of productivity costs (14,15).
The published literature contains few studies of costs in SLE. Although differences in methodologies make comparisons difficult, a discussion of some of these studies will help put our results in perspective. One of the most comprehensive is the Tri-National Study by Clarke et al, in which health resource use was compared among SLE patients attending tertiary care clinics in 3 different countries: the US, Canada, and the UK (11). In order to facilitate comparisons among patients from the 3 countries, this analysis valued resources using only Canadian prices. Mean cumulative direct costs per patient over 4 years in the US, Canada, and the UK were $20,244, $15,845, and $17,647, respectively (2002 Canadian dollars). Therefore, annual direct costs in the US were estimated at approximately $5,061 (2002 Canadian dollars) or $3,347 (2004 US dollars). Our study used American prices, which are often much higher than prices in other countries (28), and has likely resulted in more accurate costs for persons with SLE living in the US. In the only other study assessing health resource use in SLE patients from the US, Nichol and colleagues assessed the association between ethnicity, resource use, and direct medical costs in a population-based study of California Medicaid patients with SLE (29). This study demonstrated that all ethnic groups incurred similar monthly costs at study entry (approximately $900 in 2002 US dollars); however, over 3 years, Hispanics had increasingly lower monthly costs (approximately $200 at 3 years), whereas the costs incurred by whites and African Americans remained similar to the baseline level.
Health care costs of several rheumatic diseases were compared in a study by Huscher et al using a German national database (14). This study showed that patients with SLE incurred mean annual direct costs of €3,191, whereas patients with RA incurred costs of €4,737, patients with ankylosing spondylitis €3,676, and patients with psoriatic arthritis €3,156. Direct costs were considerably lower in patients with SLE than in patients with RA; however, this difference was due in large part to differences in the cost of medications. Patients with RA incurred mean total medication costs of €1,843, representing 38.9% of total direct costs, whereas patients with SLE incurred mean total medication costs of €850, representing 26.6% of total direct costs. Although there were important differences in the methodologies used and the health care systems studied, medication costs represented a very similar percentage of total direct costs in our study (25.7%). However, as seen in RA, the proportion of direct costs attributable to medications will likely increase in the future, because newer and more expensive therapies are introduced in the treatment of SLE (7).
A comparison of our estimated health care costs with national average per capita expenditures for a similar array of services highlights the high costs incurred in the care of persons with SLE. In 2004, the average per capita expenditure in the US for hospital and nursing home care, physician and clinical services, other professional services, and prescription drugs was $4,482 (30). In our study, persons with SLE incurred approximately 3-fold greater direct health care costs than the national per capita average. Participants with the lowest level of disease activity incurred almost twice this national average, whereas those with the highest level of disease activity incurred 4 times the national average per capita cost.
A number of published studies have evaluated work productivity among persons with SLE, while only 2 have calculated productivity costs. Again, methodologies among studies differ considerably and comparisons should be made with caution. In a followup to the Tri-Nation Study by Clarke et al described above, productivity costs over a 4-year period were calculated for patients with SLE from the US, Canada, and the UK. To facilitate comparisons, Canadian age- and sex-matched average wages were used to estimate costs for subjects from all 3 countries (12). Over 4 years, cumulative costs due to diminished productivity in paid labor in the US, Canada, and the UK were $56,745, $38,642, and $42,213 (2002 Canadian dollars), respectively. Therefore, annual productivity cost in the US was estimated at approximately $14,186 (2002 Canadian dollars) or $9,384 (2004 US dollars). In that study, participants were queried about the number of hours they missed from their current work schedule and the number of additional hours they would work if not ill. In contrast, our study, which showed mean productivity costs of $8,659, assessed the difference between the actual number of hours participants reported working in the year their SLE was diagnosed and the number of hours they currently work. Our methods allowed us to capture both decreases and increases in productivity, which may explain the somewhat lower productivity costs in our study. In fact, for certain participants with low levels of disease activity, work productivity actually increased since SLE diagnosis. The study by Huscher et al, making assessments based on sick leave and permanent work disability, estimated even higher productivity costs (14). Patients with SLE incurred mean annual productivity costs of €11,220, which converts to approximately $10,485 (2004 US dollars).
A number of studies have assessed work disability in SLE without calculating associated productivity costs (31,32). Two German studies assessed employment rates of individuals with SLE and compared them with other rheumatic conditions and with the general population. After appropriate matching, an equal proportion of SLE and RA patients (46%) remained employed (32). Standardized employment ratios (SERs; ratio of observed to expected number of patients employed) were used to compare employment rates between individuals with SLE and a matched normal population. For individuals with a disease duration less than 6 years, employment rates did not differ from the general population; however, with greater disease duration, fewer individuals with SLE were working (SER 0.8 at 6–10 years and SER 0.68 at >10 years). Yelin et al, using data from the LOS cohort used in the present study, showed that employment declined from 74% at the time of SLE diagnosis to 48% at the time of the study (2004) (16). Among participants working at diagnosis, the proportion employed declined by 15%, 36%, and 63% after 5, 10, and 20 years, respectively.
As with all studies that rely on patient-reported data, recall bias may be a potential limitation of our study, which required participants to recall use and employment over a 1-year period. Although there is little information regarding the ideal frequency and recall period for patient-reported economic questionnaires, a shorter recall period may have been preferred. For example, in one study, patient-reported questionnaires administered every 3 months yielded results similar to payer data (33). Furthermore, we were not able to separate costs that were related to SLE from those that were not. As such, all health care costs were considered in our estimations of direct costs, and not only the additional costs incurred as a result of SLE. Although we attempted to assess costs from a societal perspective, which include all costs regardless of payer, we were not able to evaluate all cost components. Certain direct costs, such as those incurred for transportation and for diagnostic tests obtained in the outpatient setting, were not included. Our analysis of productivity costs did not capture diminished productivity in nonpaid work such as housework and childrearing, which may be particularly relevant in diseases that affect a predominantly female population (10). Furthermore, our study design did not allow us to provide estimates of productivity costs using the friction method, which assumes that the economic cost to society is limited to the period required to replace the disabled employee, and typically generates lower estimates. Finally, although participants of this study reside in 41 different states, the majority (72%) resides in California, and so inference beyond California is limited.
In summary, SLE imposes a considerable financial burden, not only because of the health care resources that are consumed in the management of this complex disease, but also because of the loss of productivity due to work disability. The hope is that efforts to improve the care and treatment of persons with SLE will improve outcomes and help offset these considerable personal and societal costs.
Acknowledgments
This study was carried out in part in the General Clinical Research Center, Moffitt Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources (grant 5-M01-RR-00079), US Public Health Service. Dr. Panopalis’ work was supported by the Canadian Institutes of Health Research Fellowship. Dr. Criswell’s work and the Rosalind Russell Medical Research Center for Arthritis were supported by the NIH (grants K24-AR0-2175 and R01-AR-44804). Dr. Yelin’s work was supported by the State of California Lupus Fund, the Arthritis Foundation, the Agency for Healthcare Research and Quality/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1-R01-HS0-13893), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant P60-AR0-53308).
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Panopalis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Panopalis, Yazdany, Hersh, Criswell, Yelin.
Acquisition of data. Trupin, Criswell, Yelin.
Analysis and interpretation of data. Panopalis, Yazdany, Gillis, Trupin, Hersh, Katz, Yelin.
Manuscript preparation. Panopalis, Yazdany, Gillis, Julian, Hersh, Criswell, Katz.
Statistical analysis. Panopalis, Yazdany, Trupin.
REFERENCES
- 1.Yelin E, Callahan LF for the National Arthritis Data Work Group. The economic cost and social and psychological impact of musculoskeletal conditions. Arthritis Rheum. 1995;38:1351–1362. doi: 10.1002/art.1780381002. [DOI] [PubMed] [Google Scholar]
- 2.Yelin E, Cisternas MG, Pasta DJ, Trupin L, Murphy L, Helmick CG. Medical care expenditures and earnings losses of persons with arthritis and other rheumatic conditions in the United States in 1997: total and incremental estimates. Arthritis Rheum. 2004;50:2317–2326. doi: 10.1002/art.20298. [DOI] [PubMed] [Google Scholar]
- 3.Yelin E, Herrndorf A1, Trupin L, Sonneborn D. A national study of medical care expenditures for musculoskeletal conditions: the impact of health insurance and managed care. Arthritis Rheum. 2001;44:1160–1169. doi: 10.1002/1529-0131(200105)44:5<1160::AID-ANR199>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Ward MM, Javitz HS, Yelin EH. The direct cost of rheumatoid arthritis. Value Health. 2000;3:243–252. doi: 10.1046/j.1524-4733.2000.34001.x. [DOI] [PubMed] [Google Scholar]
- 5.Lajas C, Abasolo L, Bellajdel B, Hernandez-Garcia C, Carmona L, Vargas E, et al. Costs and predictors of costs in rheumatoid arthritis: a prevalence-based study. Arthritis Rheum. 2003;49:64–70. doi: 10.1002/art.10905. [DOI] [PubMed] [Google Scholar]
- 6.Ruof J, Hulsemann JL, Mittendorf T, Handelmann S, von der Schulenburg JM, Zeidler H, et al. Costs of rheumatoid arthritis in Germany: a micro-costing approach based on healthcare payer’s data sources. Ann Rheum Dis. 2003;62:544–549. doi: 10.1136/ard.62.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum. 2003;48:2750–2762. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford) 2005;44:1531–1537. doi: 10.1093/rheumatology/kei049. [DOI] [PubMed] [Google Scholar]
- 9.Maetzel A, Li LC, Pencharz J, Tomlinson G, Bombardier C. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis. 2004;63:395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke AE, Penrod J, St Pierre Y, Petri MA, Manzi S, Isenberg DA, et al. for the Tri-Nation Study Group. Underestimating the value of women: assessing the indirect costs of women with systemic lupus erythematosus. J Rheumatol. 2000;27:2597–2604. [PubMed] [Google Scholar]
- 11.Clarke AE, Petri M, Manzi S, Isenberg DA, Gordon C, Senecal JL, et al. for the Tri-Nation Study Group. The systemic lupus erythematosus Tri-Nation study: absence of a link between health resource use and health outcome. Rheumatology (Oxford) 2004;43:1016–1024. doi: 10.1093/rheumatology/keh229. [DOI] [PubMed] [Google Scholar]
- 12.Panopalis P, Petri M, Manzi S, Isenberg DA, Gordon C, Senecal JL, et al. The Tri-Nation Study Group. The systemic lupus erythematosus Tri-Nation study: cumulative indirect costs. Arthritis Rheum. 2007;57:64–70. doi: 10.1002/art.22470. [DOI] [PubMed] [Google Scholar]
- 13.Clarke AE, Petri MA, Manzi S, Isenberg DA, Gordon C, Senecal JL, et al. for the Tri-Nation Study Group. An international perspective on the well being and health care costs for patients with systemic lupus erythematosus. J Rheumatol. 1999;26:1500–1511. [PubMed] [Google Scholar]
- 14.Huscher D, Merkesdal S, Thiele K, Zeidler H, Schneider M, Zink A. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65:1175–1183. doi: 10.1136/ard.2005.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutcliffe N, Clarke AE, Taylor R, Frost C, Isenberg DA. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:37–47. doi: 10.1093/rheumatology/40.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57:56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Labor, Bureau of Labor Statistics. How the government measures unemployment. 2001 URL: http://www.bls.gov/cps/cps_htgm.htm.
- 20.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–286. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 21.Yazdany J, Yelin EH, Panopalis P, Trupin L, Julian L, Katz PP. Validation of the Systemic Lupus Erythematosus Activity Questionnaire in a large observational cohort. Arthritis Rheum. 2008;59:136–143. doi: 10.1002/art.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. 2007 URL: http://www.hcup-us.ahrq.gov/home.jsp.
- 24.Bamezai A, Melnick G, Nawathe A. The cost of an emergency department visit and its relationship to emergency department volume. Ann Emerg Med. 2005;45:483–490. doi: 10.1016/j.annemergmed.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Fleming T. Red Book: pharmacy’s fundamental reference. Montvale (NJ): Thomson; 2005. [Google Scholar]
- 26.US Renal Disease Data System. Costs of CKD and ESRD. 2005 URL: http://www.usrds.org/2007/pdf/11_econ_07.pdf?zoom_highlight=Costs+of+CKD+and+ESRD.
- 27.US Bureau of Labor Statistics. National Compensation Survey: occupational wages in the United States, July 2004. 2005 URL: http://www.bls.gov/ncs/home.htm.
- 28.Anderson GF, Reinhardt UE, Hussey PS, Petrosyan V. It’s the prices, stupid: why the United States is so different from other countries. Health Aff (Millwood) 2003;22:89–105. doi: 10.1377/hlthaff.22.3.89. [DOI] [PubMed] [Google Scholar]
- 29.Nichol MB, Shi S, Knight TK, Wallace DJ, Weisman MH. Eligibility, utilization, and costs in a California Medicaid lupus population. Arthritis Rheum. 2004;51:996–1003. doi: 10.1002/art.20819. [DOI] [PubMed] [Google Scholar]
- 30.US Census Bureau. Table 125: health services and supplies. Per capita expenditures by object: 1990 to 2005. 2008 URL: http://www.census.gov/compendia/statab/tables/08s0125.pdf.
- 31.Mau W, Listing J, Huscher D, Zeidler H, Zink A. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol. 2005;32:721–728. [PubMed] [Google Scholar]
- 32.Zink A, Fischer-Betz R, Thiele K, Listing J, Huscher D, Gromnica-Ihle E, et al. for the German Collaborative Arthritis Centers. Health care and burden of illness in systemic lupus erythematosus compared to rheumatoid arthritis: results from the National Database of the German Collaborative Arthritis Centers. Lupus. 2004;13:529–536. doi: 10.1191/0961203304lu1054oa. [DOI] [PubMed] [Google Scholar]
- 33.Merkesdal S, Ruof J, Huelsemann JL, Mittendorf T, Handelmann S, Mau W, et al. Indirect cost assessment in patients with rheumatoid arthritis (RA): comparison of data from the health economic patient questionnaire HEQ-RA and insurance claims data. Arthritis Rheum. 2005;53:234–240. doi: 10.1002/art.21080. [DOI] [PubMed] [Google Scholar]