Synthesis of multimodal nanoprobes for use as diagnostic and therapeutic agents is a goal in the emerging field of nanomedicine.1 The medical use of radionuclides has evolved and increased during the last thirty years.2 β-emitters have the advantage of a relatively short penetration range thus providing localized ionizing radiation and the possibility of targeted applications. β–emitters show promise in the treatment of a wide variety of conditions from joint pain to tumors.3 Delivery mechanisms for radionuclides have focused on chelating agents.3 Unfortunately, due to chemical interactions or decay events, the radionuclide or the decay product may be released.
Incarceration of atoms inside fullerene cages, e. g., clusters of several atoms inside an Ih C80 cage,4 provides an ideal delivery platform for nanomedicines, since the metal ion is isolated from the biosystem. Our previous work on the trimetallic nitride templated endohedral metalofullerenes (TNT EMF) demonstrated Lu3N@C80 as an x-ray contrast agent5 and Gd3N@C80 as an MRI contrast agent with relaxivities 30-40 times higher than commercial MRI agents.6 Other groups have reported radiolabeled endohedral metallofullerenes (R-EMF), but there is a paucity of reports describing multimodal nanoprobes.7 For example, Diener and coworkers have reported preparation of 212Pb@C60, an α emitting potential radiopharmaceutical, but without a targeting modality.7c
In this communication, we describe the encapsulation of the β–emitter 177Lu in a fullerene cage and demonstrate that for a period of at least one half-life (6.7 days) the encapsulated 177Lu3+ ions are not released. It should be noted that 177Lu has an emission spectrum that includes gamma radiation which is detectable, for example, by SPECT imaging. We also demonstrate that this agent can be conjugated with an interleukin-13 (IL-13) peptide that is designed to target an overexpressed receptor in Glioblastoma Multiforme tumors. 8
The TNT EMF was synthesized in a quartz Kräschmer-Huffman electric generator that can be controlled behind a radiation shield.9 Graphite rods containing Lu2O3 and radioactive 177LuCl3 were vaporized. Then the chamber was remotely washed with a toluene spray, driving the products to the bottom of the quartz reactor where the soot particles were collected in a filter. The solution was collected and purified through a Merrifield resin column.10
The inclusion of the radiolabel was confirmed by high performance liquid chromatography (HPLC) with UV-vis and radioactivity detectors (see SI) with a radiolabeled yield averaged over several experiments of ~0.02 % The retention times with both detectors matched that of non-radioactive Lu3N@C80.
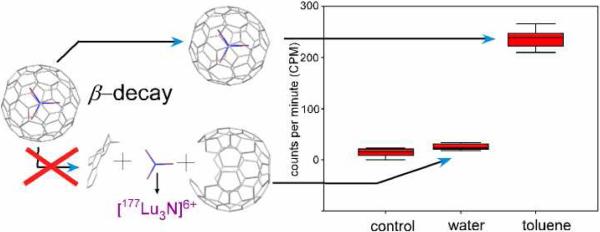
A necessary characteristic for this nanomedical agent is that the cage remains intact during the radioactive decay. The cage integrity was investigated by allowing a toluene solution containing 177LuxLu(3-x)N@C80 to equilibrate with water for a 177Lu half-life (6.7 days). Samples were extracted from each solvent layer and counted on a gamma counter, along with deionized water control samples (Figure 1). The toluene extract showed a mean of 240 counts per minute (cpm), while the water extract and the control were not significantly different, thus demonstrating that the 177Lu was retained in the toluene solvent layer. This result corroborates findings for other R-EMFs,7 and shows that the cage is robust enough to survive a β-decay process.
Figure 1.
Left: illustration of possible cage breakdown or retention due to β-decay events. Right: box plots corresponding to the control, aqueous and toluene extracts of 177LuxLu(3-x)N@C80 after 6.7 days.
In Glioblastoma Multiforme, the most common and lethal brain tumor in humans (median survival ~1 year), an interleukin-13 (IL-13) receptor is over-expressed but not in normal brain tissue.8 The receptor for IL-13 in glioma cells is the IL-13Rα2 receptor.11 Thus, a peptide was chosen for conjugation to the 177LuxLu(3-x)N@C80 that possesses the binding sequence to the IL-13 receptor and a fluorescent tag.
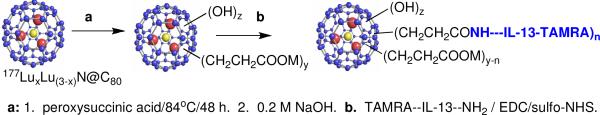
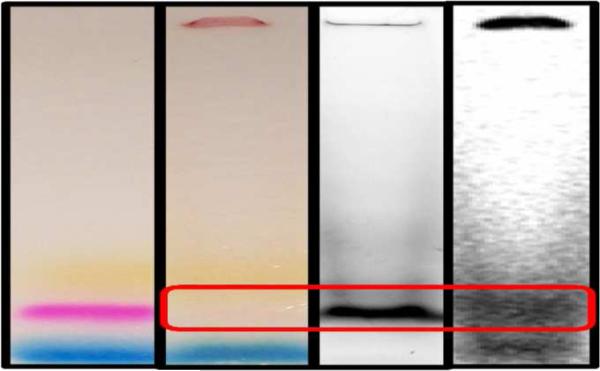
177LuxLu(3-x)N@C80 was functionalized and conjugated to the IL-13 peptide (Scheme 1) via previously reported methods.12,13 Successful conjugation was verified by polyacrylamide gel electrophoresis (PAGE) separation and co-localization of signals from each component. As seen in Figure 2 (from left to right), the first lane is a visible image of the TAMRA (tetramethyl-6-carboxyrhodamine) labeled IL-13 peptide alone showing the pink color of the TAMRA tag between the blue and yellow loading buffer dyes. Lane 2 is a visible image of the 177LuxLu(3-x)N@C80-TAMRA-IL-13 peptide reaction product run on the same gel, and lanes 3 and 4 are fluorescent and autoradiograph images of lane 2, respectively. While the visible location of the 177LuxLu(3-x)N@C80-TAMRA-IL-13 peptide is difficult to see, the fluorescent signal from the TAMRA is clearly seen in lane 3 and a corresponding radioactive signal from the 177Lu is seen in lane 4.
Scheme 1.
Functionalization and conjugation of 177LuxLu(3-x)N@C80. with TAMRA labeled IL-13 peptide. Z = ~26, y = ~16, n = 1 or 2.
Figure 2.
PAGE Images. From left to right: TAMRA labeled IL-13 peptide alone, visible lane; 177LuxLu(3-x)N@C80-TAMRA-IL-13 peptide lanes: visible, fluorescent and autoradiograph. Red rectangle shows the 177LuxLu(3-x)N@C80-TAMRA-IL-13 peptide conjugate.
All of these signals in lanes 2-4 (red box) are aligned adjacent to the TAMRA labeled IL-13 peptide alone in lane 1, with proper alignment being confirmed by the loading buffer dyes and the residual reaction product that became insoluble and remained at the top of the gel. It is also evident that the conjugation reaction leaves some unconjugated 177LuxLu(3-x)N@C80 (bottom, lane 4), which can be removed by HPLC.
In this paper, we have reported the successful incorporation of 177Lu into the endohedral metallofullerene Lu3N@C80 cage using a modified Krätschmer-Huffman apparatus that allows remote preparation and extraction in an atmospherically controlled environment. We also show that the radiolabeled 177Lu ions are not readily removed from the fullerene cage and we demonstrate potential targeting with a conjugated IL-13 protein. This nanoparticle delivery platform provides flexibility to meet a wide range of other radiotherapeutic and radiodiagnostic applications (e.g., with 166Ho and 90Y).
Supplementary Material
Acknowledgments
We are grateful for support from the National Institutes of Health (VCU/VT NCI Platform Grant R01 CA119371), the National Science foundation (NIRT DMR-0507083), the Center for Innovative Technology, Commonwealth of Virginia; the Virginia Commonwealth Technology Research Fund (CTRF). We also appreciate the assistance of Tom Wertalik, glassblower, who constructed the quartz apparatus.
Footnotes
Supporting information available. Synthetic details and characterization data (HPLC and MS) are included. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, Bonn GK. Int. J. Nanomed. 2007;2:639–49. [PMC free article] [PubMed] [Google Scholar]
- 2.Volkert W, Hoffman T. Chem. Rev. 1999;99:2269–2292. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]; Kowalsky RJ, Falen SW. Radiopharmaceuticals in nuclear pharmacy and nuclear medicine. 2nd ed. APhA, American Pharmacists Association; Washington, D.C.: 2004. [Google Scholar]
- 3.Kneifel S, Bernhardt P, Uusijärvi H, Good S, Plasswilm L, Buitrago-Téllez C, Müller-Brand J, Mäcke H, Merlo A. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1388–95. doi: 10.1007/s00259-006-0351-8. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson S, Rice G, Glass T, Harich K, Cromer F, Jordan MR, Craft J, Hadju E, Bible R, Olmstead MM, Maitra K, Fisher AJ, Balch AL, Dorn HC. Nature. 1999;401:55–57. [Google Scholar]
- 5.Iezzi E, Duchamp J, Fletcher K, Glass T, Dorn H. Nano Lett. 2002;2:1187–1190. [Google Scholar]
- 6.Fatouros PP, Corwin F, Chen Z-J, Broaddus W, Tatum J, Kettenmann B, Ge Z, Gibson HW, Russ J, Leonard A, Duchamp J, Dorn HC. Radiology. 2006;240:756–64. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 7.a Grushko Y, Khodorkovski M, Kozlov V, Shilin V, Grachev S, Artamonova T. Fullerenes, Nanotubes and Carbon Nanostructures. 2006;14:249–259. [Google Scholar]; b Akiyama K, Haba H, Tsukada K, Asai M, Toyoshima A, Sueki K, Nagame Y, Katada M. J. Radioanal. Nucl. Chem. 2009;280:329–331. [Google Scholar]; c Diener MD, Alford JM, Kennel SJ, Mirzadeh S. J. Am. Chem. Soc. 2007;129:5131–5138. doi: 10.1021/ja068639b. [DOI] [PubMed] [Google Scholar]
- 8.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Amer. Assoc. Cancer Res. 1999:985–990. [PubMed] [Google Scholar]; Debinski W, Miner R, Leland P, Obiri NI, Puri RK. J. Biol. Chem. 1996;271:22428–22433. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]; Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Clin. Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 9.Kratschmer W, Lamb L, Fostiropoulos K, Huffman D. Nature. 1990;347:354–358. [Google Scholar]
- 10.Ge Z, Duchamp J, Cai T, Gibson HW, Dorn HC. J. Am. Chem. Soc. 2005;127:16292–16298. doi: 10.1021/ja055089t. [DOI] [PubMed] [Google Scholar]
- 11.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Madhankumar AB, Mintz A, Debinski W. Neoplasia. 2004;6:15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillmore HL, Shultz MD, Henderson S, Cooper P, Broaddus WC, Chen ZJ, Chun-Ying S, Dorn HC, Corwin F, Hirsch JI, Wilson JD, Fatouros P. Neuro-Oncology. 2009;11(5):593. [Google Scholar]
- 13.Shu C-Y, Ma X-Y, Zhang J-F, Corwin F, Sim J, Zhang E-Y, Dorn HC, Gibson HW, Fatouros P, Wang C-R, Fang X-H. Bioconj. Chem. 2008;19:651–655. doi: 10.1021/bc7002742. [DOI] [PubMed] [Google Scholar]; Shu C, Corwin F, Zhang J, Chen Z, Reid J, Sun M, Xu W, Sim J, Wang C, Fatouros P, Esker A, Gibson HW, Dorn HC. Bioconj. Chem. 2009;20:1186–1193. doi: 10.1021/bc900051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.