Abstract
Most of the gap junction proteins are regulated in part by post-translational phosphorylation. Phosphorylation has been shown to be important in gap junction assembly and turnover, and for channel function in the resting state. Connexin phosphorylation may be altered by the activation of intracellular signaling pathways in response to growth factors, tumor promoters, activated oncogenes, hormones and inflammatory mediators. In some instances altered phosphorylation has been associated with changes in connexin function and in other cases appears to be associated with changes in the levels of the connexin protein and/or mRNA. This review focuses on the role of tyrosine protein kinases in the regulation of gap junctions. The literature is most extensive for connexin43 and those studies are reviewed here. A great deal has been learned in recent years about how connexin43 is regulated by tyrosine kinase-dependent signaling pathways. These pathways are often complex and to some extent are cell type- and stimulus-dependent. Although considerable progress has been made in unraveling the cellular pathways that regulate connexin function, significant challenges remain to be addressed in identifying additional phosphorylation sites and determining the stoichiometries of the phosphorylation events that regulate connexin function and it's interaction with other cellular proteins.
Keywords: Connexin, Gap junction, Tyrosine protein kinase, Src, EGF receptor, MAP kinase
1. Introduction
Connexin proteins span the membrane four times, such that the amino terminus, carboxyl terminus and an intracellular loop are formed on the cytoplasmic side of the membrane and two extracellular loops are formed on the outside surface of the cell. Most of the connexin proteins are phosphorylated in vivo, primarily on serine residues with smaller amounts of phosphothreonine or phosphotyrosine reported for some of the connexins. Connexin43 (Cx43) is one of the most ubiquitously expressed connexins and much is known about its phosphorylation. A basal level of serine phosphorylation on at least five sites appears to be required for the assembly and function of Cx43 gap junctions [1–5]. An increasing number of studies have implicated growth factors, tumor promoters, oncogene protein kinases, hormones and inflammatory mediators in the regulation of gap junctional communication (GJC) through phosphorylation events occurring on the C-terminal portion of the protein, ~ aa 236–382 [6–13]. A number of the serine kinases that target Cx43 in stimulated cells have been identified (reviewed in Ref. [14]) including PKC (Ser368 and Ser372), MAP kinases (Ser255, Ser279, and Ser282), cdc2/cyclin B (Ser255), and casein kinase I (Ser325, Ser328 and Ser330). v-Src and v-Fps are two of the tyrosine kinases that have been shown to target tyrosine residues in Cx43.
Some of the phosphorylation-induced effects on Cx43 appear to occur directly, such as the tyrosine phosphorylation of Cx43 induced by v-Src and the physical interaction of Cx43 with v-Src that is associated with the disruption of GJC [15–17]. These events lead to a chronic and dramatic down-regulation of GJC in v-Src cells [9–11]. In other instances, a tyrosine kinase such as activated c-Src has been implicated in intracellular signaling pathways that influence Cx43 function, but tyrosine phosphorylation on Cx43 has not been detected and the disruption of GJC may be transient [18–21]. This suggests that the actions of c-Src may be indirect in these cases and dependent on the activation of other kinases such as the Ser/Thr kinases, mitogen-activated protein (MAP) kinase and protein kinase C (PKC) to mediate the effects on GJC. In still other cases, protein phosphorylation does not appear to be directly involved in channel regulation, but rather there is a loss of Cx43 from gap junction plaques in the plasma membrane and this may be associated with a change in the levels of the Cx43 mRNA [22–26]. The events that lead to these changes in Cx43 expression are not well understood. Activation of some intracellular signaling pathways has been shown to up-regulate GJC, generally through an increase in Cx43 protein and mRNA. These effects may be more long-term, occurring at 6 or more hours following the activation of intracellular signaling [27,28]. The regulation of GJC appears to be complex and has been difficult to dissect, since multiple signaling pathways impinge on Cx43 in different cell types and under different stimulatory conditions. Some of the studies of the regulation of Cx43-mediated GJC by activated tyrosine protein kinases are described in the following sections of this review. They represent some of the spectrum of signaling events that are thought to be involved in the regulation of the Cx43 protein and/or GJC.
This review focuses on the tyrosine kinase-dependent phosphorylation of Cx43, because this represents one of the better-understood mechanisms involved in the regulation of GJC. Many growth factor receptors are tyrosine kinases, as are oncoprotein kinases such as v-Src and v-Fps. In addition, the inhibition of protein tyrosine-phosphatases [29] has been shown to alter Cx43 regulation. Key intracellular signaling pathways utilize the actions of tyrosine protein kinases/tyrosine protein phosphatases to exquisitely regulate the transmission of molecular signals to downstream effectors and adaptor proteins [30]. This is particularly important to the regulation of normal cell growth, hyperproliferative diseases such as cancer, gene transcription, the actions of the insulin receptor, and the synchronization of contraction in the heart and the uterus. Protein tyrosine kinases induce protein–protein interactions through the creation of phosphotyrosine binding sites for SH2- or PTB-containing proteins [31–35]. This allows transmission of a signal from an activated growth factor receptor to downstream kinases that may mediate increased serine phosphorylation on Cx43. Activation and autophosphorylation of the EGF receptor tyrosine kinase provides phosphotyrosine-docking sites for SH2-containing effector molecules like Grb2 and Shc and leads to the activation of the Ras/Raf/MEK/MAP kinase signaling pathway. In the case of v-Src, the direct phosphorylation of Cx43 on Tyr265 provides a docking site for the SH2 domain of v-Src, leading to an enhanced interaction with Cx43 and the phosphorylation of additional tyrosine sites in Cx43 by the v-Src kinase. Many of the kinases that are important to intracellular signaling pathways induce the phosphorylation of Cx43 and may be involved in the regulation of GJC. The cytoplasmic C-terminal domain of Cx43 is particularly rich in potential kinase phosphorylation target sites and regulatory binding domains that may participate in directing Cx43's interactions with other cellular proteins. Although there is a considerable body of data available on the regulation of Cx43 and GJC by the tyrosine protein kinases, there is some controversy associated with this field and there are a number of areas where additional careful studies are warranted.
This review does not cover some of the other aspects of the regulation of gap junctions by protein phosphorylation. Several recent reviews have addressed some of these other areas of importance to this field, including the role of protein phosphatases in the regulation of gap junctions [14,29,36]. We have provided some background on the subject of Cx43 phosphorylation, but have concentrated on the more recent developments and more-studied examples of the regulation of Cx43 by tyrosine protein kinases. We apologize in advance for the many studies that are not discussed here and that have also provided important contributions to the understanding of the regulation of GJC by connexin protein phosphorylation.
2. Non-receptor tyrosine protein kinases
Phosphorylation of Cx43 by the v-Src non-receptor tyrosine kinase represents one of the most-studied examples of how GJC is regulated by tyrosine protein kinases. In early work, several laboratories demonstrated that activated non-receptor tyrosine kinases, such as pp60v-src and p130gag-fps, promoted Cx43 phosphorylation on tyrosine, and this was associated with a marked disruption of gap junctional intercellular communication [9–11,13]. v-Src and v-Fps have some cellular substrates in common [37] and the Cx43 protein may be an additional biologically relevant substrate for both of these tyrosine kinases. Cx43 appeared to be phosphorylated on similar sites in cells expressing these oncoprotein kinases as demonstrated by the migration patterns of tyrosine-containing tryptic peptides of Cx43 that was isolated from 32Pi labeled cells [9].
2.1. v-Src-induced Cx43 phosphorylation
A Tyr265Phe mutant of Cx43 produced functional gap junction channels when expressed in Xenopus oocytes, but unlike the channels formed by the wild-type Cx43 protein, these channels were not closed by the co-expression of the pp60v-src kinase [13]. These data provided the first evidence of the critical role for phosphorylation at the Tyr265 site of Cx43 in the v-Src mediated disruption of GJC. Activated pp60src appeared to directly phosphorylate Cx43 as demonstrated by in vitro studies using purified proteins, and phosphorylation occurred on tryptic peptides that co-migrated with a subset of the tryptic peptides obtained from Cx43 phosphorylated in vivo in Rat1 v-Src cells [16]. Consistent with the hypothesis that Cx43 served as a direct substrate for v-Src, Cx43 co-localized with the v-Src kinase at the plasma membrane regions of cell-to-cell contact [17] and appeared to interact directly with v-Src as demonstrated by reciprocal co-immunoprecipitation studies in Rat1 v-Src cells [15,17].
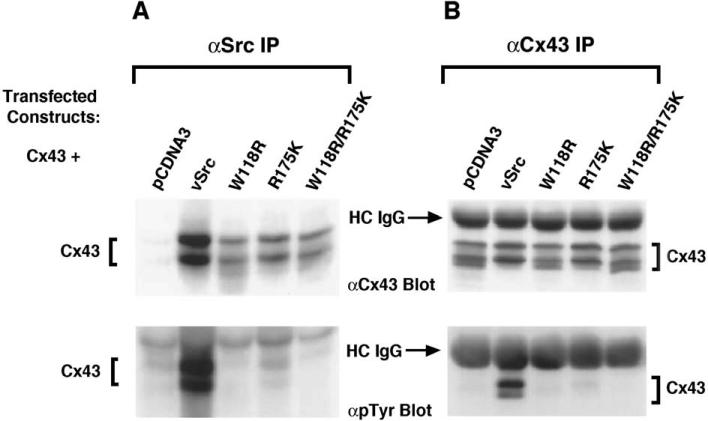
The interactions of v-Src and Cx43 were found to be dependent upon the intact SH3 and SH2 domains of v-Src, and regions in the cytoplasmic C-terminal tail of Cx43, which included a proline-rich region (Pro274–Pro284) and the phosphorylated Tyr265 site [15]. Mutations in either the SH3 or the SH2 domain of v-Src greatly reduced the interaction of v-Src with Cx43 in co-transfected HEK293 cells as demonstrated by co-immunoprecipitation studies. The mutations in these domains essentially abolished the tyrosine phosphorylation of Cx43 in the v-Src expressing cells (see Fig. 1). In addition, disruption of the proline-rich region of Cx43 by the substitution of alanine for proline residues also prevented the binding interactions of Cx43 with v-Src. Furthermore, the Tyr265Phe Cx43 mutant failed to co-immunoprecipitate with v-Src and was not significantly phosphorylated on tyrosine in HEK293 cells that co-expressed the v-Src kinase [15]. This observation suggested that the Tyr265 site of Cx43 was a key target for v-Src in vivo. In contrast, the Tyr247Phe and Tyr267Phe Cx43 mutants demonstrated significant tyrosine phosphorylation on Cx43 and were co-immunoprecipitated with v-Src from HEK293 cells that expressed these Cx43 mutants and v-Src. This indicated that these two tyrosine sites in Cx43 were not likely to be required for the binding interaction with v-Src.
Fig. 1.
The intact SH3 and SH2 domains of v-Src are required to mediate the interaction of v-Src with Cx43 and to induce the tyrosine phosphorylation of Cx43. HEK293 cells were transiently transfected with Cx43 and either vector alone (pCDNA3), v-Src, or v-Src with mutations in the SH3 domain (Trp118Arg; W118R) or in the SH2 domain (Arg175Lys; R175K) or in both of these domains (W118R/R175K). v-Src (panel A) and Cx43 (panel B) were immunoprecipitated from cell lysates and subjected to SDS-PAGE and Western blotting. The membranes were probed with antibodies to Cx43, top panels orto phosphotyrosine (pTyr), lower panels. Cx43 co-precipitated with wild-type v-Src, but not with v-Src with mutations in the SH3 or SH2 domains (panel A). The Cx43 that co-precipitated with v-Src contained phosphotyrosine, as shown in the lower part of panel A. Total Cx43 immunoprecipitated from these cells was tyrosine phosphorylated in cells that expressed wild-type v-Src, but not in cells that expressed v-Src with mutations in the SH3 or SH2 domains (panel B). Reprinted from Ref. [15] with permission.
More recent studies, carried out in Cx43 knockout mouse fibroblasts that stably expressed exogenous wild-type or mutant Cx43 have confirmed the Tyr265 in Cx43 as a site that is phosphorylated by v-Src in vivo and identified the Tyr247 site as a second site that is targeted by the v-Src kinase [38]. Interestingly, phosphorylation of the Tyr265 site alone, in cells that expressed the Tyr247Phe Cx43 mutant, was not sufficient to induce channel closure in this mammalian cell system. This observation raised the possibility that Cx43 phosphorylation may occur in a processive manner with phosphorylation of the Tyr265 site occurring first, creating a binding site for the SH2 domain of v-Src, and strengthening the interaction of these proteins. This facilitates phosphorylation of Cx43 at the Tyr247 site and leads to the closure of the gap junction channels. Electro-physiological studies by Cottrell et al. [39] using these mouse fibroblasts have suggested that the reduction of GJC by the v-Src-induced tyrosine phosphorylation of Cx43 was not due to reductions in single channel amplitude and may have been attributed to a reduction in P0 (channel open probability) and changes in permselectivity.
However, the studies of Zhou et al. [40] have proposed a different mechanism for the regulation of Cx43 channel activity by v-Src. In their Xenopus oocyte system, junctional conductance established by the Tyr265Phe and Tyr247Phe single and the Tyr265Phe,Tyr247Phe double Cx43 mutants was disrupted by v-Src expressed from the injected RNA. This surprising result was interpreted as evidence that these two tyrosine sites in Cx43 were not required for the disruption of GJC induced by the v-Src kinase [40]. A quadruple serine site mutant of Cx43 (Ser255Ala, Ser257Ala, Ser279Ala, Ser282Ala Cx43), that lacked the MAP kinase phosphorylation sites, showed a reduced disruption of GJC when co-expressed with v-Src in the oocytes. This suggested a key role for MAP kinase-mediated phosphorylation of Cx43 in the regulation of GJC in the v-Src cells. Moreover, these investigators found that a MEK inhibitor, PD98059, blocked the ability of v-Src to disrupt GJC in LA25 rat fibroblasts that expressed a temperature-sensitive form of v-Src. v-Src was rapidly activated in these cells (within 5 min) by a shift to the permissive temperature. The results with the MEK inhibitor suggested that phosphorylation of Cx43 on serine, mediated by activated MAP kinase, and not the tyrosine phosphorylation of Cx43 by v-Src, was involved in the regulation of GJC in the LA25 rat fibroblasts [40].
Currently, the reasons for these very different experimental results are not clear. In the presence of acutely expressed v-Src from the transcription of microinjected RNA in oocytes or induced by a temperature shift in LA25 rat fibroblasts [40], the effects on GJC may be different than those that occurred in cells with constitutive expression of a non-temperature-sensitive v-Src, as in the mouse fibroblast system [38]. In this latter study, treatment with a MEK inhibitor, PD98059, did not block the ability of constitutively expressed v-Src to induce channel closure in the cells that expressed wild-type Cx43. This suggested that MAP kinase activation and the possible serine phosphorylation of Cx43 were not required for v-Src's actions in this cell system [38]. This conclusion was supported by additional studies that utilized Cx43 knockout mouse fibroblasts that expressed v-Src and a Cx43 mutant that lacked the MAP kinase phosphorylation sites (Ser255Ala, Ser279Ala, Ser282Ala).1 Cx43 was phosphorylated on tyrosine in these cells and GJC was dramatically disrupted. This occurred in the absence of possible phosphorylation at the MAP kinase target sites. These results have provided additional direct support for the view that MAP kinase-mediated phosphorylation of Cx43 is not required for the effects of v-Src on GJC and they have supported the role for tyrosine phosphorylation of Cx43 in the disruption of GJC in v-Src cells.
Studies that have identified the tyrosine sites of Cx43 that are targeted by v-Src and the protein domains that are required for the binding interaction of these proteins have suggested a possible model for the events that lead to the disruption of GJC [15,38]. In this model, the SH3 domain of v-Src initially binds to the proline-rich region in Cx43 (Pro274–Pro284) and brings these two proteins into close proximity (see Fig. 2). This results in phosphorylation of the Tyr265 site in Cx43 by the v-Src kinase domain and generates a second binding site in Cx43 that interacts with the SH2 domain of v-Src and stabilizes the protein interaction. The induced protein proximity leads to the processive phosphorylation of Cx43 at the Tyr247 site and this leads to the disruption of GJC. This model suggests that the phosphorylated Tyr265 site may be important as a binding site that strengthens the interaction of Cx43 with v-Src, whereas phosphorylation of Tyr247 may promote the closure of the Cx43 channels. The requirement for phosphorylation at the Tyr265 site in order to promote efficient phosphorylation of the Tyr247 site was supported by the observation, that in the absence of the Tyr265 site, Cx43 isolated from the v-Src-expressing cells contained significantly reduced levels of phosphotyrosine ( ~ 10%) compared to the wild-type protein [38] (see Fig. 3).
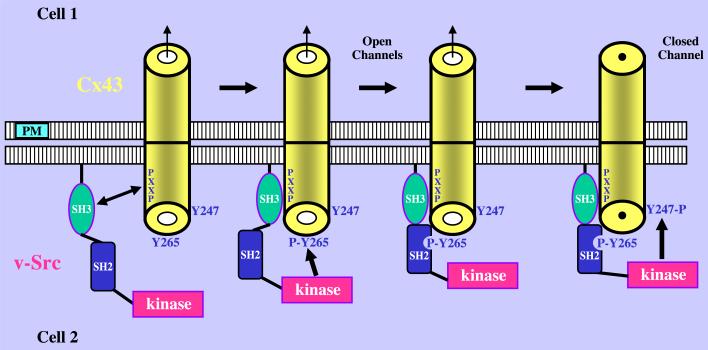
Fig. 2.
A model for Cx43's interaction with and phosphorylation by membrane localized v-Src. The interaction of Cx43 with v-Src at the plasma membrane (PM) is initiated by the binding of the SH3 domain of v-Src to the proline-rich region of Cx43 (PXXP, Pro274–Pro284). This brings the kinase domain of v-Src into proximity with the Tyr265 site of Cx43 (Y265) and allows phosphorylation at this site (P-Y265). The P-Y265 site of Cx43 provides a binding site for the SH2 domain of v-Src, strengthening the interaction between these proteins and allowing for the subsequent phosphorylation at the Tyr247 site of Cx43 (pY247). This leads to closure of the Cx43 gap junction channel (depicted as a cylinder in this model). Reprinted from Ref. [38] with permission.
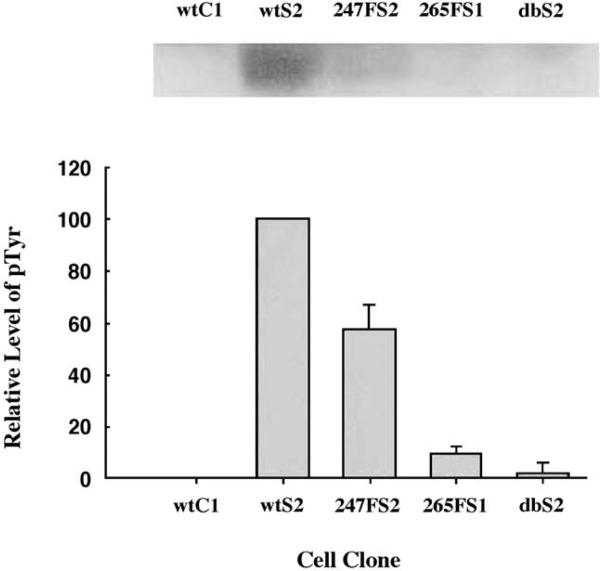
Fig. 3.
Mutation of the Tyr265 site of Cx43 greatly reduced tyrosine phosphorylation of Cx43 in v-Src cells. Cx43 was immunoprecipitated from Cx43 knockout cells expressing wild-type or mutant Cx43 under conditions of antigen excess and analyzed by SDS-PAGE and Western blotting for phosphotyrosine (pTyr) content, upper panel. The levels of pTyr in the Cx43 mutants were quantified in two experiments relative to the controls; wild-type Cx43 without v-Src (wtC1 cells, 0% pTyr) or with v-Src (wtS2 cells, 100% pTyr), see bar graph. In the absence of the Tyr265 site (265FS1 cells), tyrosine phosphorylation was greatly reduced to ~ 10% of wild-type Cx43 and was further reduced to ~2% in the double tyrosine site Cx43 mutant (dbS2 cells; Tyr265Phe, Tyr247Phe) in the v-Src cells. This demonstrated a key role for the Tyr265 site in the interactions of Cx43 with v-Src. The Tyr247Phe mutant expressed with v-Src (247FS2) demonstrated ~ 57% of the pTyr content relative to wild-type Cx43 and phosphorylation presumably occurred largely at the Tyr265 site. Reprinted from Ref. [38] with permission.
These data and the working model described above raise the question of how the phosphorylated Tyr265 and Tyr247 sites of Cx43 cooperate to induce the closure of the Cx43 channels, since phosphorylation at either site alone was not sufficient to induce channel closure [38]. Data obtained in one study has demonstrated the ability of the C-terminal tail of Cx43 to promote the closure of the gap junction channels by v-Src, when introduced as a separate peptide into Xenopus oocytes that expressed a truncated form of Cx43 (truncated at Asp245) [40]. Regions of the Cx43 cytoplasmic C-terminal tail between amino acids 241 and 280 appeared to mediate this sensitivity to v-Src. This important demonstration of a reconstituted system has provided support for the concept that Cx43 channel closure induced by v-Src phosphorylation may be mediated through a “ball-and-chain” or “particle-receptor” type of mechanism, as has been proposed for the pH- and insulin-induced Cx43 channel gating [12,41]. The specifics that underlie the mechanisms of the phosphorylation-induced Cx43 channel closure are significant issues that must be addressed in order to fully understand how GJC is regulated by protein phosphorylation.
2.2. c-Src induced Cx43 phosphorylation
In early studies, it was demonstrated that over-expression of c-Src induced a moderate fall in GJC in NIH3T3 cells, whereas the expression of a kinase-active mutant of c-Src (the Tyr527Phe mutant) induced a dramatic fall in GJC [42]. In additional studies, it was demonstrated that a constitutively active mutant of c-Src, but not a kinase-dead mutant, interacted with and phosphorylated Cx43 in co-transfected COS-7 cells [43]. GJC was disrupted in the cells that were co-transfected with wild-type Cx43 and activated c-Src and a direct interaction of Cx43 with activated c-Src was demonstrated by co-precipitation of the proteins. Mutation of the Tyr265 site to phenylalanine abolished both the tyrosine phosphorylation of Cx43 and the co-precipitation of Cx43 with activated c-Src. These studies demonstrated that Cx43 is a substrate for activated c-Src and provided additional support for the role of the Tyr265 site and tyrosine phosphorylation of Cx43 in the disruption of GJC induced by the c-Src tyrosine kinase. Toyofuku et al. [44] have demonstrated an increase in c-Src activity and tyrosine phosphorylation of Cx43 in a hamster model of cardiomyopathy. These investigators expressed activated c-Src in cardiomyocytes and demonstrated a reduction in GJC that was correlated with the tyrosine phosphorylation of the Cx43 protein. These studies indicated that c-Src tyrosine phosphorylation of Cx43 may occur in vivo and the resulting disruption of GJC may contribute to the electrical coupling abnormalities that have been observed in cardiomyopathy.
c-Src has also been implicated in the ability of G protein-coupled receptors to induce the disruption of Cx43-mediated GJC in Rat-1 cells [18]. G protein-coupled receptors lack an intrinsic kinase activity, but are coupled to downstream signaling events, such as the activation of c-Src and MAP kinase, through an interaction with heterotrimeric G proteins. In studies with lysophosphatidic acid (LPA) and thrombin, physiological activators of G protein-coupled receptor signaling, the downstream signaling events were found to be independent of Ca2+ mobilization and the activation of PKC, MAP kinase, Rho, or Ras [18]. Instead, the activated G protein-coupled receptors appeared to require the kinase activity of c-Src to induce the changes in Cx43 channel activity. These effects of G protein-coupled receptor signaling on GJC were prevented in cells that expressed a dominant negative form of c-Src, in Src-deficient fibroblasts, and in cells pretreated with tyrosine kinase inhibitors. These studies supported a key role for the activity of the c-Src kinase in mediating the effects of signaling on Cx43 function. The actions of c-Src in this experimental system may have been indirect since tyrosine phosphorylation of Cx43 was not detected [18]. In other studies, Hii et al. [45] and Warn-Cramer et al. [46] have demonstrated a role for MAP kinase in mediating the LPA-induced phosphorylation of Cx43. Whether c-Src kinase activity is required for MAP kinase activation and whether MAP kinase phosphorylation of Cx43 affects GJC in this system is not known. Additional studies will be required to identify the kinase(s) activated by G protein-coupled receptor signaling that are directly responsible for mediating Cx43 phosphorylation and to determine whether the increased Cx43 phosphorylation occurs on tyrosine or serine sites and if this phosphorylation induces channel closure.
Endothelin-1 (ET-1) has been shown to disrupt GJC and increase Cx43 phosphorylation in Rat-1 [18] and human ovarian carcinoma cells [47]. In the ovarian cells, a transient tyrosine phosphorylation of Cx43 and a decrease in gap junction plaques were also observed. ET-1 and its’ G protein-coupled receptor, ETAR, are increased in primary and metastatic ovarian carcinoma cells and the ET-1-induced disruption of GJC may contribute to the deregulation of growth in these cells. ET-1-induced disruption of GJC was blocked by pretreatment with tyrphostin 25 or the Src family kinase inhibitor, PP2, suggesting a role for an Src family kinase in the ET-1 induced tyrosine phosphorylation of Cx43.
Lipopolysaccharide (LPS) treatment of primary micro-vascular endothelial cells isolated from rat skeletal muscle stimulated a reversible tyrosine kinase-dependent reduction in gap junctional coupling [20]. This functional loss was accompanied by tyrosine phosphorylation of Cx43, as shown by immunoblotting studies with a phosphotyrosine antibody and by direct phosphoamino acid analysis. Little change was observed in the phosphoserine content of Cx43 from the treated cells [20]. Although the tyrosine kinase involved in these effects of LPS was not identified, data obtained with the use of chemical inhibitors suggested the participation of an Src family member. In addition, treating the cells with tyrosine phosphatase inhibitors accentuated the tyrosine phosphorylation of Cx43 and the disruption of GJC in this system [20].
The proinflammatory cytokine, TNFα has been reported to differentially regulate GJC in human airway epithelial cells and c-Src has been implicated in the effects on Cx43 [21]. TNFα increased the activation of c-Src in the airway epithelial cells. Expression of a dominant negative form of c-Src prevented the TNFα-induced channel closure whereas, the expression of activated c-Src abolished GJC in these cells. The transient expression of a Cx43 mutant that lacked the Src phosphorylation sites (Tyr247Ala, Tyr265Ala) in the airway cells, prevented the TNFα-induced effects on GJC. Since wild-type Cx43 was also expressed in these cells, these data are somewhat difficult to interpret. However, these studies have supported a key role for c-Src in the disruption of GJC induced by TNFα, although the tyrosine phosphorylation of Cx43 was not demonstrated [21]. Thus, it appears that both of these inflammatory agents, LPS and TNFα, induced the disruption of GJC through a c-Src kinase-dependent pathway.
Hypoxia and reoxygenation has been shown to induce a transient fall in GJC and tyrosine phosphorylation on Cx43 in human umbilical vein endothelial cells [48]. These effects were prevented by pretreatment with the tyrosine kinase inhibitor genestein and enhanced by treatment with the phosphatase inhibitor, vanadate, implicating a tyrosine kinase in the effects on Cx43. Treating hamster embryo fibroblasts with pervanadate or permolybdate, potent protein tyrosine phosphatase inhibitors, induced increased phosphotyrosine on a number of cellular proteins, including Cx43, and a decrease in the non-phosphorylated isoform of Cx43 [49]. Whether this induced phosphorylation on Cx43 was due to the inhibition of the dephosphorylation of a transient low level of tyrosine phosphorylation that occurs in normal cells or was due to the activation of an Src family kinase is not known.
Another manner in which the Src tyrosine kinase may regulate Cx43 is illustrated by the studies of Toyofuku et al. [23]. These investigators reported that the expression of an activated form of c-Src in rat neonatal cardiac myocytes inhibited the endogenous interaction that occurred between Cx43 and the tight junction-associated protein, ZO-1. The disruption of the binding of ZO-1 to Cx43 appeared to depend upon the competitive binding of the SH2 domain of activated c-Src to Cx43, since ZO-1 binding was not lost in cells that expressed activated c-Src and a Tyr265Phe Cx43 mutant. These studies indicated that the phosphorylation state of Cx43 might be important for its interaction with other cellular proteins. In other studies, Jin et al. have demonstrated that Cx43 phosphorylation is altered when the binding interaction with ZO-1 is disrupted.2 Thus, the phosphorylation-dependent loss of binding interactions or the formation of new binding interactions may result in altered regulation of the Cx43 protein. It is also interesting to note that the changes in Cx43 phosphorylation and ZO-1 binding that were observed in the HEK293 cells expressing activated c-Src, correlated with a down-regulation of the total and plasma membrane-localized Cx43 protein and with the inhibition of GJC [23]. This suggested that Cx43 stability might have been altered by the tyrosine phosphorylation of Cx43 and/or by the loss of Cx43's interaction with the ZO-1 protein.
3. Receptor tyrosine protein kinases
3.1. EGF receptor tyrosine kinase
The epidermal growth factor (EGF) receptor is a transmembrane tyrosine protein kinase that undergoes homo- or heterodimerization on ligand binding, resulting in receptor autophosphorylation and the activation of its tyrosine kinase activity [50]. Activation of the EGF receptor is important for mitogenesis and cell differentiation and in tumorigenesis, where over-expression of the receptor is associated with enhanced cell proliferation. Tyrosine phosphorylation of residues in the C-terminus of the receptor provides docking sites for the assembly of signaling complexes through the binding of SH2 and PTB domains in adaptor proteins [34]. Four family members have been described, Erb1, Erb2/neu, Erb3 and Erb4. EGF and TGFα are the primary ligands for Erb1. A ligand for Erb2 has not been identified and it is most likely activated as a heterodimer with another Erb family member. Heterodimers with Erb3 are particularly tumorigenic [51]. Erb3 and Erb4 are both activated by neuregulin (heregulin). Although Erb3 has little functional activity, it serves to activate Erb2 in a heterodimer on binding to neuregulin. Many breast cancer cell lines have also been found to over-express the EGF receptor and c-Src and these tyrosine kinases function synergistically to enhance the growth of mammary epithelial cells [52].
In many cases, the EGF-induced activation of the EGF receptor tyrosine kinase (Erb1) has resulted in a rapid and transient disruption of GJC and a transient increase in Cx43 phosphorylation. This induced phosphorylation occurred on serine residues and not on tyrosine, indicating that the EGF receptor did not phosphorylate Cx43 directly [24,53,54]. The disruption of GJC appeared to be independent of a significant loss of Cx43 from the gap junction plaques. EGF induced a disruption of Cx43 function in T51B rat liver epithelial cells that was determined to be independent of PKC activity, but required the downstream activation of MAP kinase [54]. MAP kinase phosphorylated Cx43 directly in in vitro reactions using purified proteins and phosphorylation occurred on tryptic peptides that co-migrated with a subset of the phosphotryptic peptides of Cx43 obtained from the EGF-treated cells [54]. Later studies identified Ser255, Ser279, and Ser282 of Cx43 as the target phosphorylation sites for activated MAP kinase in vitro [55] and demonstrated that these sites appeared to be targets for MAP kinase in vivo [46]. A triple phosphorylation site Cx43 mutant (Ser255Ala, Ser279Ala, Ser282Ala Cx43), introduced into cells that lacked Cx43, retained the ability to establish functional gap junction channels, but these channels were resistant to the MAP kinase-mediated disruption of GJC induced by activation of the EGF receptor [46]. Fig. 4 depicts a model for EGF receptor-induced phosphorylation of Cx43 on serine residues that is mediated by the downstream activation of MAP (Erk) kinases through the activation of the Ras/Raf/MEK/Erk signaling pathway.
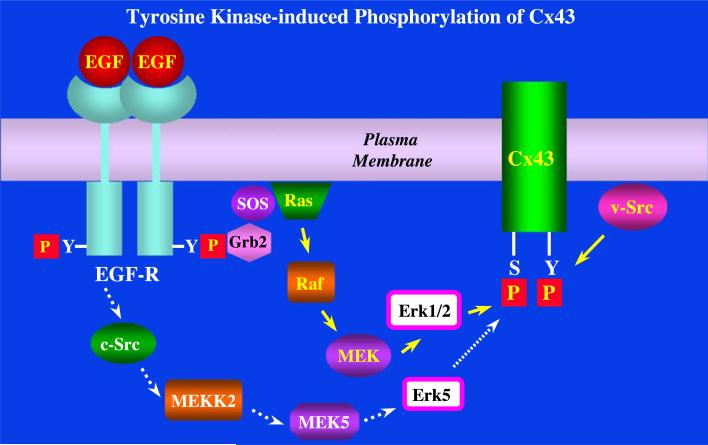
Fig. 4.
A model for Cx43's interaction with the tyrosine kinases v-Src and the EGF receptor. In this model, v-Src directly phosphorylates Cx43 on tyrosine sites in the C-terminus of the protein. Whereas, the ligand-induced activation of the EGF receptor leads to autophosphorylation of the receptor and the formation of phosphotyrosine binding sites for SH2 and PTB containing adaptor proteins such as Grb2/SOS. This leads to the activation of the Ras/Raf/MEK/Erk [34] and/or the c-Src/MEKK2/MEK5/Erk5 signaling pathways [105] and induces serine phosphorylation on Cx43 [46,55,61]. Tyrosine phosphorylation of Cx43 by v-Src or serine phosphorylation of Cx43 by EGF receptor-induced activation of downstream kinases is associated with the disruption of GJC.
To address the mechanism of the EGF-induced regulation of GJC, Cottrell et al. [39] carried out electrophysiological studies in EGF-treated Cx43 knockout mouse fibroblasts that expressed exogenous Cx43. These studies indicated that the ability of the activated EGF receptor to induce gap junction channel closure was not attributed to a reduction in the unitary conductance, but rather may have been due to a reduction in P0 channel open probability [39]. Similar results were obtained in the mechanism of the v-Src-induced channel closure. However, the closure of Cx43 channels by activated PKC appeared to result from a reduction in the channel unitary conductance [56]. These studies have suggested that Cx43 channel function may be regulated differently by phosphorylation events that occur at different sites in the C-terminal tail of Cx43.
In another study, Cx43 hemichannels were reconstituted into unilamellar lipid vesicles and were determined to be permeable to sucrose or lucifer yellow dye. When these vesicles were dephosphorylated by treatment with calf intestinal phosphatase, the permeability of the liposomes was increased. Treatment of the phosphatase-treated liposomes with purified, activated MAP kinase (Erk2) induced Cx43 phosphorylation and this resulted in a reduction in the permeability of the Cx43 hemichannels in the reconstituted system [57]. These data confirmed that Cx43 was a direct target for activated MAP kinase and that MAP kinase-mediated phosphorylation on Cx43 was sufficient to down-regulate Cx43 function.
In other studies, Leykauf et al. [58] examined Cx43 phosphorylation in EGF-treated WB-F344 rat liver epithelial cells using a phospho-specific peptide antibody that recognized the phosphorylated Ser279 and Ser282 sites of Cx43. This antibody recognized the most phosphorylated isoform of Cx43 that was isolated from the EGF-treated cells. Pretreatment with an EGF receptor inhibitor prevented the increased Cx43 phosphorylation and pretreatment with the PD98059 MEK inhibitor partially inhibited the EGF-induced Cx43 phosphorylation. These data were consistent with MAP kinase-mediated phosphorylation of Cx43 at the Ser279 and Ser282 sites in the EGF-treated cells. This antibody was used, together with an antibody that recognized all forms of Cx43, for confocal studies of Cx43 localization and these studies demonstrated that differentially phosphorylated isoforms of Cx43 coexisted in the plasma membrane [58]. Interestingly, treating these cells with anisomycin, which is known to activate the stress-activated kinase p38 MAP kinase, but not Erk1/2 kinases, also induced the weak recognition of a Cx43 band by the phospho-specific peptide antibody. This suggested that the Ser279 and/or Ser282 sites of Cx43 might also be a target for other non-Erk1/2 kinases [58]. This antibody will be very useful in examining the site-specific phosphorylation of Cx43 following the activation of different cellular signaling pathways that induce the activation of MAP kinase.
Polontchouk et al. [59] have also suggested a role for Erk1/2 and p38 MAP kinase in the regulation of Cx43 in cardiomyocytes following the activation of the endothelin 1 and angiotensin II G protein-coupled receptors. In addition, JNK, another stress-activated kinase, has been implicated in the regulation of Cx43 in cardiomyocytes, but this appears to occur through the down-regulation of the Cx43 protein. This decrease in GJC may be a contributing factor for cardiac dysfunction in the failing heart [60]. These studies have supported a role for other MAP kinase family members in the regulation of Cx43.
A very recent study by Cameron et al. [61] has raised questions about the role of MAP kinase in mediating the disruption of GJC in EGF-treated cells. These investigators reported that Erk5/BMK-1 (big MAP kinase-1) also phosphorylates Cx43 on the Ser255 site and that it is this phosphorylation event that is associated with the disruption of GJC in the EGF-treated cells. In light of reports that the PD98059 MEK inhibitor, originally thought to be specific for MEK1, also inhibits MEK5 and the downstream activation of Erk5 [62], these investigators examined the role for Erk5 in the EGF-induced disruption of GJC. They found that pretreatment with the PD98059 inhibitor blocked Erk1/2 and Erk5 activation in EGF-treated HEK293 cells that exogenously expressed Cx43. The expression of a constitutively active form of MEK5, but not a constitutively active form of MEK1, induced uncoupling of GJC in these cells. Erk5 was also shown to interact directly with Cx43 in vivo by co-immunoprecipitation studies in over-expressing cells [61]. Whether Erk1/2 and Erk5 both phosphorylate Cx43 in vivo under different conditions or in different cell types remains to be determined (see Fig. 4). These kinases have similar active sites but appear to differ in their substrate specificities. Previous reports have shown that cdc2/cyclinB also induces the phosphorylation of Cx43 at the Ser255 site in mitotic cells [63], and this may be correlated with the down-regulation of GJC during the G2/M phase of the cell cycle. Thus, there appears to be a number of protein kinases that can target the Ser255 site of Cx43. This serine site may be an important regulatory site in Cx43 that is targeted by different kinases, at different stages of the cell cycle, and by the activation of different intracellular signaling pathways.
To further complicate the understanding of how the activation of the EGF receptor regulates GJC, EGF treatment has been reported to enhance GJC in human kidney epithelial K7 cells [22]. In these cells, TPA and EGF both induced the activation of p42 MAP kinase and induced similar patterns of phosphorylation on Cx43, but these agents had opposite effects on GJC. The enhancement of GJC induced by EGF occurred after 2–3 h and thus, may have involved altered Cx43 protein synthesis or transport rather than the increased serine phosphorylation on Cx43, which appeared to occur rapidly and to be complete after approximately 15 min.
In addition, Diez et al. [64] have reported that the EGF receptor, isolated from rat liver membranes, directly phosphorylated Cx32 isolated from the same membrane preparations on tyrosine residues. Tyrosine phosphorylation appeared to be very inefficient in this cell-free system and contamination with another tyrosine kinase in these preparations is a possibility. It remains to be seen whether the EGF receptor directly phosphorylates Cx32 in vivo under physiological conditions and whether this phosphorylation regulates Cx32 function.
3.2. MAP kinase-mediated serine phosphorylation of Cx43
The phosphorylation of Cx43 by MAP kinase, activated downstream of a receptor tyrosine kinase or v-Src, has been discussed in the preceding paragraphs. Other studies have described the abilities of diverse stimuli to activate MAP kinase and induce Cx43 phosphorylation. Treating WB-F344 rat liver epithelial cells with TPA activated Erk1/2, increased Cx43 phosphorylation, and blocked dye transfer in these cells [65]. The down-regulation of dye coupling by TPA was correlated with an apparent loss of gap junction plaques from the plasma membrane rather than with Cx43 channel gating. TPA treatment also reduced GJC in IAR6.1 rat liver epithelial cells [24]. The TPA-induced loss of GJC was correlated with the increased phosphorylation of Cx43 in these cells. However, the effects of treatment on the internalization of Cx43 from the plasma membrane regions were not addressed in this study. Treatment with vitamin K3 (menadione) has been shown to decrease GJC in WB-F344 rat liver epithelial cells through a mechanism that involves the activation of the EGF receptor and MAP kinase and this resulted in increased phosphorylation of Cx43 [25]. The effects of vitamin K3 treatment on GJC appeared to occur in the absence of the loss of Cx43 from the plasma membrane. EGF has also been reported to increase the expression of Cx43 in porcine granulosa cells [26]. This change in Cx43 protein levels occurred in the absence of changes in Cx43 phosphorylation and may be involved in the up-regulation of GJC in early folliculogenesis.
3.3. PDGF receptor tyrosine kinase
Disruption of GJC induced by the activated platelet-derived growth factor (PDGF) receptor is rapid and transient and appears to be complex in terms of the possible downstream effectors, since both PKC and MAP kinase have been reported to be necessary to disrupt Cx43 function [66]. Disruption of GJC was correlated with the increase in Cx43 phosphorylation and required the tyrosine kinase activity of the PDGFβ receptor. Down-regulation of the TPA-sensitive forms of PKC or treatment with the PD98059 MEK inhibitor to down-regulate MAP kinase both reduced the PDGF-induced effects on GJC and Cx43 phosphorylation. More recent reports have suggested that the PDGF-induced disruption of Cx43 function did not rely solely on Cx43 phosphorylation or on the activation of MAP kinase, but may have required additional signaling pathways [67,68]. The site(s) of serine phosphorylation induced by the activated PDGFβ receptor signaling pathways has not been characterized.
PDGF treatment also induced a rapid and transient disruption of GJC in mesangial cells of the kidney glomerulus [69]. This was correlated with the activation of Erk1/2 and the tyrosine phosphorylation of Cx43. PI3-kinase inhibitors blocked the tyrosine phosphorylation of Cx43 and the activation of Erk in this system, suggesting a role for PI3 kinase in the regulation of Cx43 in the PDGF-induced signaling in these cells. The kinase involved in the PDGF-induced tyrosine phosphorylation of Cx43 in these cells was not identified. Further studies will be necessary to elucidate the complex molecular mechanisms that contribute to the regulation of Cx43 following the activation of the PDGF receptor and to identify the sites in Cx43 that are phosphorylated by PDGF receptor-induced intracellular signaling.
3.4. VEGF receptor tyrosine kinase
Vascular endothelial growth factor (VEGF) binds to and activates the tyrosine kinase activity of the VEGF receptor 2 (Flk-1 or KDR). The VEGF receptor is highly related to the PDGF receptor family. Endothelial cells express multiple connexins, including Cx43, Cx40 and Cx37. In a manner that is similar to the EGF stimulation of epithelial cells [53,54], VEGF-A treatment of human umbilical vein endothelial cells resulted in a rapid and transient reduction in GJC that correlated with increased Cx43 phosphorylation, and this phosphorylation appeared to occur on serine or threonine residues [19]. Altered Cx43 localization at the plasma membrane was not observed in this study. Activation of the VEGF receptor appeared to involve the downstream activation of an Src family member and MAP kinase and both of these kinases appeared to have a role in the induction of Cx43 phosphorylation. The disruption of channel activity in the VEGF-A-treated cells was also dependent upon the activation of the c-Src tyrosine kinase and MAP kinase [19]. The ability of the connexin proteins to form Cx43/Cx40 mixed gap junction channels further complicates the regulation of GJC in vascular endothelial cells [70,71]. Mixed connexin channels may be subject to the regulation of GJC by pathways that target either the Cx40 or Cx43 proteins. Additional studies will be required to further characterize VEGF receptor-induced intracellular signaling and the importance of this signaling pathway in regulating GJC in vascular endothelial cells.
3.5. FGF receptor tyrosine kinase
Fibroblast growth factor (FGF) is a large family of heparin-binding proteins that have potent mitogenic activity for neural cells, cardiomyocytes and endothelial cells [6,72,73]. Basic FGF (b-FGF or FGF-2) plays a role in wound healing, angiogenesis, and cell proliferation and may also participate in regulating GJC in the injured myocardium. b-FGR has been shown to co-localize with Cx43 at the plasma membrane in gap junction plaques in the intercalated discs of cardiomyocytes and in the rat brain [74,75]. These data suggested, but did not prove a physical association of these two proteins. b-FGF has been shown to have both long- and short-term effects on Cx43 [73]. Long-term treatment (>6 h) stimulated GJC in cardiac fibroblasts and up-regulated Cx43 mRNA and protein levels [27]. In contrast, short-term treatment (30 min) with b-FGF was associated with a fall in GJC, without a change in the Cx43 protein levels or loss of Cx43 from the plasma membrane. This fall in GJC was associated with increased Cx43 phosphorylation on serine, although a tyrosine kinase inhibitor prevented these effects on GJC. Tyrosine phosphorylation on Cx43 was not detected in this study, but a fraction of the Cx43 in the b-FGF treated myocytes was precipitated with a phosphotyrosine antibody. These data suggested that b-FGF induced the association of Cx43 with a phosphotyrosine-containing protein(s) [27]. Whether or not this protein might be the c-Src kinase that is activated by FGF receptor-dependent signaling is not known.
In another study, FGF-1 or FGF-2 increased GJC in primary cultures of embryonic chick lens cells without detectable increases in the synthesis or assembly of gap junctions [28]. FGF-2 induced a sustained activation of Erk in the lens cells that was necessary for the increase in GJC. This effect of sustained Erk activation on GJC differs from the short-term transient activation of Erk that has been associated with the disruption of GJC in other cell systems.
3.6. Insulin receptor tyrosine kinase
Insulin or insulin-like growth factor (IGF) activates the insulin receptor tyrosine kinase and induces the disruption of Cx43 channels in a paired oocyte system [12]. The effects of insulin on GJC were not observed with the expression of a Cx43 mutant that was truncated at Met257 and thus lacked the C-terminal cytoplasmic tail of Cx43. As mentioned previously, the free carboxyl-terminal tail of Cx43, expressed as a separate peptide, reconstituted the insulin-induced disruption of GJC in oocytes that expressed a truncated form of Cx43. These studies supported a “ball-and-chain” type model for Cx43 channel gating. Amino acids in the region between Gly261 and Pro280 of Cx43 appeared to be required for this effect on GJC. This region contains the MAP kinase recognition sequence for Ser279 phosphorylation and part of the recognition sequence for MAP kinase phosphorylation at the Ser282 site of Cx43 [12]. Activation of the insulin receptor is known to induce MAP kinase activation [76], however, it is not known whether the participation of MAP kinase was required for the insulin-induced effects on GJC in the oocyte expression system.
IGF induced the translocation and activation of PKCγ, increased serine phosphorylation of Cx43, and induced a fall in GJC in rabbit lens epithelial cells [77]. PKCγ translocation induced an association between Cx43 and PKCγ as shown by co-immunoprecipitation studies. IGF treatment increased diacylglycerol (DAG) and this stimulated PKCγ translocation and the increased phosphorylation of Cx43 [77]. It is not known whether PKCγ directly phosphorylates Cx43 in this system or whether MAP kinase participates in the induced Cx43 serine phosphorylation.
4. Mechanism(s) of Cx43 channel closure
The critical importance of phosphorylation of the tyro-sine sites in Cx43 by v-Src was underscored by a recent report that examined electrical coupling [39]. The actions of v-Src on Cx43 most likely promoted channel closure by decreasing P0 channel open probability, rather than by diminishing channel unitary conductance, as was found to be the case in the actions of PKC on Cx43 [56,78]. Cottrell et al. [39] also detected differences in the dye selectivity profiles of the phosphotyrosine-containing Cx43 channels in the v-Src cells. These authors proposed a model for further testing that suggests that PKC-mediated phosphorylation of Cx43 at the distal Ser 368 site may induce a conformational change at the carboxyl-terminus of the protein that somehow affects the channel pore and alters unitary conductance. Whereas, the phosphorylation induced by c-Src or MAP kinase, occurring at the proximal end of the carboxyl-tail leads to effects on channel open probability. A challenge for future studies will be to design ways to test this model and to address the question of the physiological significance of the different ways in which Cx43 is regulated by phosphorylation events that occur at different molecular sites in the C-terminal domain of the protein.
5. Phosphorylation of other connexins by tyrosine protein kinases
Less is known about the regulation of other connexins by tyrosine protein kinases. As mentioned previously, there is one report that the EGF receptor can directly phosphorylate Cx32 in a cell-free system [64], but whether this occurs in vivo is unknown. Traub et al. [79] have reported that murine Cx37 expressed in HeLa cells is phosphorylated mainly on serine with less phosphorylation on tyrosine and little phosphorylation on threonine. Junctional conductance was not inhibited in these cells. Hertlein et al. [80] found that murine Cx45 expressed in HeLa cells also contained some phosphotyrosine and phosphothreonine, but was primarily phosphorylated on serine ( ≥ 90%) at nine sites in the C-terminus of the protein. These cells were coupled through gap junctions, so the level of tyrosine phosphorylation or the site(s) of tyrosine phosphorylation did not interfere with GJC. These studies indicate that other connexins can be phosphorylated on tyrosine, at least when expressed in transformed cells, and this phosphorylation is not necessarily associated with a disruption of GJC.
Treatment with pervanadate enhanced the tyrosine phosphorylation on Cx45 in the HeLa cells and increased phosphorylation was associated with a 44% fall in junctional conductance [81]. Increased Cx45 phosphorylation was not blocked in cells pretreated with the PD98059 MEK inhibitor and EGF did not induce a phosphorylation increase on Cx45, indicating that MAP kinases were not likely to be involved in the pervanadate-induced phosphorylation. The increased phosphorylation on Cx45 presumably occurred through the inhibition of tyrosine phosphatase activity and the increased level of tyrosine phosphorylation or the induced sites of phosphorylation led to the disruption of GJC. The sites for tyrosine phosphorylation in Cx45 have not been characterized. Regulation of channel function in this case appeared to be through the modulation of channel open probability [81] as has also been shown for the v-Src induced disruption of GJC by tyrosine phosphorylation of Cx43 [39].
6. Regulation by protein phosphatases
The regulation of connexin phosphorylation by protein phosphatases is another important aspect of intracellular signaling that affects the connexin proteins. Phosphatases attenuate the activity of protein kinases and terminate activities that are transiently induced by different stimulatory events. Phosphatases are themselves subject to regulation by post-translational modifications, secondary messengers, targeting subunits, inhibitory proteins, and subcellular localization [35]. All of these regulatory events may be altered by the activation of intracellular signaling pathways. Phosphatases are thought to be involved in the trafficking and degradation of connexins and in the regulation of phosphorylation-induced channel gating, and they may also be important in regulating the interaction of connexins with other cellular proteins. The regulation of connexins by protein phosphatases is not covered in this review, but has been critically reviewed elsewhere [29,82].
7. Connexin regulation in transformed cells
A number of studies have examined the role of connexins in the regulation of growth in transformed cells [83–87]. Loss of connexin expression or function has been associated with increased rates of cell proliferation and loss of anchorage-dependent growth and the re-expression of connexins in transformed cells has been shown to slow cell proliferation. Many of these studies have examined homologous cell–cell communication. However, another important aspect is how transformed cells communicate with surrounding “normal” cells. Cai et al. [88] found that the co-culture of EVC304 endothelial cells with human breast cancer cells induced a rapid and transient fall in GJC and the appearance of phosphotyrosine on Cx43. These authors speculated that this fall in GJC might contribute to extravasation of tumor cells during metastasis. Others have suggested that disrupted GJC, together with effects on cell adhesion molecules, may be important in determining the metastatic potential of transformed cells [89,90]. Co-culture studies have shown that non-transformed cells can decrease the ability of transformed cells to grow as foci [91]. Goldberg et al. [92] used anti-sense technology to demonstrate that these growth suppressive effects of co-culture required the presence of Cx43 and/or GJC.
Some polyetherurethane materials used in bioimplants have been shown to induce tumors in rats [93] and to inhibit GJC in cultures of V79 Chinese hamster fibroblasts [94]. Ichikawa and Tsuchiya [95] examined the effects of these biomaterials in Balb/c 3T3 A31-1-1 cells transfected with Cx43 and found a decreased effect of polyetherurethanes on GJC in cells that expressed a Tyr265Phe Cx43 mutant compared to cells that expressed wild-type Cx43. They concluded that phosphorylation of Tyr265 of Cx43 was a key step in the reduction of GJC induced by the exposure of the cells to these materials and suggested that tyrosine phosphorylation of Cx43 and down-regulation of GJC may contribute to the tumorigenic potential of these biomaterials.
Qin et al. [96] found that the expression of Cx43 or Cx26 in MDA-MB-231 human breast tumor cells did not achieve the expected increases in GJC, but did suppress tumor growth when the cells were implanted into the mammary fat pads of nude mice. Cx43 was cytoplasmically localized in the cells and not found in gap junction plaques. Both the Cx43 and Cx26 over-expressing cells demonstrated decreased levels of FGF receptor-3 as compared to the parental cells and the authors suggested that connexins had a GJC-independent effect on the regulation of genes involved in cell growth. Krutovskikh et al. [97] found that the expression of a dominant negative transport-deficient mutant of Cx43 to down-regulate GJC in a rat bladder carcinoma cell line did not significantly enhance the growth of the transfected cells in nude mice as expected. This also suggested a growth suppressive function of Cx43 that was independent of GJC.
Moorby and Patel [98] found that transient expression of only the C-terminal cytoplasmic tail portion of Cx43 (aa 243–382) in Neuro2a cells was sufficient to suppress cell growth. The C-terminal tail protein was distributed throughout the cytoplasm suggesting that membrane localization of the protein and GJC were not required for the effects on cell growth. Zhang et al. [99] also reported that full-length Cx43 is not required for growth suppressive effects. These authors found that the C-terminal portion of the protein (aa 147–382, containing transmembrane sequences, but lacking the N-terminus, first extracellular loop, and the intracellular loop) was sufficient to reduce the proliferation of U2OS osteosarcoma cells. The expression of this N-terminally truncated Cx43 reduced the levels of Skp2, a human F-box protein that regulates ubiquitination of the p27 cell cycle inhibitor when expressed in U2OS or COS-7 cells. They proposed that Cx43 might function as a tumor-suppressor gene by targeting Skp2 and increasing the levels of p27 in a manner that was independent of GJC.
However, studies by Omori and Yamasaki [100] have demonstrated that Cx43 without the C-terminal tail (truncated at aa 239), when expressed in HeLa cells, suppressed anchorage-independent cell growth and delayed the appearance of tumors in nude mice, without an effect on cell proliferation. In these studies, it was the loss of the C-terminal tail of Cx43 that induced a negative cell growth control capacity on Cx43. Clearly the effects of Cx43 on cell proliferation and tumorigenic potential are complex and may be somewhat cell type-specific. It is not clear in these studies utilizing the expression of different deletion mutants of Cx43, how the mutant proteins were post-translationally modified and how this affected their localization, turnover and interactions with other cellular proteins.
8. Future directions
There are a number of outstanding issues that need to be addressed to achieve a better understanding of the regulation of connexins by protein phosphorylation induced by the activation of tyrosine kinase-dependent intracellular signaling pathways. New sites of phosphorylation and additional kinases that target connexins have been uncovered in recent years and have added to our knowledge of how phosphorylation regulates GJC [38,101,102]. Follicle-stimulating hormone (FSH) has been reported to induce the phosphorylation of Cx43 at four sites near the C-terminus at Ser365, Ser368, Ser369, and Ser373 and to increase Cx43 protein levels [102]. A Cx43 mutant that lacked all of these serine sites demonstrated severely reduced dye transfer activity when expressed in HeLa cells, suggesting a functional role for some or all of these serine sites. Casein kinase I phosphorylation of Cx43 on the Ser325, Ser328 and/or Ser330 sites appears to be involved in the assembly of gap junctions [14,101]. Whereas, the tyrosine phosphorylation of Cx43 by v-Src and the serine phosphorylation of Cx43 by MAP kinase appear to be important in the regulation of P0 channel open probability [39]. In the case of PKC-induced phosphorylation of Cx43 at the Ser368 site a reduction was found in the channel unitary conductance [56]. These studies begin to address the mechanics of channel regulation by protein phosphorylation. However, it is still not clear what these alterations in the electrophysiological properties of gap junctions mean in terms of the biological consequences of connexin phosphorylation. And, in those instances where connexin phosphorylation does not appear to be required for the disruption of GJC, there may be other consequences of protein phosphorylation, such as the creation or disruption of binding sites that affect the ability of Cx43 to provide a scaffolding function that is important to the assembly of protein complexes that may be involved in mediating Cx43's biological effects.
In addition to effects on channel gating and gap junction assembly, connexin phosphorylation may affect protein stability and regulate protein degradation by proteosomal and lysosomal mechanisms [103,104]. Much like the serine phosphorylation of β-catenin induced by glycogen synthase kinase 3 (GSK3), which targets β-catenin for proteosomal degradation, phosphorylation destabilized Cx43 and targeted the protein for proteosomal degradation in lens epithelial cells [103]. Treating the cells with proteosomal inhibitors stabilized the phosphorylated form of the Cx43 protein in these cells. Thus, protein stability is another manner is which connexin function may be regulated by protein phosphorylation events, but this is an area in which much is currently unknown.
Another aspect of connexin phosphorylation for which there is very little data is the issue of the stoichiometry of phosphorylation. Although specific sites of phosphorylation have been identified, and in the case of v-Src-induced tyrosine phosphorylation of Cx43, this phosphorylation appears to down-regulate GJC through a reduction in P0, we do not know the stoichiometry of the phosphorylation events required to induce this effect. A connexin channel is made up of 12 connexin proteins and at this point we have no idea how many of these molecules are phosphorylated in vivo. Nor do we know how many phosphorylation events are sufficient to alter channel properties or to alter connexin protein turnover. In one study of v-Src-induced Cx43 phosphorylation, essentially all of the Cx43 in the cell lysates was removed by repeated immunoprecipitations with a phosphotyrosine antibody [11]. This suggested that all Cx43 molecules were tyrosine-phosphorylated in cells expressing a highly active tyrosine kinase. But this does not answer the question of how many phosphorylation events are minimally required to disrupt GJC. In the studies of Huang et al. [21], the transient expression of the Tyr265-Ala Cx43 mutant in airway epithelial cells blocked the TNFα-induced disruption of GJC that appeared to be c-Src-dependent. Since these cells also endogenously expressed wild-type Cx43, channel closure may have been blocked by preventing tyrosine phosphorylation on some but perhaps not all, of the connexin proteins in a gap junction channel. This may mean that full phosphorylation of the available sites in all connexins comprising a channel was not required for the functional effects.
An additional aspect of the regulation of gap junctions that needs further study is the mechanism of channel gating. Several studies have demonstrated a reconstituted system of channel closure with the expression of the C-terminal tail of Cx43 as a separate peptide in oocytes that expressed truncated forms of Cx43 [12,40]. These data are consistent with a “particle-receptor” model for channel closure. However, this does not rule out the possible contributions of connexin-interacting proteins to channel regulation. Moreover, very little is known about the possible contributions of other parts of the connexin protein that may serve to form the “receptor” (such as the intracellular loop) in mediating channel regulation. Clearly, these are several areas in the field of connexin research that await additional research or significant conceptual or technical breakthroughs to extend our understanding of how GJC is regulated by the phosphorylation of connexins induced by the activation of intracellular signaling pathways.
Acknowledgements
Studies carried out in the authors’ laboratories were supported by grants from the NIH (RR16453-02 to Charles Boyd; BWC, project P.I. and CA52098 to AFL), by an ACS Institutional Grant (BWC) and grants from the American Heart Association, Hawaii Affiliate to AFL (0051599Z) and Rui Lin (HIFW-17-98). The authors thank Dr. Darren Park for reviewing the manuscript.
Footnotes
Warn-Cramer, B.J. et al., manuscript in preparation.
Jin, C. et al., manuscript submitted for publication.
References
- 1.Lampe PD. Analyzing phorbol ester effects on gap junction communication: a dramatic inhibition of assembly. J. Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laird DL, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J. Cell Biol. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 4.Cooper CD, Solan JL, Dolejsi KK, Lampe PD. Analysis of connexin phosphorylation sites. Methods. 2000;20:196–204. doi: 10.1006/meth.1999.0937. [DOI] [PubMed] [Google Scholar]
- 5.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J. Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepper MS, Meda P. Basic fibroblast growth factor increases junctional communication and connexin 43 expression in microvascular endothelial cells. J. Cell. Physiol. 1992;153:196–205. doi: 10.1002/jcp.1041530124. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado PE, Rose B, Loewenstein WR. Growth factors modulate junctional cell-to-cell communication. J. Membr. Biol. 1988;106:203–210. doi: 10.1007/BF01872158. [DOI] [PubMed] [Google Scholar]
- 8.Matesic DF, Rupp HL, Bonney WJ, Ruch RJ, Trosko JE. Changes in gap-junction permeability, phosphorylation, and number mediated by phorbol ester and non-phorbol-ester tumor promoters in rat liver epithelial cells. Mol. Carcinog. 1994;10:226–236. doi: 10.1002/mc.2940100407. [DOI] [PubMed] [Google Scholar]
- 9.Kurata WE, Lau AF. p130gag-fps disrupts gap junctional communication and induces phosphorylation of connexin43 in a manner similar to that of pp60v-src. Oncogene. 1994;9:329–335. [PubMed] [Google Scholar]
- 10.Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol. Cell. Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filson AJ, Azarnia R, Beyer EC, Loewenstein WR, Brugge JS. Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth Differ. 1990;1:661–668. [PubMed] [Google Scholar]
- 12.Homma N, Alvarado JL, Coombs W, Stergiopoulos K, Taffet SM, Lau AF, Delmar M. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ. Res. 1998;83:27–32. doi: 10.1161/01.res.83.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyro-sine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. J. Biochem. Cell Biol. 2004 doi: 10.1016/S1357-2725(03)00264-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J. Biol. Chem. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- 16.Loo LWM, Berestecky JM, Kanemitsu MY, Lau AF. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J. Biol. Chem. 1995;270:12751–12761. doi: 10.1074/jbc.270.21.12751. [DOI] [PubMed] [Google Scholar]
- 17.Loo LW, Kanemitsu MY, Lau AF. In vivo association of pp60v-src and the gap-junction protein connexin 43 in v-src-transformed fibroblasts. Mol. Carcinog. 1999;25:187–195. [PubMed] [Google Scholar]
- 18.Postma FR, Hengeveld T, Alblas J, Giepmans BN, Zondag GC, Jalink K, Moolenaar WH. Acute loss of cell – cell communication caused by G protein-coupled receptors: a critical role for c-Src. J. Cell Biol. 1998;140:1199–1209. doi: 10.1083/jcb.140.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J. Cell. Sci. 2001;114:1229–1235. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- 20.Lidington D, Tyml K, Ouellette Y. Lipopolysaccharide-induced reductions in cellular coupling correlate with tyrosine phosphorylation of connexin 43. J. Cell. Physiol. 2002;193:373–379. doi: 10.1002/jcp.10179. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Dudez T, Scerri I, Thomas MA, Giepmans BN, Suter S, Chanson M. Defective activation of c-Src in cystic fibrosis airway epithelial cells results in loss of tumor necrosis factor-alpha-induced gap junction regulation. J. Biol. Chem. 2003;278:8326–8332. doi: 10.1074/jbc.M208264200. [DOI] [PubMed] [Google Scholar]
- 22.Vikhamar G, Rivedal E, Mollerup S, Sanner T. Role of Cx43 phosphorylation and MAP kinase activation in EGF induced enhancement of cell communication in human kidney epithelial cells. Cell Adhes. Commun. 1998;5:451–460. doi: 10.3109/15419069809005603. [DOI] [PubMed] [Google Scholar]
- 23.Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 24.Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543–1550. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- 25.Klotz L-O, Patak P, Ale-Agha N, Buchczyk DP, Abdelmohsen K, Gerber PA, von Monfort C, Sies H. 2-methyl-1,4-naphthoquinone, vitamin K3, decreases gap-junctional intercellular communication via activation of the epidermal growth factor receptor/extracellular signal-regulated kinase cascade. Cancer Res. 2002;62:4922–4928. [PubMed] [Google Scholar]
- 26.Bolamba D, Floyd AA, McGlone JJ, Lee VH. Epidermal growth factor enhances expression of connexin 43 protein in cultured porcine preantral follicles. Biol. Reprod. 2002;67:154–160. doi: 10.1095/biolreprod67.1.154. [DOI] [PubMed] [Google Scholar]
- 27.Doble BW, Kardami E. Basic fibroblast growth factor stimulates connexin-43 expression and intercellular communication of cardiac fibroblasts. Mol. Cell. Biochem. 1995;143:81–87. doi: 10.1007/BF00925930. [DOI] [PubMed] [Google Scholar]
- 28.Le AC, Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 2001;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herve JC, Sarrouilhe D. Modulation of junctional communication by phosphorylation: protein phosphatases, the missing link in the chain. Biol. Cell. 2002;94:423–432. doi: 10.1016/s0248-4900(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 30.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 31.Kuriyan J, Cowburn D. Modular peptide recognition domains in eukaryotic signaling. Annu. Rev. Biophys. Biomol. Struct. 1997;26:259–288. doi: 10.1146/annurev.biophys.26.1.259. [DOI] [PubMed] [Google Scholar]
- 32.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 33.Margolis B, Borg JP, Straight S, Meyer D. The function of PTB domain proteins. Kidney Int. 1999;56:1230–1237. doi: 10.1046/j.1523-1755.1999.00700.x. [DOI] [PubMed] [Google Scholar]
- 34.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 35.Hunter T. Signaling—2000 and beyond. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 36.Cruciani V, Mikalsen S-O. Connexins, gap junctional intercellular communication and kinases. Biol. Cell. 2002;94:433–443. doi: 10.1016/s0248-4900(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 37.Kamps MP, Sefton BM. Most of the substrates of oncogenic viral tyrosine protein kinases can be phosphorylated by cellular tyrosine protein kinases in normal cells. Oncog. Res. 1988;3:105–115. [PubMed] [Google Scholar]
- 38.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphor-ylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src and mitogen-activated protein kinase-induced reduction of gap junction communication. Am. J. Physiol., Cell Physiol. 2003;284:C511–C520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L, Kasperek EM, Nicholson BJ. Dissection of the molecular basis of pp60v-src induced gating of connexin 43 gap junction channels. J. Cell Biol. 1999;144:1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azarnia R, Reddy S, Kmiecik TE, Shalloway D, Loewenstein WR. The cellular src gene regulates junctional cell-to-cell communication. Science. 1988;239:398–400. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- 43.Giepmans NG, Hengeveld T, Postma FR, Moolenaar WH. Interaction of c-Src with gap junction protein connexin-43. J. Biol. Chem. 2001;276:8544–8549. doi: 10.1074/jbc.M005847200. [DOI] [PubMed] [Google Scholar]
- 44.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M. Functional role of c-Src in gap junctions of the cardiomyopathic heart. Circ. Res. 1999;85:672–681. doi: 10.1161/01.res.85.8.672. [DOI] [PubMed] [Google Scholar]
- 45.Hii CST, Oh S-Y, Schmidt SA, Clark KJ, Murray AW. Lysophosphatidic acid inhibits gap-junctional communication and stimulates phosphorylation of connexin-43 in WB cells: possible involvement of the mitogen-activated protein kinase cascade. Biochem. J. 1994;303:475–479. doi: 10.1042/bj3030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J. Biol. Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- 47.Spinella F, Rosano L, DiCastro V, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 decreases gap-junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J. Biol. Chem. 2003;278:41294–41301. doi: 10.1074/jbc.M304785200. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YW, Morita I, Nishida M, Murota SI. Involvement of tyrosine kinase in the hypoxia/reoxygenation-induced gap junctional intercellular communication abnormality in cultured human umbilical vein endothelial cells. J. Cell. Physiol. 1999;180:305–313. doi: 10.1002/(SICI)1097-4652(199909)180:3<305::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 49.Mikalsen SO, Husoy T, Vikhamar G, Sanner T. Induction of phosphotyrosine in the gap junction protein, connexin43. FEBS Lett. 1997;401:271–275. doi: 10.1016/s0014-5793(96)01489-5. [DOI] [PubMed] [Google Scholar]
- 50.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 51.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 52.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lau AF, Kanemitsu MY, Kurata WE, Danesh S, Boynton AL. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol. Biol. Cell. 1992;3:865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanemitsu MY, Lau AF. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoyl 13-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Mol. Biol. Cell. 1993;4:837–848. doi: 10.1091/mbc.4.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J. Biol. Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 56.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim DY, Kam Y, Koo SK, Joe CO. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J. Biol. Chem. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- 58.Leykauf K, Durst M, Alonso A. Phosphorylation and subcellular distribution of connexin43 in normal and stressed cells. Cell Tissue Res. 2003;311:23–30. doi: 10.1007/s00441-002-0645-5. [DOI] [PubMed] [Google Scholar]
- 59.Polontchouk L, Ebelt B, Jackels M, Dhein S. Chronic effects of endothelin 1 and angiotensin II on gap junctions and intercellular communication in cardiac cells. FASEB J. 2002;16:87–89. doi: 10.1096/fj.01-0381fje. [DOI] [PubMed] [Google Scholar]
- 60.Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, Wang Y. c-Jun N-terminal kinase activation mediates down-regulation of connexin43 in cardiomyocytes. Circ. Res. 2002;91:640–647. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- 61.Cameron SJ, Malik S, Akaike M, Lerner-Marmarosh N, Yan C, Lee JD, Abe JI, Yang J. Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase 1/ERK5 but not ERK1/2 kinase activation. J. Biol. Chem. 2003;278:18682–18688. doi: 10.1074/jbc.M213283200. [DOI] [PubMed] [Google Scholar]
- 62.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 63.Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J. Cell. Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- 64.Diez JA, Elvira M, Villalobo A. The epidermal growth factor receptor tyrosine kinase phosphorylates connexin32. Mol. Cell. Biochem. 1998;187:201–210. doi: 10.1023/a:1006884600724. [DOI] [PubMed] [Google Scholar]
- 65.Ruch RJ, Trosko JE, Madhukar BV. Inhibition of connexin43 gap junctional intercellular communication by TPA requires ERK activation. J. Cell. Biochem. 2001;83:163–169. doi: 10.1002/jcb.1227. [DOI] [PubMed] [Google Scholar]
- 66.Hossain MZ, Ao P, Boynton AL. Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase C and mitogen-activated protein kinase. J. Cell. Physiol. 1998;176:332–341. doi: 10.1002/(SICI)1097-4652(199808)176:2<332::AID-JCP11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 67.Hossain MZ, Jagdale AB, Ao P, Boynton AL. Mitogen-activated protein kinase and phosphorylation of connexin43 are not sufficient for the disruption of gap junctional communication by platelet-derived growth factor and tetradecanoylphorbol acetate. J. Cell. Physiol. 1999;179:87–96. doi: 10.1002/(SICI)1097-4652(199904)179:1<87::AID-JCP11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 68.Hossain MZ, Jagdale AB, Ao P, Kazlauskas A, Boynton AL. Disruption of gap junctional communication by the platelet-derived growth factor is mediated via multiple signaling pathways. J. Biol. Chem. 1999;274:10489–10496. doi: 10.1074/jbc.274.15.10489. [DOI] [PubMed] [Google Scholar]
- 69.Yao J, Morioka T, Oite T. PDGF regulates gap junction communication and connexin 43 phosphorylation by PI 3-kinase in mesangial cells. Kidney Int. 2000;57:1915–1926. doi: 10.1046/j.1523-1755.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 70.Cottrell GT, Wu Y, Burt JM. Functional characteristics of heteromeric Cx40 – Cx43 gap junction channel formation. Cell Adhes. Commun. 2001;8:193–197. doi: 10.3109/15419060109080722. [DOI] [PubMed] [Google Scholar]
- 71.Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am. J. Physiol., Cell Physiol. 2002;282:C1469–C1482. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- 72.Reuss B, Dermietzel R, Unsicker K. Fibroblast growth factor 2 (FGF-2) differentially regulates connexin (cx) 43 expression and function in astroglial cells from distinct brain regions. Glia. 1998;22:19–30. [PubMed] [Google Scholar]
- 73.Doble BW, Chen Y, Bosc DG, Litchfield DW, Kardami E. Fibroblast growth factor-2 decreases metabolic coupling and stimulates phosphorylation as well as masking of connexin43 epitopes in cardiac myocytes. Circ. Res. 1996;79:647–658. doi: 10.1161/01.res.79.4.647. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto T, Kardami E, Nagy JI. Basic fibroblast growth factor in rat brain: localization to glial gap junctions correlates with connexin43 distribution. Brain Res. 1991;554:336–343. doi: 10.1016/0006-8993(91)90213-f. [DOI] [PubMed] [Google Scholar]
- 75.Kardami E, Stoski RM, Doble BW, Yamamoto T, Hertzberg EL, Nagy JI. Biochemical and ultrastructural evidence for the association of basic fibroblast growth factor with cardiac gap junctions. J. Biol. Chem. 1991;266:19551–19557. [PubMed] [Google Scholar]
- 76.Grigorescu F, Baccara MT, Rouard M, Renard E. Insulin and IGF-1 signaling in oocyte maturation. Horm. Res. 1994;42:55–61. doi: 10.1159/000184146. [DOI] [PubMed] [Google Scholar]
- 77.Lin D, Boyle DL, Takemoto DJ. IGF-I-induced phosphorylation of connexin 43 by PKCgamma: regulation of gap junctions in rabbit lens epithelial cells. Invest. Ophthalmol. Visual Sci. 2003;44:1160–1168. doi: 10.1167/iovs.02-0737. [DOI] [PubMed] [Google Scholar]
- 78.Kwak BR, Van Veen TAB, Analbers LJS, Jongsma HJ. TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels. Exp. Cell Res. 1995;220:456–463. doi: 10.1006/excr.1995.1337. [DOI] [PubMed] [Google Scholar]
- 79.Traub O, Hertlein B, Kasper M, Eckert R, Krisciukaitis A, Hulser D, Willecke K. Characterization of the gap junction protein connexin37 in murine endothelium, respiratory epithelium, and after transfection in human HeLa cells. Eur. J. Cell Biol. 1998;77:313–322. doi: 10.1016/S0171-9335(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 80.Hertlein B, Butterweck A, Haubrich S, Willecke K, Traub O. Phosphorylated carboxy terminal serine residues stabilize the mouse gap junction protein connexin45 against degradation. J. Membr. Biol. 1998;162:247–257. doi: 10.1007/s002329900362. [DOI] [PubMed] [Google Scholar]
- 81.van Veen TAB, van Rijen HVM, Jongsma HJ. Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc. Res. 2000;46:496–510. doi: 10.1016/s0008-6363(00)00047-x. [DOI] [PubMed] [Google Scholar]
- 82.Cruciani V, Kaalhus O, Mikalsen SO. Phosphatases involved in modulation of gap junctional intercellular communication and dephosphorylation of connexin43 in hamster fibroblasts: 2B or not 2B? Exp. Cell Res. 1999;252:449–463. doi: 10.1006/excr.1999.4650. [DOI] [PubMed] [Google Scholar]
- 83.Loewenstein WR, Rose B. The cell – cell channel in the control of growth. Semin. Cell Biol. 1992;3:59–79. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- 84.Yamasaki H, Naus CG. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199–1213. doi: 10.1093/carcin/17.6.1199. [DOI] [PubMed] [Google Scholar]
- 85.Mesnil M. Connexins and cancer. Biol. Cell. 2002;94:493–500. doi: 10.1016/s0248-4900(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 86.Naus C. Gap junctions and tumour progression. Can. J. Physiol. Pharm. 2002;80:136–141. doi: 10.1139/y02-009. [DOI] [PubMed] [Google Scholar]
- 87.Trosko JE. The role of stem cells and gap junctional intercellular communication in carcinogenesis. J. Biochem. Mol. Biol. 2003;36:43–48. doi: 10.5483/bmbrep.2003.36.1.043. [DOI] [PubMed] [Google Scholar]
- 88.Cai J, Jiang WG, Mansel RE. Gap junctional communication and the tyrosine phosphorylation of connexin 43 in interaction between breast cancer and endothelial cells. Int. J. Mol. Med. 1998;1:273–278. [PubMed] [Google Scholar]
- 89.Jiang WG, Bryce RP, Mansel RE. Gamma linolenic acid regulates gap junction communication in endothelial cells and their interaction with tumour cells, Prostaglandins. Leukot. Essent. Fat. Acids. 1997;56:307–316. doi: 10.1016/s0952-3278(97)90575-5. [DOI] [PubMed] [Google Scholar]
- 90.Hotz-Wagenblatt A, Shalloway D. Gap junctional communication and neoplastic transformation. Crit. Rev. Oncog. 1993;4:541–558. [PubMed] [Google Scholar]
- 91.Sakamoto TY, Takagi H, Tsuchiya T, Shoji A, Miyazaki K, Umeda M. Inhibition of focus formation of transformed cloned cells by contact with non-transformed BALB/c 3T3 A31-1-1 cells. Cancer Lett. 1999;136:159–165. doi: 10.1016/s0304-3835(98)00318-8. [DOI] [PubMed] [Google Scholar]
- 92.Goldberg GS, Martyn KD, Lau AF. A connexin43 antisense vector reduces the ability of normal cells to inhibit the foci formation of transformed cells. Mol. Carcinog. 1994;11:106–114. doi: 10.1002/mc.2940110208. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura A, Kawasaki Y, Takada K, Aida Y, Kurokama Y, Kojima S, Shintani H, Matsui M, Nohmi T, Matsuoka A, et al. Difference in tumor incidence and other tissue responses to polyetherurethanes and polydimethylsiloxane in long-term subcutaneous implantation into rats. J. Biomed. Mater. Res. 1992;26:631–650. doi: 10.1002/jbm.820260506. [DOI] [PubMed] [Google Scholar]
- 94.Nakaoka R, Tsuchiya T, Nakamura A. The inhibitory mechanism of gap junctional intercellular communication induced by polyethylene and the restorative effects by surface modification with various proteins. J. Biomed. Mater. Res. 2001;57:567–574. doi: 10.1002/1097-4636(20011215)57:4<567::aid-jbm1203>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 95.Ichikawa A, Tsuchiya T. Studies on the tumor promoting mechanism of hard and soft segment models of polyetherurethane: Tyr265 phosphorylation of connexin43 is a key step in the GJIC inhibitory reaction induced by polyetherurethane. J. Biomed. Mater. Res. 2002;62:157–162. doi: 10.1002/jbm.10239. [DOI] [PubMed] [Google Scholar]
- 96.Qin H, Shao Q, Curtis H, Galipeau J, Belliveau DJ, Wang T, Alaoui-Jamali MA, Laird DW. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 2002;277:29132–29138. doi: 10.1074/jbc.M200797200. [DOI] [PubMed] [Google Scholar]
- 97.Krutovskikh VA, Troyanovsky SM, Piccoli C, Tsuda H, Asamoto M, Yamasaki H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene. 2000;19:505–513. doi: 10.1038/sj.onc.1203340. [DOI] [PubMed] [Google Scholar]
- 98.Moorby C, Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp. Cell Res. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- 99.Zhang YW, Nakayama K, Morita I. A novel route for connexin 43 to inhibit cell proliferation: negative regulation of S-phase kinase-associated protein (Skp 2) Cancer Res. 2003;63:1623–1630. [PubMed] [Google Scholar]
- 100.Omori Y, Yamasaki H. Gap junction proteins connexin32 and connexin43 partially acquire growth-suppressive function in HeLa cells by deletion of their C-terminal tails. Carcinogenesis. 1999;20:1913–1918. doi: 10.1093/carcin/20.10.1913. [DOI] [PubMed] [Google Scholar]
- 101.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J. Biol. Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 102.Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Lett. 2002;531:132–136. doi: 10.1016/s0014-5793(02)03441-5. [DOI] [PubMed] [Google Scholar]
- 103.Girao H, Pereira P. Phosphorylation of connexin 43 acts as a stimuli for proteasome-dependent degradation of the protein in lens epithelial cells. Mol. Vis. 2003;9:24–30. [PubMed] [Google Scholar]
- 104.Laird DW. The life cycle of a connexin: gap junction formation, removal, and degradation. J. Bioenerg. Biomembranes. 1996;28:311–318. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- 105.Sun W, Wei X, Kesavan K, Garrington TP, Fan R, Mei J, Gelf SM, Gelf EW, Johnson GL. MEK kinase 2 and adaptor protein Lad regulate extracellular signal-regulated kinase 5 activation by epidermal growth factor via Src. Mol. Cell. Biol. 2003;23:2298–2308. doi: 10.1128/MCB.23.7.2298-2308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]