Abstract
Purpose
There is continuing controversy over the most appropriate treatment for screen-detected and clinically localised prostate cancer and increasing interest in monitoring such men initially, with radical treatment targeted at cancers showing signs of progressive potential but while still curable. Current evidence on monitoring protocols and biomarkers used to predict disease progression was systematically reviewed.
Methods
MEDLINE and Excerpta Medica (EMBASE) bibliographic databases were searched from 1988 to October 2004, supplemented by manual searches of reference lists, focusing on studies reporting monitoring of men with localised prostate cancer.
Results
48 potentially eligible papers were found, but only five studies (451 men) restricted entry criteria to men with clinically localised (T1-2) prostate cancer. Monitoring protocols varied with little consensus, although the majority used PSA levels and digital rectal examination, with some adding re-biopsy to assess progression. Actuarial probabilities of freedom from disease progression at 4-5 years of follow-up were 67%-72%. However, up to 50% of men abandoned monitoring within 2 years, largely because of anxiety related to rising PSA levels rather than objective evidence of disease progression. There was no robust evidence to support the use of PSA doubling times or velocity to identify men in whom disease may progress. Studies were characterised by small sample size, short-term follow-up, observer bias, and uncertain validity around variable definitions of progression.
Conclusions
Current evidence suggests that some form of monitoring would be a suitable treatment option for men with localised prostate cancer, but there is little consensus over what markers should be used in such a programme, or how ‘progression’ should be properly defined. The search for a method that safely identifies men with prostate cancer who could avoid radical intervention must continue.
Keywords: Review Literature, Prostatic Neoplasms, Prognosis, Disease Progression, Natural History, Prostate Specific Antigen
Introduction
Annually, over 500,000 men worldwide are diagnosed with prostate cancer, accounting for 10% of all male incident cancers1, and it is rapidly becoming the most common cancer in men. Autopsy studies show that cancerous cells can be found in the prostates of 30-40% of men at age 60, rising to 60-70% by age 80, yet for a 50-year-old US man the lifetime risks of clinical or fatal prostate cancer are estimated to be only 9.5% and 2.9 %, respectively2. The dilemma is that, although most cancers detected by screening are clinically confined to the prostate, and hence potentially curable, current screening tests cannot differentiate between cancers that have low biological likelihood of progression from those with more aggressive potential3. Furthermore, there is uncertainty over the effectiveness of radical surgical and radiotherapy treatments for screen-detected disease4. Screening may thus result in substantial overdiagnosis and overtreatment of clinically insignificant prostate cancer.
The doubts surrounding the benefits of screening and early radical treatment have led, in recent years, to an increasing use of monitoring (variously termed active monitoring, surveillance, watchful waiting) as a therapeutic option4. This involves regular follow-up using one or more of the following investigations for those with clinically localised cancers: PSA testing, digital rectal examination (DRE), review of symptoms, and sometimes transrectal ultrasound (TRUS) guided re-biopsy. These investigations aim to determine which cancers should be treated by potentially curative interventions and when. This differs from traditional ‘watchful waiting’ regimes where follow-up typically waited for the development of systemic disease and the therapeutic goal was palliation. Appropriate targeting of active monitoring requires markers that can differentiate between indolent tumours and those with aggressive potential suitable for radical curative treatment4. However, the most appropriate frequency and form of follow-up in patients choosing active monitoring remains undefined3. We undertook a systematic review of the literature to identify and review studies conducted in the PSA-testing era (after 1988) that have followed up men initially managed conservatively (watchful waiting or active monitoring), documented their risk of progression and related this to potential markers of disease progression.
Methods
MEDLINE and EMBASE bibliographic databases were searched between 1988 and October 2004 using the following combination of mesh headings and text word search terms: exp Prostatic Neoplasms/ or (prostat$ adj5 neoplas$).tw. or (prostat$ adj5 cancer$).tw. AND exp Disease Progression/ or exp Survival Analysis/ or exp Natural History/ or (expectant$ adj5 manage$).tw. or (conservative$ adj5 manage$).tw. or (active adj5 surveillance).tw. or (watchful adj5 waiting).tw. or (watch adj5 wait).tw. or (watchful adj5 observation).tw. or (active$ adj5 monitor$).tw. or (defer$ adj5 treatment).tw. Reference lists of eligible studies and review articles were also searched.
Studies were included if they involved men with localised prostate cancer that was initially managed conservatively and if potential biomarkers of disease activity were related to an objective clinical, pathological or biochemical assessment of whether or not the disease had progressed. Eligibility criteria and follow-up protocols, definitions of progression, triggers for recommending treatment, the relationship between biomarkers and progression, the proportion of men undergoing active treatment, and the reasons for treatment were documented.
Reports of active monitoring with curative intent that investigated predictors of the subsequent use of radical treatments in the absence of predefined objective measures of disease progression were included separately, with data abstracted on the proportion of men subsequently choosing radical treatment and their reasons for abandoning active monitoring.
Results
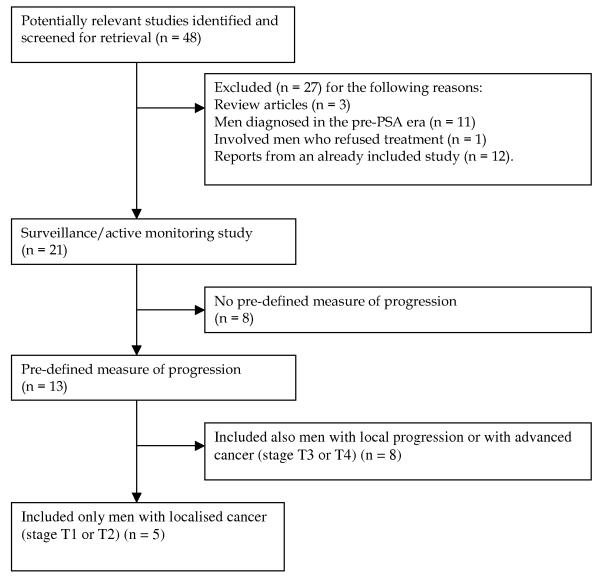
The search resulted in 2946 papers in total, of which 48 were potentially eligible (Figure 1). Of these, 27 were excluded (list of papers available on request). Of the 21 remaining studies, eight appeared to be offering active monitoring protocols with curative intent but without predefined objective measures of progression5-12 (Appendix 1). All eight were based on retrospective case note reviews and were small scale (median sample size = 186.5 men; range: 49 to 1,158); six were limited to men with localised (stage T1-T2) disease5-10. These reports showed that 22%-73.2% of men abandoned active monitoring within 2-5 years6-12, with patient preference the most commonly cited factor by physicians6,9,10. Higher baseline PSA and tumour stage11, and short PSA doubling times (< 2-3 years)6,7 were associated with higher subsequent rates of active treatment, while older age8,11 and adverse pre-treatment social circumstances8 were associated with lower rates of choosing active treatment.
Figure 1. Flow diagram showing the number of studies included and excluded from the review.
Appendix 2: Characteristics, eligibility criteria, monitoring protocols, definitions of progression and outcomes in studies with predefined measures of disease progression which included men with locally advanced or metastatic (T3-T4) disease, ordered by length of follow-up, 1988-2004 (continued overleaf).
| 1st author, setting (year published) |
N (yrs recruited) |
Status | Eligibility | Median age ± SD (range), yrs |
Median initial PSA, ng/ml (range) |
Stage (%) | Monitoring protocol |
|---|---|---|---|---|---|---|---|
| Neulander, Florida, USA (2000)21 |
54 (1991- 1998) |
P | Men electing ‘watchful waiting’ to be treated only when disease progressed |
76.4 (63-88) |
7 | T1a (7%); T1c (56%); T2 (31%); T3 (6%) |
3-4 monthly DRE, PSA & assessment of voiding function. |
| Kakehi, Japan (2002)18 | 78 (1997- 2000) |
R | Clinically localised cancer excluding T1a and T1b cancers. |
73.8-77.0 depending on stage (49- 91) |
8.6-48.9 depending on stage |
T1c (60%); T2a (8%); T2b (27%); T3 (5%). |
PSA, DRE, imaging, evidence of metastases |
| Bangma, Rotterdam (1995)25 | 29 (-) | R | Histologically confirmed cancer; no metastases; estimated survival > 1 yr. |
74 yrs (58- 85); |
- | T1a (52%); T2 (38%); T3 (10%) |
Twice yearly PSA, DRE, alk phos; serial transrectal ultrasound volumetry. |
| Stephenson, McGill University, (2002)17 |
94 (-) | R | Localised cancer managed “expectantly” due to pt choice, limited life expectancy or presumed insignificant cancer |
69 (51-86) | 7.4 (0.9-25.2) | T1a/b (5%); T1c (48%) T2a-b (42%); T3 (4%) |
Serial PSA and DRE at 3-6 mo intervals; 45 pts underwent repeat prostatic biopsies on average 2 years apart. |
| Schmid, Stanford University Medical Centre (1995)24 |
43 (1985- 1989) |
? | Histologically-proved prostate cancer managed conservatively. |
71 (51-83) | 6.7 & 30.8 for organ-confined & non-organ confined disease, respectively |
Organ confined: 65% Not organ confined: 35% |
Serial serum PSA. |
| Arai, Kitasato University Hospital, Japan (2001)20,30 |
64 (1994- 1999) |
P | Biopsy confirmed localised prostate cancer |
75 (53-87) |
9.1 (0.2-242) | T1a/b (4%); T1c (44%) T2 (46%); T3 (6%) |
3-6 monthly DRE, PSA, TRUS; annual bone scan. |
| McLaren, British Columbia Cancer Agency (BCCA) (1998)22 |
113 (-) | R = 62%; P = 48% |
Men placed on a watchful waiting programme; no prior hormonal or surgical treatment. |
75 yrs (49- 85) |
5.8 (0.2-21) | T1a/b (21%); T1c (31%); T2 (42%); T3 (6%) |
“…no fixed follow-up schedule; …generally seen every 3-6 months” |
| McIntyre, Manchester (2001)19 |
51 (-) | R | Bone-scan negative, treated by watchful waiting. |
78 | - | Bone-scan negative |
6 monthly; “multiple bone scans”. |
| 1st author, setting (yr published) |
Definition of progression | Median follow- up (range) |
% progressed (median time; range) |
Probability of freedom from progression |
Probability of freedom from treatment |
Reasons for abandoning monitoring |
|---|---|---|---|---|---|---|
| Neulander21 | Local stage progression detected by DRE and/or biochemical progression (PSA increase 25-50%/yr) or systemic progression when metastases detected. |
47 mo (12-72) | 52% (35 mo*; 12-69) |
- | - | Of 28 progressors: 10 = PSA; 11 local; 4 = PSA + local; 3 mets. |
| Kakehi18 | 3 consecutively “elevated” (undefined) PSA levels or local progression defined on basis of DRE, imaging or metastases. |
37.5 mo (8.6-73.9) | 15% | - | - | Overall, 12 withdrew due to clinical or PSA progression; of 53 T1c or T2a men 7 withdrew (clinical progression: 3; PSA rise: 4). |
| Bangma25 | Local progression: symptomatic, increase in T category, increase in prostate size on DRE by 25%, increase in ultrasound measured volume > 40%; metastatic progression (new bone lesion). |
39*mo (11-73). | 45% (31 mo). None had metastases. |
- | 79% | Of 13 pts with progression, 6 started treatment (5 for subjective symptoms; 1 for objective progression only) |
| Stephenson17 | Indicators of progression were: development of metastases; worsening clinical stage by DRE; histologic progression on repeat biopsy (defined as: a) increase in no. of +ve cores; b) progression to > 50% cancer in a core; c) change in Gleason score ≥ 2; d) change in Gleason category [scores categorised as 2-4, 5-6, 7, 8-10]). |
33 mo (12-121). | 23% (2 men developed bone metastases) |
- | - | - |
| Schmid24 | Increase in prostate size on DRE and positive bone scans. | 26 mo (12-63) | None had evidence of bony metastases. |
- | - | - |
| Arai20,30 | Local clinical progression: an increase in T stage; increase in prostate size of ≥ 25% by DRE; or > 50% volume increase by TRUS; or distant disease progression: skeletal (new bone lesions on bone scans or X-rays) or soft-tissue (on biopsy) metastases. |
22 mo (6-68) | 13% (41.5 mo; 18-51) |
- | - | Of 8/64 ‘progressors’, 3 had skeletal metastases, 5 local progression and 1 remains untreated.. |
| McLaren22 | Clinical progression – an increase in palpable disease or T classification. |
21 mo (0-306) | 37% (2/113 with metastases) |
Overall survival at 2 & 5 yrs 92% & 68% respectively |
- | - |
| McIntyre19 | Bone scan for metastases; PSA levels | 21 mo | None had evidence of skeletal metastases. |
- | - | - |
BPH: benign prostatic hyperplasia; P: prospective case-series; R: retrospective case-series; TURP: transurethral resection of prostate.
mean value (± SD if given)
Alk phos: alkaline phosphatase; DRE: digital rectal examination; fPSA: free prostate specific antigen; lnPSA: natural log PSA; mo: months; PSA: Prostate specific antigen; TRUS: transrectal ultrasound scan; TURP: transurethral resection of prostate; UTI: urinary tract infection.
The 13 remaining studies had pre-defined objective measures of progression13-25, but eight included men with advanced (stage T2-T3) disease followed-up with palliative intent (Appendix 2). The remaining five reports were limited to localised (stage T1-T2) prostate cancer13-16,23, involving a total of 451 men (median: 78; range: 27 to 206). The following review focuses on these five studies (Table 1)13-16,23, two of which were retrospective case-note reviews13,15 and three were prospective case series14,16,23.
Table 1. Characteristics, eligibility criteria, monitoring protocols, definitions of progression and outcomes in studies with predefined measures of disease progression and restricted to men with localised (T1-T2) disease, ordered by length of follow-up, 1988-2004 (continued overleaf).
| 1st author, setting (year published) |
N (yrs recruited) |
Design | Eligibility | Median age ± SD (range), yrs |
Median initial PSA, ng/ml (range) |
Stage (%) | Monitoring protocol |
|---|---|---|---|---|---|---|---|
| Chen, Taichung Veterans Hospital, Taiwan, (2003)15 |
52 (1983- 1996) |
R | Men undergoing TURP for clinically diagnosed BPH with stage T1a cancer. |
71.5* ± 7 | - | T1a (100%) | 3-6 monthly DRE & PSA. |
| Patel, Baylor College of Medicine & Memorial Sloan Kettering Cancer Centre, USA (2004)13 |
88 (1984- 2001) |
R | Clinically localised cancer; Gleason score ≤ 7; eligible for radical treatment; no significant co- morbidities. |
65.3* (44-79) |
5.9 (0.09-30.2) | T1a/b (20%) T1c (58%) T2a-c (22%) |
DRE & PSA every 3 mo for yr 1 & every 6 mo afterwards. TRUS guided sextant biopsy at 6 mo & if DRE/TRUS or PSA abnormalities. PSA velocity calculated from 3 values over 12 mo. |
| Choo, Toronto (2002)16,28,29 | 206 (1995- 2000) |
P | T1b-T2b N0M0, Gleason ≤ 7, PSA ≤ 15 ng/ml |
70 (49-84) |
6.5 (0.3-14.6) | T1b (6%); T1c (57%) T2a (24%); T2b (13%) |
Follow-up every 3 mo for 2 yrs & every 6 mo afterwards with PSA and DRE. TRUS every 6 mo; rpt biopsy at 12-18 mo; bone scan at 12 mo for 1st 2 yrs (then every 24 mo or 12 mo if PSA > 15 ng/ml). |
| Mohler, University of North Carolina (1997)23 |
27 (1991- 1996) |
P | All pts with clinical stage T1c disease. | 69* ± 5 yrs | 5.3 ± 9.8* | T1c (100%) | Follow-up at 3 mo and then 6 monthly with clinical examination, PSA, creatinine and haematocrit. |
| Khan, John Hopkins University USA, (2003)14,26,27 |
78 (1992- 2002) |
P | T1c, PSA density < 15 ng/ml/cm3; Gleason < 7; no grade 4/5; < 3 cores involved with cancer; ≤ 50% involvement of any core |
65 ± 4.5 (50-74) |
4.6 ± 2.3 (0.8-11.9) | T1c (100%) | Twice yearly total and %fPSA, DRE, annual TRUS biopsy (12 cores). |
| 1st author | Definition of progression | Median follow-up (range) |
% progressed (median time; range) |
Probability of freedom from progression |
Probability of freedom from treatment |
Reasons for abandoning monitoring |
|---|---|---|---|---|---|---|
| Chen15 | Abnormal DRE and/or progressive elevation of PSA “proved” by transrectal needle biopsy, or appearance of metastatic disease. |
7.3 yrs (0.5- 15) |
8% | 72% at 5 yrs 60% at 10 yrs |
- | 3 (6%) progressed to stage T2a disease; 1 (2%) progressed to bony mets. |
| Patel13 | Progression defined as a total of ≥ three points awarded for: i) Gleason score increases (increases of 1, > 1 or any new Gleason pattern 4 or 5 awarded 1, 2, and 3 points, respectively); ii) PSA velocity (> 0.75 ng/ml/year in 12 or 24 months awarded 2 and 3 points, respectively); iii) DRE/TRUS findings (increasing old lesion, new lesion not biopsy proven and new biopsy proven lesion awarded 1, 2, and 3 points, respectively); iv) biopsy (bilateral or multifocal cancer, and greater than four cores with cancer awarded 2 and 3 points, respectively). |
44.1 mo (7- 172) |
25% (45 mo; 7-84) |
95% at 1 yr 88% at 2 yrs 67% at 5 yrs 56% at 10 yrs |
93% at 1 yr 84% at 2 yrs 58% at 5 yrs 41% at 10 yrs |
31 (35%) withdrew (17 objective progression; 7 pt anxiety; 7 pt anxiety + ‘ominous features’) (no mets reported). |
| Choo16,28,29 | Any of i) PSA progression (co-existance of PSA doubling time < 2 years + final PSA > 8ng/ml + p < 0.05 on regression of lnPSA on time); ii) clinical progression (based on DRE, symptoms requiring TURP, ureteric obstruction, bone scan, radiologic/clinical evidence of metastases); iii) histological progression (Gleason score upgraded to 8 or greater). |
29 mo (2-66) | 17% | 81% (± 6.2) at 2 yrs; 67% (+/− 12%) at 4 yrs. |
67% (± 7.3) at 2 yrs 48% (± 10.7) at 4 yrs |
69 (33%) withdrew: 15 = clinical progression; 16 = PSA; 5 = histological; 23 = pt request; 6=protocol violation; 4 = other death (no mets). |
| Mohler23 | Clinical criteria: development of palpable disease on DRE, gross haematuria, UTI, symptoms of bladder outlet obstruction, evidence of metastatic disease on physical examination or radiography; Biochemical criteria: increase in serum PSA on 3 consecutive measures and total increase ≥ 5 ng/ml. |
23 mo (6- 62)* |
33% (23 mo) | - | 85% | 15% (4/27) of men withdrew due to PSA progression (no mets). |
| Khan14,26,27 | Progression defined as unfavourable repeat 12-core biopsy findings: Gleason score ≥ 7; or any Gleason pattern 4 or 5; or ≥ 3 cores involved with cancer; or > 50% involvement of any core with cancer. |
≈ 23 mo27 | 29% (20 mo) | - | 76% | (no mets reported). |
BPH: benign prostatic hyperplasia; DRE: digital rectal examination; %fPSA: percent free PSA; P: prospective case-series; R: retrospective case-series; TRUS: trans-rectal ultrasound guided biopsy; TURP: transurethral resection of prostate.
mean value (± SD if given)
Eligibility criteria (Table 1)
The average age of the men was between 65-71.5 years, but only two studies restricted the upper age to circa 75 years to include those potentially eligible for radical treatment14,23. The proportion of men with stage T1c was 100% in two studies14,23, 55-60% in two studies13,16, and 0% in one study including just T1a disease15. In three studies, histological criteria were specified for inclusion13,14,16, and one study required a PSA density < 15 ng/ml/cm3 for a participant to be eligible14. In all but one study15, the men were followed up for less than 5 years on average.
Definitions of disease progression
The active monitoring studies revealed different protocols for monitoring (Table 2) and diagnosing disease progression (Table 3). All protocols included serial PSA levels and DRE assessment, with three including repeated transrectal ultrasound-guided biopsies13,14,16, and others a variety of clinical measures (Table 1). Progression rates for T1c-T2 disease ranged between 17-33% with little clear relationship to median duration of follow up, mean age or median initial PSA level. A large proportion of men underwent radical treatment without clinical evidence of progression, usually because of anxiety or withdrawal prompted by ‘PSA progression’. Patel, for example, showed that of 31 men switching to radical treatment, only 17 met the criteria for progression13.
Table 2. Summary of measures used in monitoring protocols for men with clinically localised prostate cancer in the five reviewed studies.
| Measure | Number of studies | Frequency of measure |
|---|---|---|
| Serial PSA levels | 513-16,23 | 3-6 monthly |
| Serial DRE | 513-16,23 | 3-6 monthly |
| Routine repeat TRUS guided biopsy | 313,14,16 | Every 6 months (n=2); annual (n=1) |
| PSA velocity | 113 | Calculated from 3 PSA values measured over 12 months |
| % free PSA | 114 | Twice yearly |
| Serial bone scans | 116 | Every 12-24 months |
| Creatinine, haeamtocrit | 123 | Every 6 months |
Table 3. Summary of measures used in active monitoring studies to define disease progression requiring radical treatment.
| Measures used to define disease progression | Number of studies |
|---|---|
| Local stage progression | |
| Increase in T category detected by DRE | 413,15,16,23 |
| Development of symptoms requiring TURP | 116 |
| Ureteric obstruction | 116 |
| Gross haematuria | 123 |
| UTI | 123 |
| Symptoms of bladder outlet obstruction | 123 |
| Biopsy findings | |
| Increase in Gleason score of 1 or more | 313,14,16 |
| New Gleason pattern 4 or 5 | 213,14 |
| Presence of bilateral or multifocal cancer | 113 |
| Increased number of cores involved with cancer | 213,14 |
| > 50% involvement of any core with cancer | 114 |
| Biochemical progression | |
| Progressive elevation of PSA (undefined) | 115 |
| Increase in PSA on 3 consecutive measures & total increase ≥ 5 ng/ml | 123 |
| PSA velocity (> 0.75 ng/ml/year) | 113 |
| Co-existence of PSA doubling time < 2 years + final PSA > 8ng/ml + p < 0.05 on regression of lnPSA on time |
116 |
| Systemic progression | |
| Development of metastases | 315,16,23 |
In the Johns Hopkin’s series, 13% (9/70) of men exhibited a change in Gleason score of ≤ 6 to ≥ 7 on repeat biopsy26. In 8 of these men the change occurred within 15 months of initial sampling. Patel found that 23% of men with localised prostate cancer had worse Gleason scores within 6 months of initial biopsy, while 61% had no cancer detected at repeat biopsy13. In a case series which included 4% of men with stage T3, a total of 53% (24/45) of men who had a routine repeat sextant biopsy had evidence of progression at a median of 33 months17. Two of the five eligible studies followed-up men using a combination of both clinical (DRE / radiological / clinical evidence of metastases) and biochemical (PSA) criteria, but did not include routine histological surveillance15,23.
The short-term probability of metastases in the five studies of localised cancer was low: in four, there was no evidence of metastatic progression after a median of between 23-44.1 months of follow-up13,14,16,23; in men with T1a cancer followed up for a median of 7.3 years, 1 man (2%) progressed to bony metastases after 12 years15. In two of the eight case-studies with more advanced disease (Appendix 2), 3/6420 and 2/11322 men developed skeletal metastases after a median of 21-22 months follow-up.
PSA monitoring
All five studies of localised cases included PSA measures in their monitoring protocols13-16,23 (Table 2). One study included twice yearly percent free/total (f/t) PSA measurements14, though it is not clear how these measures were used to trigger further investigation. Another included PSA velocity calculated from at least three values over a period of 12 months; a PSA velocity > 0.75 ng/ml/year was included in a composite definition of progression (described in Table 1)13.
Four studies diagnosed progression using PSA criteria 13,15,16,23 (Table 3), including PSA velocity13 and PSA doubling time16. In Choo’s series, the median PSA doubling time was 6.68 years and the proportion of men with fast PSA doubling times (< 2 years) reported by both Patel and Choo was 11%13,16. 12%-46% of men had negative doubling times13,16 and 42% had PSA doubling times > 10 years16, suggesting limited impact of low volume cancers on serial PSA in a large number of men.
There is some evidence that biopsy criteria are more likely to result in a diagnosis of progression than PSA criteria. In Patel’s series, the predominant factors determining a diagnosis of progression were i) the biopsy criteria (accounting for 57% of diagnosed progression, due to increased Gleason score in 30% and increased cancer volume in 27%), ii) PSA velocity > 0.75 ng/ml/year (36%), and iii) clinical findings on DRE or TRUS (7%)13. Progression rates were lower in the two studies which included measures of PSA (25% progressed in a median of 44.1 months13; 17% in 29 months16) than in a study based solely on unfavourable repeat biopsy findings (29% in 23 months)14.
Factors associated with disease progression
Actuarial probabilities of freedom from disease progression at 4-5 years of follow-up were 67%-72%13,15,16. The following section reviews the evidence on potential prognostic factors associated with progression of prostate cancer.
Clinico-pathological measures at baseline
Three studies showed associations of baseline Gleason score15, stage13 and prostate volume14 with clinical progression. However, two of these studies and another, larger study, found no associations of age14,16, Gleason score13,16 or tumour stage16 with progression. These null findings are not simply because studies were underpowered, since the largest study found no associations16, but may reflect variable protocols and definitions of progression.
Static PSA measures
Associations of baseline serum PSA with clinical progression were observed in some13,14 but not all studies16. In a case-series of T1c disease, men with baseline %fPSA > 20% had an 87% lower odds of progression (95% CI: 35% to 99%; p = 0.004) versus men with %fPSA ≤ 20% (n=67)14. All men received an annual biopsy, so the results are unlikely to be due to closer scrutiny of men with initial adverse levels of %fPSA. In univariable analyses, histological progression was associated with greater PSA density, lower gland volume and lower mean %fPSA14,27. A model with a gland volume ≤ 40.5 cc3 followed by %fPSA ≤ 19.5%, or gland volume > 40.5 cc3 followed by total PSA > 7.95 ng/ml generated 82% sensitivity and 83% specificity to select men with progressive potential based on repeat biopsy14. Patel found no evidence of an association between PSA density and progression (p = 0.5)13.
Dynamic PSA measures
Three dynamic markers of prostate cancer progression were investigated: a) PSA doubling time; b) PSA velocity (PSA levels per unit of time); and c) rate of change of f/tPSA over time. Patel found no association of PSA doubling time with progression (p = 0.3), even though PSA velocity was incorporated in the definition of progression13. Choo found that median PSA doubling times differed in men with clinical or histological progression (5.4 and 3.4 years, respectively) compared with no clinical or histological progression (7.4 and 7.5 years, respectively), but the numbers of men with progression was small (n = 29/231), effect estimates were imprecise and the results could have arisen by chance (p = 0.97 and 0.31, respectively)16,28. None of the men with PSA doubling times less than two years had positive bone scans16, but longer follow-up would be required to assess their prognosis.
Greater PSA velocity was associated with histological progression, but there was extensive overlap in values amongst those who progressed (mean: 1.1 ng/ml/yr, range: 0.0-5.2) versus those who did not (mean: 0.35 ng/ml/yr, range: 0.0-3.3; p = 0.03)14,27, limiting its accuracy as a prognostic marker. A combination of %fPSA, PSA velocity and gland volume may distinguish men with favourable versus unfavourable histological features on repeat biopsy (sensitivity of 65% at 90% specificity)14.
Rate of change of f/tPSA above the median (i.e > - 0.0096 / year) was associated with 85% progression-free survival at 5 years versus 58% for men below the median (p = 0.071)16,29. However, 43% of men were defined as having progressed on the basis of PSA doubling time, so the apparent association between rate of change of f/tPSA and progression-free survival may reflect the correlation between these two different measures of PSA velocity (r = −0.18; p = 0.021).
Six of the eight studies which included men with T3-T4 disease (Appendix 2) investigated the role of PSA doubling time as a marker of prostate cancer progression17,18,20,22,24,25. Rapid PSA doubling times or higher PSA velocity have been associated with a poor prognosis in some of these studies18,20,22,30, but there were limitations. In one report, associations disappeared after controlling for other prognostic factors20, including mean nuclear volume20,30, in another, rising PSA levels were a criterion for defining progression so the relationship carries little meaning, and in a third, PSA doubling time was not associated with clinical progression in an analysis ignoring PSA results obtained 2-12 months prior to the date of progression22. This latter study suggests an observer bias: knowledge of the latest available PSA results (i.e. whether rising or stable) may have influenced the diagnosis of clinical progression. Others showed no relationship between PSA change over time or rapid PSA doubling times and disease progression17,25.
Two of the studies which included advanced cases (Appendix 2) identified other potential markers: mean nuclear volume (MNV) 20 and the presence of circulating prostate cells19. These studies require replication. No association was found between mean annual increase in prostatic volume and clinical progression in a study involving 29 men25.
Discussion
We investigated the protocols and markers used in the PSA-testing era to predict and define prostate cancer progression, but just five observational studies of men with localised (T1-T2) prostate cancer were identified up to 2004. These reports involved a total of only 451 men followed up for less than five years. A key challenge for monitoring programmes is to reach an appropriate balance between enabling men with slow-growing localised prostate cancer to avoid radical intervention whilst also ensuring that those whose disease will progress to threaten the length and/or quality of their life can be identified and offered the opportunity of cure. The only consensus in the current literature is that PSA levels and DRE should be assessed. The timing of these assessments is not agreed, and different forms of PSA criteria are used, including its velocity13, doubling time13,16, total level and free/total ratio19. Three of the studies also recommended repeated transrectal biopsies13,14,16, but timings varied from six monthly to annually, and protocols differed in number of cores obtained. There is even less consensus over the definition of disease progression and/or need for active intervention (Table 3). All studies employed combinations of criteria, sometimes in complex or highly refined detail, and somewhat inevitably reported wide-ranging levels of progression (8-33%).
In men with clinically evident prostate cancer diagnosed in the pre PSA era, initial Gleason score was strongly associated with prostate cancer survival31. However, apart from the study by Neulander21, standard clinico-pathological criteria at the time of diagnosis (serum PSA, stage, or grade) were not strongly associated with disease progression in the monitoring studies reviewed here. This paradox may be related to the different patient populations being considered: clinically evident prostate cancer diagnosed in the pre PSA era31 compared to men with predominantly T1 localised prostate cancer on active monitoring protocols. Studies using re-biopsy in their protocols and histological definitions of progression (e.g. changes in Gleason score or pattern, or increase in number or proportion of cores with cancer) reported high rates of progression within the first 2-3 years of follow-up13,17,26. The sampling error associated with transrectal ultrasound-guided biopsy is well known32. The large proportion of observed changes in histology occurring within a very short time span suggest that the findings of “progression” on repeat biopsy probably reflect initial under-sampling of a higher-grade component rather than true dedifferentiation, casting doubt on the reliability of re-biopsies as a tool to diagnose progression.
Four of the five monitoring studies used some form of PSA change to identify ‘progression’13,15,16,23, making statistical associations between PSA-based biomarkers and progression meaningless. These studies effectively assumed that a rapidly rising PSA should be an indication for treatment and eliminated the opportunity to observe whether the cancer would have progressed based on more objective criteria. It has been suggested that fast PSA doubling times (e.g. < 1-2 years) can be used to identify men whose disease may progress. There was no evidence, however, from the studies included in this review to support this: some men show no evidence of progression despite fast PSA doubling times13,28 and some cancers that progress do so in spite of stable PSA levels17. In the Baltimore Longitudinal Study of Ageing, there was little difference in PSA doubling times measured on serial frozen sera up to 12-26 years before diagnosis in those who subsequently developed metastatic disease compared with those who did not progress 5.5-12.3 years after diagnosis (p = 0.47)33, but this study was based on only 16 cases. The absence of an association between fast PSA doubling times and progression seems counterintuitive but the hypothesis is based on inference - loss of growth control is a key step leading to prostate cancer progression, serum PSA levels are proportional to tumour volume (r2 = 0.49)34, and faster PSA doubling times are associated with advanced disease35. A recent study of 1,095 men with predominantly T1c cancer and baseline PSA levels < 10 ng/ml offers more compelling evidence: those who had a PSA velocity > 2.0 ng/ml/yr (highest quartile) in the year before diagnosis had a 9.8 (95% CI: 2.8-34.3) fold increase risk of death from prostate cancer after radical prostatectomy versus men in the lowest three quartiles (PSA velocity < 2.0 ng/ml/yr)36. Nevertheless the role of PSA velocity in active monitoring protocols remains undefined.
In the Baltimore Longitudinal Study of Ageing37 serum f/tPSA was lower in men diagnosed with aggressive compared with non-aggressive disease at the visit nearest the diagnosis (8% vs 19%; p = 0.006), 5 years (8% vs 16%; p = 0.003) and 10 years (9% vs 23%; p = 0.008) before the diagnosis37. At 10-15 yrs before diagnosis, total PSA levels were similar in both groups, suggesting that f/tPSA levels may be predictive of tumour behaviour at a time when total PSA levels provide no information on tumour aggressiveness37. Baseline percent free PSA14 and rate of change of f/tPSA29 were associated with disease progression in the active monitoring studies reviewed here, but these findings require replication in well designed studies in other populations. The rate of change of f/tPSA over time appears to provide different information to total PSA doubling time because the correlation between these two measures is weak (r = 0.18)29. A combination of %fPSA, PSA velocity and gland volume may predict cancers with progressive potential14 but the use of prediction rules based on these variables, and the outcomes, need to be tested in a clinical setting.
Measurement error due to biological or laboratory variation may attenuate underlying associations between PSA doubling times and progression. This problem is compounded because the studies reviewed generally involved fewer than 100 men, were of less than 5 years duration, with progression rates around 20%, leading to low statistical power to detect clinically important effects. There is also uncertainty over the number of PSA measurements and the duration of PSA monitoring needed to calculate PSA doubling times or velocity with precision. Differences in results between studies may reflect the wide variability in the PSA doubling times of a given patient depending on the methods used in its calculation5,12,18: short-term changes in PSA correlate only moderately with overall PSA velocity (r = 0.418)5; PSA doubling time was underestimated by 2 years in 18% of men when calculated in a period of one year or less18; and there are only moderate correlations (r = 0.53-0.56) between short and long-term estimations of PSA doubling time12. Thus, an initial increase in PSA may not truly reflect an individual’s cancer growth rate10.
A large proportion of men abandoning active monitoring without objective evidence of progression. ‘Patient anxiety’13, ‘request’16, or ‘withdrawal’ because of rise in PSA13,16,23 were the most common reasons for abandoning monitoring in up to one third of patients. Between 1992-1994 and 1998-2000 there was a 42% decrease in rates of active monitoring in the USA, with the most rapid decrease in patients with the most favourable baseline clinical variables38. The clinical meaning of findings from re-biopsy or regular PSA tests is uncertain, but reporting these to patients is highly likely to raise anxiety, adversely affect quality of life and make it difficult for patients to remain on monitoring.
The real dilemma with monitoring is the risk faced by patients of missing the window of opportunity to achieve cure when it is necessary, as it is possible that the first sign of progressing disease may also mean that it is no longer curable. It is this concern which has to date hampered most monitoring studies, as the real natural history of the disease has not been studied. It would appear, however, that the current protocols including PSA rise and grade change following biopsy, probably cause too many men to ‘opt out’ of monitoring, but these measures may be of limited value in determining cancers that are biologically active. There is a clear need to develop accurate, clinically-relevant, markers of disease progression that can unambiguously provide reassurance or initiate therapy as appropriate. As molecular techniques improve, novel prognostic markers may be developed. Currently, very few markers have been identified in surveillance studies conducted before or during the PSA era (mean nuclear volume20, angiogenesis39, VEGF and neuroendocrine expression40; p53 nuclear protein accumulation41; MIB-1 antibody42; DNA ploidy43; α-catenin44; and c-erbB-2 oncogene45). Most have been tested in single studies only and without replication it is not possible to rule out chance, bias or confounding as alternative explanations of reported associations.
Appendix 1: Summary of active monitoring studies without a predefined measure of disease progression (1988-2004) (ordered by stage & publication yr).
| 1st author, setting (year published) |
N (yrs recruited) |
Eligibility | Median age ± SD (range) |
Median initial PSA, ng/ml (range) |
Stage (%); median Gleason score |
Median follow-up (range) |
% choosing active treatment† during follow-up |
Reasons for abandoning active monitoring |
|---|---|---|---|---|---|---|---|---|
| LOCALISED DISEASE ONLY (STAGE T1-T2) | ||||||||
| El-Geneidy, Portland VA Medical Centre (2004)6 |
175 (1993- 2000) |
T1-T2 NXM0 | 71 (50- 86) |
6.9 (0.5-76.5) | T1 or T2 (31% stage T1c); 6 |
3.3 yrs (0.1-8.6) |
22% | PSA increase only: 32%; signs of local tumour progression: 8%; pt wish, no evidence of progression: 45%; unknown: 16%. PSA DT < 3 yrs (in 23% of pts) was associated with 3 fold increased rate of active treatment (p < 0.02; multivariable analysis). |
| Carter, Centre for Prostate Disease Research, (2003)7 |
313 (1991- 2002) |
Age ≤ 70; Gleason ≤ 6; no grade 4; PSA ≤ 20 ng/ml; ≤ 3 + ve cores; stage ≤ 2 |
65 (41- 70) |
5.1 (0.5-20) | Low grade, localised (60% stage T1c); 5 |
3.8 yrs (0.5-10.5) |
57.3% and 73.2% within 2 and 4 yrs respectively |
PSA DT < 2 yrs (in 22% of pts) was associated with > 3 fold increased rate of active treatment (p < 0.0001; multivariable analysis). |
| Meng, CaPSURE database (2003)8 |
457 (1989- 2001) |
Stage ≤ 3a N0M0; expectant initial treatment |
- | - | Localised (46% stage T1); - |
- | 41% at median 1.7 yrs. Only 15% went from low- intermediate / high risk on PSA criteria |
In multivariable models, men aged ≥ 75, < college educated, income < $30K, were 65%, 37% & 58% less likely, respectively, to abandon watchful waiting. Those with absolute PSA change from base ≥ 2 ng/ml had 3 fold rate of active treatment. |
| Zeitman, Massachusetts General Hospital (2001)9 |
198 (1990- 1999) |
T1-T2NXM0, PSA < 20 ng/ml |
71 | 6.6 | Localised (21% stage T1c); - |
3.4 | 57% of those alive by 5 yrs; 74% of those alive by 7 yrs. |
Of treated pts 81% believed the physician initiated therapy because of PSA increase or a nodule; physicians recorded advocating treatment due to clinical or biochemical progression in 24%. |
| Nam,Toronto, (1998)10 |
141 (1990- 1995) |
Clinically localised | 69 (53- 80) |
- | Localised disease (stage not stated); - |
1.7 yrs (0.2-4.1) |
48% underwent radical surgery |
Patient preference (none based on PSA velocity). |
| Gerber, Univ of Chicago, (1998)5 |
49 (1989- 1996) |
Stage T1-T2 | 71.9 ± 7.0 (55- 84) |
12.3 ±11.1* (1.1-49.0) |
Localised (T1c: 47%); - |
32 mo* (12-69). |
12% of those alive & not lost to follow-up. |
Patient preference – not based on uniform criteria. |
| INCLUDES LOCALLY ADVANCED/DISTANT METASTATIC DISEASE (STAGE T3-T4) | ||||||||
| Ross, McGill University, Canada, (2004)12. |
142 (1994- ?) |
Clinically localised prostate cancer |
69* (51- 87) |
8.1 (0.23-41) | T1-T3 (54% stage T1c); 57% Gleason score = 5-6 |
3.95 yrs (0.3-13) |
28% | “The decision…was on an individual basis. No specific criteria..” |
| Wu, Dept of Defence Centre for Prostate Disease Research (2004)11 |
1,158 (1990- 2001) |
Expectant initial treatment; no metastases |
70.9 | 6.4 | T1-T4 (53.6% T1; 41.7% T2); 37.8% Gleason = 5-6 |
2.8 yrs (0.8-11.3) |
39.1% | In multivariable models, LogPSA and clinical stage were positively associated, and age at diagnosis was inversely associated, with active treatment |
DRE: digital rectal examination; CaPSURE: Cancer of the Prostate Strategic Urologic Research Endeavour. PSA DT: Prostate specific antigen doubling time.
Radical prostatectomy, radiotherapy, hormonal treatment.
mean value used
Reference List
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. European Journal of Cancer. 2001;37(Supplement 8):4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Whitmore WF. Localised prostatic cancer: management and detection issues. Lancet. 1994;343(8908):1263–7. doi: 10.1016/s0140-6736(94)92156-3. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberger M, Partin A. Progress toward Identifying Aggressive Prostate Cancer. N Engl J Med. 2004;351(2):180–1. doi: 10.1056/NEJMe048119. [DOI] [PubMed] [Google Scholar]
- 4.Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet. 2003;361(9363):1122–8. doi: 10.1016/S0140-6736(03)12890-5. [DOI] [PubMed] [Google Scholar]
- 5.Gerber GS, Gornik HL, Goldfischer ER, Chodak GW, Rukstalis DB. Evaluation of changes in prostate specific antigen in clinically localized prostate cancer managed without initial therapy. Journal of Urology. 1998;159(4):1243–6. [PubMed] [Google Scholar]
- 6.El-Geneidy M, Garzotto M, Panagiotou I, Hsieh YC, Mori M, Peters L, et al. Delayed therapy with curative intent in a contemporary prostate cancer watchful-waiting cohort. BJU Int. 2004;93(4):510–5. doi: 10.1111/j.1464-410x.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 7.Carter CA, Donahue T, Sun L, Wu H, McLeod DG, Amling C, et al. Temporarily deferred therapy (watchful waiting) for men younger than 70 years and with low-risk localized prostate cancer in the prostate-specific antigen era. J Clin Oncol. 2003;21(21):4001–8. doi: 10.1200/JCO.2003.04.092. [DOI] [PubMed] [Google Scholar]
- 8.Meng MV, Elkin EP, Harlan SR, Mehta SS, Lubeck DP, Carroll PR. Predictors of treatment after initial surveillance in men with prostate cancer: results from CaPSURE. Journal of Urology. 2003;170(6 Pt 1):2279–83. doi: 10.1097/01.ju.0000094190.46523.b2. [DOI] [PubMed] [Google Scholar]
- 9.Zietman AL, Thakral H, Wilson L, Schellhammer P. Conservative management of prostate cancer in the prostate specific antigen era: the incidence and time course of subsequent therapy. Journal of Urology. 2001;166(5):1702–6. [PubMed] [Google Scholar]
- 10.Nam RK, Klotz LH, Jewett MA, Danjoux C, Trachtenberg J. Prostate specific antigen velocity as a measure of the natural history of prostate cancer: defining a ’rapid riser’ subset. British Journal of Urology. 1998;81(1):100–4. doi: 10.1046/j.1464-410x.1998.00523.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Sun L, Moul JW, Wu HY, McLeod DG, Amling C, et al. Watchful waiting and factors predictive of secondary treatment of localized prostate cancer. Journal of Urology. 2004;171(3):1111–6. doi: 10.1097/01.ju.0000113300.74132.8b. [DOI] [PubMed] [Google Scholar]
- 12.Ross PL, Mahmud S, Stephenson AJ, Souhami L, Tanguay S, Aprikian AG. Variations in PSA doubling time in patients with prostate cancer on ’watchful waiting’: Value of short-term PSADT determinations. Urology. 2004;64(2):323–8. doi: 10.1016/j.urology.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Patel M, De Concini DT, Lopez-Corona E, Ohori M, Wheeler T, Scardino PT. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. Journal of Urology. 2004;171(4):1520–4. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 14.Khan MA, Carter HB, Epstein JI, Miller MC, Landis P, Walsh PW, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? Journal of Urology. 2003;170(6 Pt 1):2274–8. doi: 10.1097/01.ju.0000097124.21878.6b. [DOI] [PubMed] [Google Scholar]
- 15.Chen WM, Yang CR, Ou YC, Cheng CL, Kao YL, Ho HC, et al. Clinical outcome of patients with stage T1a prostate cancer. Journal of the Chinese Medical Association. 2003;66(4):236–40. [PubMed] [Google Scholar]
- 16.Choo R, Klotz L, Danjoux C, Morton GC, DeBoer G, Szumacher E, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. Journal of Urology. 2002;167(4):1664–9. [PubMed] [Google Scholar]
- 17.Stephenson AJ, Aprikian AG, Souhami L, Behlouli H, Jacobson AI, Begin LR, et al. Utility of PSA doubling time in follow-up of untreated patients with localized prostate cancer. Urology. 2002;59(5):652–6. doi: 10.1016/s0090-4295(02)01526-1. [DOI] [PubMed] [Google Scholar]
- 18.Kakehi Y, Kamoto T, Shiraishi T, Kato T, Tobisu K, Akakura K, et al. Correlation of initial PSA level and biopsy features with PSA-doubling time in early stage prostate cancers in Japanese men. European Urology. 2002;41(1):47–53. doi: 10.1016/s0302-2838(01)00020-3. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre IG, Clarke RB, Anderson E, Clarke NW, George NJ. Molecular prediction of progression in patients with conservatively managed prostate cancer. Urology. 2001;58(5):762–6. doi: 10.1016/s0090-4295(01)01358-9. [DOI] [PubMed] [Google Scholar]
- 20.Arai Y, Egawa S, Kuwao S, Ogura K, Baba S. The role of volume-weighted mean nuclear volume in predicting tumour biology and clinical behaviour in patients with prostate cancer undergoing watchful waiting. BJU Int. 2001;88(9):909–14. doi: 10.1046/j.1464-4096.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- 21.Neulander EZ, Duncan RC, Tiguert R, Posey JT, Soloway MS. Deferred treatment of localized prostate cancer in the elderly: the impact of the age and stage at the time of diagnosis on the treatment decision. BJU Int. 2000;85(6):699–704. doi: 10.1046/j.1464-410x.2000.00569.x. [DOI] [PubMed] [Google Scholar]
- 22.McLaren DB, McKenzie M, Duncan G, Pickles T. Watchful waiting or watchful progression?: Prostate specific antigen doubling times and clinical behavior in patients with early untreated prostate carcinoma. Cancer. 1998;82(2):342–8. doi: 10.1002/(sici)1097-0142(19980115)82:2<349::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Mohler JL, Williams BT, Freeman JA. Expectant management as an option for men with stage T1c prostate cancer: a preliminary study. World Journal of Urology. 1997;15(6):364–8. doi: 10.1007/BF01300184. [DOI] [PubMed] [Google Scholar]
- 24.Schmid HP. Tumour markers in patients on deferred treatment: prostate specific antigen doubling times. Cancer Surveys. 1995;23:157–67. [PubMed] [Google Scholar]
- 25.Bangma CH, HOP WCJ, Schroder FH. Serial prostate specific antigen measurements and progression in untreated confined (stages T0 to 3NxM0, grades 1 to 3) carcinoma of the prostate. Journal of Urology. 1995;154(4):1403–6. [PubMed] [Google Scholar]
- 26.Epstein JI, Walsh PC, Carter HB. Dedifferentiation of prostate cancer grade with time in men followed expectantly for stage T1c disease. Journal of Urology. 2001;166(5):1688–91. [PubMed] [Google Scholar]
- 27.Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. Journal of Urology. 2002;167(3):1231–4. [PubMed] [Google Scholar]
- 28.Choo R, Klotz L, Deboer G, Danjoux C, Morton GC. Wide variation of prostate-specific antigen doubling time of untreated, clinically localized, low-to-intermediate grade, prostate carcinoma. BJU Int. 2004;94(3):295–8. doi: 10.1111/j.1464-410X.2004.04926.x. [DOI] [PubMed] [Google Scholar]
- 29.Do V, Choo R, De Boer G, Klotz L, Danjoux C, Morton G, et al. The role of serial free/total prostate-specific antigen ratios in a watchful observation protocol for men with localized prostate cancer. BJU Int. 2002;89(7):703–9. doi: 10.1046/j.1464-410x.2002.02737.x. [DOI] [PubMed] [Google Scholar]
- 30.Vollmer RT, Egawa S, Kuwao S, Baba S. The dynamics of prostate specific antigen during watchful waiting of prostate carcinoma: a study of 94 Japanese men. Cancer. 2002;94(6):1692–8. doi: 10.1002/cncr.10443. [DOI] [PubMed] [Google Scholar]
- 31.Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280(11):975–80. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 32.Stroumbakis N, Cookson MS, Reuter VE, Fair WR. Clinical significance of repeat sextant biopsies in prostate cancer patients. Urology. 1997;49(3, Supplement 1):113–8. doi: 10.1016/s0090-4295(97)00178-7. [DOI] [PubMed] [Google Scholar]
- 33.Cadeddu JA, Pearson JD, Partin AW, Epstein JI, Carter HB. Relationship between changes in prostate-specific antigen and prognosis of prostate cancer. Urology. 1993;42(4):383–9. doi: 10.1016/0090-4295(93)90362-e. [DOI] [PubMed] [Google Scholar]
- 34.Stamey TA, Kabalin JN. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. I. Untreated patients. Journal of Urology. 1989;141(5):1070–5. doi: 10.1016/s0022-5347(17)41174-8. [DOI] [PubMed] [Google Scholar]
- 35.Pearson JD, Carter HB. Natural history of changes in prostate specific antigen in early stage prostate cancer. Journal of Urology. 1994;152(5 Pt 2):1743–8. doi: 10.1016/s0022-5347(17)32375-3. [DOI] [PubMed] [Google Scholar]
- 36.D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA Velocity and the Risk of Death from Prostate Cancer after Radical Prostatectomy. New England Journal of Medicine. 2004;351(2):125–35. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 37.Carter HB, Partin AW, Luderer AA, Metter EJ, Landis P, Chan DW, et al. Percentage of free prostate-specific antigen in sera predicts aggressiveness of prostate cancer a decade before diagnosis. Urology. 1997;49(3):379–84. doi: 10.1016/s0090-4295(96)00629-2. [DOI] [PubMed] [Google Scholar]
- 38.Harlan SR, Cooperberg MR, Elkin EP, Lubeck DP, Meng MV, Mehta SS, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. Journal of Urology. 2003;170(5):1804–7. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 39.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. British Journal of Cancer. 1998;78(7):940–4. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borre M, Nerstrom B, Overgaard J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin Cancer Res. 2000;6(5):1882–90. [PubMed] [Google Scholar]
- 41.Borre M, Stausbol-Gron B, Overgaard J. p53 accumulation associated with bcl-2, the proliferation marker MIB-1 and survival in patients with prostate cancer subjected to watchful waiting. Journal of Urology. 2000;164(3 Pt 1):716–21. doi: 10.1097/00005392-200009010-00023. [DOI] [PubMed] [Google Scholar]
- 42.Borre M, Bentzen SM, Nerstrom B, Overgaard J. Tumor cell proliferation and survival in patients with prostate cancer followed expectantly. Journal of Urology. 1998;159(5):1609–14. doi: 10.1097/00005392-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 43.Borre M, Hofyer M, Nerstrom B, Overgaard J. DNA ploidy and survival of patients with clinically localized prostate cancer treated without intent to cure. Prostate. 1998;36(4):244–9. doi: 10.1002/(sici)1097-0045(19980901)36:4<244::aid-pros5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Crundwell MC, Arkell DG, Gearty J, Phillips SM. Genetic alterations in incidentally diagnosed, transitional zone prostate cancer: a seven year follow-up. Journal of Urology. 1997;158(4):1568–75. [PubMed] [Google Scholar]
- 45.Arai Y, Yoshiki T, Yoshida O. c-erbB-2 oncoprotein: a potential biomarker of advanced prostate cancer. Prostate. 1997;30(3):195–201. doi: 10.1002/(sici)1097-0045(19970215)30:3<195::aid-pros8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]