Abstract
Key features of clathrin-mediated endocytosis have been conserved across evolution. However, endocytosis in Saccharomyces cerevisiae is completely dependent on a functional actin cytoskeleton, while actin appears to be less critical in mammalian cell endocytosis. We reveal that the fundamental requirement for actin in early stages of yeast endocytosis is to provide a strong framework to support force generation required to direct the invaginating plasma membrane into the cell against turgor pressure. By providing osmotic support, pressure differences across the plasma membrane were removed and this reduced the requirement for actin-bundling proteins required for normal endocytosis. Conversely, increased turgor pressure in specific yeast mutants correlated with decreased rate of endocytic patch invagination.
Endocytosis is a highly regulated and essential process in the majority of eukaryotic cells. It is required for recycling of plasma membrane lipids and trafficking proteins, and for uptake or down-regulation of cell-surface receptors. During endocytosis the plasma membrane invaginates into the cell resulting in the production of a vesicle that is then able to fuse with endosomes. Work in the model organism Saccharomyces cerevisiae has led to significant advances in our understanding of the distinct stages that take place during endocytosis in vivo. It is now widely believed that the broad stages of coat assembly (early), invagination (mid) and scission/inward movement (late) are largely conserved across evolution, and that in many cases direct homologues of proteins are responsible for carrying out the same steps in the process 1. One notable difference between yeast and mammalian cells is that F-actin is absolutely required during plasma membrane invagination in yeast. Sequestering monomeric actin with latrunculin, or stabilisation with jasplakinolide both block yeast endocytosis 2-4. In mammalian cells, while actin is known to localise to sites of endocytosis, blocking its function does not generally prevent invagination, though the later stage of vesicle scission can be inhibited 5-7. This observed difference between the systems has led to questions over the degree to which the yeast model can be more generally applied.
In this study we aimed to address the reason for the observed differences in actin requirement. Considering the fundamental nature of the cells involved we reasoned that the primary distinction is the cell wall. This is needed in yeast to prevent lysis due to the cell's internal turgor pressure. This led us to hypothesise that an F-actin framework is needed to support the force generation required to pull membrane into the cell against this turgor pressure.
To investigate the possibility that turgor pressure is a major reason for the F-actin requirement, we used cells that lack the actin-bundling proteins Sac6 and Scp1 that normally allow cells to cross link F-actin and generate a strong meshwork during endocytic invagination. In cells lacking these proteins the majority of endocytic assembly events fail, with both reduced invagination and post-scission movement 8. If this actin framework is only required to support force generation for directing the invagination into the cell against the cell's own internal pressure, then the requirement for the actin bundling proteins would be lost if we reduce turgor pressure.
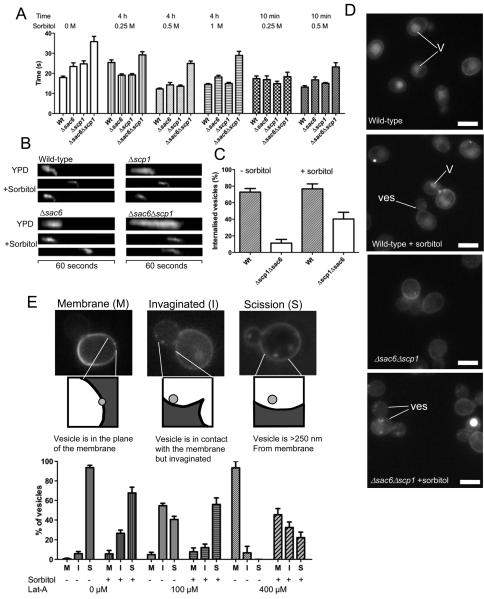
To test this we assessed the lifetime of GFP-Abp1 in patches at the plasma membrane in wild-type cells and in cells lacking either one (Δsac6, Δscp1) or both (Δsac6Δscp1) actin-bundling proteins. GFP-Abp1 is widely used as a reporter of mid to late stages of endocytosis and marks the stage at which actin is assembled at the endocytic site. To reduce the effects of turgor pressure, sorbitol was added to the medium. Using a range of sorbitol concentrations (0.25 - 1 M) and incubation times (10 minutes and 4 hours) we observed that additon of sorbitol could fully rescue the endocytic lifetime defects of both single mutants and substantially rescue the double mutant defect. Rescue was fully evident at just 10 minutes with 0.5 M sorbitol indicating that gene expression is not required for the effect. To demonstrate that reduction in lifetime also corresponded to a rescue in invagination, endocytic patches were studied using kymographs. Kymographs are generated by collating images of the same fluorescent spot taken in a time lapse series. Thus, they are able to provide information on spatial position over time. Time is represented in the x-axis and distance moved (i.e invagination) by any movement in the y-axis. As shown in figure 1B, inward movement of the patches can be clearly seen in both single and double mutants. Quantification of the rescue reveals a significant increase in the number of endocytic patches that are now able to internalise (figure 1C). Finally, the effect of sorbitol on fluid phase uptake of a fluorescent dye, Lucifer yellow was followed. Again the double mutant Δsac6Δscp1 shows almost no uptake of the dye. In the presence of sorbitol interestingly while uptake into vesicles can be observed, movement of these to the vacuoles appears inhibited (figure 1D). This indicates that while sorbitol can alleviate functions associated with turgor pressure at the plasma membrane, once vesicles have formed in the cell they continue to require bundled or crosslinked actin to move away from the membrane.
Figure 1. Alleviation of turgor pressure rescues a requirement for bundled actin during endocytosis.
(A) Wild-type yeast, or strains lacking either actin bundling protein Δsac6, or Δscp1 or both Δsac6Δscp1 were transformed with a marker of actin in endocytosis (GFP-Abp14). Sorbitol was added at either 0.25, 0.5, or 1 M to the cells for either 4 hours or 10 minutes and the effect on lifetimes of GFP-Abp1 measured. Number of patches assessed for each sample ≥30 in ≥4 cells. (B) Kymographs from these strains illustrating the effect of sorbitol on lifetime and behaviour of the patches. (C) The proportion of GFP-Abp1 patches showing inward movement was quantified for wild-type and the Δsac6Δscp1 strain. Number of patches assessed for each sample ≥45 in ≥8 cells (D) Actin-bundling mutants affect uptake of the fluid phase marker Lucifer yellow. Addition of sorbitol increases the proportion of cells showing uptake of the stain into vesicles (Ves) and endosomes in cells but few cells still show vacuolar (V) staining indicating a post-scission requirement for actin that is not affected by sorbitol. Bar = 5 μM. (E). The effect of increasing latrunculin-A concentration on endocytosis and partial rescue of the effect by sorbitol. Cells were treated with either 100 or 400 μM Latrunculin-A (or the control with DMSO alone), for 10 minutes, prior to addition of sorbitol for 10 minutes, and Lucifer yellow for 20 minutes. Spots that were visualized were categorised as indicated - (M) for spots still in the plane of the plasma membrane; (I) for spots that have moved out of the membrane but are still contiguous in terms of the lucifer yellow signal; (S) for spots that have successfully undergone scission and are at least 250 nm from the membrane. Number of spots assessed for each sample ≥140 in ≥60 cells.
While a requirement for actin-bundling during invagination and fluid phase endocytosis can be rescued by addition of sorbitol, there could still be a requirement for F-actin alone. To address this, cells were treated with 400 μM latrunculin-A, which caused complete disassembly of actin patches3. Sorbitol was then added to cells and uptake of Lucifer yellow was followed. Given that movement to the vacuole was not observed, uptake was categorised as unsuccessful (when bright patches were still in the plane of the membrane (M)); invaginated, when the patch had moved out of the plane but was still contiguous (I), or successful when a region with no fluorescence could be seen between the vesicle and the plasma membrane (S). As shown, (figure 1E) while addition of latrunculin-A blocks successful uptake of the fluid phase marker, this can be significantly rescued by addition of sorbitol. This demonstrates that even a complete absence of F-actin can be at least partially rescued by addition of sorbitol. Analysis of these data also indicate that while addition of sorbitol appears to assist the invagination step of endocytosis, it does infact have a slight inhibitory effect on scission and post-scission stages. This might explain why a previous study, assessing endocytic uptake rates (rather than analysis of distinct stages), showed an inhibition of endocytosis under hypertonic conditions 9.
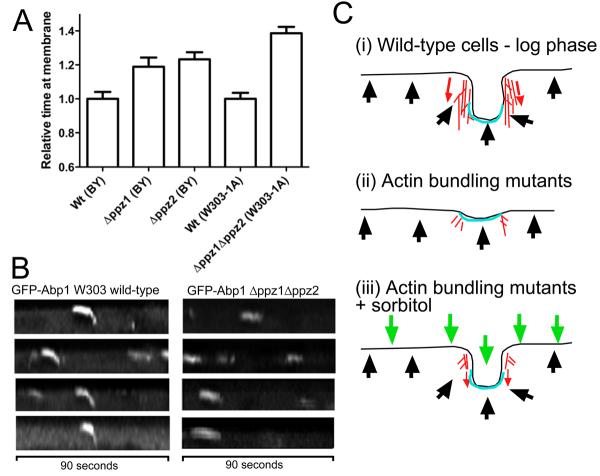
While the data strongly supports a link between turgor and a requirement for actin during the invagination stage of endocytosis, an important test of the hypothesis is that increasing turgor pressure should then make invagination more difficult. This could be manifested as an increase in lifetime of endocytic patches, and potentially as an increased rate of failed internalisation events. To address this possibility we made use of mutant yeast strains reported to have an increase in cell turgor pressure. These are strains lacking the non-essential type 1 protein phosphatases Ppz1 and Ppz2. Ppz deficient strains have increased steady state K+ levels and K+ is the major determinant of turgor pressure in S.cerevisiae 10, 11. In strains lacking either ppz gene alone or lacking both, the lifetime of GFP-Abp1 was measured. Deletion of the ppz genes led to a marked increase in lifetime of the GFP-Abp1 endocytic protein at the site of endocytosis (figure 2A). Furthermore, assessment of successful invaginations revealed that loss of both of these type I phosphatases markedly reduced the chance of successful invagination from 82.5% to 10%. Kymographs also reveal differences with a clear increase in the non-motile stage of endocytosis (figure 2B).
Figure 2. Increases in turgor pressure are detrimental to endocytic invagination.
(A). Deletion of the type I phosphatase genes ppz1 and ppz2 have been linked to an increase in turgor pressure10. Strains lacking either or both ppz1 and ppz2 were transformed with GFP-Abp1 and the lifetimes of GFP in the endocytic patch complexes measured. Because we were unable to generate a viable Δppz1Δppz2 double mutant in the Euroscarf strain used for our other experiments, this mutant was obtained from L.Yenush 10 and compared directly to its W303 background parent. Because the timings of W303 and BY4741 wild-type strains are different for endocytic markers, the timings here are relative to each parental strain. Differences between wild-type cells and all mutants are statistically relevant. In t-test comparisons: wild-type to Δppz1, P value 0.02; wt to Δppz2, P value 0.005; and wild type (W303) to Δppz1Δppz2, P value ≤0.0001. Number of patches assessed for each sample ≥30 in ≥4 cells. (B) Kymographs from wild-type and Δppz1Δppz2 strains illustrating the marked effect of their deletion on endocytic invagination. (C) A model to illustrate the findings. (i) In wild-type cells in exponential phase the inward force generation by actin in association with its bundling proteins is sufficient to overcome the outward force of turgor pressure. (ii) in actin-bundling mutants little invagination is observed due to the inability of a sufficiently strong actin meshwork to support the required inward force. (iii) Addition of sorbitol effectively balances out cell turgor pressure and now the requirement for actin during invagination is substantially alleviated. Actin - red; endocytic coat - blue; turgor - black arrows; external osmotic force - green arrows.
A shift to hypotonic conditions also increases turgor and under such conditions yeast cells pump glycerol from the cell using the Fps1 transporter to restore osmotic balance. The behaviour of GFP-Abp1 patches was analysed in wild type cells and in a strain deleted for fps1, before and after exposure to hypotonic conditions (see materials and methods). While GFP-Abp1 lifetime was not significantly affected under these hypotonic conditions, there was a dramatic increase in the proportion of assembly events that did not culminate in successful invagination. The parental wild-type strain showed only 9% of failed internalisation events, compared with 47% failed events after 15 minutes incubation in hypotonic conditions. The Δfps1 strain showed 41% failed events before shifting to hypotonic conditions and following the shift, no patches were observed to internalise (n ≥ 30).
Together these data support the idea that increased turgor pressure and an impaired ability to be able to respond to such changes (as in Δfps1) is detrimental to the ability of a cell to invaginate its plasma membrane as required for endocytosis.
Given the data presented, a simple model can be put forward, whereby a robust actin meshwork is required to support inward growth of plasma membrane in endocytosis due to the turgor pressure across the yeast plasma membrane (figure 2C). Alleviation of the pressure reduces the requirement for actin, while increased pressure makes invagination more difficult.
Overall our data clearly demonstrate that turgor pressure is a key factor in the requirement in yeast cells for a robust actin meshwork to form during endocytosis. While the endocytic coat contains several proteins that are capable of deforming a membrane (e.g. ENTH domain proteins), it cannot ensure inward growth of that membrane. Only with the force of actin polymerisation driven by the Arp2/3 complex and the type-I myosins, and the binding of this F-actin into bundles and crosslinked meshworks, can a membrane be successfully invaginated such that it can be pinched off to form a vesicle. Our data also shows that following scission actin is still required for movement of the vesicles away from the membrane. The alleviation of the pressure differences across the plasma membrane effectively make yeast more similar to a mammalian cell in having a greater requirement for actin at later, rather than the early invaginating, stages of endocytosis.
Supplementary Material
Acknowledgements
We thank Viki Allan, Elizabeth Smythe and Steve Winder for discussions and critical reading of the manuscript, and Lynne Yenush (Valencia, Spain) for yeast strains. KRA is a Senior non-clinical MRC Fellow (G0601600). SA is supported by a BBSRC studentship. The light microscopy-imaging centre at the University of Sheffield was funded by a grant from the Wellcome Trust (GR077544AIA).
Footnotes
Online Supplementary Material includes methodology and details of strains used in this study.
References
- 1.Smythe E, Ayscough KR. J. Cell Sci. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- 2.Ayscough KR. Curr.Biol. 2000;10:1587–1590. doi: 10.1016/s0960-9822(00)00859-9. [DOI] [PubMed] [Google Scholar]
- 3.Ayscough KR, et al. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaksonen M, Sun Y, Drubin DG. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 5.Merrifield CJ, Feldman ME, Wan L, Almers W. Nat Cell Biol. 2002;4:691–8. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 6.Merrifield CJ, Perrais D, Zenisek D. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Yarar D, Waterman-Storer CM, Schmid SL. Mol Biol Cell. 2005;16:964–75. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheorghe DM, et al. J. Biol. Chem. 2008;283:15037–15046. doi: 10.1074/jbc.M710332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitacre JL, Davis DA, Toenjes KA, Brower SM, Adams AEM. Genetics. 2001;157:533–43. doi: 10.1093/genetics/157.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchan S, Bernal D, Serrano R, Yenush L. Euk. Cell. 2004;3:100–107. doi: 10.1128/EC.3.1.100-107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yenush L, Mulet JM, Arino J, Serrano R. EMBO J. 2002;21:920–929. doi: 10.1093/emboj/21.5.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.