Abstract
Background
Few studies have examined the overall effect of maternal fish intake during pregnancy on child development or examined whether the developmental benefits of maternal fish intake are greater in infants breastfed for a shorter duration.
Objective
We aimed to study associations of maternal prenatal fish intake and breastfeeding duration with child developmental milestones.
Design
We studied 25 446 children born to mothers participating in the Danish National Birth Cohort, a prospective population-based cohort study including pregnant women enrolled between 1997 and 2002. Mothers reported child development by a standardized interview, which we used to generate developmental scores at ages 6 and 18 mo. We used multivariate cumulative ordinal logistic regression to evaluate the odds of higher developmental scores associated with maternal fish intake and breastfeeding, after adjustment for child age, sex, and growth; maternal size and pregnancy characteristics; and parental education and social status.
Results
Higher maternal fish intake and greater duration of breast-feeding were associated with higher child developmental scores at 18 mo [odds ratio: 1.29 (95% CI: 1.20, 1.38) for the highest versus the lowest quintile of fish intake, and 1.28 (1.18, 1.38) for breast-feeding for ≥10 mo compared with breastfeeding for ≤1 mo]. Associations were similar for development at 6 mo. Associations of fish intake with child development did not differ by breastfeeding duration.
Conclusions
Maternal fish intake during pregnancy and the duration of breastfeeding are independently associated with better early child development. Future research and consumption guidelines, incorporating nutritional benefits as well as contaminant risks, should consider the overall effect of prenatal fish consumption on child development.
INTRODUCTION
Fetal life and early infancy are critical periods for brain development. Early-life exposure to toxicants such as ethanol and lead may result in persistent deficits in cognition and development (1, 2). In addition, evidence is accumulating that early nutrition may program later neurodevelopment (3).
In particular, some investigators have examined whether early postnatal exposure to the long-chain n–3 polyunsaturated fatty acid docosahexaenoic acid (DHA) benefits later development. DHA, whose primary dietary source is fish and other seafood, is an essential structural component of the brain (4). DHA is also present in breast milk but not, until the past few years, in infant formula. Higher intake of DHA in infancy from breastfeeding (5–7) or from random assignment to an infant formula supplemented with DHA (8, 9) may benefit cognitive development.
Fewer investigators have examined whether the mother’s prenatal n–3 fatty acid status is associated with child development (10). The available studies have been consistent in finding improved development in infants and children with higher maternal prenatal DHA intake, whether estimated by more frequent fish intake (11–13), supplementation with fish oil (14, 15), or higher maternal or umbilical cord blood concentrations (16, 17). To date, no studies have examined possible interactions of maternal fish or DHA intake during pregnancy with breastfeeding.
In the present study, we examined associations of maternal fish consumption during pregnancy and the duration of infant breastfeeding with attainment of child developmental milestones reported at 6 and 18 mo of age. We used data from the Danish National Birth Cohort (DNBC), a large population-based study of pregnant women and their children. We hypothesized that fish intake and breastfeeding duration would each be directly associated with attainment of developmental milestones. We also hypothesized that the benefit of maternal fish intake would be greatest among children who breastfed for a shorter duration, because they would have less opportunity for postnatal DHA intake.
SUBJECTS AND METHODS
Subjects
The DNBC enrolled 101 042 pregnant women from 1997 to 2002; this group represented ≈ 30% of all deliveries in Denmark during these years. Study aims and protocols were reported previously (18, 19). Briefly, general practitioners throughout Denmark recruited women at their initial prenatal visit, usually in weeks 6–12 of pregnancy. Enrolled participants completed computer-assisted telephone interviews at gestation weeks 12 and 30 as well as at 6 and 18 mo after delivery, and they also completed mailed food-frequency questionnaires (FFQs) at gestation week 25. Study instruments are publicly available (20).
Of the 92 676 women with liveborn singleton infants, 50 276 completed both the initial interview and the FFQ during pregnancy. Of the 28 277 mothers who completed the 18-mo post-partum interview, we studied the 25 446 who responded at 18.0–20.9 mo and who had complete data for all previously specified covariates—namely maternal age, social status, marital status, parity, smoking and alcohol use during pregnancy; maternal and paternal education; and child gestational age, birth weight, sex, and breastfeeding. We also studied the 35 557 mothers who completed the 6-mo postpartum interview, of whom we included the 28 958 who responded between 5.0 and 6.9 mo after delivery and who provided information on all previously specified covariates, as above. Women included in this analysis did not differ markedly from those not included with respect to mean fish intake (26.5 and 26.9 g/d. respectively), age (29.3 and 29.1 y, respectively), or parity (53.5% and 52.8% parous, respectively), but the included women had somewhat longer mean breastfeeding duration (8.0 and 7.6 mo, respectively) and were less likely to be single (0.9% and 2.8%, respectively) and to smoke cigarettes during pregnancy (23.5% and 28.0%, respectively) than were the unincluded women. Included children had slightly greater gestation length (40.1 and 39.9 wk, respectively) and birth weight z score (0.24 and 0.20, respectively) than did unincluded children.
All participants provided written informed consent. The Regional Scientific Ethics Committee for the municipalities of Copenhagen and Frederiksberg approved all study protocols, and all procedures were in accordance with the Declaration of Helsinki.
Ascertainment of prenatal diet
A detailed description of the dietary assessment used in the DNBC was published previously (19, 21). At gestation week 25, enrolled women were mailed a self-administered semiquantitative FFQ, which contained >360 questions about the intake of foods and supplements during the previous month, including the intake of fish as a main meal and of fish with bread. This food-frequency questionnaire was modified from one used at the Danish Cancer Registry (22), and it has been validated in a subset of women against 7-d weighed food diaries and blood biomarkers (23). We calculated total fish intake (in g/d) by using assumptions about standard portion sizes. For our primary analyses, we analyzed fish intake in quintiles. In addition, we analyzed fish intake as a continuous exposure in weekly servings and also according to current US recommendations for weekly fish intake during pregnancy, with the categories of no fish (0 g/wk), 1–2 servings/wk (1–340 g/wk), or ≥3 servings/wk (>340 g/wk) (12, 24).
Ascertainment of breastfeeding
At the 6-mo postpartum interview, mothers reported whether they had never breastfed, were currently breastfeeding, or had stopped breastfeeding. Those who were no longer breastfeeding reported the child’s age when daily breastfeeding stopped. At the 18-mo postpartum interview, mothers reported whether they had breastfed their child beyond 6 mo and, if so, whether they were still breastfeeding or they reported the child’s age at discontinuation of breastfeeding. We analyzed breastfeeding duration as the duration of any breastfeeding, not distinguishing between exclusive and supplemented breastfeeding, in categories of ≤1, 2–3, 4–6, 7–9, or ≥10 mo.
Developmental milestones
The primary outcome was total development at 18 mo. At the 18-mo postpartum interview, mothers answered (yes or no) to 9 questions about whether the child could climb stairs, remove his or her socks and shoes, drink from a cup, be occupied for 15 min without adult participation, fetch an object when requested, write or draw, orient a book correctly, use word-like sounds, and put 2 words together. Mothers also reported the ages (in mo) at which the child could first sit unsupported and could walk unassisted and the total number of words the child could currently say. We excluded questionnaires with “don’t know” or missing responses to any of the questions. We created a total developmental scale with 1 point for each yes answer on the yes-or-no questions or for being in the highest decile for the 3 continuous responses (sat at <5 mo, walked at <10.5 mo, and says >60 words). We also a priori created overlapping subscales for motor (sat at <5 mo, walked at <10.5 mo, climb stairs, remove socks or shoes, drink from cup, fetch object, color, and orient book) and social or cognitive (remove shoes, drink from cup, fetch object, occupy self, color, hold book, use words, say >60 words, and use 2 words together) milestones.
Mothers also reported child development at 6 mo. At this interview, mothers answered 13 yes-or-no questions about the child’s current development: whether the child could hold up his or her head, sit with a straight back, roll back to front, sit unsupported, look in the direction of sounds or voices, throw a toy to the floor, make sounds while playing (other than crying), imitate sounds, reach for objects, crawl, seek contact with the parent (by reaching or making sounds), express dislikes, and bring an object to his or her mouth. We created a total developmental scale based on the responses to these 13 questions, assigning 1 point for each “yes” answer. In addition, a priori we created overlapping subscales for motor (hold head up, sit, roll, sit unsupported, look in the direction of sounds, throw toy, reach for object, crawl, reach for parent, and bring object to mouth) and social or cognitive (look at sounds, make sounds, imitate sounds, reach for object, reach for parent, and express dislikes) milestones.
Because of the small numbers of subjects with low scores, we collapsed the lowest categories of each developmental scale. At 18 mo, the ranges of possible scores were 5–12 for total, 3–8 for motor, and 4–9 for social or cognitive development. At 6 mo, the ranges of possible scores were 6–13 for total, 6–10 for motor, and 3–6 for social or cognitive development.
Covariates
Using data from the pregnancy interviews, we determined maternal age, parity, prepregnancy BMI, marital status, maternal and paternal education, history of parental school problems, and social class based on the higher of mother’s or father’s employment status. We calculated gestational age from the last menstrual period reported by the mother at study recruitment (gestation week 6–10) or from the expected date of delivery provided by the woman during the second telephone interview (gestation week 30), which was most often based on ultrasound results (21). The date of birth was extracted from the Danish Civil Registration System. The midwife who attended the child’s birth recorded birth weight, birth length, and head circumference. We calculated sex-specific weight and head circumference z scores at birth on the basis of published reference data (25). From the 6-mo postpartum interview, we determined whether there had been maternal smoking or alcohol use (or both) during pregnancy and the occurrence or nonoccurrence of postpartum depression. At the 18-mo postpartum interview, mothers reported the child’s weight, height, and head circumference, which had been measured by the general practitioner at routine 5- and 12-mo well-child visits and recorded in a booklet kept by the mother.
Statistical analysis
We examined parent and child characteristics according to quintiles of maternal prenatal fish consumption for the 25 446 children for whom we obtained outcome data at age 18 mo. We tested for trends across quintiles of fish intake by using ANOVA for continuous characteristics and Mantel-Haentzel chi-square tests for categorical characteristics.
We performed multivariate, cumulative, ordinal, logistic regression analysis for each of the 3 outcomes (motor, social or cognitive, and total development) at the 2 ages (6 and 18 mo). This method provides an estimate of the effect of an exposure on the likelihood of obtaining any given score or higher compared with any lower score. Unlike in typical ordinal logistic regression, which provides distinct effect estimates for each individual category compared with a reference category, this method provides a single pooled estimate. An odds ratio (OR) >1 indicates that an exposure is associated with a higher developmental score, and an OR <1 indicates that an exposure is associated with a lower score.
We included as covariates maternal and child factors determined a priori to be of interest— namely, infant sex, gestation length, age at questionnaire completion, and birth weight z score; maternal age, parity, marital status, prepregnancy BMI, smoking or alcohol use during pregnancy, and postpartum depression; and parental social class, education, and learning difficulties. All covariates were modeled as presented in Table 1. For continuous or dichotomous characteristics, we report the P value for the exposure. For categorical characteristics, we report the P value for trend across categories of the exposure. We did not include child weight or head circumference at 5 and 12 mo because of many missing values, although, in an analysis of the subset of infants with available data, further adjustment for these measures did not appreciably change results (data not shown).
TABLE 1.
Parent and child characteristics according to maternal prenatal fish consumption among 25 446 children in the Danish National Birth Cohort
| Maternal prenatal fish intake |
|||||
|---|---|---|---|---|---|
| Characteristics | Overall | Lowest quintile | Middle quintile | Highest quintile | P1 |
| Maternal | |||||
| Fish intake (g/d) | 26.6 ± 22.72 | 5.4 ± 3.3 | 22.3 ± 2.5 | 58.6 ± 30.2 | — |
| Age (y) | 29.3 ± 4.1 | 28.2 ± 4.1 | 29.4 ± 4.0 | 30.0 ± 4.2 | <0.0001 |
| Socioeconomic status (%) | <0.0001 | ||||
| High | 23.3 | 16.6 | 24.3 | 27.7 | |
| Medium | 32.5 | 28.8 | 33.6 | 32.7 | |
| Skilled | 28.0 | 33.8 | 27.1 | 24.3 | |
| Unskilled | 10.1 | 13.9 | 9.3 | 9.2 | |
| Unemployed | 1.2 | 1.9 | 1.0 | 1.1 | |
| Student | 4.8 | 5.1 | 4.7 | 5.0 | |
| Nulliparous (%) | 47.5 | 52.9 | 46.8 | 43.0 | <0.0001 |
| Unmarried (%) | 0.9 | 1.4 | 0.8 | 0.7 | 0.0008 |
| Any alcohol use (%) | 58.0 | 50.0 | 61.2 | 60.7 | <0.0001 |
| Postpartum depression (%) | 3.5 | 3.9 | 3.1 | 3.7 | 0.45 |
| Education (%) | <0.0001 | ||||
| <9th grade | 0.3 | 0.5 | 0.1 | 0.3 | |
| 9th grade | 6.5 | 8.4 | 5.6 | 5.7 | |
| 10th grade | 25.9 | 32.9 | 25.0 | 22.2 | |
| Graduated from 2-y high school3 | 13.7 | 12.9 | 13.5 | 14.5 | |
| Graduated from high school | 53.7 | 45.3 | 55.8 | 57.3 | |
| Prepregnancy BMI (kg/m2) | <0.0001 | ||||
| ≤18.5 (%) | 4.1 | 4.2 | 3.6 | 4.8 | |
| >18.5 to 325 (%) | 68.0 | 62.5 | 69.8 | 70.5 | |
| ≥25 to <30 (%) | 19.7 | 22.0 | 19.7 | 17.6 | |
| ≥30 to <35 (%) | 6.1 | 8.1 | 5.3 | 5.3 | |
| ≥35 to <40 (%) | 1.6 | 2.4 | 1.1 | 1.4 | |
| ≥40 (%) | 0.6 | 0.9 | 0.5 | 0.5 | |
| Smoking | <0.0001 | ||||
| Nonsmoker (%) | 76.1 | 72.4 | 77.6 | 77.7 | |
| Occasional smoker (%) | 12.3 | 12.3 | 12.1 | 12.7 | |
| <15 cigarettes/d (%) | 9.8 | 12.2 | 8.7 | 9.5 | |
| ≥15 cigarettes/d (%) | 1.7 | 3.1 | 1.5 | 1.1 | |
| Academic school problems for either parent (%) | 30.2 | 33.7 | 29.2 | 29.7 | <0.0001 |
| Paternal | |||||
| Education (%) | <0.0001 | ||||
| <9th grade | 2.2 | 2.5 | 2.0 | 1.9 | |
| 9th grade | 16.4 | 20.4 | 14.3 | 14.4 | |
| 10th grade | 35.8 | 40.5 | 37.0 | 33.2 | |
| Graduated from 2-y high school3 | 5.9 | 5.1 | 6.1 | 6.2 | |
| Graduated from high school | 39.7 | 31.5 | 40.6 | 44.4 | |
| Child | |||||
| Gestation length (wk) | 40.1 ± 1.7 | 40.0 ± 1.7 | 40.1 ± 1.7 | 40.1 ± 1.6 | 0.15 |
| Birth weight z score | 0.24 ± 1.0 | 0.19 ± 1.0 | 0.24 ± 1.0 | 0.26 ± 1.0 | <0.0001 |
| Head circumference at birth (z score) | 0.38 ± 1.6 | 0.36 ± 1.6 | 0.35 ± 1.3 | 0.39 ± 1.8 | 0.46 |
| Female (%) | 49.7 | 49.3 | 49.5 | 50.4 | 0.67 |
| Breastfeeding duration (mo) | 7.9 ± 4.6 | 7.0 ± 4.7 | 8.1 ± 4.5 | 8.6 ± 4.7 | <0.0001 |
| Age at 18-mo interview (mo) | 19.2 ± 0.7 | 19.2 ± 0.7 | 19.2 ± 0.7 | 19.2 ± 0.7 | 0.11 |
| Motor development score at 18 mo | <0.0001 | ||||
| 3 (%) | 2.7 | 3.5 | 2.5 | 2.1 | |
| 4 (%) | 10.6 | 11.6 | 10.9 | 9.0 | |
| 5 (%) | 38.9 | 38.4 | 38.7 | 38.2 | |
| 6 (%) | 37.9 | 36.2 | 38.2 | 39.1 | |
| 7 (%) | 9.0 | 8.9 | 8.4 | 10.3 | |
| 8 (%) | 1.3 | 1.5 | 1.4 | 1.3 | |
| Social or cognitive development score at 18 mo | <0.0001 | ||||
| 4 (%) | 4.1 | 5.0 | 3.7 | 3.3 | |
| 5 (%) | 13.4 | 14.8 | 13.6 | 11.6 | |
| 6 (%) | 30.1 | 30.0 | 30.5 | 29.2 | |
| 7 (%) | 30.8 | 30.3 | 30.6 | 32.2 | |
| 8 (%) | 16.6 | 15.3 | 16.5 | 17.7 | |
| 9 (%) | 5.0 | 4.7 | 5.2 | 6.0 | |
| Total development score at 18 mo | <0.0001 | ||||
| 5 (%) | 4.5 | 5.7 | 4.1 | 3.5 | |
| 6 (%) | 12.0 | 12.8 | 12.3 | 10.4 | |
| 7 (%) | 26.2 | 25.7 | 26.9 | 24.6 | |
| 8 (%) | 28.5 | 28.1 | 27.9 | 30.2 | |
| 9 (%) | 19.0 | 18.1 | 18.8 | 19.8 | |
| 10 (%) | 8.0 | 7.6 | 8.2 | 9.2 | |
| 11 (%) | 1.7 | 1.6 | 1.5 | 2.0 | |
| 12 (%) | 0.2 | 0.3 | 0.2 | 0.3 | |
P value from chi-square test or ANOVA for trend across all 5 quintiles of fish intake.
x̄ ± SD (all such values).
Standard duration of high school in Denmark.
Because we did not observe any evidence of a differential effect of fish intake by child sex (P = 0.26 at 6 mo and P = 0.84 at 18 mo for fish intake × child sex interaction), we present data from boys and girls together. We also looked for a potential interaction between the effects of fish intake and breastfeeding by using a multiplicative interaction term and stratified analyses. We used SAS software (version 9.1; SAS Inc, Cary, NC) for all analyses.
RESULTS
Among the 25 446 mother-child pairs with information on 18-mo development, mean maternal fish intake was 26.6 (range: 0–493.9) g/d. Maternal fish intake increased from a mean of 5.4 (range: 0–10.5) g/d in the lowest quintile of intake to 22.3 (range: 18.2–26.8) g/d in the middle quintile and 58.6 (range: 39.4–493.9) g/d in the highest quintile. Thus, on average, women in the lowest quintile consumed <1 fish serving/wk, those in the middle quintile consumed ≈1.5 fish servings/wk, and those in the highest quintile consumed ≈3.5 fish servings/wk. Most (86.3%) of the women reported consuming 1–2 fish servings/wk (1–340 g/wk), and 11.0% consumed ≥3 fish servings/wk (>340 g/wk), whereas only 2.8% of women never consumed fish (0 g/wk).
The fish species most frequently consumed by women in the DNBC were cod, plaice, salmon, herring, and mackerel. The median mercury content of these species, according to the Danish food monitoring program, ranges from 0.034 to 0.049 ppm (26). These fish types account for ≈85% of the total seafood intake in the study population. Species with high mercury content, such as shark and king mackerel, are not commonly consumed in Denmark.
Compared with mothers in the lowest quintile of prenatal fish intake, those in the highest quintile were slightly older, more likely to be of high socioeconomic status, more likely to be high school graduates, and more likely to use alcohol but less likely to be nulliparous or unmarried and to smoke cigarettes during pregnancy (Table 1). The mean duration of any breastfeeding was 7.9 mo. Mothers with higher fish intake had infants who were heavier at birth and who tended to breastfeed for a longer duration.
Child motor, social or cognitive, and total development scores also varied by maternal fish intake (Table 1). For example, 5.7% of children with mothers in the lowest quintile of fish intake had the lowest total development score at 18 mo, whereas only 3.5% of children with mothers in the highest quintile of fish intake had the lowest total development score (Table 1).
In general, participant characteristics were associated with child development in the anticipated directions (Table 2). Thus, predictors of a higher child developmental score at 18 mo included having a mother who was younger and (previously) nulliparous or who did not drink alcohol during pregnancy, having a father with higher education, being female, and being born after longer gestation or with higher birth weight (Table 2). One association went in the direction opposite that anticipated (namely, that children of mothers who smoked cigarettes had higher developmental scores); this finding was mainly based on higher motor development (data not shown), which was perhaps due to the fact that children born to smoking mothers are more likely to be hyperactive (27).
TABLE 2.
Associations of parent and child characteristics with child total developmental score at 18 mo among 25 446 children in the Danish National Birth Cohort
| Odds ratio (95% CI)1 | P2 | |
|---|---|---|
| Maternal | ||
| Age (per 5-y increment) | 0.92 (0.89, 0.95) | <0.0001 |
| Socioeconomic status | 0.67 | |
| High | 1.00 (referent) | |
| Medium | 1.00 (0.94, 1.07) | |
| Skilled | 0.96 (0.90, 1.03) | |
| Unskilled | 0.96 (0.87, 1.05) | |
| Unemployed | 0.99 (0.80, 1.22) | |
| Student | 1.06 (0.95, 1.19) | |
| Nulliparous | 1.22 (1.16, 1.28) | <0.0001 |
| Unmarried | 1.24 (0.97, 1.57) | 0.08 |
| Education | 0.26 | |
| <9th grade | 1.08 (0.70, 1.70) | |
| 9th grade | 0.99 (0.90, 1.09) | |
| 10th grade | 0.95 (0.90, 1.01) | |
| Graduated from 2-y high school | 0.98 (0.92, 1.05) | |
| Graduated from high school | 1.00 (referent) | |
| Prepregnancy BMI (kg/m2) | 0.29 | |
| ≤18.5 | 1.04 (0.93, 1.16) | |
| >18.5 to <25 | 1.00 (referent) | |
| ≥25 to <30 | 1.01 (0.95, 1.07) | |
| ≥30 to <35 | 1.12 (1.02, 1.23) | |
| ≥35 to <40 | 0.94 (0.79, 1.13) | |
| ≥40 | 1.07 (0.81, 1.42) | |
| Smoking | <0.0001 | |
| Nonsmoker | 1.00 (referent) | |
| Occasional smoker | 1.06 (0.99, 1.14) | |
| <15 cigarettes/d | 1.14 (1.05, 1.23) | |
| ≥15 cigarettes/d | 1.23 (1.03, 1.46) | |
| Any alcohol use | 0.91 (0.87, 0.96) | 0.0001 |
| Postpartum depression | 1.11 (0.98, 1.25) | 0.10 |
| Either parent had academic school problems | 1.03 (0.98, 1.08) | 0.21 |
| Paternal | ||
| Education | <0.0001 | |
| <9th grade | 0.78 (0.66, 0.91) | |
| 9th grade | 0.86 (0.80, 0.92) | |
| 10th grade | 0.86 (0.81, 0.91) | |
| Graduated from 2-y high school | 1.05 (0.95, 1.16) | |
| Graduated from high school | 1.00 (referent) | |
| Child | ||
| Gestation length (wk) | 1.13 (1.12, 1.15) | <0.0001 |
| Birth weight (z score) | 1.09 (1.07, 1.12) | <0.0001 |
| Head circumference at birth (z score) | 0.99 (0.97, 1.00) | 0.05 |
| Female | 1.77 (1.69, 1.85) | <0.0001 |
| Age at 18-mo interview (mo) | 1.34 (1.30, 1.39) | <0.0001 |
Odds ratios from cumulative ordinal logistic regression represent the likelihood of attaining a higher developmental score, and they are adjusted for all characteristics in table as well as maternal fish intake and breastfeeding duration.
P value for trend for exposures with > 2 categories.
In the unadjusted analysis, higher maternal fish intake was associated with a higher total developmental score at age 18 mo (unadjusted OR: 1.28; 95% CI: 1.19, 1.36 for the highest compared with the lowest quintile of fish intake). Adjustment for parent and child characteristics did not markedly influence estimates. For example, the effect of maternal fish intake in the highest compared with the lowest quintile was 1.27 (95% CI: 1.18, 1.36) after adjustment for child characteristics and 1.30 (95% CI: 1.21, 1.40) after additional adjustment for parental characteristics. Further adjustment for other potential maternal dietary predictors of child development, namely iron and folic acid intakes, did not alter estimates for fish consumption (data not shown).
After additional adjustment for breastfeeding duration, higher maternal prenatal fish intake remained associated with higher child developmental scores at 18 mo, with an OR of 1.29 (95% CI: 1.20, 1.38) for the highest compared with the lowest quintile (Table 3). When we instead analyzed fish intake according to current guidelines for intake during pregnancy, as was done previously (12), ORs for higher total development at 18 mo were 0.98 (95% CI: 0.85, 1.12) for 1–340 g/wk and 1.20 (1.04, 1.40) for >340 g/wk, compared with 0 g/wk. When we expressed fish intake as a continuous exposure, the OR for higher development was 1.49 (95% CI: 1.33, 1.66) for each additional fish serving/wk. Estimates of the associations of prenatal fish intake with motor and social or cognitive development were generally similar, and approximately the same for milestones reported at 6 mo as for those reported at 18 mo (OR for higher total development at 6 mo: 1.25; 95% CI: 1.17, 1.34 for highest compared with lowest quintile of fish intake) (Table 3).
TABLE 3.
Associations of maternal prenatal fish intake [by quintile (Q)] and duration of infant breastfeeding with attainment of developmental milestones at ages 6 mo (n = 28 958) and 18 mo (n = 25 446) among children in the Danish National Birth Cohort
| Subjects | Median fish intake | Median breastfeeding duration | Motor development | Social or cognitive development | Total development | |
|---|---|---|---|---|---|---|
| n (%) | g/d | mo | ||||
| Maternal fish intake (quintiles) | ||||||
| 6-mo outcomes | ||||||
| Q1 | 5744 (19.8) | 5.9 | — | 1.0 (referent)1 | 1.0 (referent) | 1.0 (referent) |
| Q2 | 5873 (20.3) | 14.5 | — | 0.98 (0.92, 1.05) | 1.0 (0.93, 1.07) | 0.99 (0.92, 1.05) |
| Q3 | 5913 (20.4) | 22.2 | — | 1.03 (0.97, 1.11) | 1.07 (0.99, 1.15) | 1.05 (0.99, 1.13) |
| Q4 | 5823 (20.1) | 32.2 | — | 1.05 (0.98, 1.12) | 1.18 (0.09, 1.27) | 1.09 (1.02, 1.17) |
| Q5 | 5605 (19.4) | 50.8 | — | 1.17 (1.09, 1.25) | 1.33 (1.23, 1.44) | 1.25 (1.17, 1.34) |
| 18-mo outcomes | ||||||
| Q1 | 5038 (19.8) | 5.9 | — | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Q2 | 5143 (20.2) | 14.4 | — | 1.00 (0.93, 1.07) | 1.00 (0.94, 1.08) | 0.99 (0.93, 1.07) |
| Q3 | 5117 (20.1) | 22.2 | — | 1.08 (1.00, 1.16) | 1.11 (1.04, 1.19) | 1.09 (1.01, 1.17) |
| Q4 | 5152 (20.3) | 32.3 | — | 1.11 (1.03, 1.19) | 1.15 (1.07, 1.24) | 1.14 (1.06, 1.22) |
| Q5 | 4996 (19.6) | 50.7 | — | 1.24 (1.15, 1.33) | 1.28 (1.19, 1.37) | 1.29 (1.20, 1.38) |
| Breastfeeding duration (mo) | ||||||
| 6-mo outcomes | ||||||
| ≤ 1 mo | 2829 (9.8) | — | 0 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| 2–3 mo | 2564 (8.9) | — | 3 | 1.11 (1.00, 1.22) | 1.09 (0.98, 1.21) | 1.12 (1.02, 1.23) |
| 4–6 mo | 4393 (15.2) | — | 5 | 1.09 (1.00, 1.19) | 1.07 (0.97, 1.18) | 1.11 (1.02, 1.21) |
| >6 mo | 19172 (66.2) | — | 10 | 1.10 (1.02, 1.19) | 1.12 (1.03, 1.22) | 1.12 (1.04, 1.20) |
| 18-mo outcomes | ||||||
| ≤1 mo | 2704 (10.6) | — | 0 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| 2–3 mo | 2416 (9.5) | — | 3 | 1.15 (1.04, 1.27) | 1.13 (1.02, 1.24) | 1.16 (1.05, 1.28) |
| 4–6 mo | 4696 (18.5) | — | 5 | 1.09 (1.00, 1.20) | 1.03 (0.95, 1.13) | 1.05 (0.96, 1.14) |
| 7–9 mo | 7078 (27.8) | — | 8 | 1.08 (1.00, 1.18) | 1.15 (1.06, 1.25) | 1.11 (1.02, 1.21) |
| ≥10 mo | 8552 (33.6) | — | 12 | 1.26 (1.16, 1.37) | 1.27 (1.17, 1.38) | 1.28 (1.18, 1.38) |
Odds ratios; 95% CIs in parentheses (all such values). Odds ratios from cumulative ordinal logistic regression represent the likelihood of attaining a higher developmental score, and they are adjusted for maternal age, prepregnancy BMI, parity, smoking, alcohol use, postpartum depression, and marital status; maternal and paternal school problems, education, and social class; and child birth weight z score, head circumference at birth z score, gestational age, sex, age at outcome assessment, fish intake (breastfeeding estimates), and breastfeeding (fish intake estimates).
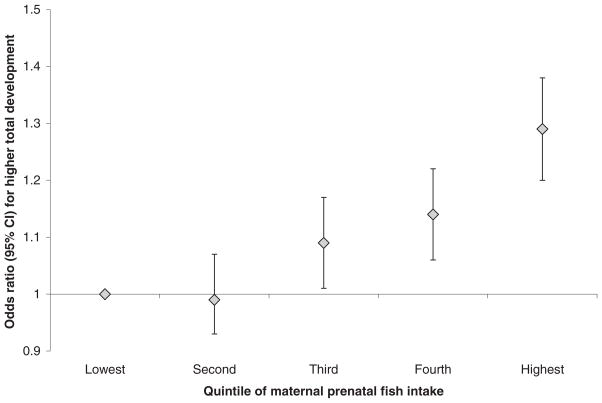
The adjusted ORs for the association of maternal prenatal fish intake (in quintiles) with attainment of a greater number of developmental milestones at age 18 mo are shown in Figure 1. Estimates were similar for the lowest and second quintile and then increased across the 3 highest quintiles of intake (Figure 1).
FIGURE 1.
Associations of maternal prenatal fish intake, in quintiles, with total developmental milestones attained by children at 18 mo of age. n = 25 446 children in the Danish National Birth Cohort. Odds ratios from cumulative ordinal logistic regression analysis represent the likelihood of attaining a higher developmental score and are adjusted for maternal, child, and parental sociodemographic characteristics as well as for breastfeeding duration.
Longer duration of breastfeeding was also associated with better development at age 18 mo. The effect estimate was minimally changed after adjustment for parent and child characteristics; the unadjusted OR was 1.33 (95% CI: 1.23, 1.43); after adjustment for parent and child characteristics and for breast-feeding duration of ≥10 mo compared with ≤1 mo, the OR was 1.29 (95% CI: 1.19, 1.40). After additional adjustment for fish intake, longer breastfeeding duration remained associated with a greater number of attained developmental milestones (OR: 1.28; 95% CI: 1.18, 1.38 for breastfeeding ≥10 mo compared with ≤1 mo). The effect of breastfeeding on the social or cognitive outcomes appeared similar to that on the motor outcomes (Table 3). Associations were somewhat weaker for outcomes assessed at 6 mo than for those assessed at 18 mo (Table 3).
Because we were concerned that we were not adequately accounting for the effects of differences in maternal characteristics according to fish intake, we next restricted our analyses to the children of mothers who had normal prepregnancy BMI and high socioeconomic status, who were nonsmokers and married, and who had no postpartum depression. In this subset of 11 274 children with 18-mo outcome data, the OR was 1.26 (95% CI: 1.12, 1.40) for the highest compared with the lowest quintile of maternal prenatal fish intake, and 1.22 (95% CI: 1.05, 1.41) for breastfeeding ≥10 mo compared with ≤1 mo.
Among women who breastfed for >6 mo (61.4% of the population), the association of prenatal fish intake with their infant’s total developmental score at 18 mo (OR: 1.34; 95% CI: 1.23, 1.48 for the highest compared with the lowest quintile) was, if anything, slightly stronger than the effect estimate among those who breastfed up to 6 mo (OR: 1.23; 95% CI: 1.10, 1.38). However, the interaction term for breastfeeding and fish intake did not suggest any effect modification (P = 0.77).
DISCUSSION
In this large, population-based cohort of pregnant women and their children in Denmark, we observed a benefit of higher maternal fish consumption during pregnancy on attainment of developmental milestones at 6 and 18 mo. Longer duration of breastfeeding also was associated with improved development. The effects of maternal prenatal fish consumption and breast-feeding duration were independent of each other. Contrary to our original hypothesis, the benefit of maternal fish intake was not greater in the children breastfed for a shorter duration.
Because of the proven neurotoxic effects of organic mercury (28, 29), which contaminates fish, federal advisory bodies in the United States have recommended that pregnant women limit their intake of seafood during pregnancy to 340 g (≤2 servings)/wk (24). However, similar to data from other prospective cohort studies (11, 12), the results from the present study do not show any overall detrimental effect of prenatal fish intake on developmental milestones, but, rather, they show that higher maternal fish intake is associated with better early development. Thus, for the amount and type of fish intake observed in this population, the nutrient benefits of prenatal fish intake for child development appear to outweigh the toxicant harms.
Expert panels in the United States and Europe have advised that pregnant women consume a minimum of 200 mg DHA/d (30, 31). Most women do not consume this much DHA from fish and other dietary sources, and thus supplements may be a reasonable alternative. Some studies have found improved development in children of women randomly assigned to receive prenatal DHA supplements, although the doses (1–2 g/d) were substantially higher than the recommended amount (14, 15). However, it is possible that DHA from fish, in combination with other nutrients, may be more beneficial for nervous system development than is DHA from a supplement (32).
The large study population in the DNBC allows for narrow confidence limits, which in the present study indicate that it is unlikely that higher prenatal fish intake, even above the range currently recommended, is associated with any adverse effect on early childhood development. However, with such a large number of participants, it was not feasible to perform in-person outcome measurements, such as a validated test of child development. Previous research indicated that the attainment of developmental milestones in infancy, even within the range of normal development, predicts cognitive outcomes in adulthood, such as educational attainment (33), executive function (34), and intelligence quotient (35). However, future studies of subgroups of children in the DNBC at older ages that include more-detailed cognitive assessments and studies in other cohorts with detailed information on maternal prenatal fish intake will be helpful for refining estimates of the influence of prenatal fish intake on child development.
Other advantages of the present study include prospective data collection and the assessment of a number of potential predictors of child development. However, developmental milestones were ascertained solely by maternal report. It is possible that mothers who consumed more fish or breastfed for a longer duration were more likely to report attained milestones, thus biasing results. In previous studies, parental reports of ages at which developmental milestones were achieved were related to later intelligence quotient and social achievement (33, 36). In addition, as with any observational study, unmeasured confounding is a concern. In particular, we did not have a measure of parental intelligence or an assessment of the home environment. A recent study suggested that maternal intelligence may account for much of the observed cognitive benefit of breastfeeding (37), although, in that population from the United States, the prevalence and duration of breastfeeding were low, and breast-milk DHA concentrations also were likely to have been low. Other studies that also accounted for maternal intelligence found persistent benefits of breastfeeding (6). In the present study, adjustment for several previously identified physical and sociodemographic predictors of child development, including maternal body mass index, parental education, marital status, and social class and child growth, influenced results only minimally, and restricting the sample to a more homogeneous subset also did not diminish the observed benefit of fish intake.
We did not have any data on biomarkers of exposure to environmental pollutants, such as methyl mercury or polychlorinated biphenyls, both of which contaminate fish and may harm development (11, 28, 29). Maternal seafood consumption and subsequent transfer via the placenta or breast milk are the primary routes of early-life exposure to these pollutants (38). We expect that adjustment for the adverse influence of these toxicants would suggest even stronger developmental benefits of maternal fish consumption and breastfeeding (11, 29). It is possible, however, that women who consume types of fish that have higher toxicant contamination, or less DHA, than were consumed by the women in the DNBC may experience less of a benefit or even perhaps overall harm from higher prenatal fish intake. Therefore, we recommend that pregnant women, to maximize the benefits and minimize the risks, consider toxicant concentrations in choosing fish to consume.
In conclusion, the present study adds to the growing body of evidence that greater maternal fish consumption during pregnancy and a longer duration of breastfeeding are associated with more favorable child development. We support ongoing efforts to promote breastfeeding to optimize a variety of health outcomes, including development. To allow mothers to make the best choices for their children’s development, future studies of prenatal diet should incorporate detailed information on fish intake as well as information on both nutrient and toxicant exposures.
Acknowledgments
We acknowledge the contributions of the Managerial Team of the Danish National Birth Cohort: Jørn Olsen (Chair), Mads Melbye, Anne Marie Nybo Andersen, Sjurdur F Olsen, Thorkild IA Sørensen, and Peter Aaby.
Footnotes
Supported by the Danish National Research Foundation, the Danish Pharmaceutical Association, the Danish Ministry of Health, the Danish National Board of Health, Statens Serum Institut, BIOMED, the March of Dimes Birth Defects Foundation, the Danish Heart Association, the Danish Medical Research Council, and Sygekassernes Helsefond (to the Danish National Birth Cohort); by the Early Nutrition Programming Project [(EARNEST) Project No. FOOD-CT-2005-007036]; and by grant no. HD44807 from the National Institutes of Health and a fellowship from the American Scandinavian Foundation, Inger and Jens Bruun Foundation (both to EO). In addition, the March of Dimes Birth Defects Foundation supported collaboration between the Maternal Nutrition Group at the Statens Serum Institut and Harvard Medical School.
The authors’ responsibilities were as follows—EO and SFO: study conception and design; EO, MLØ, MWG, VKK, TIH, MS, DCB, MH-A, KFM, and SFO: analysis and interpretation of data; EO and DCB: drafting of the manuscript; EO, MLØ, TIH, and DCB: statistical analysis; EO and SFO: obtained funding; MLØ, MWG, VKK, TIH, MS, MH-A, KFM, and SFO: critical revision of the manuscript; and VKK and SFO: acquisition of data. EO and MLØ had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. EO and SFO also take responsibility for the whole content of the manuscript. MWG has received grant support from Mead Johnson Nutritionals, and DCB served as a member of an expert panel for a study conducted by the Harvard Center for Risk Analysis (with support from the National Food Producers Association) that evaluated the benefits and risks of seafood consumption. None of the other authors had a personal or financial conflict of interest.
References
- 1.Spohr HL, Willms J, Steinhausen HC. Fetal alcohol spectrum disorders in young adulthood. J Pediatr. 2007;150:175–9. 179, e1. doi: 10.1016/j.jpeds.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environ Res. 2006;100:284–93. doi: 10.1016/j.envres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Lucas A. Programming by early nutrition. J Nutr. 1998;128(suppl):401S–6S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- 4.Crawford MA. Lipids and development of the human brain. Biochem Soc Trans. 1976;4:231–3. doi: 10.1042/bst0040231. [DOI] [PubMed] [Google Scholar]
- 5.Horta BL, Bahl R, Martines JC, Victora CG. Evidence of the long-term effects of breastfeeding; systematic reviews and meta-analyses. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 6.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr. 1999;70:525–35. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. The association between duration of breastfeeding and adult intelligence. JAMA. 2002;287:2365–71. doi: 10.1001/jama.287.18.2365. [DOI] [PubMed] [Google Scholar]
- 8.Heird WC. The role of polyunsaturated fatty acids in term and preterm infants and breastfeeding mothers. Pediatr Clin North Am. 2001;48:173–88. doi: 10.1016/s0031-3955(05)70292-3. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JT, Bellinger DC, Connor WE, Shaywitz BA. A quantitative analysis of prenatal intake of n–3 polyunsaturated fatty acids and cognitive development. Am J Prev Med. 2005;29:366–74. doi: 10.1016/j.amepre.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Hadders-Algra M, Bouwstra H, van Goor SA, Dijck-Brouwer DA, Muskiet FA. Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. J Perinat Med. 2007;35(suppl):S28–34. doi: 10.1515/JPM.2007.034. [DOI] [PubMed] [Google Scholar]
- 11.Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–80. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Radesky JS, Wright RO, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–81. doi: 10.1093/aje/kwn034. (Epub 2008 Mar 18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F45–50. doi: 10.1136/adc.2006.099085. [DOI] [PubMed] [Google Scholar]
- 15.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n–3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 16.Colombo J, Kannass KN, Shaddy DJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–67. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouwstra H, Dijck-Brouwer J, Decsi T, et al. Neurologic condition of healthy term infants at 18 months: positive association with venous umbilical DHA status and negative association with umbilical transfatty acids. Pediatr Res. 2006;60:334–9. doi: 10.1203/01.pdr.0000233043.16674.1d. [DOI] [PubMed] [Google Scholar]
- 18.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29:300–7. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 19.Olsen SF, Mikkelsen TB, Knudsen VK, et al. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21:76–86. doi: 10.1111/j.1365-3016.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 20. [accessed 11 February 2007];Danish National Birth Cohort. Internet: http://www.ssi.dk/sw9314.asp.
- 21.Halldorsson TI, Meltzer HM, Thorsdottir I, Knudsen V, Olsen SF. Is high consumption of fatty fish during pregnancy a risk factor for fetal growth retardation? A study of 44,824 Danish pregnant women. Am J Epidemiol. 2007;166:687–96. doi: 10.1093/aje/kwm133. [DOI] [PubMed] [Google Scholar]
- 22.Tjonneland A, Overvad K, Haraldsdottir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20:906–12. doi: 10.1093/ije/20.4.906. [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen TB, Osler M, Olsen SF. Validity of protein, retinol, folic acid and n–3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr. 2007;9:771–8. doi: 10.1079/phn2005883. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services. [accessed 20 July 2004];What you need to know about mercury in fish and shellfish. 2004 March; Internet: http://www.cfsan.fda.gov/3dms/admehg3.html.
- 25.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–29. [PubMed] [Google Scholar]
- 26.Fromberg A, Larsen EH, Hartkopp H, et al. Part 1: Chemical contaminants. Søborg, Denmark: Danish Institute for Food and Veterinary Research; 2005. [accessed 1 February 2008]. Food monitoring, 1998–2003. Internet: http://www.foedevarestyrelsen.dk/forside.htm. [Google Scholar]
- 27.Obel C, Linnet KM, Henriksen TB, et al. Smoking during pregnancy and hyperactivity-inattention in the offspring—comparing results from three Nordic cohorts. Int J Epidemiol. 2008 Feb 2; doi: 10.1093/ije/dym290. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 29.Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–7. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koletzko B, Cetin I, Thomas Brenna J. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–7. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- 31.Kris-Etherton PM, Innis S. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107:1599–611. [PubMed] [Google Scholar]
- 32.Hadders-Algra M. Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J Perinat Med. 2008;36:101–9. doi: 10.1515/JPM.2008.029. [DOI] [PubMed] [Google Scholar]
- 33.Taanila A, Murray GK, Jokelainen J, Isohanni M, Rantakallio P. Infant developmental milestones: a 31-year follow-up. Dev Med Child Neurol. 2005;47:581–6. [PubMed] [Google Scholar]
- 34.Murray GK, Veijola J, Moilanen K, et al. Infant motor development is associated with adult cognitive categorisation in a longitudinal birth cohort study. J Child Psychol Psychiatry. 2006;47:25–9. doi: 10.1111/j.1469-7610.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- 35.Murray GK, Jones PB, Kuh D, Richards M. Infant developmental milestones and subsequent cognitive function. Ann Neurol. 2007;62:128–36. doi: 10.1002/ana.21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capute AJ, Shapiro BK, Palmer FB, Ross A, Wachtel RC. Cognitive-motor interactions. The relationship of infant gross motor attainment to IQ at 3 years. Clin Pediatr (Phila) 1985;24:671–5. doi: 10.1177/000992288502401201. [DOI] [PubMed] [Google Scholar]
- 37.Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945. doi: 10.1136/bmj.38978.699583.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goyer R, Aposhian V, Arab L, et al. Toxicological effects of methyl-mercury. Washington, DC: National Academy Press; 2000. [Google Scholar]