Abstract
Objective
Forgetting to take medications is an important cause of nonadherence. This study evaluated factors associated with forgetting to take medications in a large cohort of persons with systemic lupus erythematosus (SLE) participating in the University of California, San Francisco Lupus Outcomes Study (LOS). Relationships among adherence problems and service utilization (outpatient visits, emergency department visits, and hospitalizations) were also evaluated.
Methods
The cohort consisted of 834 LOS participants who provided self-reported frequency of forgetting to take medications as directed. Predictors of adherence and service utilization patterns included self-reported sociodemo-graphics, disease-related characteristics (e.g., disease activity, recent SLE flare), and mental health characteristics (Center for Epidemiologic Studies Depression Scale and cognitive function screen). Health care utilization patterns included the presence and quantity of visits to rheumatologists, primary care physicians, other care providers, emergency departments, and hospitalizations.
Results
Forty-six percent of the LOS cohort reported forgetting to take medications at least some of the time. Depressive symptom severity was a strong predictor of adherence difficulties (odds ratio [OR] 1.04, 95% confidence interval [95% CI] 1.02–1.05; P < 0.0001) after accounting for all other predictors. Persons reporting adherence difficulties had significantly greater numbers of outpatient rheumatology and primary care visits, and were more likely to visit the emergency department (OR 1.45, 95% CI 1.04–2.04; P = 0.03).
Conclusion
Depression may be an important cause of medication adherence problems, and difficulties with adherence are significantly associated with high-cost service utilization, specifically emergency department visits. In an era of rapidly evolving treatments for lupus, identifying patients at risk for adherence problems may decrease medical expenditures and improve patient outcomes in SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a complex condition with multiorgan involvement that can have a profound impact on an individual’s quality of life (1). Recently, we have seen a dramatic decrease in all-cause mortality among patients with SLE (2) that can be partially attributed to treatment advances that delay disease progression and minimize organ damage. For many SLE patients, however, these improved treatment regimens are complex, and treatment adherence problems leave patients at risk for poorer health outcomes.
Behavioral causes of nonadherence can be conceptually divided into 2 classes. First, intentional causes include those instances when a patient chooses not to follow a treatment recommendation. This can occur for a number of reasons (e.g., patient-doctor communication and trust, patient beliefs, or side effects). Conversely, unintentional adherence problems occur when a patient cannot follow treatment recommendations due to circumstances that are out of their control (e.g., poor comprehension, language barrier, or barriers related to physical disabilities). Forgetting to take medications is also considered to be unintentional, and is a common cause of medication adherence problems across a number of medical populations (3–7). Depression is often associated with patient reports of forgetting to take medications; however, studies of forgetfulness and adherence have not been conducted in patients with SLE (8,9). Cognitive impairment may also contribute to adherence problems in SLE, and one study observed that poorer performance on a memory test was associated with nonadherence (i.e., missing medications) among African American patients (10).
Consequences of adherence problems in SLE have been inadequately studied. Existing studies suggest that poor adherence is associated with poor SLE prognosis (e.g., renal outcomes [11,12]). With respect to service utilization, one study found an association between medication adherence and increased emergency hospitalizations among patients with SLE (13); however, an earlier study showed no relationship among adherence and hospitalizations (11). In other chronic conditions, problems in adherence are predictive of increased health care utilization, particularly high-cost services such as emergency department utilization and hospitalizations (14–17).
The first goal of this investigation was to examine factors associated with medication adherence in a large cohort of persons with SLE, with a particular focus on patient-related factors determined to be predictive in the literature. Specifically, symptoms of depression appear to be an important mediator of adherence; however, this has never been investigated in patients with SLE. Identifying patient- and disease-related factors associated with medication adherence difficulties would provide targets for both prevention and intervention to improve health outcomes. Second, we evaluated relationships between adherence and service utilization patterns (provider visits, emergency department visits, and hospitalizations) over a 1-year period. Understanding the influence of adherence problems on health care utilization could facilitate the identification of specific patient-related characteristics associated with reliance on high-cost services.
SUBJECTS AND METHODS
Data source and subjects
The Lupus Outcomes Study (LOS) is a prospective longitudinal study of 982 individuals with SLE whose diagnoses were confirmed by medical chart review prior to enrollment, using American College of Rheumatology criteria (18). Details about enrollment and data collection for this study have been reported previously (19). Briefly, subjects were recruited through academic medical centers, community rheumatology offices, nonclinical sources including patient support groups and conferences, and other forms of publicity. Of 1,265 people contacted for the study, 78% completed at least 1 interview. In each of the 2 followup interviews, 92% of the eligible subjects in the prior wave participated. There were 20 (2.0%) deaths among study participants during this time. Additionally, 22 (2.2%) participants withdrew for health reasons, 63 (6.4%) declined further participation, and 35 (3.6%) were lost to followup. The present analysis incorporated responses from the third wave of data, where medication adherence was evaluated.
The research protocol was approved by the University of California, San Francisco Committee on Human Research. All participants gave their informed consent to be part of the study. LOS interviews are conducted annually by trained telephone interviewers. Interviews average 50 minutes and consist mainly of validated measures of SLE disease activity and manifestations; general, physical, and mental health status; and disability, employment, and sociodemographic characteristics (20).
Measures
Self-reported medication nonadherence
The medication item from the Cognitive Symptoms Inventory (21) was used to evaluate medication forgetfulness, one source of unintentional adherence. Participants reported the frequency of forgetting to take medications as directed on a 4-point scale (never a problem, sometimes a problem, a problem most of the time, a problem all of the time). Participants were classified into 2 categories according to the frequency of self-reported forgetting medication: those reporting that forgetting medications was never a problem, and those who reported that it was a problem at least some of the time.
Disease characteristics
Disease activity was assessed using the Systemic Lupus Activity Questionnaire (SLAQ) (22,23), a validated, patient-reported questionnaire assessment of disease activity in SLE. In addition, disease activity was evaluated by the presence versus absence of self-reported SLE disease flare within the past 3 months. Disease duration was calculated as the number of years since diagnosis of SLE. Finally, medication status was evaluated using the total number of self-administered medications currently prescribed.
Mental health characteristics
Depressive symptom severity was evaluated using the Center for Epidemiologic Studies Depression Scale (CES-D), with a score range of 0–60 (24). Patients were also screened for cognitive dysfunction by telephone using the Hopkins Verbal Learning Test-Revised (HVLT-R), a brief measure of verbal learning and memory (25). Two scores utilized in this study include the HVLT-R learning score, which is the total of all verbal material learned over 3 trials, and the HVLT-R percent retention delay score, which consists of the proportion of previously learned material retained after a delay.
Health care utilization
Health care utilization variables included self-reported number of visits in the past year to health care providers in the following categories: 1) rheumatology, 2) primary care, 3) visits to other subspecialty care providers and/or other allied health professionals, 4) visits to the emergency department (presence versus absence), 5) number of visits to an emergency department, 6) hospitalizations (presence versus absence), and 7) number of hospitalizations.
Data analytic plan
Group comparisons (paired t-test, chi-square analyses) were conducted between medication adherent versus nonadherent groups across demographic, disease-related, and mental health characteristics. Next, hierarchical logistic regressions were estimated to determine predictors of medication nonadherence. Independent variables were entered sequentially into the model in the following order: 1) demographics, 2) disease-related characteristics, 3) cognitive functioning, and 4) CES-D depression severity score. Finally, hierarchical logistic regression models predicting service utilization were estimated with independent variables entered in the following steps: 1) demographics, 2) disease-related characteristics, 3) mental health characteristics, and 4) patient-reported adherence. Scales of disease activity and depression (i.e., SLAQ and CES-D) frequently overlapped in their symptom profiles (e.g., somatic symptoms). In order to avoid compromising psychometric integrity of either measure, measures were not modified and subscales were not used. Instead, tests of multicollinearity as suggested by Maruyama (26) were conducted among predictor variables to determine indication of overlap and high correlations among predictor variables.
RESULTS
Predictors of adherence difficulties
A total of 834 LOS participants provided information on medication adherence, and 45.6% reported forgetting to take medications at least some of the time. Demographic, disease-related, and mental health characteristics by adherence status are shown in Table 1. Patients whose economic status was below poverty level were significantly more likely to report adherence difficulties (P = 0.03). In addition, being female was associated with adherence problems, although this did not reach statistical significance (P > 0.05).
Table 1.
Demographic, disease-related, and mental health characteristics among Lupus Outcomes Study participants reporting medication adherence problems*
| Adherent (n = 454) |
Nonadherent (n = 380) |
t-test or χ2 | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean ± SD years | 49.64 ± 13.82 | 48.33 ± 11.45 | 1.50 | 0.14 |
| At or above high school education | 87.0 | 84.5 | 1.09 | 0.32 |
| Female | 89.6 | 93.7 | 4.33 | 0.05 |
| White | 54.4 | 45.6 | 0.01 | 0.96 |
| Hispanic/Latino | 48.7 | 51.3 | 1.12 | 0.33 |
| African American | 58.3 | 48.7 | 0.40 | 0.59 |
| Asian | 59.7 | 40.3 | 0.93 | 0.34 |
| Below poverty status | 10.0 | 15.3 | 5.25 | 0.03† |
| Married/with partner | 21.6 | 21.6 | 0.00 | 1.00 |
| Disease characteristics | ||||
| Disease duration, mean ± SD years | 15.91 ± 9.40 | 13.37 ± 7.32 | 4.37 | < 0.0001† |
| SLAQ score, mean ± SD | 8.01 ± 5.98 | 12.05 ± 7.31 | −8.61 | < 0.0001† |
| Disease flare in past 3 months | 39.8 | 56.5 | 22.55 | < 0.0001† |
| Number of medications, mean ± SD | 2.67 ± 1.61 | 2.94 ± 1.68 | −2.28 | 0.02† |
| Mental health characteristics, mean ± SD | ||||
| CES-D total score | 11.69 ± 10.89 | 20.21 ± 13.29 | −9.94 | < 0.0001† |
| HVLT-R learning score | 26.06 ± 5.39 | 24.88 ± 5.43 | 3.11 | 0.002† |
| HVLT-R percent retention delay score | 92.96 ± 16.06 | 88.72 ± 20.06 | 3.28 | 0.001† |
Values are the percentage unless otherwise indicated. SLAQ = Systemic Lupus Activity Questionnaire; CES-D = Center for Epidemiologic Studies Depression Scale; HVLT-R = Hopkins Verbal Learning Test-Revised.
Significant.
Several disease characteristics were significantly associated with adherence difficulties, including shorter disease duration (P < 0.0001), higher SLAQ score (P < 0.0001), recent disease flare (P < 0.0001), and a greater number of self-administered medications (P = 0.02). In addition, severity of depressive symptoms was associated with self-reported adherence (P < 0.0001), as was poorer performance on the 2 screening measures of cognitive functioning, including the HVLT-R learning score (P = 0.002) and HVLT-R percent retention delay score (P = 0.001).
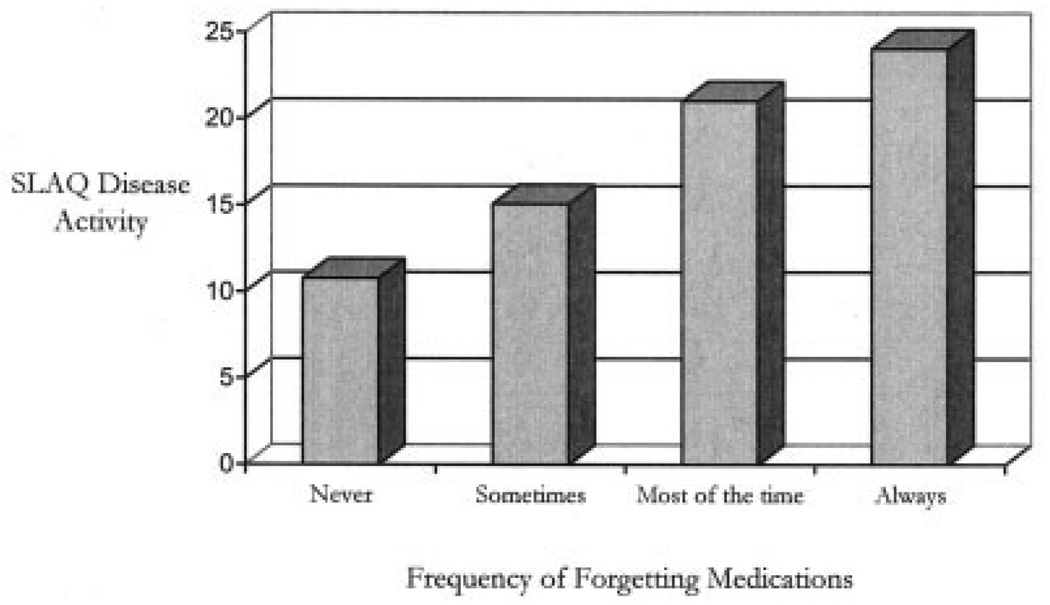
In lieu of the dichotomized variable, participants were also grouped according to their responses on the 4-point adherence scale and assigned a group number (1 = never, 2 = sometimes, 3 = most of the time, and 4 = always). SLAQ scores were compared across participant levels of forgetting medication. Univariate analyses of variance suggest that patients who reported increasing levels of forgetting reported significantly higher disease activity, as measured by the SLAQ (Figure 1). Post hoc Bonferroni corrections yielded significant group differences among all adherence groups with the exception of groups 3 versus 4 (results not shown). SLAQ scores increased monotonically with increases in the frequency of forgetting to take medications.
Figure 1.
Systemic Lupus Activity Questionnaire (SLAQ) disease activity scores among medication adherence groups.
A logistic regression analysis predicting adherence revealed that severity of depressive symptoms was an independent predictor of adherence problems after controlling for sociodemographic, disease-related, and cognitive function characteristics (odds ratio [OR] 1.04, 95% confidence interval [95% CI] 1.02–1.05; P < 0.0001) (Table 2). In the adjusted analyses, disease activity as measured by the SLAQ (OR 1.05, 95% CI 1.02–1.07; P < 0.0001) and disease duration (OR 0.96, 95% CI 0.94–0.98; P < 0.0001) remained significant independent predictors in this model.
Table 2.
Stepwise multivariate logistic regression predicting patient-reported medication nonadherence*
| Step 1: model with demographics |
Step 2: model with demographics and disease characteristics |
Step 3: model with demographics, disease characteristics, and cognitive functioning |
Step 4: model with demographics, disease characteristics, cognitive functioning, and depressive symptom severity |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age, years | 0.99 (0.98–1.00) | 0.23 | 1.00 (0.98–1.01) | 0.43 | 0.99 (0.98–1.01) | 0.23 | 1.00 (0.98–1.01) | 0.55 |
| Education† | 0.72 (0.47–1.13) | 0.15 | 0.85 (0.53–1.35) | 0.49 | 0.98 (0.60–1.60) | 0.93 | 1.05 (0.64–1.73) | 0.85 |
| Ethnicity‡ | 1.14 (0.83–1.57) | 0.02 | 1.06 (0.75–1.49) | 0.20 | 1.13 (0.80–1.60) | 0.14 | 1.51 (0.83–2.76) | 0.18 |
| Female | 1.95 (1.11–3.43) | 0.43 | 1.47 (0.82–2.66) | 0.75 | 1.56 (0.86–2.82) | 0.50 | 1.15 (0.81–1.64) | 0.44 |
| Below poverty status | 1.62 (0.99–2.64) | 0.05 | 1.10 (0.65–1.87) | 0.71 | 1.05 (0.62–1.78) | 0.86 | 0.99 (0.58–1.70) | 0.97 |
| Married/with partner | 0.91 (0.62–1.33) | 0.62 | 0.87 (0.58–1.29) | 0.48 | 0.89 (0.60–1.34) | 0.59 | 0.84 (0.56–1.27) | 0.40 |
| Disease duration, years | 0.96 (0.94–0.98) | < 0.0001 | 0.96 (0.94–0.98) | < 0.0001 | 0.96 (0.94–0.98) | < 0.0001 | ||
| SLAQ score | 1.08 (1.05–1.11) | < 0.0001 | 1.08 (1.05–1.10) | < 0.0001 | 1.05 (1.02–1.07) | < 0.0001 | ||
| Recent disease flare | 1.00 (0.70–1.44) | 0.98 | 1.04 (0.70–1.44) | 0.98 | 0.98 (0.68–1.41) | 0.89 | ||
| Number of medications | 1.05 (0.95–1.16) | 0.34 | 1.04 (0.94–1.15) | 0.45 | 1.03 (0.93–1.14) | 0.58 | ||
| HVLT-R learning score | 0.98 (0.95–1.01) | 0.18 | 0.99 (0.96–1.02) | 0.54 | ||||
| HVLT-R percent retention delay score |
0.99 (0.98–1.00) | 0.17 | 0.99 (0.98–1.00) | 0.18 | ||||
| CES-D total score | 1.04 (1.02–1.05) | < 0.0001 | ||||||
OR = odds ratio; 95% CI = 95% confidence interval; SLAQ = Systemic Lupus Activity Questionnaire; HVLT-R = Hopkins Verbal Learning Test Revised; CES-D = Center for Epidemiologic Studies Depression Scale.
Less than high school versus high school graduate and beyond.
White versus other.
Predictors of health care utilization
Group comparisons (t-est or chi-square analyses) showed that participants who reported difficulties with adherence had increased numbers of rheumatology visits (mean ± SD 4.34 ± 6.93 versus 3.24 ± 3.19; P = 0.005) and primary care visits (mean ± SD 4.78 ± 6.27 versus 3.80 ± 6.43; P = 0.03) when compared with persons who did not report adherence difficulties, whereas group differences across other health care visits did not reach statistical significance (mean ± SD 22.43 ± 32.42 versus 18.52 ± 26.14; P = 0.06) (Table 3). Participants with adherence difficulties were also significantly more likely to visit an emergency department (55.3% versus 44.7%; P < 0.0001) and had more emergency department visits in a 1-year period when compared with those without adherence difficulties (mean ± SD 1.31 ± 3.04 versus 0.74 ± 3.68; P = 0.02). Evaluating responses to the entire adherence scale (i.e., forgetting never, sometimes, most of the time, and always) in lieu of the dichotomized variable, descriptive analyses showed that 83% of patients who reported forgetting medications all of the time required emergency department services compared with 32% of patients who reported that forgetting medications was not a problem, 46% of those reporting adherence problems some of the time, and 41% of those reporting adherence problems most of the time. Hospitalizations did not differ between dichotomized nonadherence and adherence groups (presence of a hospitalization 47.6% versus 52.4%; P = 0.56 and mean ± SD number of hospitalizations 1.77 ± 2.20 versus 1.53 ± 1.04; P = 0.33).
Table 3.
Health care utilization among patients who reported medication nonadherence*
| Adherent (n = 454) |
Nonadherent (n = 380) |
t-test or χ2 | P | |
|---|---|---|---|---|
| Rheumatology visits | 3.24 ± 3.19 | 4.34 ± 6.93 | −2.84 | 0.005 |
| Primary care visits | 3.80 ± 6.43 | 4.78 ± 6.27 | −2.20 | 0.03 |
| Other health care visits | 18.52 ± 26.14 | 22.43 ± 32.42 | −1.9 | 0.06 |
| Visiting the emergency department, % | 44.7 | 55.3 | 20.77 | < 0.0001 |
| Number of emergency department visits | 0.74 ± 3.68 | 1.31 ± 3.04 | −2.45 | 0.02 |
| Hospitalized, % | 52.4 | 47.6 | 0.44 | 0.56 |
| Number of hospitalizations | 1.53 ± 1.04 | 1.77 ± 2.20 | −0.98 | 0.33 |
Values are the mean ± SD unless otherwise indicated.
Medication adherence did not significantly predict the number of outpatient medical visits (rheumatology visits or primary care visits) in hierarchical regression models. Significant predictors of the number of rheumatology visits included younger age (P < 0.0001), being married/with partner (P = 0.003), total number of self-administered medications (P < 0.0001), and SLAQ score (P < 0.0001). Together, these variables accounted for 12% (F = 7.27, P < 0.0001) of the variance in the number of rheumatology visits. Predictors of the number of primary care visits included being female (P = 0.02), white ethnicity (P = 0.001), CES-D depressive symptom severity score (P = 0.003), and SLAQ score (P < 0.0001). All variables predicted 14% (F = 9.24, P < 0.0001) of the variance.
Hierarchical logistic regression analyses (Table 4) revealed that medication adherence was an independent predictor of whether participants visited the emergency department (OR 1.45, 95% CI 1.04–2.04; P = 0.03) after controlling for all demographic, disease-related, and mental health characteristics. Disease activity (SLAQ score OR 1.06, 95% CI 1.03–1.09; P < 0.0001) remained a significant predictor in the final model, whereas both measures of cognitive functioning (HVLT-R learning score OR 0.97, 95% CI 0.94–1.00; P = 0.05 and HVLT-R percent retention delay score OR 0.99, 95% CI 0.98–1.00; P = 0.06) did not reach statistical significance in the final adjusted model. Finally, medication adherence did not independently predict hospitalizations or number of hospitalizations in our SLE cohort.
Table 4.
Stepwise multivariate logistic regression predicting emergency department visits in a 1-year period*
| Step 1: model with demographics |
Step 2: model with demographics and disease characteristics |
Step 3: model with demographics, disease characteristics, and mental health characteristics |
Step 4: model with demographics, disease characteristics, mental health characteristics, and self–reported adherence |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age, years | 1.00 (0.99–1.01) | 0.97 | 1.00 (0.98–1.01) | 0.43 | 0.99 (0.98–1.01) | 0.19 | 0.99 (0.98–1.01) | 0.21 |
| Education† | 0.82 (0.52–1.28) | 0.38 | 0.88 (0.56–1.41) | 0.60 | 1.11 (0.68–1.81) | 0.68 | 1.11 (0.69–1.84) | 0.67 |
| Female | 1.41 (0.79–2.51) | 0.24 | 1.19 (0.66–2.17) | 0.56 | 1.29 (0.71–2.36) | 0.41 | 1.25 (0.69–2.31) | 0.47 |
| Ethnicity‡ | 1.04 (0.75–1.45) | 0.80 | 1.01 (0.71–1.42) | 0.97 | 1.12 (0.78–1.59) | 0.54 | 1.11 (0.79–1.61) | 0.58 |
| Below poverty status | 1.98 (1.22–3.22) | 0.006 | 1.46 (0.87–2.44) | 0.46 | 1.35 (0.80–2.27) | 0.26 | 1.35 (0.82–2.32) | 0.26 |
| Married/with partner | 1.22 (0.83–1.79) | 0.32 | 1.20 (0.81–1.79) | 1.37 | 1.26 (0.84–1.89) | 0.26 | 1.28 (0.85–1.91) | 0.13 |
| Disease duration, years | 1.01 (0.99–1.03) | 0.27 | 1.01 (0.99–1.03) | 0.22 | 1.02 (0.99–1.04) | 0.13 | ||
| SLAQ score | 1.07 (1.04–1.09) | < 0.0001 | 1.06 (1.03–1.09) | < 0.0001 | 1.06 (1.03–1.09) | < 0.0001 | ||
| Recent disease flare | 0.89 (0.62–1.28) | 0.53 | 0.89 (0.61–1.29) | 0.53 | 0.89 (0.63–1.33) | 0.53 | ||
| Number of medications | 1.10 (1.00–1.22) | 0.05 | 1.09 (0.99–1.20) | 0.09 | 1.08 (0.99–1.20) | 0.11 | ||
| HVLT-R learning score | 0.97 (0.94–1.00) | 0.06 | 0.97 (0.94–1.00) | 0.06 | ||||
| HVLT-R percent retention delay score |
0.99 (0.98–1.00) | 0.05 | 0.99 (0.98–1.00) | 0.06 | ||||
| CES-D total score | 1.00 (0.99–1.02) | 0.52 | 1.01 (0.99–1.02) | 0.78 | ||||
| Forgetting medications | 1.45 (1.04–2.04) | 0.03 | ||||||
OR = odds ratio; 95% CI = 95% confidence interval; SLAQ = Systemic Lupus Activity Questionnaire; HVLT-R = Hopkins Verbal Learning Test-Revised; CES-D = Center for Epidemiologic Studies Depression Scale.
Less than high school or high school graduate and beyond.
White versus other.
Analyses of multicollinearity were also conducted using 5 indicators as suggested by Maruyama (26). These analyses suggest that although our predictor variables were not completely independent and one indicator was slightly suggestive, 4 of the 5 indicators of multicollinearity we examined were completely negative, suggesting that multicollinearity is likely not a major impediment to our analyses or the inferences that we draw from them.
DISCUSSION
In this study, we evaluated factors associated with forgetting to take medications, an important source of medication nonadherence. In our cohort of 834 persons with SLE, 45% reported that forgetting to take medications was a problem at least some of the time. These rates are roughly equivalent to adherence rates reported in studies of patients with SLE (11), rheumatoid arthritis (27), and other chronic conditions, including hypertension (28) and asthma (29).
Reduced disease duration, but not age, was associated with adherence, suggesting that patients may be at greater risk for adherence problems early in the disease process. In addition, increased disease activity and recent disease flares were associated with decreased adherence. Increased complexity of medication regimen, at least for the number of self-administered medications, was also associated with reduced adherence; however, the absolute number of self-administered medications differed only slightly among groups. Decreased performance on learning and memory tasks was also associated with patient-reported adherence, but cognitive functioning did not remain significant after accounting for other variables. Given the prevalence of cognitive dysfunction in lupus, cognitive status warrants further exploration as to whether these impairments may be important to assess as a potential mediator of a patient’s ability to follow treatment guidelines, or as an indicator of the need for adherence aids.
Severity of depressive symptoms was strongly associated with patient-reported adherence, and was an independent predictor after accounting for all other disease-related and sociodemographic characteristics. These findings are consistent with studies in other conditions (8,30), and suggest that symptoms of depression are important factors influencing medication forgetfulness. In the clinic setting, we are reminded of the importance of identifying patients with clinically significant depressive symptoms, because these patients may be less likely to adhere to our treatment recommendations. Previous studies also observed considerable improvements in adherence after treatment for depression (31).
Studies in lupus vary with respect to the presence of a relationship between adherence and service utilization (e.g., hospitalizations) (11,13). In our study, before adjustment, patient-reported forgetfulness was associated with a greater number of rheumatology visits, primary care visits, and emergency department visits in a 1-year period. Adherence was not associated with the presence or number of inpatient hospitalizations. Even after adjustment for important covariates such as depression and other patient and disease characteristics, there remained greater emergency department utilization by participants with adherence problems. Eighty-three percent of participants who reported the most severe difficulties in adherence required emergency department visits compared with a range of 30–46% of patients reporting less difficulties with adherence. We posit that decreased adherence may lead to increased emergency department usage, but perhaps these emergency department visits do not necessarily result in subsequent hospitalizations.
This study is not without limitations. We conducted a cross-sectional study and inferences on causality cannot be made. We found strong associations among depression, adherence, and increased health care utilization rates even after controlling for disease activity. There is clearly a relationship between disease activity and adherence. Worsening disease activity may be a cause or a consequence of poor medication adherence. In addition, poor adherence and increasing service utilization may each be a byproduct of increasing disease activity. Likewise, higher numbers of prescribed medications may be an indication of increased disease severity. An additional limitation to this investigation is the self-reported assessment of disease activity. Optimally, patient-reported disease activity and flares would be interpreted in conjunction with objective markers of disease activity assessed by a physician and/or serologic studies. In addition, adherence difficulties to specific medications were not evaluated in this study, and this information would more fully inform the health status costs of adherence problems. For example, the health status costs of forgetting medications such as vitamin supplements may be less critical than those costs of forgetting prescribed immunosuppressant medications. Prospective studies evaluating adherence and health outcomes are warranted.
Other unintentional and intentional sources of adherence were not measured in this study, including factors related to financial constraints, comprehension, patient concerns, distrust, perceived side effects, etc. Patients are active participants in their medical care and in making decisions about treatment choices. A more comprehensive appreciation of these additional factors influencing adherence will become increasingly important in the care of persons with SLE. The measure of adherence we selected was a single question assessing how often participants forget to take their medications as directed. Some studies suggest that patients are not always accurate self-reporters of adherence (32). Recent validation studies in other conditions, however, suggest that for a majority of patients, self-reported adherence corresponded strongly with adherence measured by an electronic monitoring device (33,34). In addition, our rates of self-reported nonadherence are consistent with adherence rates using other measures in related studies, suggesting the possibility that participants provided more candid responses to our survey interviewers as compared with their health care providers. Physician-rated adherence is also frequently used; however, this method has also been determined to be commonly inaccurate (35). In addition, more objective measures of patient adherence (e.g., pharmacy records, pill counts, electronic monitoring devices, biochemical or serologic measurement) are not widely available. Despite the potential psychometric limitations, our intent was to utilize an assessment of adherence with strong face validity that could be easily used in any clinical setting. The true cause of forgetting medication is unknown in this investigation and patients may volitionally forget medications that perhaps have greater financial costs or side effects, or conversely, their forgetting may be entirely unintentional. Performance on a neuropsychological memory task predicted adherence in this study, which may causally suggest at least some unintentional sources of forgetting due to cognitive problems. We show important relationships among this measure, patient characteristics, and health care utilization, suggesting that perhaps there are feasible means to accurately determine adherence in the clinic.
Lupus treatments are rapidly evolving, and many factors will play into the likelihood of a patient’s willingness and ability to use their medications as directed. Addressing adherence problems could decrease patient care expenditures and potentially provide one important strategy to improve patient outcomes.
REFERENCES
- 1.Abu-Shakra M, Mader R, Langevitz P, Friger M, Codish S, Neumann L, et al. Quality of life in systemic lupus erythematosus: a controlled study. J Rheumatol. 1999;26:306–309. [PubMed] [Google Scholar]
- 2.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–2557. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 3.Burra TA, Chen E, McIntyre RS, Grace SL, Blackmore ER, Stewart DE. Predictors of self-reported antidepressant adherence. Behav Med. 2007;32:127–134. doi: 10.3200/BMED.32.4.127-134. [DOI] [PubMed] [Google Scholar]
- 4.Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, et al. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulloch AG, Adair CE, Patten SB. Forgetfulness: a role in noncompliance with antidepressant treatment. Can J Psychiatry. 2006;51:719–722. doi: 10.1177/070674370605101110. [DOI] [PubMed] [Google Scholar]
- 6.Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102:43–49. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 7.Rand CS. Measuring adherence with therapy for chronic diseases: implications for the treatment of heterozygous familial hypercholesterolemia. Am J Cardiol. 1993;72:68D–74D. doi: 10.1016/0002-9149(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 8.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Arch Intern Med. 2005;165:2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosley-Williams A, Lumley MA, Gillis M, Leisen J, Guice D. Barriers to treatment adherence among African American and white women with systemic lupus erythematosus. Arthritis Rheum. 2002;47:630–638. doi: 10.1002/art.10790. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med. 1991;91:345–353. doi: 10.1016/0002-9343(91)90151-m. [DOI] [PubMed] [Google Scholar]
- 12.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology (Oxford) 2006;45:1144–1147. doi: 10.1093/rheumatology/kel039. [DOI] [PubMed] [Google Scholar]
- 13.Rojas-Serrano J, Cardiel MH. Lupus patients in an emergency unit: causes of consultation, hospitalization and outcome. A cohort study. Lupus. 2000;9:601–606. doi: 10.1191/096120300678828785. [DOI] [PubMed] [Google Scholar]
- 14.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 15.Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25:2958–2971. doi: 10.1016/s0149-2918(03)80347-8. [DOI] [PubMed] [Google Scholar]
- 16.Lew KH, Chang EY, Rajagopalan K, Knoth RL. The effect of medication adherence on health care utilization in bipolar disorder. Manag Care Interface. 2006;19:41–46. [PubMed] [Google Scholar]
- 17.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 19.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57:56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malouff JM, Schutte NS, Ramerth W. Evaluation of a short form of the POMS-depression scale. J Clin Psychol. 1985;41:389–391. doi: 10.1002/1097-4679(198505)41:3<389::aid-jclp2270410314>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Pincus T. A self-report cognitive symptoms inventory to assess patients with rheumatic diseases: results in eosinophiliamyalgia syndrome (EMS), fibromyalgia, rheumatoid arthritis (RA), and other rheumatic diseases [abstract] Arthritis Rheum. 1996;39(Suppl 9):S261. [Google Scholar]
- 22.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–286. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 23.Yazdany J, Yelin E, Panopalis P, Trupin L, Julian L, Katz P. Validation of the Systemic Lupus Erythematosus Activity Questionnaire in a large observational cohort. Arthritis Rheum. 2008;59:136–143. doi: 10.1002/art.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D scale: a self-report depression measure for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Brandt J, Benedict RH. Hopkins verbal learning test: revised. Lutz (FL): Psychological Assessment Resources; 2001. [Google Scholar]
- 26.Maruyama GM. Basics of structural equation modeling. Thousand Oaks (CA): Sage; 1997. [Google Scholar]
- 27.Pullar T, Peaker S, Martin MF, Bird HA, Feely MP. The use of a pharmacological indicator to investigate compliance in patients with a poor response to antirheumatic therapy. Rheumatology (Oxford) 1988;27:381–384. doi: 10.1093/rheumatology/27.5.381. [DOI] [PubMed] [Google Scholar]
- 28.Heller RF, Rose G, Pedoe HD, Christie DG. Blood pressure measurement in the United Kingdom Heart Disease Prevention Project. J Epidemiol Community Health. 1978;32:235–238. doi: 10.1136/jech.32.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid D, Abramson M, Raven J, Walters HE. Management and treatment perceptions among young adults with asthma in Melbourne: the Australian experience from the European Community Respiratory Health Survey. Respirology. 2000;5:281–287. doi: 10.1046/j.1440-1843.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 30.Rieckmann N, Kronish IM, Haas D, Gerin W, Chaplin WF, Burg MM, et al. Persistent depressive symptoms lower aspirin adherence after acute coronary syndromes. Am Heart J. 2006;152:922–927. doi: 10.1016/j.ahj.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Mohr DC, Goodkin DE, Likosky W, Gatto N, Baumann KA, Rudick RA. Treatment of depression improves adherence to interferon β-1b therapy for multiple sclerosis. Arch Neurol. 1997;54:531–533. doi: 10.1001/archneur.1997.00550170015009. [DOI] [PubMed] [Google Scholar]
- 32.Spector SL, Kinsman R, Mawhinney H, Siegel SC, Rachelefsky GS, Katz RM, et al. Compliance of patients with asthma with an experimental aerosolized medication: implications for controlled clinical trials. J Allergy Clin Immunol. 1986;77:65–70. doi: 10.1016/0091-6749(86)90325-8. [DOI] [PubMed] [Google Scholar]
- 33.Zeller A, Schroeder K, Peters TJ. An adherence self-report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J Clin Epidemiol. 2008;61:282–288. doi: 10.1016/j.jclinepi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder K, Fahey T, Hay AD, Montgomery A, Peters TJ. Adherence to antihypertensive medication assessed by self-report was associated with electronic monitoring compliance. J Clin Epidemiol. 2006;59:650–651. doi: 10.1016/j.jclinepi.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Norell SE. Accuracy of patient interviews and estimates by clinical staff in determining medication compliance. Soc Sci Med E. 1981;15:57–61. doi: 10.1016/0271-5384(81)90063-6. [DOI] [PubMed] [Google Scholar]