Abstract
Objective
To identify the prevalence of disability in a wide range of valued life activities (VLAs) among individuals with systemic lupus erythematosus (SLE), 1-year changes in such disability, and predictors of and changes in VLA disability.
Methods
Data were from 2 waves of a cohort of 829 individuals with SLE interviewed annually by telephone. VLA disability was assessed using a scale rating the difficulty of performing 21 activities. Scores were also calculated for subscales corresponding to obligatory, committed, and discretionary activities. Changes in VLA disability from baseline to 1-year followup were assessed. Sociodemographic and disease status measures were examined as predictors of and changes in VLA disability using multiple regression analyses.
Results
Almost half of the subjects were unable to perform ≥1 VLA at baseline. Almost all (91%) reported ≥1 VLA affected by SLE. One-quarter of the subjects experienced a significant increase in the number of activities they were unable to perform; approximately half experienced significant increases in the number of activities affected and in difficulty scores. Proportions of individuals whose disability increased and whose disability decreased were roughly equivalent. Disease status measures accounted for 62–72% of the variation in VLA difficulty. More severe disease status was predictive of increases in VLA difficulty; few predictors of improvements were identified.
Conclusion
VLA disability was common, with more disability noted in committed and discretionary activities than in obligatory activities. Because VLA disability has been linked to psychological well-being in previous studies, identification of factors that may protect against such disability is important.
Introduction
Disability from systemic lupus erythematosus (SLE) is not well characterized, possibly because of the variability in the clinical manifestations and disease severity of SLE. However, studies have suggested that SLE is associated with significant disability. In one study, individuals reported that SLE compromised their activities of daily living to the extent that almost two-thirds experienced a permanent or periodic inability to perform activities at home or work (1). Rates of work disability among persons with SLE are high (1–4), with a 3-fold to 4-fold increased risk of work disability; the higher rate appears among individuals with neuropsychiatric manifestations (5). Social relationships and sexual functioning also appear to be affected by SLE (1,6–8). A number of studies have examined quality of life among persons with SLE using a variety of measures that include assessments of physical and/or role functioning (9–15), and these studies have generally found impaired functioning.
We present data on disability in valued life activities (VLAs; which are a wide range of life activities deemed to be important by the individual) among adults with SLE, using the disablement model of Verbrugge and Jette (16) as a framework. Previous studies have shown that VLA disability is more closely linked to perceptions of quality of life and satisfaction with function than with measures of more basic functioning (17,18). Increases in VLA disability have also been linked to development of psychological distress (19,20). To our knowledge, no studies of VLA disability have been undertaken in SLE.
The disability model developed by Nagi, later amended by the Institute of Medicine and then expanded by Verbrugge and Jette in their model of the disablement process, is one of the primary models used in disability research (16,21,22). This model encompasses 4 components: pathology (biochemical and physiologic abnormalities, or disease, injury, or congenital/developmental conditions); impairments (dysfunctions or significant abnormalities in specific body systems that can have consequences for physical, mental, or social functioning); functional limitations (restrictions in performing generic, fundamental physical and mental actions used in daily life in many circumstances); and disability (difficulty performing activities of daily life). The disablement process was described as a pathway progressing from pathology to impairments to functional limitations to disability.
When assessing disability, Verbrugge proposed that life activities be grouped into 3 categories (16,23,24): obligatory activities, required for survival and self-sufficiency; committed activities, associated with one's principal productive social roles; and discretionary activities, such as socializing, engaging in leisure time activities and pastimes, or other activities that individuals engage in for relaxation and pleasure.
In addition to presenting data on disability in a wide range of life activities among adults with SLE, we also identified factors that are associated with VLA disability and with changes in VLA disability over a 1-year period.
Patients and Methods
Sample
The sample for the present study was drawn from the initial interview (baseline) and the first annual followup of the University of California at San Francisco (UCSF) Lupus Outcomes Study (LOS). Participants in the LOS had formerly participated in a study of genetic risk factors for SLE outcomes (25,26) and were recruited from both clinical and community-based sources, including UCSF-affiliated clinics (22%), non-UCSF rheumatology offices (11%), lupus support groups and conferences (26%), and newsletters, Web sites, and other forms of publicity (41%). Between September 2002 and August 2003, we conducted baseline interviews with 897 participants. In 2004, we conducted a second annual interview with 832 (93%) of the 897 LOS participants initially enrolled prior to the end of 2003. Of the 68 whom we did not re-interview, 11 had died, 11 were medically unable to participate in an interview, 22 declined further participation, and 24 were lost to followup. Additional details regarding the LOS have been reported by Yelin et al (4). The study was approved by the UCSF Committee on Human Research.
VLA disability
Development of the VLA disability scale for rheumatoid arthritis (RA) has been previously described in detail (27). The version used in the LOS was adapted from the RA scale with minor changes, and it included 21 activity domains covering obligatory, committed, and discretionary activities. The full text of the scale items is shown in Table 1. Activities were defined as obligatory, committed, or discretionary based on the definitions of these activity categories by Verbrugge.
Table 1. Valued life activity scale items*.
| Subscale | Item |
|---|---|
| O | 1. Sleep. |
| C | 2. Work at a job or go to school. |
| O | 3. Take care of your basic needs, such as bathing, washing, getting dressed, or taking care of personal hygiene. |
| O | 4. Walking, just to get around. (This does not include walking for exercise.) |
| O | 5. Getting around your community by car or public transportation. |
| C | 6. Housework. |
| C | 7. Preparing meals and cooking. |
| C | 8. Shopping and doing errands. |
| C | 9. Taking care of other family members, such as your spouse or parent, or other people close to you. |
| C | 10. Other work around the house, such as making minor home repairs or working in the garage fixing things. |
| D | 11. Participating in activities with your children/grandchildren. |
| D | 12. Participating in religious or spiritual activities. |
| D | 13. Having friends and family members visit you in your home. |
| D | 14. Visiting with friends or family members in their home. |
| D | 15. Participating in leisure activities OUTSIDE your home, such as playing cards or bingo, or going to movies/restaurants. |
| D | 16. Going to parties, celebrations, or other social events. |
| D | 17. Traveling out of town. |
| D | 18. Working on hobbies or crafts or creative activities, such as sewing, woodwork, or painting. |
| D | 19. Physical recreational activities, such as dancing, playing golf, or bowling. |
| D | 20. Participating in vigorous physical recreational activities, such as walking for exercise, jogging, bicycling, swimming, or water aerobics. |
| D | 21. Intimate relations with partner. |
Patients answered the question: “Because of your lupus, do you have no difficulty, some difficulty, a great deal of difficulty, or are you unable to…?” Additional responses were: “not important to me” or “not applicable to me.” O = obligatory; C = committed; D = discretionary.
Assessment of disability with the VLA scale represents advancement over previous instruments in 3 ways. First, a wide spectrum of activities is included in the VLA scale, ranging from obligatory activities, such as self-care, to discretionary activities, such as recreation and social participation. Second, the VLA scale takes personal value into account. Activities that are not applicable to an individual (e.g., “taking care of children” if the individual has no children) or are not important to the individual (e.g., “cooking” if the spouse does all of the cooking) are not included in scoring. Finally, unlike most disability indices, the VLA scale asks respondents to attribute performance difficulties to the health condition under study.
In the telephone interview, participants rated the difficulty of performing 21 life activities with a 4-point scale corresponding to the response scale of the Health Assessment Questionnaire (where 0 = no difficulty and 3 = unable to perform) (28). Activities that participants deemed unimportant to them, or that they did not do for reasons unrelated to lupus, were not rated and were not included in scoring.
Three types of VLA summary measures were calculated: the number of activities that individuals were completely unable to perform because of SLE, the number of activities that were affected by SLE (at any level of difficulty or inability to perform), and the average difficulty score. These scores were calculated for the total VLA scale, and for the obligatory, committed, and discretionary subscales.
Predictors of VLA disability
Potential predictors of VLA disability were selected based on the Verbrugge and Jette model, and included both functional limitations and measures of health and disease status, representing the impairments stage of the disablement model (27,29).
The following measures of health and disease status were used: rating of perceived lupus disease activity during the past 3 months on a scale of 0 (no activity) to 10 (extremely active) (30); self-report of a lupus flare in the 3 months prior to interview; presence of cardiovascular, renal, central nervous system, or peripheral vascular conditions (each of these was coded as a dummy variable); number of other comorbid conditions (i.e., hypertension, diabetes, cancer, and lung disease); use of immunosuppressant medications in the past 12 months; fatigue severity, based on the vitality scale of the Short Form 36 (SF-36) health survey (scores range from 0–100, and were reversed so that higher scores reflected greater fatigue) (31); and self-reported cognitive function, based on the Medical Outcomes Study Cognitive Function scale (which is composed of 6 items; scores range from 0–100, with higher scores reflecting better function) (32).
Functional limitations were assessed with the 10-item Physical Function (PF) subscale of the SF-36 (31). Scores on the PF subscale range from 0–100, with lower scores reflecting more limitations.
Covariates
In addition to the variables described above, the following sociodemographic variables were included in analyses: age, sex, race (white non-Hispanic compared with others), and education (high school education or less compared with others).
Statistical analysis
Descriptive demographic and disease-related statistics were compiled. Differences in the baseline characteristics of individuals who were and were not available for followup were assessed with t-tests or chi-square analyses. Frequency distributions, means, and SDs of VLA summary scores were calculated.
Changes in VLA scores from baseline to followup were calculated. The proportion of individuals whose VLA scores exhibited a clinically meaningful increase or decrease, defined as increases (or decreases) in difficulty of ≥0.5 SD, was tabulated. Norman et al have presented evidence that minimally important changes in health-related quality of life scales among individuals with chronic diseases are consistently ∼0.5 SD (33).
To assess the sensitivity to change of the VLA scores, 2 methods were used. In the first, changes in VLA scores from baseline to followup were compared with changes in the Physical Component Summary (PCS) score of the Short Form 12 health survey (34), the SF-36 PF subscale score, and the lupus activity rating. Subjects were placed into 1 of 3 categories according to whether their VLA difficulty score increased (worsened), decreased (improved), or did not exhibit clinically meaningful change. Differences in PCS, PF, and lupus activity rating among these 3 groups were tested with analyses of variance. The second method of assessing sensitivity to change used the standardized response mean (SRM) (35). The SRM was calculated as the mean change in scores divided by the SD of the change. Because the disability scores of some individuals in the sample increased and scores of others decreased, it is likely that the overall SRM underestimated the responsiveness. Therefore, the SRM was calculated separately for the groups characterized as worse, the same, and improved.
The disablement model suggests that measures of disease status would predict functional limitations (in this case, PF subscale score), and that, in turn, functional limitations would predict disability. Therefore, we first conducted a multiple linear regression model with PF score as the dependent variable and with each of the disease status measures as independent variables, including sociodemographic variables as covariates. Next, factors associated with baseline VLA disability were identified using 2 multiple linear regression analyses, with VLA difficulty scores as dependent variables and measures of disease status and functional limitations as independent variables. The first analysis included rating of lupus activity; recent lupus flare; presence of cardiovascular, renal, central nervous system, or peripheral vascular disease; presence of other comorbidities; use of immunosuppressant medications; fatigue; self-reported cognitive function; and age, sex, race, education, and duration of lupus. The second analysis retained all of these variables and added the SF-36 PF score.
To identify baseline predictors of increase (or, in other analyses, decrease) in VLA disability from baseline to followup, multiple logistic regression analyses were conducted, including all of the baseline measures of disease status and functional limitations. An exception was the flare variable. For these analyses, the followup rating of flare in the past 3 months was used instead of the baseline rating. In the analysis to identify predictors of a worsening of VLA disability, subjects whose VLA disability increased were compared with those whose VLA disability was unchanged, omitting subjects whose VLA disability decreased. Similarly, to identify predictors of improvement in VLA disability, subjects whose VLA disability decreased were compared with those with no change, omitting those whose VLA disability increased. Two regression models were estimated, first including sociodemographic and disease-related characteristics alone, and then adding the PF score. Each of these regression models also included the baseline VLA disability score.
Sensitivity analyses
Two sensitivity analyses were performed. In the first, additional definitions of VLA disability (number of VLAs affected and number of VLAs that individuals were unable to perform) were used in regression models to identify predictors of changes in disability. In the second, a larger change in VLA difficulty (1 SD instead of 0.5 SD) was used as the criterion for change in the analyses to identify predictors of change.
Results
Subject characteristics
Three subjects were excluded from analysis because of incomplete data. The majority of subjects (91.4%) were women. At baseline, mean age was 47.2 years and mean duration of SLE was 12.7 years. Additional characteristics are shown in Table 2. The 829 individuals who were available for followup (the analysis sample) were more commonly female (92.3% of those with followup versus 80.9% of those with no followup; P < 0.001) and white non-Hispanic (70.8% versus 52.9%; P < 0.001), had more education (19.4% with high school or less versus 32.4%; P = 0.02), had lupus of shorter duration (12.6 years versus 14.8 years; P = 0.04), and were less likely to report renal disease (7.4% versus 16.2%; P = 0.02) or peripheral vascular disease (15.0% versus 26.5%; P = 0.02).
Table 2. Subject characteristics at baseline*.
| Available for followup | ||||
|---|---|---|---|---|
| Characteristics | Total (n = 897) |
Yes† (n = 829) |
No (n = 68) |
P‡ |
| Sociodemographic | ||||
| Age at interview, mean ± SD years | 47.2 ± 13.1 | 47.2 ± 12.8 | 47.8 ± 16.2 | 0.68 |
| Female, n (%) | 820 (91.4) | 765 (92.3) | 55 (80.9) | 0.00 |
| Married/partner, n (%) | 545 (60.8) | 503 (60.7) | 42 (61.8) | 0.49 |
| White, non-Hispanic, n (%) | 623 (69.5) | 587 (70.8) | 36 (52.9) | 0.00 |
| Education: high school graduate or less, n (%) | 183 (20.4) | 161 (19.4) | 22 (32.4) | 0.02 |
| Health-related and SLE-related | ||||
| Duration of SLE, mean ± SD years | 12.7 ± 8.6 | 12.6 ± 8.5 | 14.8 ± 8.9 | 0.04 |
| Comorbidities, n (%) | 0.05 | |||
| 1 | 327 (36.5) | 307 (37.0) | 20 (29.4) | |
| ≥2 | 312 (34.8) | 279 (33.7) | 33 (48.5) | |
| Rating of lupus disease activity | 3.1 (4.3) | 3.1 (4.3) | 3.5 (4.6) | 0.35 |
| Cardiovascular disease, n (%) | 46 (5.2) | 39 (4.8) | 7 (10.6) | 0.08 |
| Renal disease, n (%) | 72 (8.0) | 61 (7.4) | 11 (16.2) | 0.02 |
| CNS manifestations, n (%) | 222 (24.7) | 207 (25.0) | 15 (22.1) | 0.66 |
| Peripheral vascular disease, n (%) | 142 (15.8) | 124 (15.0) | 18 (26.5) | 0.02 |
| Major medications, n (%) | 247 (27.4) | 225 (27.1) | 22 (32.4) | 0.40 |
| SF-36 PF subscale score, mean ± SD | 58.1 ± 30.8 | 58.6 ± 30.6 | 52.0 ± 32.7 | 0.09 |
| SF-36 Fatigue subscale score, mean ± SD | 56.4 ± 23.0 | 56.5 ± 23.0 | 55.9 ± 23.8 | 0.85 |
SLE = systemic lupus erythematosus; CNS = central nervous system; SF-36 = Short Form 36 health survey; PF = Physical Function.
Individuals who participated in both the baseline and followup interviews comprised the primary analysis sample.
By t-test or chi-square analysis comparing individuals who remained in the study for followup versus those who did not.
Prevalence and severity of disability in VLAs
Each activity was affected by lupus for at least one-quarter of the sample (Table 3). At baseline, the activities least commonly affected were 2 obligatory activities: basic self-care (28.0% reported this activity being affected) and getting around the community (30.7%). At least half of the sample reported 12 of the 21 activities to be affected. The activities most frequently affected by lupus were vigorous physical activities (83.9%), home repairs (79.4%), sleep (72.9%), paid work (70.7%), and housework (67.8%).
Table 3. Disability ratings for valued life activities (n = 829).
| Affected, % | Unable, % | Difficulty rating, mean ± SD | ||||
|---|---|---|---|---|---|---|
| Activities | Baseline | Followup | Baseline | Followup | Baseline | Followup |
| Obligatory | ||||||
| Basic self-care | 28.0 | 27.8 | 0.4 | 0.1 | 0.33 ± 0.57 | 0.32 ± 0.54 |
| Car/transit | 30.7 | 31.8 | 2.5 | 2.8 | 0.42 ± 0.71 | 0.43 ± 0.72 |
| Walk to get around | 42.5 | 44.4 | 1.0 | 1.4 | 0.53 ± 0.70 | 0.56 ± 0.71 |
| Sleep | 72.9 | 73.9 | 2.3 | 5.5 | 1.08 ± 0.81 | 1.13 ± 0.86 |
| Committed | ||||||
| Cook | 48.1 | 50.3 | 2.4 | 3.3 | 0.63 ± 0.76 | 0.67 ± 0.79 |
| Family care | 55.9 | 58.0 | 5.7 | 8.6 | 0.78 ± 0.85 | 0.87 ± 0.93 |
| Shopping/errands | 59.1 | 61.0 | 3.7 | 3.8 | 0.83 ± 0.83 | 0.83 ± 0.81 |
| Housework | 67.8 | 68.9 | 6.9 | 8.6 | 1.02 ± 0.90 | 1.06 ± 0.92 |
| Paid work/school | 70.7 | 72.5 | 31.5 | 32.3 | 1.45 ± 1.21 | 1.50 ± 1.20 |
| Repair work | 79.4 | 80.7 | 25.0 | 28.7 | 1.55 ± 1.08 | 1.64 ± 1.09 |
| Discretionary | ||||||
| Religious/spiritual activities | 36.0 | 35.5 | 3.1 | 3.0 | 0.49 ± 0.76 | 0.46 ± 0.73 |
| Entertain others | 38.7 | 43.9 | 1.7 | 1.4 | 0.48 ± 0.68 | 0.54 ± 0.69 |
| Leisure out | 41.4 | 41.9 | 3.5 | 3.4 | 0.54 ± 0.75 | 0.54 ± 0.75 |
| Visiting others | 48.0 | 50.1 | 3.1 | 2.4 | 0.64 ± 0.79 | 0.68 ± 0.79 |
| Intimate relations with partner | 48.0 | 48.8 | 5.3 | 4.3 | 0.69 ± 0.86 | 0.46 ± 0.73 |
| Hobbies | 51.4 | 54.2 | 6.8 | 7.9 | 0.75 ± 0.89 | 0.80 ± 0.92 |
| Travel | 53.0 | 56.1 | 7.0 | 7.5 | 0.82 ± 0.93 | 0.87 ± 0.94 |
| Activities with children | 60.7 | 60.7 | 2.6 | 3.6 | 0.80 ± 0.78 | 0.81 ± 0.80 |
| Parties/events | 60.5 | 62.4 | 5.6 | 7.0 | 0.87 ± 0.87 | 0.91 ± 0.89 |
| Volunteer work | 66.2 | 66.2 | 19.2 | 20.7 | 1.18 ± 1.10 | 1.21 ± 1.12 |
| Vigorous physical activities | 83.9 | 83.1 | 31.0 | 33.7 | 1.70 ± 1.07 | 1.73 ± 1.10 |
Ratings of inability to perform activities were much less common, with fewer than 10% reporting inability to perform most activities. Notable exceptions were paid work (31.5% were unable to perform) and vigorous physical activities (31.0%). In general, activities affected for the fewest subjects had the lowest difficulty scores, and activities affected for the most subjects had the highest difficulty scores.
At baseline, ≥1 VLA was affected for 91% of the sample (Table 4). The proportion with ≥1 activity affected was 78.0% for obligatory activities, 81.9% for committed activities, and 85.6% for discretionary activities. Similar findings were noted at followup.
Table 4. Valued life activity (VLA) summary scores at baseline, followup, and changes from baseline to followup (n = 829).
| All activities (n = 21) |
Obligatory (n = 4) |
Committed (n = 6) |
Discretionary (n = 11) |
|
|---|---|---|---|---|
| Affected | ||||
| ≥1 VLA, baseline, % | 91.0 | 78.0 | 81.9 | 85.6 |
| ≥1 VLA, followup, % | 91.4 | 78.9 | 81.7 | 85.5 |
| Number of activities at baseline, mean ± SD | 10.4 ± 6.9 | 1.7 ± 1.4 | 3.5 ± 2.3 | 5.2 ± 3.7 |
| Number of activities at followup, mean ± SD | 10.7 ± 7.1 | 1.8 ± 1.4 | 3.6 ± 2.3 | 5.4 ± 3.9 |
| Increase in number of activities, % | 43.9 | 24.4 | 29.6 | 37.4 |
| Decrease in number of activities, % | 39.6 | 23.8 | 25.0 | 32.0 |
| Unable | ||||
| ≥1 VLA, baseline, % | 45.8 | 5.5 | 36.7 | 36.7 |
| ≥1 VLA, followup, % | 48.7 | 9.3 | 38.0 | 38.0 |
| Number of activities at baseline, mean ± SD | 1.5 ± 2.6 | 0.1 ± 0.3 | 0.7 ± 1.1 | 0.8 ± 1.5 |
| Number of activities at followup, mean ± SD | 1.7 ± 2.7 | 0.1 ± 0.3 | 0.7 ± 1.2 | 0.8 ± 1.5 |
| Increase in number of activities, % | 28.0 | 6.8 | 18.7 | 21.0 |
| Decrease in number of activities, % | 22.2 | 3.3 | 14.6 | 19.2 |
| Difficulty | ||||
| Baseline, mean ± SD | 0.8 ± 0.6 | 0.6 ± 0.5 | 1.0 ± 0.8 | 0.8 ± 0.7 |
| Followup, mean ± SD | 0.9 ± 0.7 | 0.6 ± 0.5 | 1.1 ± 0.8 | 0.8 ± 0.7 |
| Increase ≥0.5 SD, % | 25.0 | 31.6 | 25.9 | 25.1 |
| Decrease ≥0.5 SD, % | 22.6 | 30.6 | 23.3 | 22.7 |
Almost half of the participants were unable to perform ≥1 VLA because of lupus at baseline (Table 4). While the proportion reporting that they were unable to perform an obligatory activity was rather small (5.5%), over one-third were unable to perform ≥1 committed (36.7%) or discretionary (36.7%) activities. Again, similar findings were noted at followup.
The average ± SD VLA difficulty score at baseline was 0.8 ± 0.6. The lowest mean difficulty rating was for obligatory activities (0.6), and the highest was for committed activities (1.0). Followup ratings were similar.
Changes in VLA disability
Substantial portions of the sample reported changes in VLA disability, both increases and decreases, from baseline to followup (Table 4). Of the sample, 43.9% reported more VLAs affected at followup than at baseline, with the largest increase being in discretionary activities (37.4%). Slightly more than one-quarter (28%) of subjects reported an increase from baseline to followup in the number of VLAs they were unable to perform. The number of individuals with increases in the number of activities they were unable to perform was the lowest for obligatory activities (6.8%), more than double that for committed activities (18.7%), and the greatest for discretionary activities (21.0%). One-quarter of the participants' VLA difficulty scores increased by ≥0.5 SD from baseline to followup.
Improvement was noted almost as commonly as worsening. Approximately 40% of subjects reported fewer VLAs affected at followup, with the largest improvement noted in discretionary activities (32.0%). A decrease in the number of activities that were unable to be performed was reported by 22.2% of the subjects, with the largest improvements in committed (14.6%) and discretionary (19.2%) activities. The VLA difficulty scores of approximately one-quarter (22.6%) of the sample decreased by ≥0.5 SD.
Sensitivity to change
Similar proportions of subjects experienced declines in functioning as measured by the VLA disability (24.9%), the PCS (24.6%), and the SF-36 PF scales (25.0%), as well as by ratings of disease activity (25.0%). Similar proportions of subjects experienced improvements in functioning according to the VLA disability scale (22.6), the PCS scale (22.7), the SF-36 PF scale (22.6%), and the ratings of disease activity (22.7%). Individuals whose VLA difficulty scores increased (worsened) exhibited declines in functioning as measured by the PCS and PF scales and increases in disease activity ratings (Figure 1). Conversely, those whose VLA difficulty score decreased (improved) exhibited improvements in functioning and decreases in ratings of disease activity. In each case, significant differences (P < 0.0001) were noted among the VLA change groups.
Figure 1.
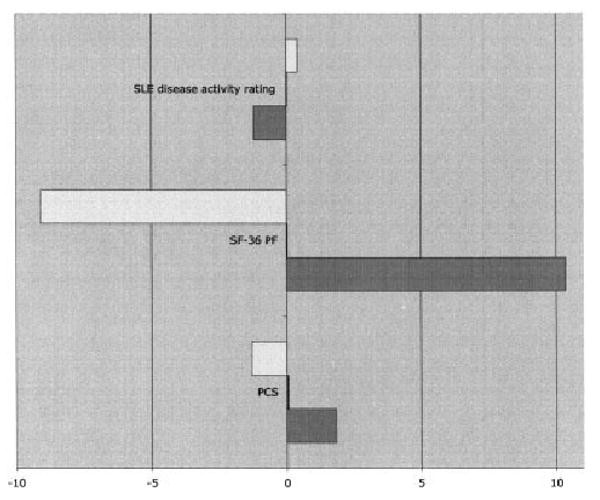
Responsiveness of valued life activity (VLA) disability scores. Values to the left of the 0 point on the graph represent scores that decreased from baseline to followup on the systemic lupus erythematosus (SLE) disease activity rating, the Short Form 36 (SF-36) health survey's Physical Function (PF) subscale, and the SF-36 Physical Component Summary (PCS) subscale; values to the right represent scores that increased. Open bars represent the scores on these scales for individuals whose VLA disability worsened, solid bars (which are barely visible) represent the scores for individuals whose VLA disability did not change, and shaded bars represent the scores for individuals whose VLA disability improved.
The overall SRM was 0.08. However, as noted above, improvements in disability were almost as common as declines, and the concurrent effects of the improvements and declines would be expected to neutralize the overall SRM. The average changes in the groups were 0.49, 0.01, and −0.44, and the SRMs were 1.96, 0.10, and −1.65, for the worse, same, and improved groups, respectively, indicating a high degree of sensitivity to change (35).
Predictors of VLA disability
The disablement model posits that impairments (represented by disease status characteristics in these analyses) lead to functional limitations (represented by PF score), which in turn lead to disability. In testing the first part of this model, several disease measures (rating of lupus activity, and presence of ≥2 comorbid conditions, central nervous system, or peripheral vascular involvement) were significantly associated with the PF score (data not shown). In addition, older age, female sex, low education, and nonwhite race/ethnicity were also significantly associated with the PF score. The entire model accounted for 40% of the variance in PF score.
When the next component of the disablement model (the identification of predictors of disability) was tested, several measures (age, female sex, comorbid conditions, rating of greater lupus activity, presence of central nervous system disease, poorer cognitive function, and greater fatigue) were significantly associated with VLA disability (model 1, model R2 = 0.62) (Table 5). Adding the PF score to the regression model (model 2) significantly increased the model R2 to 0.72, but the PF score did not appear to mediate the relationship of any of the disease status variables with VLA disability. In other words, the factors noted above remained significantly associated with VLA disability after addition of PF score to the regression model.
Table 5. Correlates and predictors of changes in VLA difficulty scores*.
| Change in VLA disability, baseline to followup | ||||||
|---|---|---|---|---|---|---|
| Baseline VLA disability† | Increase (worsening)† | Decrease (improvement)‡ | ||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Age | 0.004§ | - | 1.02 (1.00, 1.03)¶ | 1.01 (1.00, 1.03) | - | - |
| Female | 0.18 | 0.08 | - | - | - | - |
| Duration of lupus | - | - | 0.98 (0.96, 1.00)¶ | 0.98 (0.96, 1.00)¶ | - | - |
| Education, high school or less | - | - | - | - | - | - |
| Race, white non-Hispanic | - | - | - | - | - | - |
| Comorbidities, 1 | - | - | - | - | - | - |
| Comorbidities, ≥2 | 0.16# | 0.08¶ | - | - | - | - |
| SLE flare** | - | - | 1.60 (1.06, 2.40)¶ | 1.60 (1.06, 2.38)§ | - | - |
| Rating of lupus activity | 0.06# | 0.04# | - | - | - | - |
| Cardiovascular disease | - | - | - | - | - | - |
| Renal disease | - | - | - | - | ||
| CNS disease | 0.07¶ | 0.05 | - | - | - | - |
| Peripheral vascular disease | - | - | - | - | - | - |
| Use of immunosuppressant medications | - | - | - | - | - | - |
| Cognitive function score†† | −0.05# | −0.04# | - | - | - | - |
| Fatigue score†† | 0.12# | 0.07# | 1.24 (1.10, 1.38)# | 1.22 (1.09, 1.37)§ | - | - |
| SF-36 PF subscale score† | Not included | −0.01# | Not included | - | Not included | - |
| Baseline VLA | Not included | Not included | - | - | 4.42 (2.57, 7.61)# | 5.01 (2.66, 9.46)# |
| Model‡‡ | R2 = 0.62 | R2 = 0.72 | R2 = 0.14 | R2 = 0.15 | R2 = 0.27 | R2 = 0.27 |
Only independent variables significant at P ≤ 0.05 are shown. In each case, model 2 is identical to model 1 except for the addition of the SF-36 Physical Function subscale score. VLA = valued life activity; SLE = systemic lupus erythematosus; CNS = central nervous system; SF-36 = Short Form 36 health survey; PF = Physical Function.
Analyses compare those whose VLA disability worsened with those whose VLA disability did not change.
Analyses compare those whose VLA disability improved with those whose VLA disability did not change.
0.05 < P ≤ 0.01
P ≤ 0.05
0.01 < P ≤ 0.001
SLE flare at baseline for cross-sectional analysis. SLE flare at followup for longitudinal analysis of increase/decrease in VLA disability.
Higher scores reflect better cognitive function, greater fatigue, and better physical function. Odds ratios are per 10-point increment in score.
At baseline, the R2 for each model is adjusted. At followup, the R2 for each model is the Nagelkerke R2 approximation.
Greater age, shorter duration of lupus, recent lupus flare, greater fatigue at baseline, and lower baseline VLA disability were each independent predictors of a clinically meaningful increase in VLA difficulty from baseline to followup. Adding baseline PF score to the model increased the predictive power of the model only slightly (χ2 = 2.38, P > 0.05). The PF score did not appear to mediate the effects of other predictors.
When PF score was not included in the regression model, individuals who had higher VLA difficulty scores at baseline had greater odds of VLA disability improvement at followup (odds ratio 4.4; 95% confidence interval 2.6, 7.6). Adding the PF score to the model did not significantly increase the predictive power of the model (χ2 = 1.72, P > 0.05).
Sensitivity analyses
Analyses using the other definitions of VLA disability yielded results that were not substantially different from those based on VLA difficulty. Results from analyses examining a 1 SD instead of a 0.5 SD increase in VLA difficulty were also not substantially different from the primary analyses. However, when predicting a 1 SD decrease in disability, use of immunosuppressant medications was no longer predictive; otherwise, there were no meaningful differences in the models.
Discussion
VLA disability is common among individuals with SLE. Approximately half of this sample was unable to perform ≥1 VLA because of SLE, and almost all reported ≥1 VLA affected by SLE. Obligatory activities were the least affected, but there was little difference between effects on committed and discretionary activities. Approximately 35–40% were unable to perform ≥1 committed and discretionary activity, suggesting that these activities are the ones most commonly given up. Whether this is a voluntary relinquishment to allow time and/or energy for other activities, or whether these activities are lost because of functional limitations or the increased physical demands of these activities, requires further examination. The higher difficulty ratings seen for committed activities may be an indication that these activities, necessary for meeting life roles, require more effort and thus leave less time and effort for discretionary activities. This hypothesis is consistent with previous reports that when dealing with disability, people may give up some activities in order to have time and energy for others (36,37).
Why is it important to consider disability in VLAs? Most disability research has focused on basic activities of daily living (ADL; e.g., personal hygiene, transfers), instrumental activities of daily living (IADL; e.g., preparing meals), and employment, corresponding to obligatory and some committed activities, and has thus ignored a great deal of daily life, particularly valued discretionary activities (23). This emphasis reflects assumptions by researchers that ADL, IADL, and employment are a priori more important and that the difficulty in doing them is thus more significant (23). These assumptions may, in fact, not be true. Some activities are more important or more meaningful to individuals than others, and the person-specific meaning, i.e., value, attached to activities may affect the impact of disability. The importance of individual priorities and values, and the failure of common functional assessments to take these individual values into account, has been recognized by some researchers, and studies have shown that a large proportion of activities that individuals deem to be important are outside the realm of ADL, IADL, and employment (38–40). Functioning in discretionary, valued activities may also be more strongly linked to satisfaction with function than more basic ADL-type levels of functioning (17).
Performance of VLAs also appears to be linked to psychological well-being more strongly than limitations in general function. For example, persons with RA who reported high levels of depressive symptoms performed fewer VLAs than those who did not report depressive symptoms, and the loss of VLAs has been shown to be a stronger predictor of the subsequent onset of new depressive symptoms than decline in basic function (19,41). Another consideration is that as more effective treatments become available, patient goals will likely expand beyond simple preservation of ADL. Measurement of a wider range of life activities coincides with these new expectations.
There are potential limitations to this study. It is possible that our assessment of VLAs was incomplete. In fact, as a result of open-ended queries about other activities that have been affected by SLE, a new version of the VLA assessment is being developed and tested, to which additional life activities, such as taking care of household business, going to appointments, or taking care of pets, have been added. While this new measure may be more sensitive, there is no reason to believe that the overall tenor of these results would change as a result. The considerable change in VLA disability scores, both improvement and worsening, may suggest some unreliability in the measure. However, roughly equivalent proportions of subjects experienced either worsening or improvement on all 4 measures examined, and results of the sensitivity to change analyses indicated that individuals who experienced clinically meaningful changes in VLA disability scores also experienced consistent changes in other measures of functioning and disease status.
It is also possible that factors other than those included here may affect VLA disability. For example, VLA disability may be more common among individuals with cognitive dysfunction. While we included a self-reported measure of cognitive symptoms, a more objective neurocognitive assessment may be a better indicator. Unfortunately, such neurocognitive measures were not available at baseline for this cohort, although such data are now being collected, enabling examination of this association in the future. The LOS cohort may be unrepresentative of individuals with SLE in some way. However, the LOS is among the largest cohorts of individuals with SLE and, because participants were recruited from a variety of sources rather than solely through an academic medical center or tertiary care center, it is probable that the distribution of disease severity and other relevant characteristics is more similar to a random sample of individuals with SLE than subjects exclusively from clinical environments. Finally, although the VLA measure was found to be highly sensitive to change, we found that the best predictor of improvement was high disability at baseline. This may suggest either that individuals' scores tended to regress to the mean or that there may be limitations to the scale.
In summary, disability in VLAs is very common among individuals with SLE. Changes in VLA disability, both worsening and improvement, are also common. More severe disease at baseline appears to predict worsening of VLA disability, but few predictors of improvement in VLA disability were identified. In other conditions, VLA disability has been a powerful predictor of the development of depression (18–20,42). Future research in SLE should include identification of the relationship between VLA disability and psychological distress, and particularly of factors that may protect against psychological distress following VLA disability.
Acknowledgments
The authors gratefully acknowledge Rosemary Prem, Jessica Spry, and Janet Stein for conducting the LOS telephone interviews, and the rest of the LOS study group for their helpful comments during the preparation of this manuscript.
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P60-AR-053308), and by the State of California Lupus Fund.
Footnotes
Because Drs. Katz and Yelin are Editors of Arthritis Care & Research, review of this article was handled by the Editor of Arthritis & Rheumatism.
Author Contributions: Dr. Katz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Katz, Yelin.
Acquisition of data. Yelin, Trupin.
Analysis and interpretation of data. Katz, Morris, Yazdany, Trupin.
Manuscript preparation. Katz, Yelin.
Statistical analysis. Katz, Morris.
References
- 1.Boomsma MM, Bijl M, Stegeman CA, Kallenberg CG, Hoffman GS, Tervaert JW. Patients' perceptions of the effects of systemic lupus erythematosus on health, function, income, and interpersonal relationships: a comparison with Wegener's granulomatosis. Arthritis Rheum. 2002;47:196–201. doi: 10.1002/art.10341. [DOI] [PubMed] [Google Scholar]
- 2.Partridge AJ, Karlson EW, Daltroy LH, Lew RA, Wright EA, Fossel AH, et al. Risk factors for early work disability in systemic lupus erythematosus: results from a multicenter study. Arthritis Rheum. 1997;40:2199–206. doi: 10.1002/art.1780401214. [DOI] [PubMed] [Google Scholar]
- 3.Karlson EW, Daltroy LH, Lew RA, Wright EA, Partridge AJ, Roberts WN, et al. The independence and stability of socioeconomic predictors of morbidity in systemic lupus erythematosus. Arthritis Rheum. 1995;38:267–73. doi: 10.1002/art.1780380217. [DOI] [PubMed] [Google Scholar]
- 4.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57:56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsen A, Bengtsson AA, Nived O, Ryberg B, Sturfelt G. Outcome of neuropsychiatric SLE within a defined Swedish population: increased morbidity but low mortality. Rheumatology. 2002;41:1308–12. doi: 10.1093/rheumatology/41.11.1308. [DOI] [PubMed] [Google Scholar]
- 6.Curry S, Levine S, Corty E, Jones P, Kurit D. The impact of systemic lupus erythematosus on women's sexual functioning. J Rheumatol. 1994;21:2254–60. [PubMed] [Google Scholar]
- 7.Liang MH, Rogers M, Larson M, Eaton HM, Murawski BJ, Taylor JE, et al. The psychosocial impact of systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1984;27:13–9. doi: 10.1002/art.1780270102. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe L, Denton F, Schrieber L. Validity of the disease repercussion profile in patients with systemic lupus erythematosus. Lupus. 2004;13:428–35. doi: 10.1191/0961203303lu1037oa. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Shakra M, Mader R, Langevitz P, Friger M, Codish S, Neumann L, et al. Quality of life in systemic lupus erythematosus: a controlled study. J Rheumatol. 1999;26:306–9. [PubMed] [Google Scholar]
- 10.Da Costa D, Dobkin P, Fitzcharles M, Fortin P, Beaulieu A, Zummer M, et al. Determinants of health status in fibromyalgia: a comparative study with systemic lupus erythematosus. J Rheumatol. 2000;27:365–72. [PubMed] [Google Scholar]
- 11.Dobkin PL, Da Costa D, Dritsa M, Fortin PR, Senecal JL, Goulet JR, et al. Quality of life in systemic lupus erythematosus patients during more and less active disease states: differential contributors to mental and physical health. Arthritis Rheum. 1999;12:401–10. doi: 10.1002/1529-0131(199912)12:6<401::aid-art8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Khanna S, Pal H, Pandey R, Handa R. The relationship between disease activity and quality of life in systemic lupus erythematosus. Rheumatology (Oxford) 2004;43:1536–40. doi: 10.1093/rheumatology/keh376. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon GS, Roseman J, Bartolucci AA, Friedman AW, Moulds JM, Goel N, et al. LUMINA Study Group Systemic lupus erythematosus in three ethnic groups. II. Features predictive of disease activity early in its course. Arthritis Rheum. 1998;41:1173–80. doi: 10.1002/1529-0131(199807)41:7<1173::AID-ART5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Daltroy LH, Lew RA, Wright EA, Patridge AJ, Fossel AH, et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:47–56. doi: 10.1002/art.1780400108. [DOI] [PubMed] [Google Scholar]
- 15.Panopolis P, Petri M, Manzi S, Isenberg D, Gordon C, Senecal J, et al. The systemic lupus erythematosus tri-nation study: longitudinal changes in physical and mental well-being. Rheumatology. 2005;44:751–5. doi: 10.1093/rheumatology/keh580. [DOI] [PubMed] [Google Scholar]
- 16.Verbrugge L, Jette A. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 17.Katz PP, Yelin EH, Lubeck D, Buatti M, Wanke LA. Satisfaction with function: what type of function do rheumatoid arthritis patients value most? Arthritis Rheum. 2001;44:S185. abstract. [Google Scholar]
- 18.Katz P, Yelin E, Eisner M, Earnest G, Blanc P. Performance of valued life activities reflected asthma-specfiic quality of life more than general physical function. J Clin Epidemiol. 2004;57:259–67. doi: 10.1016/j.jclinepi.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Katz PP, Yelin EH. The development of depressive symptoms among women with rheumatoid arthritis: the role of function. Arthritis Rheum. 1995;38:49–56. doi: 10.1002/art.1780380108. [DOI] [PubMed] [Google Scholar]
- 20.Katz PP, Yelin EH. Activity loss and the onset of depressive symptoms: do some activities matter more than others? Arthritis Rheum. 2001;44:1194–202. doi: 10.1002/1529-0131(200105)44:5<1194::AID-ANR203>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Nagi S. Disability concepts revisited: implications for prevention. In: Pope A, Tarlov A, editors. Disability in America: toward a national agenda for prevention. Washington, DC: National Academy Press; 1991. pp. 309–26. [Google Scholar]
- 22.Verbrugge L. The iceberg of disability. In: Stahl S, editor. The Legacy of longevity: health and health care in later life. Newbury Park (CA): Sage Publications; 1990. pp. 55–75. [Google Scholar]
- 23.Verbrugge L. Disability. Rheum Dis Clin North Am. 1990;16:741–61. [PubMed] [Google Scholar]
- 24.Verbrugge L, Gruber-Baldini A, Fozard J. Age differences and age changes in activities: Baltimore Longitudinal Study of Aging. J Gerontol Soc Sci. 1996;51B:S30–41. doi: 10.1093/geronb/51b.1.s30. [DOI] [PubMed] [Google Scholar]
- 25.Freemer M, King TJ, Criswell L. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. 2006;65:581–4. doi: 10.1136/ard.2005.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorburn C, Prokunina-Olsson L, Sterba K, Lum R, Seldin M, Alarcon-Riquelme M, et al. Association of PCDC1 genetic variation with risk and clinical manifestations of systemic lupus erythematosus in a multiethnic cohort. Genes Immun. 2007;8:279–87. doi: 10.1038/sj.gene.6364383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz P, Morris A, Yelin E. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis. 2006;65:763–9. doi: 10.1136/ard.2005.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 29.Escalante A, Haas RW, del Rincon I. A model of impairment and functional limitation in rheumatoid arthritis. BMC Musculoskelet Disord. 2005;6:16. doi: 10.1186/1471-2474-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlson E, Daltroy L, Rivest C, Ramsey-Goldman R, Wright E, Patrtridge A, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 31.Ware JJ, Snow K, Kosinski M, Gandek B. SF-36 Health Survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 32.Stewart A, Ware JJ, Sherbourne C, Wells K. Psychological distrss/well-being and cognitive functioning measures. In: Stewart A, Ware JJ, editors. Measuring functioning and well-being: the Medical Outcomes Study approach. Durham, North Carolina: Duke University Press; 1992. pp. 102–42. [Google Scholar]
- 33.Norman G, Sloan J, Wyrwich K. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. J Clin Epi. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 34.Ware J, Kosinski M, Keller S. SF-12: How to score the SF-12 Physical and Mental Health Summary Scores. Boston: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 35.Liang M, Fossel A, Larson M. Comparisons of 5 health status instruments for orthopedic evaluation. Med Care. 1990;28:632–42. doi: 10.1097/00005650-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Badley EM, Rothman LM, Wang PP. Modeling physical dependence in arthritis: the relative contribution of specific disabilities and environmental factors. Arthritis Care Res. 1998;11:335–45. doi: 10.1002/art.1790110505. [DOI] [PubMed] [Google Scholar]
- 37.Gignac MA, Cott C, Badley EM. Adaptation to chronic illness and disability and its relationship to perceptions of independence and dependence. J Gerontol B Psychol Sci Soc Sci. 2000;55:P362–72. doi: 10.1093/geronb/55.6.p362. [DOI] [PubMed] [Google Scholar]
- 38.Hewlett S, Smith A, Kirwan J. Values for function in rheumatoid arthritis: patients, professionals, and public. Ann Rheum Dis. 2001;60:928–33. doi: 10.1136/ard.60.10.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoeven A, Boers M, van der Linden S. Validity of the MACTAR questionnaire as a functional index in a rheumatoid arthritis clinical trial. J Rheumatol. 2000;27:2801–9. [PubMed] [Google Scholar]
- 40.Tugwell P, Bombardier C, Buchanan W, Goldsmith C, Grace E, Hanna B. The MACTAR Patient Preference Disability Questionnaire: an individualized functional priority approach for assessing improvement in physical disability in clinical trials in rheumatoid arthritis. J Rheumatol. 1987;14:446–51. [PubMed] [Google Scholar]
- 41.Katz PP, Yelin EH. Life activities of persons with rheumatoid arthritis with and without depressive symptoms. Arthritis Care Res. 1994;7:69–77. doi: 10.1002/art.1790070205. [DOI] [PubMed] [Google Scholar]
- 42.Katz P, Eisner M, Yelin E, Trupin L, Earnest G, Balmes J, et al. Functioning and psychological status among individuals with COPD. Qual Life Res. 2005;14:1835–43. doi: 10.1007/s11136-005-5693-3. [DOI] [PubMed] [Google Scholar]


