Abstract
Phosphoglucose isomerase/autocrine motility factor (PGI/AMF) is a housekeeping gene product/cytokine which catalyzes a step in glycolysis and gluconeogenesis, and acts as a multi-functional cytokine associated with aggressive tumors. PGI/AMF has been correlated significantly with breast cancer progression and poor prognosis in breast cancer. We show here that ectopic expression of PGI/AMF induced epithelial-to-mesenchymal transition (EMT) in MCF10A normal human breast epithelial cells, and inhibition of PGI/AMF expression triggered mesenchymal-to-epithelial transition (MET) in aggressive mesenchymal-type human breast cancer MDA-MB-231 cells. EMT in MCF10A cells was demonstrated by morphological changes and loss of E-cadherin/ß-catenin-mediated cell-cell adhesion, which is concomitant with the induction of the E-cadherin transcriptional repressor Snail and proteosome-dependent degradation of ß-catenin protein. Molecular analysis showed that PGI/AMF suppressed epithelial marker expressions and enhanced mesenchymal marker expressions. Silencing of PGI/AMF expression by RNA interference in MDA-MB-231 cells induced the reverse processes of EMT including altered cell shape, gain of epithelial marker and reduction of mesenchymal marker, e.g. MET. Taken together, the results demonstrate the involvement of PGI/AMF in both EMT and MET: overexpression of PGI/AMF induces EMT in normal breast epithelial cells and reduction of PGI/AMF expression led to MET in aggressive breast cancer cells. These results suggest for the first time that PGI/AMF is a key gene to both EMT in the initiating step of cancer metastasis and MET in the later stage of metastasis during breast cancer progression.
Introduction
Phosphoglucose isomerase (PGI; EC 5.3.1.9) is responsible for the interconversion of glucose 6-phosphate and fructose 6-phosphate during glycolysis and is involved in glucogenesis (1). PGI is a multifunctional enzyme which serves as autocrine motility factor (AMF) (2), as neuroleukin (3), as maturation factor (4), as sperm antigen-36 (5), and as myofibril-bound serine proteinase inhibitor (6), that is, PGI is a secretable protein that extracellularly behaves as a cytokine following binding to its seven-transmembrane receptor gp78 (7). Aberrations in PGI expression or activities due to mutations or deletions are of significant clinical significance leading to hereditary nonspherocytic hemolytic anemia disease (8). It has been reported that PGI/AMF expression is associated with tumor metastasis and invasion, and its presence in the serum and urine is of prognostic value associated with cancer progression including breast cancer (9-11).
Epithelial and mesenchymal cells express different phenotypic characteristics and functions. Conversion between the epithelial and mesenchymal cells is an essential mechanism for numerous developmental processes (12, 13). Epithelial-to-mesenchymal transition (EMT) is a developmental mechanism implicated in the progression of primary tumors towards metastases. During metastatic conversion, epithelial cells acquire the ability to invade the surrounding tissue and disseminate into secondary organs, and the acquisition of migratory and invasive properties by epithelial cells may be associated with the gain of mesenchymal characteristics and the loss of epithelial features (14, 15). These phenomena were reported to be regulated in part by several growth factors and cytokines, like transforming growth factor-ß, fibroblast growth factor, and hepatocyte growth factor to name but a few (16-18).
EMT and the reverse transition from a mesenchymal to an epithelial phenotype (MET) are fundamental processes of embryonic development, and recently it was suggested that cancer cells probably utilize this MET process during the later stages of metastasis (19, 20). Switching between these two phenotypes allows the escape from the primary tumor as it enables epithelial-like cells to colonize and grow at distant sites to form metastases: EMT occurs during an early stage of cancer progression, while MET is an important step for metastasis to allow colonization of secondary sites (19, 20). Little is known about how EMT and MET occur and/or are regulated during cancer progression or what internal and external signals trigger these changes in cancer cells. Previously it was shown that down-regulation of PGI/AMF expression initiated MET in aggressive HT1080 human lung fibrosarcoma cells (21), and in order to study this phenomenon further we examined the potential dynamic role of PGI/AMF during EMT↔MET processes in human breast cell lines.
Materials and Methods
Reagents and antibodies
Anti-vimentin and -cytokeratin (AE1/AE3) were from DakoCytomation (Carpinteria, CA). Anti-E-cadherin, -ß-catenin, and -fibronectin were purchased from Transduction Laboratories (Lexington, KY). Anti-Snail was from Santa Cruz Biotechnology (Santa Cruz, CA). MG132, anti-ß-actin, -cyclin D1, -glycogen synthase kinase-3ß (GSK-3ß), and -ubiquitin were obtained from Sigma (St. Louis, MO). Anti-PGI/AMF was provided by Pfizer, Inc. (New York, NY). Recombinant human PGI/AMF was created as a glutathione S-transferase fusion protein (22).
Cell culture
MCF10A human breast epithelial cells were obtained from the Karmanos Cancer Institute (21, 23) and were maintained in DMEM-F12 medium supplemented with 0.1 μg/ml cholera toxin, 0.02 μg/ml epidermal growth factor, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% horse serum (21). Human breast cancer cell line MDA-MB-231 was kindly provided by Dr. Erik W. Thompson (Vincent T. Lombardi Cancer Research Center, Georgetown University Medical Center, Washington, D. C.) and were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin. Cultures were maintained at 37°C in an air-5% CO2 incubator.
Transfections
Full-length PGI/AMF cDNA generated by PCR amplification was inserted into a mammalian expression vector pcDNA3.1zeo (+) (Invitrogen, Carlsbad, CA) at a HindIII and an EcoRI sites. MCF10A cells were transfected with PGI/AMF cDNA using Lipofectamine 2000 reagent according to the manufacturer's protocol (Invitrogen). Isolation of single clones of the stable transfectants was accomplished by adding Zeocin (Invitrogen) to the culture medium at 300 μg/ml.
To design specific small interfering RNA (siRNA) targeting PGI/AMF, several sequences from different parts of the human PGI/AMF gene were selected using siRNA Target Finder available at http://www.ambion.com. The siRNA duplexes targeted against PGI/AMF were synthesized by Dharmacon, Inc. (Lafayette, CO). Twenty-four hours after inoculation of MDA-MB-231 cells, siRNA duplex transfections were performed using Lipofectamine 2000 reagent according to the manufacturer's protocol. The efficiency of PGI/AMF silencing was analyzed after 24h of transfection by RT-PCR and immunoblot, and the best siRNA target site for the PGI/AMF gene was selected for generating stable siRNA cell lines. The target sequence for the PGI/AMF siRNA was 5′-AATGGTACCGCGAGCACCGCT-3′. The specificity of the sequence was verified by a BLAST search of the public databases. pSilencer 3.1-H1 neo expression vector (Ambion, Inc. Austin, TX) that produce siRNA targeted against PGI/AMF (named siPGI/AMF) was also prepared according to the manufacturer's protocol. In brief, two oligonucleotides (sense, 5′-GATCCGTGGTACCGCGAGCACCGCTTTCAAGAGAAGCGGTGCTCGCGGTACCATTTTTTGGAAA-3′ and antisense, 5′-AGCTTTTCCAAAAAATGGTACCGCGAGCACCGCTTCTCTTGAAAGCGGTGCTCGCGGTACCACG-3′) were synthesized chemically, and the annealed oligonucleotides were then subcloned into the BamHI and HindIII sites of the pSilencer 3.1-H1 neo vector. MDA-MB-231 cells were transfected using the Lipofectamine 2000 reagent with PGI/AMF siRNA containing plasmids and pSilencer vector control containing no siRNA. Stably expressed single clones were established by G418 selection (700 μg/ml).
Protein extraction
For whole cell lysates, cells were washed twice with phosphate-buffered saline (PBS) and collected by scraping. Cell pellets were lysed in cold precipitation assay buffer (lysis buffer; 20 mM Tris-Hcl, pH 7.4, 150 mM NaCl, 10 mM EDTA, 1% of Nonidet P-40, Triton X-100, sodium deoxycholate) containing 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Samples were clarified by centrifugation (15,000 rpm in 4 °C for 30 min). Mem-PER eukaryotic membrane protein extraction reagent kit (Pierce Biotechnology, Rockford, IL) was used for extracting the membrane proteins, and NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology) were used to separate cytoplasmic and nuclear fractions according to the manufacturer's protocol. Protein concentrations of each sample were determined using Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA).
Immunoblotting and immunoprecipitation
Equal amounts of the proteins were separated on SDS-PAGE gels and transferred to 0.2 μm polyvinylidene fluoride membrane (Osmonics Inc., Minnetonka, MN) at 15 V, 30 mA overnight at 4 °C. The membrane was blocked with 0.1% casein solution in 0.2× PBS for 1 h at room temperature and then incubated with primary antibody and secondary antibodies conjugated with fluorophore (IRDye 800 antibodies; Rockland Immunochemical, Gilbertsville, PA, or Alexa-Fluor 680 antibodies; Molecular Probes, Eugene, OR). Blots were visualized by using the LI-COR Bioscience Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Density of each band was quantitated with NIH Image software.
For immunoprecipitation, cell lysates containing equal amounts of protein were immunoprecipitated at 4°C for 1 h with various antibodies bound to protein G-Sepharose (Amersham Biosciences, Piscataway, NJ). After extensive washing with lysis buffer, proteins were eluted with SDS sample buffer (10 mM Tris, pH 8.0, 1mM ethylene diamine tetraacetic acid, 1.0% SDS, 9.6% glycerol, 0.002% bromphenol blue, 0.2% 2-mercaptoethanol), followed by boiling, and subjected to immunoblotting.
Reverse transcription-PCR (RT-PCR)
Total RNA was extracted using TRIzol Reagent (Invitrogen). The cDNA for PCR template was generated by using First-strand cDNA Synthesis Kit (Amersham Biosciences) as recommended in the manufacturers' protocols. For quantitative evaluation of the amplified product, PCR encompassing 20-40 cycles was preliminarily performed to determine the most suitable number of amplifications for each reaction. Each PCR cycle consisted of: 1 min at 95 °C, 1 min at 60 °C and 2 min at 72 °C for E-cadherin, ß-catenin and ß-actin. PCR-amplified products were electrophoresed in 1 % agarose gel and stained with ethidium bromide. The primer sets were as follows: for E-cadherin, forward: 5′-GCTGGAGATTAATCCGGACA-3′ and reverse; 5′-ACCTGAGGCTTTGGATTCCT-3′; for ß-catenin, forward: 5′-GAAACGGCTTTCAGTTGAGC-3′ and reverse: 5′-CTGGCCATATCCACCAGAGT-3′; for ß-actin, forward: 5′-TGACGGGGTCACCCACACTGTGCCCAT-3′ and reverse: 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′. Each expression was standardized using ß-actin signal as an internal control.
Cell dissociation assay
Cell dissociation assay was performed as described (24). The cells were detached from culture plates using a rubber policeman, passed through Pasteur pipets 30 times, and then fixed in 1% glutaraldehyde in PBS. The extent of cell dissociation was represented by the index Np/Nc, i.e., number of disrupted particles (Np) per total number of cells (Nc) obtained by counting cells.
Promoter reporter assays
The human Snail promoter sequence from −1047 to +66 was amplified by PCR from human HCT-116 genomic DNA isolated by using AccuPrep Genomic DNA extraction kit (Bioneer, Alameda, CA). The fragment was inserted into pGL3 (Promega, Madison, WI) using KpnI and HindIII sites. Snail promoter construct was transiently transfected into the indicated cell lines using Lipofectamine 2000 reagent (Invitrogen). Forty-eight hours after transfection, luciferase assays were performed with the dual-luciferase reporter assay system according to the manufacturer's protocol (Promega). pRL-TK plasmid (Promega) was cotransfected to normalize transfection efficiency, and firefly-luciferase activity was normalized by renilla-luciferase activity.
Immunofluorescence
Cells seeded on coverslips were fixed/permeabilized with ice-cold methanol/acetone (1:1) for 5 min at −20°C. The cells were blocked with 3.0% BSA/PBS for 30 min, then labeled with primary antibodies in 0.1% BSA/PBS overnight at 4°C, followed by incubation with fluorescent secondary antibodies in the dark. To detect nuclei, the cells were co-stained with 4′,6′-diamidino-2-phenylindole (DAPI). Fluorescent images were analyzed in an Olympus fluorescence microscope using a X400 lens.
Statistical analysis
Data are expressed as means ± SD. Comparisons between the groups was determined using unpaired t test. P<0.05 was considered statistically significant.
Results
PGI/AMF induces a mesenchymal-like morphological conversion
The normal human breast epithelial cell line MCF10A was stably expressed with either PGI/AMF (MCF10A-PGI/AMF cells) or control vectors (MCF10A-control cells). The expression of PGI/AMF was confirmed by Western blotting and immunocytochemistry (Fig. 1AB). Parental and MCF10A-control cells exhibited a characteristic epithelial cobblestone-like morphology. In contrast, PGI/AMF transfectants lost their ability to grow as a monolayer and acquired a long spindle-like, fibroblastic morphology (Fig. 1C). This morphologic change implied that the MCF10A-PGI/AMF cells have undergone transition from epithelial cells to mesenchymal-like cells.
Figure 1.
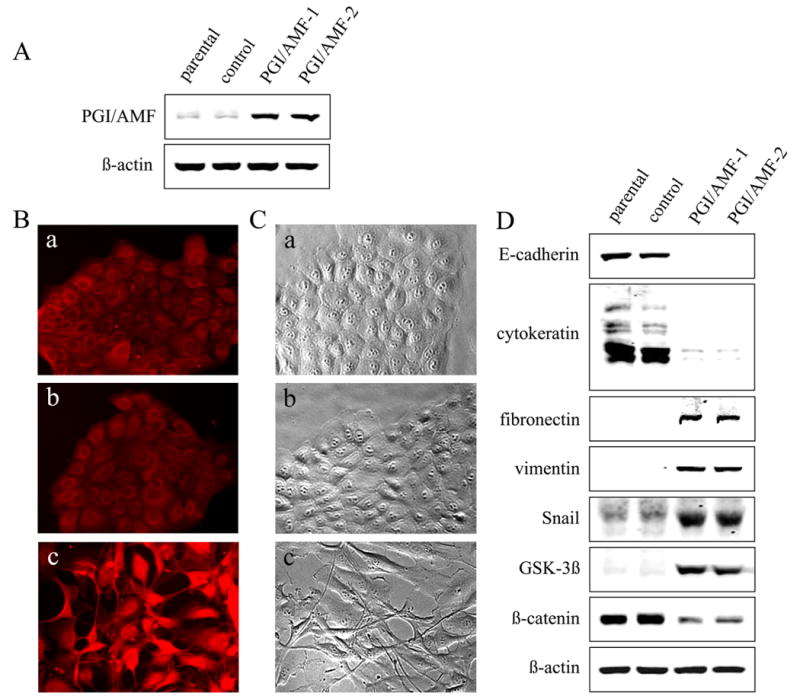
Ectopic expression of PGI/AMF induces a transformation from epithelial cells into fibroblast-like cells and a switch of expression from epithelial to mesenchymal marker proteins. A, MCF10A cells were stably transfected with plasmid containing PGI/AMF cDNA (PGI/AMF) or control plasmid as described in Materials and Methods. Cells were analyzed by immunoblot analysis for PGI/AMF and ß-actin expression. Representative results of three different experiments are shown. B, cells were fixed and processed for immunofluorescence with PGI/AMF antibody. a, MCF10A parental cells; b, control cells; c, PGI/AMF-1 cells. C, cells were cultured on the plastic dishes, and taken photographs under phase contrast microscopy. a, MCF10A parental cells; b, control cells; c, PGI/AMF-1 cells. Similar images were obtained using PGI/AMF-2 cells (data not shown). D, loss of the epithelial markers and gain of the mesenchymal markers. Cells were analyzed by immunoblot analysis for several protein expressions associated with EMT. Representative results of three different experiments are shown.
Next, to determine whether molecular changes associated with EMT could be detected, we examined the expression of epithelial and mesenchymal marker proteins by Western blotting. Consistent with the morphologic changes, the protein expression level of epithelial markers, E-cadherin and cytokeratin, was remarkably decreased upon transfection of PGI/AMF (Fig. 1D). In contrast, the expression of mesenchymal markers, fibronectin and vimentin, was strongly induced in response to PGI/AMF expression (Fig. 1D). In addition, significant inductions of Snail expression, an E-cadherin repressor and also an EMT inducer, were observed in the PGI/AMF cells as compared to the control cells (>7-fold relative to the control). PGI/AMF overexpression up-regulated GSK-3ß expression and down-regulated its downstream target ß-catenin expression. Taken together, these findings indicate that PGI/AMF overexpression is accompanied by the loss of epithelial and the gain of mesenchymal markers, and strongly show that PGI/AMF plays a crucial role in the regulation of the EMT.
Increased PGI/AMF suppresses cell-cell association
Reportedly E-cadherin and ß-catenin could form a complex in adherens junctions in cells, which provides cell-cell adhesion strength (25). In the present study, we revealed that E-cadherin and ß-catenin expressions were decreased by PGI/AMF overexpression (Fig. 1D). Therefore, we tested the translocation of ß-catenin in response to overexpression of PGI/AMF by separating membrane, cytoplasmic, and nuclear fractions of MCF10A cell transfectants. PGI/AMF expression was associated with down-regulation of cytosolic (<0.6-fold) and nuclear (<0.4-fold) expression of ß-catenin, and completely inhibited membrane accumulation of ß-catenin (Fig. 2A). To analyze E-cadherin/ß-catenin complex formation, the cell lysate was immunoprecipitated with E-cadherin antibody and then immunoblotted by E-cadherin or ß-catenin antibody, respectively. The protein amount of E-cadherin in MCF10A-PGI/AMF cells was significantly decreased and E-cadherin-associated ß-catenin was completely eliminated in MCF10A-PGI/AMF cells (Fig. 2B), indicating that E-cadherin/ß-catenin complex formation disappeared. The cell lysate was also immunoprecipitated with ß-catenin antibody followed by immunoblotting with either E-cadherin or ß-catenin antibody, and similar results were obtained (data not shown). Consistent with the disorganization between E-cadherin and ß-catenin, PGI/AMF overexpression weakened the cell-cell association (Fig. 2C).
Figure 2.
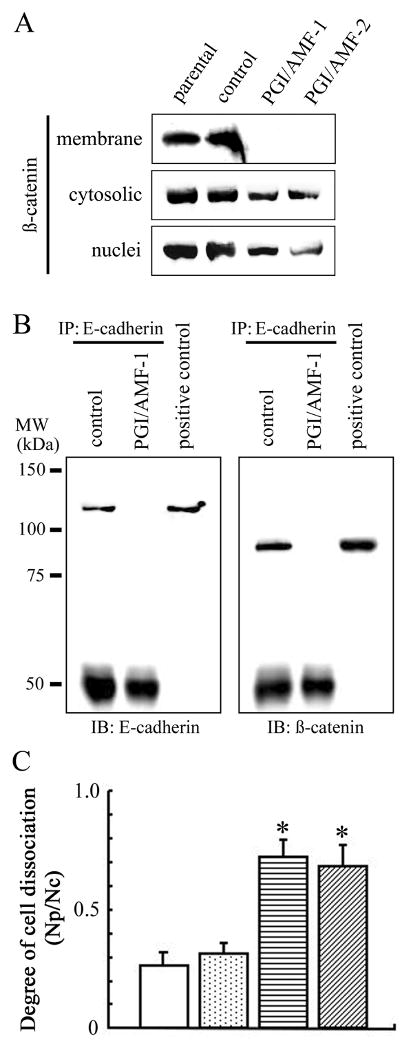
Ectopic expression of PGI/AMF disrupts cell-cell adherens junctions. A, immunoblot analysis for ß-catenin expression in membrane, cytoplasmic, and nuclear fractions of cells. Representative results of three different experiments are shown. B, E-cadherin/ß-catenin complex formation in control and PGI/AMF-overexpressed cells. Cell lysates were immunoprecipitated with anti-E-cadherin followed by immunoblot analysis for E-cadherin (left panel) and ß-catenin (right panel) expression. IP, immunoprecipitation. IB, immunoblotting. MW, molecular weight. Positive control, whole-cell lysate from parental MCF10A cells. Similar results were obtained using PGI/AMF-2 cells (data not shown). C, cell dissociation. Cells were detached from culture plates, passed through Pasteur pipets 30 times and observed under microscope. The degree of cell dissociation (the number of particles (Np)/the number of total cells (Nc)) was calculated by analyzing at least 300 cells from each sample. The data are presented as mean ± SD for triplicate determinations. *, P<0.05 compared with control cells. Open columns, MCF10A parental cells; dotted columns, control cells; horizontal-hatched columns, PGI/AMF-1 cells; diagonal-hatched columns, PGI/AMF-2 cells.
Mechanisms for regulation of E-cadherin and ß-catenin expression in PGI/AMF overexpressed cells
Next, we investigated the mechanisms controlling E-cadherin and ß-catenin expression in PGI/AMF-overexpressed cells. As shown in Fig. 3A, PGI/AMF overexpression decreased mRNA expression level of E-cadherin but did not affect that of ß-catenin. The transcription factor Snail has been described as a direct repressor of E-cadherin expression during development and carcinogenesis (26, 27). We have shown that Snail expression was markedly up-regulated in MCF10A-PGI/AMF cells (Fig. 1D), therefore the promoter activity of Snail was further examined. Snail promoter activity was significantly higher in MCF10A-PGI/AMF cells than in control cells (Fig. 3B). This implies that PGI/AMF leads to an increase in Snail gene transcription, followed by a decrease in E-cadherin expression.
Figure 3.

Mechanisms controlling E-cadherin and ß-catenin expression in PGI/AMF-overexpressed cells. A, cells were analyzed by RT-PCR for E-cadherin, ß-catenin and ß-actin expression. Representative results of three different experiments are shown. B, promoter activities of Snail gene. The human Snail promoter reporter plasmid and control plasmid expressing renilla luciferase were cotransfected into cells. After 48 h, luciferase assays were performed as described in Materials and Methods. All reported firefly-luciferase activities were normalized by renilla-luciferase activities. The data are presented as mean ± SD for triplicate determinations. *, P<0.05 compared with control cells. Open columns, MCF10A parental cells; dotted columns, control cells; horizontal-hatched columns, PGI/AMF-1 cells; diagonal-hatched columns, PGI/AMF-2 cells. C, cells were treated with or without MG132 (50 μM) for 8 h and lysates were analyzed by immunoblot analysis for ß-catenin expression. Representative results of three different experiments are shown. D, cells were treated with or without NH4Cl (25 mM) for 8 h and lysates were analyzed by immunoblot analysis for ß-catenin expression. Representative results of three different experiments are shown.
It has been suggested that ß-catenin is translocated into the nucleus or degraded through a proteasome-mediated pathway while EMT occurs (28). Because no nuclear accumulation of ß-catenin in MCF10A-PGI/AMF cells was observed (Fig. 2A), we studied whether PGI/AMF affects the proteasomal pathway. The proteasome inhibitor MG132 restored ß-catenin expression in MCF10A-PGI/AMF cells, and control and MCF10A-PGI/AMF cells showed a similar expression level of ß-catenin after the treatment with MG132 (Fig. 3C). ß-catenin expression was not affected by the treatment with NH4Cl, an inhibitor of lysosomal function, either in control or MCF10A-PGI/AMF cells (Fig. 3D). On the other hand, reduced E-cadherin expression in PGI/AMF-overexpressed cells was not affected by MG132 or NH4Cl treatment (Fig. 3CD). These data indicate that PGI/AMF down-regulates E-cadherin/ß-catenin complex formation by accelerating Snail promoter activity followed by inhibiting E-cadherin expression, and enhancing ß-catenin protein degradation through the proteasome-dependent pathway.
PGI/AMF treatment stimulates ß-catenin expression
Unexpectedly, PGI/AMF secretion in MCF10A-PGI/AMF cells was decreased (Supplementary Figure 1A), and cell growth and migration that were regulated by secreted PGI/AMF were suppressed (Supplementary Figure 1BC). Based on the data depicted in Fig. 1 and Supplementary Figure 1, we examined whether extracellular addition of PGI/AMF stimulated cellular activities in MCF10A-PGI/AMF cells. First, we examined the protein expressions in the MCF10A cell transfectants treated with or without PGI/AMF, and observed an increased expression of ß-catenin in the presence of PGI/AMF protein (Fig. 4A). Immunofluorescence experiments revealed that ß-catenin staining was localized at areas of cell–cell contact in control cells, and remarkably weaker staining was observed in MCF10A-PGI/AMF cells (Fig. 4Bg-i). Moreover, ß-catenin was slightly concentrated in the nucleus in MCF10A-PGI/AMF cells after PGI/AMF treatment (Fig. 4Bi). PGI/AMF addition didn't affect E-cadherin expressions (Fig. 4Ba-c). As shown in Supplementary Figure 2A, addition of 10 ng/ml of PGI/AMF into MCF10A-PGI/AMF cell culture increased cell growth rate and 1 ng/ml of PGI/AMF showed a little stimulation. We also determined that PGI/AMF treatment on MCF10A-PGI/AMF cells enhanced cell migration (Supplementary Figure 2B). The cell morphology and the EMT-related protein expressions, such as Snail, were not changed by PGI/AMF treatment (data not shown). These findings suggest that PGI/AMF signaling controls ß-catenin expression and its relocation followed by cell growth and motility.
Figure 4.
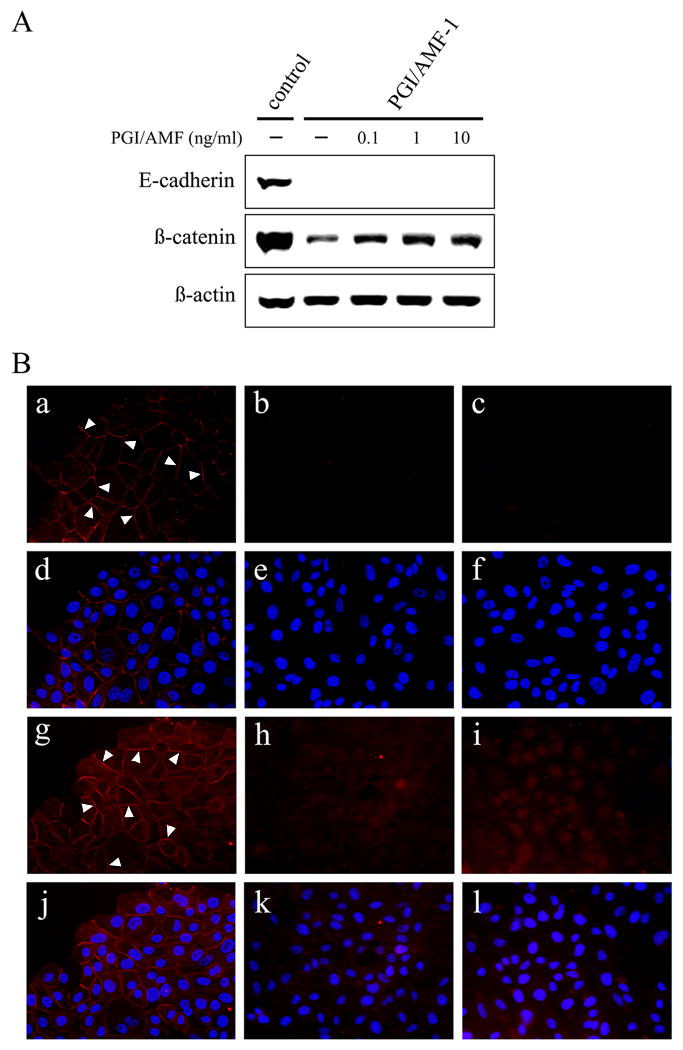
PGI/AMF treatment results in ß-catenin accumulation. A, cells were cultured in the absence or presence of various concentrations of PGI/AMF for 24 h, and then were analyzed by immunoblot analysis for E-cadherin and ß-catenin expression. Representative results of three different experiments are shown. B, E-cadherin (a-f) and ß-catenin (g-l) in cells treated with 10 ng/ml PGI/AMF for 24 h were visualized by immunofluorescence (red color) following staining for nucleus with DAPI (blue color, d-f and j-l). a, d, g, j, MCF10A control cells; b, e, h, k, PGI/AMF-1 cells; c, f, i, l, PGI/AMF-1 cells treated with 10 ng/ml PGI/AMF. Arrow indicates cell junction. Similar images were obtained using PGI/AMF-2 cells (data not shown).
Down-regulation of PGI/AMF leads to mesenchymal-to-epithelial transition in aggressive breast cancer cells
It has been proposed that breast cancer cells might undergo a transition from the epithelial to the mesenchymal phenotype (29, 30). Based on their phenotype and invasiveness, the breast cancer cell lines could be classified as epithelial-like and mesenchymal-like (30). Among the mesenchymal-like cell lines, MDA-MB-231 cells are highly invasive and exhibit mesenchymal cell phenotype. To verify our results shown above, we knocked down PGI/AMF in MDA-MB-231 cells by gene silencing using siRNA. Transfection of PGI/AMF siRNA induced a significant morphological transformation in which the cells acquired a rounded and less elongated shape (Fig. 5A) and markedly reduced the protein level of PGI/AMF in MDA-MB-231 cells (Fig. 5B). Consistent with the morphologic changes, the expression of the mesenchymal marker fibronectin was remarkably reduced and the epithelial marker E-cadherin was strongly induced (Fig. 5B). In addition, ß-catenin expression was also suppressed (Fig. 5B). Moreover, Snail promoter activity was significantly reduced in siPGI/AMF cells compared to control cells which is consistent with E-cadherin and Snail expressions (Fig. 5C). We observed similar effects by using an alternative target sequence siRNA against PGI/AMF (data not shown). These results also support that PGI/AMF is involved in regulating cellular morphology, and decline of PGI/AMF expression could induce MET in cancer cells.
Figure 5.
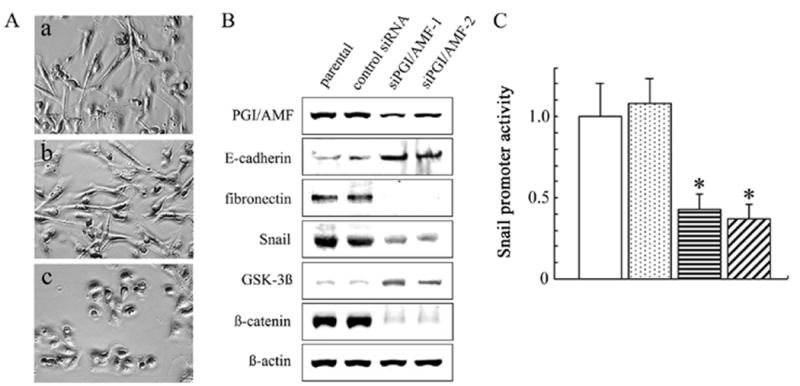
PGI/AMF knockdown in human breast cancer MDA-MB-231 cells. A, MDA-MB-231 cells were stably transfected with plasmid containing PGI/AMF-specific siRNA (siPGI/AMF) or control plasmid (control siRNA) as described in Materials and Methods. Cells were cultured on the plastic dishes, and taken photographs under phase contrast microscopy. a, MDA-MB-231 parental cells; b, control cells; c, siPGI/AMF-1 cells. Similar images were obtained using siPGI/AMF-2 cells (data not shown). B, cells were analyzed by immunoblot analysis for several protein expressions associated with EMT. Representative results of three different experiments are shown. C, promoter activities of Snail gene. The human Snail promoter reporter plasmid and control plasmid expressing renilla luciferase were cotransfected into cells. After 48 h, luciferase assays were performed as described in Materials and Methods. All reported firefly-luciferase activities were normalized by renilla-luciferase activities. The data are presented as mean ± SD for triplicate determinations. *, P<0.05 compared with control cells. Open columns, MDA-MB-231 parental cells; dotted columns, control cells; horizontal-hatched columns, siPGI/AMF-1 cells; diagonal-hatched columns, siPGI/AMF-2 cells.
Discussion
In the present study, we demonstrate that PGI/AMF regulates both EMT and MET in normal epithelial cells and in aggressive breast cancer cells. We indeed show that (i) PGI/AMF cDNA transfection induced morphological change into mesenchymal-like MCF10A cells, (ii) PGI/AMF controlled EMT-related proteins, (iii) PGI/AMF disrupted the E-cadherin/ß-catenin cell adhesion complex, and (iv) PGI/AMF-specific siRNA led to MET an in invasive breast cancer cell line.
Our data revealed that the expression of E-cadherin was decreased in PGI/AMF-overexpressing breast epithelial cells and increased in PGI/AMF knockdown breast cancer cells. Altered expression of cell adhesion molecules are considered to play a critical role in the invasive process. The cell-cell adhesion molecule E-cadherin-mediated cell interactions are essential for embryogenesis and tissue architecture by forming intercellular junction complexes and establishing cell polarization (31), and E-cadherin loss is believed to contribute to both cancer development and progression (32, 33). Control of E-cadherin gene transcription is the main mechanism to account for down-regulation of this protein. Several transcriptional factors have been shown to directly suppress the expression of E-cadherin and promote the acquisition of a mesenchymal phenotype. Among them, it has been highlighted that the up-regulation of Snail is one such example (26, 27). Snail-knockout mice die during gastrulation due to the failure of the E-cadherin downregulation (34). Expression of Snail in epithelial cells decreases E-cadherin levels and induces a complete EMT, and interference with expression leads to increased levels (26, 27). The results from our present study showed that E-cadherin expression was regulated through Snail expression by PGI/AMF, which mediates Snail promoter. Since Snail is upstream of molecules involved in cell migratory and invasive properties (26, 35, 36), these findings might indicate that the Snail/E-cadherin pathway plays an important role in stimulated motility by PGI/AMF (21).
ß-catenin is associated not only with E-cadherin but also with the APC (adenomatous polyposis coli) multi-protein complex, and the ß-catenin signal pathway is required for development and is frequently activated in cancer (37, 38). In the absence of Wnt signals, ß-catenin/APC complex is phosphorylated by GSK-3ß and targeted for ubiquitin/proteasome-mediated degradation. ß-catenin degradation is blocked by binding Wnt ligands to Frizzled receptors and activating Disheveled. Excess ß-catenin enters the nucleus where it cooperates with the transcription factor TCF/LEF and promotes the expression of several target genes, such as cyclin D1. In this study, we reveal that PGI/AMF is associated with GSK-3ß and ß-catenin regulations. Moreover, treatment with PGI/AMF results in accumulation of ß-catenin in the nucleus. Although further investigation is still necessary to address the implication of PGI/AMF and the Wnt/ß-catenin pathway, PGI/AMF is clearly involved in this signaling.
During the sequential in vivo progression of cancer, breast cancer cells undergo phenotype alterations. These alterations include the loss of epithelial-like features, and the gain of more aggressive and invasive mesenchymal-like traits (30). This EMT process in breast cancer cells has been studied, and the cell lines could be classified into epithelial and mesenchymal groups based on their phenotype and invasiveness (30, 39, 40). The epithelial group expresses a high amount of epithelial markers, and are weakly invasive. These “epithelial-like” cells grow as interconnected colonies with a cobblestone-like appearance on plastic. Breast cancer cell lines in this group includes MCF-7 and T-47D; Mesenchymal group cells do not express the epithelial markers found in the “epithelial-like” group, but in contrast exhibited a high level of markers also found in mesenchymal cells. Most of these lines had a fibroblastoid phenotype on plastic and grew as colonies in Matrigel. They are highly invasive in vitro. Breast cancer lines in this “mesenchymal-like” group includes MDA-MB-231 and BT-549. We demonstrated here that PGI/AMF knockdown induced MET on “mesenchymal-like” MDA-MB-231 cells. We also observed similar effects by PGI/AMF knockdown on BT549 cells (data not shown), suggesting that PGI/AMF could be one of potent regulators of MET in breast cancer.
EMT is a process implicated in the normal development and the conversion of early stage cancer to invasive/aggressive phenotype, in which epithelial cell layers lose polarity and cell-cell contact and undergo dramatic cytoskeletal remodeling (12-15). Recent data highlight the conversion of epithelial cancer cells to a more mesenchymal-like state to facilitate cell invasion and metastasis. The reverse conversion MET is thought to be required to generate a proliferative state and form metastases resembling the primary tumor at distant sites (12, 13, 19, 20). This suggests that cellular plasticity, the ability to undergo EMT and subsequently MET in the appropriate microenvironments, is a key feature of a successful metastatic cell. Though some transcription factors such as Snail have been reported in EMT and MET regulation (41, 42), more details about these phenomena still remain to be identified. Here we showed that PGI/AMF contributes to EMT in breast epithelial cells and MET in mesenchymal-like breast cancer cells. We also revealed that PGI/AMF controlled Snail expression which is critical for EMT and MET. Appropriate expression of PGI/AMF may regulate EMT and MET events in each step of breast cancer progression.
Understanding the EMT and/or MET programs may provide novel strategies aimed at preventing the development of metastasis, for example, targeting MET may inhibit the development of solid tumor metastases by trapping disseminated tumor cells in a state of micrometastasis. Here we propose the signaling pathway of PGI/AMF-induced EMT events including morphological changes, cell growth, and cell migration. Endogenous PGI/AMF activates Snail and therefore down-regulates its target gene E-cadherin and other epithelial marker proteins, up-regulates fibronectin and other mesenchymal marker proteins, leading to morphological changes. On the other hand, exogenous PGI/AMF signaling though gp78 inactivates GSK-3ß, resulting in up-regulation of ß-catenin expression, which increases cell growth. Cell migration is also promotes by exogenous PGI/AMF. Although further studies are required to examine the complex network that regulates EMT/MET by PGI/AMF, control of PGI/AMF would provide a new opportunity in the targeting latent micrometastases.
Supplementary Material
Acknowledgments
Grant support: NIH/NCI R01 CA51714 (A. Raz).
We thank V. Powell for her editing of the manuscript.
References
- 1.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–50. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996;56:2960–3. [PubMed] [Google Scholar]
- 3.Gurney ME, Apatoff BR, Spear GT, Baumel MJ, Antel JP, Bania MB, Reder AT. Neuroleukin: a lymphokine product of lectin-stimulated T cells. Science. 1986;234:574–81. doi: 10.1126/science.3020690. [DOI] [PubMed] [Google Scholar]
- 4.Xu W, Seiter K, Feldman E, Ahmed T, Chiao JW. The differentiation and maturation mediator for human myeloid leukemia cells shares homology with neuroleukin or phosphoglucose isomerase. Blood. 1996;87:4502–6. [PubMed] [Google Scholar]
- 5.Yakirevich E, Naot Y. Cloning of a glucose phosphate isomerase/neuroleukin-like sperm antigen involved in sperm agglutination. Biol Reprod. 2000;62:1016–23. doi: 10.1095/biolreprod62.4.1016. [DOI] [PubMed] [Google Scholar]
- 6.Cao MJ, Osatomi K, Matsuda R, Ohkubo M, Hara K, Ishihara T. Purification of a novel serine proteinase inhibitor from the skeletal muscle of white croaker (Argyrosomus argentatus) Biochem Biophys Res Commun. 2000;272:485–9. doi: 10.1006/bbrc.2000.2803. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Tani M, Watanabe H, Nagamachi Y, Niinaka Y, Shiroishi T, Ohwada S, Raz A, Yokota J. The autocrine motility factor receptor gene encodes a novel type of seven transmembrane protein. FEBS Lett. 1999;456:295–300. doi: 10.1016/s0014-5793(99)00966-7. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery CJ, Bahnson BJ, Chien W, Ringe D, Petsko GA. Crystal Structure of Rabbit Phosphoglucose Isomerase, A Glycolytic Enzyme that Moonlights as Neuroleukin, Autocrine Motility Factor, and Differentiation Mediator. Biochemistry. 2000;39:955–64. doi: 10.1021/bi991604m. [DOI] [PubMed] [Google Scholar]
- 9.Gomm SA, Keevil BG, Thatcher N, Hasleton PS, Swindell RS. The value of tumour markers in lung cancer. Br J Cancer. 1988;58:797–804. doi: 10.1038/bjc.1988.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann M, Kappl A, Lang T, Brand K, Siegfried W, Paterok E. The diagnostic validity of the serum tumor marker phosphohexose isomerase (PHI) in patients with gastrointestinal, kidney, and breast cancer. Cancer Invest. 1990;8:351–6. doi: 10.3109/07357909009012053. [DOI] [PubMed] [Google Scholar]
- 11.Filella X, Molina R, Jo J, Mas E, Ballesta AM. Serum phosphohexose isomerase activities in patients with colorectal cancer. Tumour Biol. 1991;12:360–7. doi: 10.1159/000217737. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 13.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 16.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–70. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 17.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 18.Prindull G, Zipori D. Environmental guidance of normal and tumor cell plasticity: epithelial mesenchymal transitions as a paradigm. Blood. 2004;103:2892–9. doi: 10.1182/blood-2003-08-2807. [DOI] [PubMed] [Google Scholar]
- 19.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 20.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 21.Funasaka T, Hu H, Yanagawa T, Hogan V, Raz A. Down-regulation of phosphoglucose isomerase/autocrine motility factor results in mesenchymal-to-epithelial transition of human lung fibrosarcoma cells. Cancer Res. 2007;67:4236–43. doi: 10.1158/0008-5472.CAN-06-3935. [DOI] [PubMed] [Google Scholar]
- 22.Haga A, Niinaka Y, Raz A. Phosphohexose isomerase/autocrine motility factor/neuroleukin/maturation factor is a multifunctional phosphoprotein. Biochim Biophys Acta. 2000;1480:235–44. doi: 10.1016/s0167-4838(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 23.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]
- 24.Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and beta catenin is not required for the shift. J Cell Biol. 1995;131:1839–47. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 26.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 27.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 28.Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. doi: 10.1023/a:1006200102166. [DOI] [PubMed] [Google Scholar]
- 29.Ackland ML, Newgreen DF, Fridman M, Waltham MC, Arvanitis A, Minichiello J, Price JT, Thompson EW. Epidermal growth factor-induced epithelio-mesenchymal transition in human breast carcinoma cells. Lab Invest. 2003;83:435–48. doi: 10.1097/01.lab.0000059927.97515.fd. [DOI] [PubMed] [Google Scholar]
- 30.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–89. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 31.Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 1999;21:211–20. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun. 1999;2:77–85. doi: 10.1006/mcbr.1999.0155. [DOI] [PubMed] [Google Scholar]
- 33.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25:621–8. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SC, Hung MC. Cytoplasmic/nuclear shuttling and tumor progression. Ann N Y Acad Sci. 2005;1059:11–5. doi: 10.1196/annals.1339.002. [DOI] [PubMed] [Google Scholar]
- 36.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 37.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 38.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 39.Thompson EW, Paik S, Brünner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, Martin GR, Dickson RB. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–44. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 40.Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31:325–35. doi: 10.1007/BF00666165. [DOI] [PubMed] [Google Scholar]
- 41.Olmeda D, Jordá M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 2007;26:1862–74. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- 42.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


