Abstract
Objectives
Irritable bowel syndrome (IBS) clusters in families, but previous studies documented family history only from patients. We have shown that patient–relative agreement on IBS status is poor. Thus, we conducted a family case–control study with direct survey of symptoms from family members to better quantitate the aggregation of IBS in families. The aims of this study were to (i) compare the prevalence of IBS in case-relatives with control-relatives, and (ii) determine whether gender, relationship, predominant symptom, and environmental risk factors affect familial aggregation.
Methods
Outpatients with IBS, matched controls, and their first-degree relatives completed a bowel symptom questionnaire. Percent of cases and controls with a family history were compared and odds ratios were computed using χ2 -test; recurrence risks to relatives were computed using logistic regression and generalized estimating equations.
Results
Data were collected from 477 cases, 297 controls, 1,492 case-relatives, and 936 control-relatives. Probands had a median age of 50 and 78% were women. 50% of case and 27% of control families had at least another relative with IBS yielding an odds ratio of 2.75 (95% CI: 2.01–3.76, P < 0.0001). When aggregation estimates were reevaluated stratifying by relative relationship or proband gender, generational and gender effects were not observed. Familial clustering by bowel habit was weakest for diarrhea, and strongest for alternating bowel habits.
Conclusions
IBS aggregates strongly in families. The strength of the association does vary somewhat by relationship to proband, but the lack of association in spouses supports either a possible genetic etiology or a shared household environmental exposure as an underlying cause of IBS.
Introduction
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal diagnoses seen by primary care providers and gastroenterologists (1), but the pathophysiology of IBS remains poorly understood. Previous studies have shown that patients with IBS frequently report a positive family history of IBS (2,3). The underlying question of great interest is whether the clustering of IBS in families is due to shared environmental risk factors among family members or due to shared genes. Twin studies of IBS and abdominal symptoms support the concept that IBS has both genetic and environmental contributors (4–8). Because of these findings as well as other advances in the field of genetics, there is great interest in discovering disease susceptibility loci for IBS. Several candidate gene case–control association studies have been performed to date (9), but a definitive disease-causing gene or set of genes for IBS has yet to be identified and the search for an IBS gene continues. However, gene discovery is not an easy task, and may be more difficult for a clinically and likely etiologically heterogeneous disorder such as IBS.
Further studies of families with and without IBS would be extremely helpful in elucidating the genetic and environmental contributors to IBS. The available literature of family studies of IBS has several weaknesses and knowledge gaps. First, these studies have typically collected data regarding family history of IBS from the patients and not by directly asking the relatives about their symptoms and medical diagnoses (2,10). Use of proband-provided information about family history of IBS may lead to inaccurate estimates of aggregation, as bowel habits may not be common knowledge among family members. We have recently shown in a small family study that agreement between proband-reported IBS status for relatives and relative-reported IBS was poor with a κ statistic of 0.32 and 0.14 for case and case-relatives and control and control-relatives, respectively (3). Although this study showed familial aggregation, the small sample size limited firm conclusions regarding estimates of aggregation and the role symptom subtype and other risk factors have in the development of familial IBS. Thus, the true unbiased estimate of familial aggregation of IBS remains largely unknown and factors influencing familial aggregation of IBS remain to be determined. Detailed construction and study of families are needed to determine whether future genetic studies are warranted for the study of IBS.
Our broad hypothesis is that IBS is caused by both environmental and genetic risk factors, and thus, it represents a multifactorial, complex genetic disease. To support this hypothesis, we need to first observe IBS aggregates in families. To this end, we performed a large-scale family case–control study collecting bowel symptom data from cases, controls, and first-degree relatives (FDRs). The primary aim of this study was to determine whether IBS aggregates in families by comparing the prevalence of IBS in case-relatives with control-relatives in a large family-based case–control study and to obtain quantitative estimates of aggregation and recurrence risks to relatives. Confirmation of familial aggregation of IBS is important because if it is not observed, there would be little justification for pursuing further genetic studies in IBS.
Methods
Study design
The study used a family-based case–control study design conducted at a major medical center in the Upper Midwest of the United States. This project was approved by the Mayo Clinic Institutional Review Board.
Case and control (proband) recruitment
The cases of this study, prospectively recruited between July 2004 and June 2007, were adult outpatients with IBS, ages 18–70, seen at a single medical institution. Cases were identified by reviewing their indication for an appointment within the gastroenterology division, by direct referral by a treating physician, through recruitment at an IBS patient education class, or by using a research database of medical records at Mayo Clinic. This database is updated daily and is programmed to search for patients with IBS listed among their final diagnoses during the recruitment period. Controls were frequency matched to cases on age-decile, gender, and race. Controls were prospectively recruited between February 2005 and July 2007 from patients seen in the Division of General Internal Medicine and by identification of the research database, with recruitment purposefully lagging behind cases to allow for frequency matching. Recruitment of case and control probands was performed in person if being seen in clinic or by mail if identified through the computerized database. Potential controls were also asked to complete an initial one-page screening questionnaire to identify exclusion criteria before signing consent. Case and control probands were excluded if they were under 18 years of age, had difficulty reading or speaking English, were non-US residents, were prisoners, had dementia or mental retardation, had a condition requiring a legal guardian, or were adopted. Case and control probands were also excluded if they had a current diagnosis of cancer or had another gastrointestinal diagnosis (e.g., inflammatory bowel disease, celiac sprue, history of major abdominal surgery) that could produce IBS-like symptoms. Formal chart review of case and control probands was performed to confirm clinical diagnosis of IBS and rule out alternate diagnoses in cases, to exclude clinical diagnosis of IBS in controls, to screen for exclusion criteria, document relevant medical diagnoses, document social history, and review results of all gastrointestinal testing. Controls were excluded if they reported a diagnosis of IBS or if they met Rome I or II diagnostic criteria (11,12) for IBS on either the screening or study questionnaire. If consented, all case and control probands were asked to complete a self-reported bowel symptom questionnaire, provide contact information for at least one FDR, and donate 20 ml blood. Only cases and control probands who met inclusion criteria and completed all aspects of the study were included in the final sample.
Relative recruitment
Relatives that the proband provided permission to contact were mailed recruitment letters. All FDRs younger than age 18 years were excluded from contact. Mailed recruitment packets included an introductory letter, a study brochure explaining the study, a consent form, study questionnaire, and a postage-paid return envelope. The recruitment letter included a checkbox with the option to not participate. Nonresponders to mailings were mailed up to two reminder letters, for a total of three contacts.
All case and control proband participants received $15 remuneration and their choice of a health book for time spent for the study. All relative participants received $5 remuneration and their choice of a health book for their time expended for the study.
Study questionnaires
The screening questionnaire completed by controls before recruitment was a double-sided one-page questionnaire that asked about a past diagnosis of IBS and validated questions pertaining to the Rome diagnostic criteria (12). The main proband (case and control) study questionnaire contained items regarding demographics, gastrointestinal symptoms, family structure, family history of IBS, social history, medical history, dietary history, somatization, anxiety, and depression. The questionnaire was based on the following previously developed and validated survey instruments: Talley Bowel Disease Questionnaire (13), Psychosomatic Symptom Checklist (14), Beck Anxiety Index (15), Center for Epidemiological Studies Depression Scale (16), and IBS-Quality of Life (IBS-QoL) (17,18). The relative study questionnaire was a shorter version of the proband questionnaire that contained bowel symptom, somatization, and depression questions; excluded the anxiety, dietary, and IBS-QoL items; and contained abridged sections regarding family history of IBS, medical history, and social history.
Family contact information
To allow us to collect medical information on relatives (e.g., IBS status) without violating US federal privacy regulations regarding protected health information (Health Insurance Portability and Accountability Act), we collected the names and contact information for FDRs on a separate form from the study questionnaire with blank lines for name and address of family members.
Study definitions
For the primary analysis, FDRs were considered to be “affected” with IBS if they met Rome I or II criteria for IBS (12,19) or if they reported receiving a medical diagnosis of IBS, and did not report an alternate gastrointestinal diagnosis such as ulcerative colitis, Crohn's disease, microscopic colitis, lymphocytic colitis, collagenous colitis, or celiac sprue. Because they are not blood-related to probands (cases or controls), spouses were not included in calculations determining family history of probands. IBS subtypes were based on the supportive symptom combination as suggested by the Rome II criteria (12). Constipation-predominant IBS (C-IBS) was defined by meeting either Rome I or II criteria, but endorsing one or more constipation symptoms (less than three bowel movements per week, hard stools, straining) and no diarrhea symptoms (more than three bowel movements per day, loose stools, urgent stools). Conversely, diarrhea-predominant IBS (D-IBS) was defined by meeting either Rome criteria, and endorsing one or more diarrhea symptoms but no constipation symptoms. Mixed-IBS (M-IBS) was defined as meeting either Rome but not meeting criteria for C-IBS or D-IBS. Functional constipation (FC), functional diarrhea (FD), and functional bloating (FB) were also based on Rome II definitions (12). FC was defined as not meeting either Rome criteria for IBS, endorsing two or more constipation symptoms, and not endorsing loose stools. FD was defined as not meeting either Rome criteria for IBS, endorsing frequent loose stools, and no/rare abdominal pain. Functional bloating was defined as not meeting either Rome criteria for IBS, but endorsing frequent bloating or distension. Unspecified functional bowel disorder was reserved for cases who did not meet Rome criteria for IBS, endorsing some Rome symptoms, but did not meet criteria for FC, FD, or functional bloating. Abuse was defined by reporting verbal or physical abuse, or abuse was reported by the patient on chart review of their medical record. To capture lifetime and not only current experiences, we defined depression and anxiety by questionnaire reports of these diagnoses in their medical history.
Statistical analysis
Comparison of demographics and risk factors between the case probands and the control probands was completed with t-test for continuous variables and χ2 -tests for categorical variables.
Initially, a 2×2 table was generated comparing the numbers of cases with and without at least one other affected relative with IBS, to the number of controls with and without at least one other affected relative with IBS, based on relative self-report data (and not proband-provided data). The proportion (%) of cases and proportion (%) of controls with a positive family history of IBS were calculated and were compared using the χ2 -test. Odds ratios (ORs) with 95% confidence intervals were computed.
To determine if type of relationship affects aggregation, we created similar 2×2 tables for different classes of relatives. Differences in the percents were tested using logistic regression analysis, where relative IBS status was the dependent variable and proband case status was the independent variable. Analyses were performed for the following classes of relatives: (i) parents, (ii) siblings, (iii) children, (iv) mothers only, (v) fathers only, (vi) sisters only, (vii) brothers only, (viii) daughters only, and (ix) sons only. For example, the proportion of affected case-mothers was compared with the proportion of affected control-mothers, and so forth for each class of relatives listed above. For classes of relatives with more than one relative for each case or control (e.g., each proband can have only one mother but multiple sisters), a correction for relatedness was made in the analysis using generalized estimating equations. Nominal P values from all analyses are reported. All analyses were completed using SAS version 9.1 (SAS Institute, Cary, NC).
Results
Participation and subject characteristics
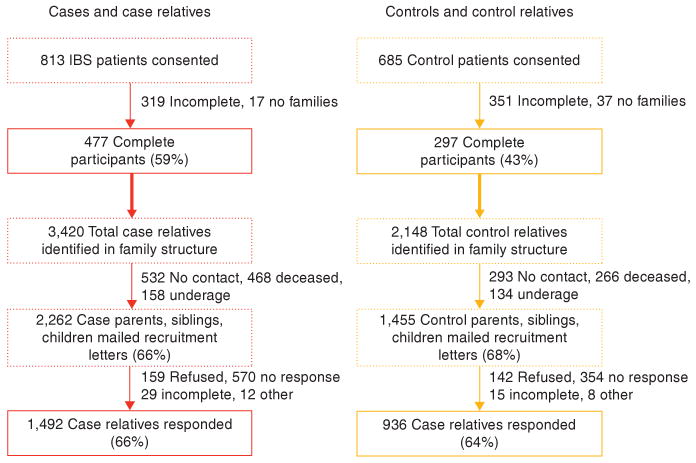
Recruitment of cases, controls, case-relatives, and control-relatives is summarized in the flow diagram illustrated in Figure 1. A total of 813 patients with IBS were consented, of whom 477 (59%) completed all aspects of the study. The distribution of predominant symptom among cases was 10% C-IBS, 27% D-IBS, 33% M-IBS, and 30% other (7% FC, 1% FD, 14% unspecified functional bowel disorder, 8% functional bloating or unspecified). Overall, 3,196 potential controls were contacted (522 in person, 2,674 by mail), of whom 685 control patients were consented, with 297 (43%) completing all aspects of the study. The final case and control groups were comparable to one another regarding age (median age, 49.5 and 50.0 years, respectively), marital status (74% vs. 73% married, 15% vs. 17% single, 13% vs. 10% other), and race (99% Caucasian for both). There was a greater preponderance of women in the cases compared with the controls (83% vs. 70%, P < 0.0001), cases had slightly lower levels of education (86% vs. 91% college and/or beyond, 13% vs. 8% high school or less, P = 0.0026), and cases had higher degrees of somatization with median scores of 1.1 compared with 0.4 in controls (P < 0.0001). Cases reported a median IBS-QOL score of 24.3 whereas controls reported a median score of 0.0 (P < 0.0001).
Figure 1.
Proband and relative recruitment flowchart.
These cases identified 3,420 family members (alive and dead) in their family structures. The average family size was seven family members per case-family. Of the 3,420 total family members, 468 (14%) were dead, 158 (5%) were underage, and 532 (16%) were not permitted contact. Of the 2,262 case-relatives mailed a recruitment letter, 1,492 (66%) agreed to participate. 2,148 control-relatives in total were identified in their family structure (average of seven family members per control-family). Of the total control-relatives, 266 (12%) were dead, 134 (6%) were underage, and 293 (14%) were not permitted contact. A total of 1,455 (68%) control-relatives were mailed recruitment letters, with a 64% overall response rate in control-relatives. Thus, contact and recruitment rates were similar in control-relatives as case-relatives (NS). The age, gender, and relationship profiles of the case-relatives and control-relatives are shown in Table 1. No differences regarding these characteristics were observed between the two groups.
Table 1. Characteristics of participating relatives by case or control status.
| Case-relatives (n =1,492) | Control-relatives (n = 936) | P value | |
|---|---|---|---|
| Age, median (range) | 51.2 (18.2, 94.0) | 51.2 (18.0, 93.2) | 0.9050 |
| Female (%) | 57 | 58 | 0.7657 |
| Relationship | |||
| Mother, n (col %) | 239 (16) | 137 (15) | 0.2755 |
| Father, n (col %) | 150 (10) | 88 (9) | 0.5455 |
| Sister, n (col %) | 371 (25) | 264 (28) | 0.0864 |
| Brother, n (col %) | 239 (16) | 165 (18) | 0.2972 |
| Daughter, n (col %) | 211 (14) | 118 (13) | 0.3068 |
| Son, n (col %) | 146 (10) | 77 (8) | 0.2355 |
| Spouse, n (col %) | 136 (9) | 87 (9) | 0.8523 |
To determine whether there were biases regarding participation by the relatives, we performed comparisons of limited proband-derived information about participating relatives with nonparticipating relatives. No difference in age was observed between the participating and nonparticipating case-relatives, nor were differences in age observed between participating and nonparticipating control-relatives. However, fathers, brothers, and sons were less likely to participate and thus, male gender was associated with nonparticipation in both case-relatives and control-relatives (P < 0.0001). In addition, participants were slightly more likely to have IBS than nonparticipants (21% vs. 16% in case-relatives, P = 0.0069 and 5% vs. 3% in control-relatives, P = 0.0125).
Familial aggregation of IBS
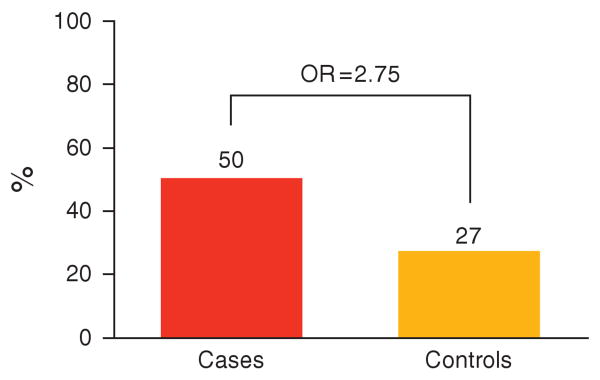
In total, 240 of 477 (50%) cases compared with 80 of 297 (27%) controls had at least one other family member with IBS by self-report (excluding the probands and spouses). Comparison of these proportions yielded a statistically significant OR of 2.75 (95% CI: 2.01–3.76; Figure 2). Overall, 327 of 1,309 (25%) case-relatives reported IBS, whereas only 97 of 820 (12%) of control-relatives reported having IBS. Comparison of these two proportions also yielded a statistically significant OR of 2.48 (95% CI: 1.90–3.25, P < 0.0001). On average, there were 0.7 affected relatives per case-family, and 0.4 affected relatives per control-family.
Figure 2.
Proportion of case and control probands with a family history of irritable bowel syndrome (IBS). OR, odds ratio.
Because 30% of our study sample included individuals with an IBS diagnosis but only met Rome criteria non-IBS functional bowel disorders, familial aggregation estimates were recalculated for cases meeting Rome criteria for IBS only. Of 330, 175 (53%) cases who met Rome criteria for IBS had another family member meeting Rome criteria for IBS or reporting a physician diagnosis of IBS, with 245 of these 928 (26%) case-relatives reporting IBS by this definition. This yielded an OR of 3.06 (95% CI: 2.19–4.28) for family history and an OR of 2.78 (95% CI: 2.10–3.69) for relatives. When definition of IBS was restricted purely to relatives meeting Rome criteria for IBS, 139 of the 330 (42%) Rome-IBS cases had another family member with IBS by Rome criteria, and 174 of 928 (19%) of their relatives reported Rome criteria-positive IBS. Compared with control relatives, this yielded an OR of 3.20 (95% CI: 2.22–4.61) and 2.98 (95% CI: 2.17–4.09) for family history and for relatives overall. In summary, as we restricted the definition of IBS from requiring either a clinical diagnosis of IBS or meeting Rome criteria for IBS among the probands (cases or controls) and their relatives, familial aggregation was consistently observed with the ORs increasing with each additional restriction.
To assess whether the aggregation estimate may have been influenced by nonparticipating relatives, we used data provided by probands regarding missing relatives to recalculate aggregation estimates. Using proxy information when self-report data were not available, we observed that 214 (45%) of cases had another family member with IBS compared with 44 (15%) of controls, yielding an OR of 4.68 (95% CI: 3.24–6.76, P < 0.0001).
Does aggregation vary by relationship to proband?
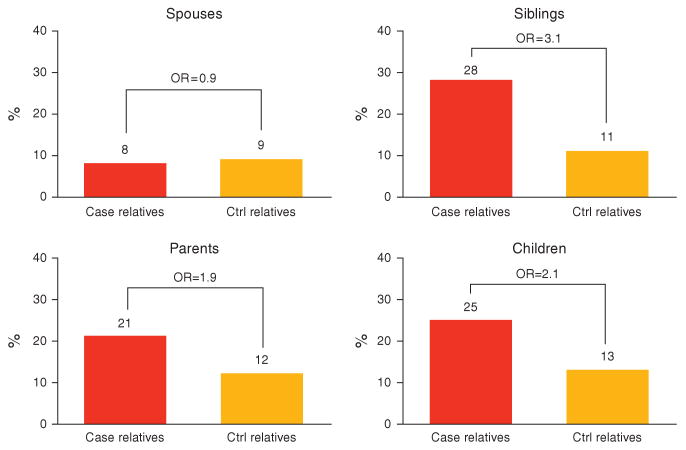
As shown in Figure 3, when the relatives are stratified by relationship to the proband (case or control), the degree of aggregation does vary by relationship. Importantly, there was no aggregation of IBS in spouses, who are not blood-related to the probands. Otherwise, all broad categories of relationships (parents, siblings, and children) yielded statistically significant ORs. However, in general, the ORs were largest in siblings (OR 3.12, 95% CI: 2.19–4.46), then children (OR 2.12, 95% CI: 1.18–3.82), and then parents (OR 1.91, 95% CI: 1.20–3.04). When specific relationships were examined, all ORs remained statistically significant, except for fathers, with ORs of 2.01 (95% CI: 1.18–3.41) for mothers, 1.69 (95% CI: 0.52–5.48) for fathers, 3.08 (95% CI: 2.05–4.63) for sisters, 4.21 (95% CI: 1.84–9.65) for brothers, 1.97 (95% CI: 1.03–3.75) for daughters, and 3.24 (95% CI: 1.06–9.92) for sons. For spouses, the OR was 0.89 (95% CI: 0.34–2.31).
Figure 3.
Aggregation estimates by relationship to proband. OR, odds ratio.
Does aggregation vary by proband gender?
When the analysis was repeated stratifying the data based on proband (case or control) gender, familial aggregation persisted irrespective of proband gender with an OR of 2.17 (95% CI: 1.61–2.92) for female probands, and 2.82 (95% CI: 1.72–4.62) for male probands. The ORs remained statistically significant for siblings irrespective of proband gender. However, for female probands, the OR for children lost statistical significance with an OR of 1.71 (95% CI: 0.89–3.27), although the OR remained significant for children for male probands (O = 4.12, 95% CI: 1.04–16.29). In addition, the OR for parents lost statistical significance for male probands (OR = 1.63, 95% CI: 0.59–4.51) but remained significant for female probands (OR = 1.94, 95% CI: 1.14–3.30).
Does aggregation vary by predominant bowel type?
To determine whether familial clustering of IBS was specific to predominant bowel type, we stratified probands (cases and controls) by predominant bowel habit. Of the 130 cases with diarrhea (D-IBS or FD), 62 had at least one other relative affected with IBS (88 of their case-relatives), of which 21 (16% of 130) had diarrhea as well (27 of their case-relatives). Of the 81 cases with constipation (C-IBS or FC), 38 had at least one other relative affected with IBS (52 case-relatives), of which 27 (33% of 81) had constipation as well (31 of their case-relatives). Of the 220 cases with mixed bowel habits (M-IBS or unspecified functional bowel disorder), 118 had at least one other relative affected with IBS (156 of their case-relatives), of which 151 (69% of 220) had mixed bowel habits (239 of their case-relatives).
Stratifying by predominant bowel type, we recalculated familial aggregation estimates (Table 2). Among probands reporting diarrhea, 16% had a positive family history with at least one other family member with D-IBS or FD, compared with 10% of controls (P = 0.10). This trend of familial aggregation by predominant bowel type was observed for constipated-IBS (33% vs. 23%, P = 0.05) and M-IBS (68% vs. 59%; P = 0.02). Similarly, when the case-relatives of a proband with diarrhea were compared with control-relatives, case-relatives were twice as likely to have diarrhea compared with control-relatives (P = 0.05). Constipation and mixed bowel habits were also more common in case-relatives than control-relatives (P = 0.02 and 0.0004 for constipation and mixed bowel habits, respectively), but the magnitude of difference was less than two-fold.
Table 2. Familial aggregation estimates by specific bowel habit.
| No. of families | % With positive family history of same bowel habit | % Relatives with same bowel habit | ||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Case-family (%) | Control-family (%) | OR (95% CI) | Case-relatives (%) | Control-relatives (%) | ORa (95% CI) | |
| Diarrhea proband | 130 | 297 | 16 | 10 | 1.65 † (0.91–3.00) | 7 | 4 | 1.80* (1.01–3.23) |
| Constipated proband | 81 | 297 | 33 | 23 | 1.72* (1.00–2.93) | 14 | 9 | 1.70* (1.10–2.61) |
| Alternating proband | 220 | 297 | 68 | 59 | 1.53* (1.06–2.20) | 41 | 31 | 1.52* (1.52–1.92) |
CI, confidence interval; OR, odds ratio.
P = 0.10.
P < 0.05.
Accounts for familial relatedness/dependency.
Can aggregation of IBS be explained by other environmental or household risk factors?
A greater proportion of cases reported intestinal infections (such as Campylobacter, Salmonella, E. coli, etc.) compared with controls (14% vs. 3%, P < 0.05), but only 7% of cases reported IBS symptom onset after an infection; 12% of cases reported symptom onset after a surgery (Table 3). Cases reported greater frequency of food sensitivity (70% vs. 23%, P < 0.05), abuse (35% vs. 13%, P < 0.05), as well as past diagnoses of depression or anxiety (48% vs. 13%, P < 0.05). Of the case probands indicating a history of abuse, 41% reported emotional abuse, 55% reported physical and emotional abuse, and 3% reported physical abuse only. Of the control probands indicating a history of abuse, 67% reported emotional abuse, 31% reported physical and emotional abuse, and 3% reported physical abuse only. These differences between cases and controls persisted after adjustment for somatization levels.
Table 3. Environmental risk factors among cases and controls, and relatives affected with IBS and unaffected relatives.
| Cases (n = 477) | Controls (n = 297) | P value | Affected relatives (n = 424) | Unaffected relatives (n =1,705) | P value | |
|---|---|---|---|---|---|---|
| Cholecystectomy, n (%) | 122 (26) | 20 (7) | < 0.0001 | NA | NA | |
| Onset after surgery, n (%) | 55 (12) | 4 (7) | 0.2851 | NA | NA | |
| Intestinal infections, n (%) | 66 (14) | 10 (3) | < 0.0001 | 37 (9) | 83 (5) | 0.0014 |
| Onset after infection, n (%) | 34 (7) | 6 (11) | 0.3747 | 2 (3) | 6 (5) | 0.6100 |
| Abuse, n (%) | 162 (35) | 36 (13) | < 0.0001 | 139 (35) | 400 (25) | < 0.0001 |
| Diet allergy/sensitivity, n (%) | 326 (70) | 67 (23) | < 0.0001 | NA | NA | |
| Dairy, n (%) | 217 (46) | 23 (8) | < 0.0001 | NA | NA | |
| Caffeinated drinks, n (%) | 219 (46) | 18 (6) | < 0.0001 | NA | NA | |
| Carbonated drinks, n (%) | 185 (39) | 18 (6) | < 0.0001 | NA | NA | |
| Fruits and vegetables, n (%) | 149 (31) | 6 (2) | < 0.0001 | NA | NA | |
| Sweetened drinks, n (%) | 98 (21) | 4 (1) | < 0.0001 | NA | NA | |
| Depression or anxiety, n (%) | 230 (48) | 39 (13) | < 0.0001 | 180 (44) | 361 (22) | < 0.0001 |
IBS, irritable bowel syndrome; NA, not available.
When comparing affected relatives (n = 424) with unaffected relatives (n = 1,705), affected relatives were again more likely to report intestinal infections (9% vs. 5%, P = < 0.05), abuse (35% vs. 25%, P < 0.05), and depression or anxiety diagnoses (44% vs. 22%, P < 0.05) compared with unaffected relatives. The type of abuse reported was similar between case-relatives and control-relatives: 64% vs. 67% emotional abuse, 34% and 30% with both emotional and physical abuse, respectively. However, the association for abuse did not persist after adjustment for somatization level using multivariable logistic regression (P = 0.2237). Median somatization levels did differ between affected and unaffected relatives (1.0 vs. 0.6, P<0.05). Symptom onset after an infection did not differ between affected and unaffected relatives (3% vs. 5%, P=0.61).
Discussion
After the sequencing of the entire genome with the Human Genome Project, the lure of a genetic basis for IBS has led to several investigators conducting candidate gene studies searching for an “IBS gene” (9). However, to date, a disease susceptibility locus for IBS has not yet been identified. In fact, the most commonly studied polymorphism in IBS, the 5-HTT LPR polymorphism in the serotonin transporter gene (SLC6A4), was recently shown in a meta-analysis to not be associated with IBS or its subtypes (20). Before allocating resources hunting for an elusive gene hiding among more than 30,000 potential genes, careful thought must be undertaken to determine (i) whether such a search is warranted, and (ii) if a search is warranted, what the best approach for finding the gene would be. One of the key initial steps in determining whether a gene search is warranted is establishing familial aggregation of the disease or phenotype of interest (21). Thus, the goal of this study was not only to collect bowel symptom data from family members to confirm familial aggregation of IBS, but also to determine whether in-depth studies of such families with IBS could also provide insight into identifying relevant risk factors that could explain or at least contribute to the development of IBS in families.
This study confirmed familial clustering of IBS. By relative self-report, 50% of cases and 27% of controls had at least one other family member with IBS. When relatives (and not probands) were the unit of interest, we observed that 25% of case-relatives and only 12% of control-relatives were affected with IBS. We have thus shown that familial IBS is approximately two times more common in patients with IBS than in individuals without IBS, and this observation held true regardless of whether we restricted IBS definition to those meeting formal Rome criteria for IBS and regardless of predominant symptom (i.e., constipation, diarrhea, or both). Furthermore, IBS appeared most common in siblings, then children, and then parents. The scientific relevance of this observation is unclear, as this could be consistent with a genetic effect or a household or an environmental effect. It is also possible that this finding was the result of relative participation or survival bias. A proband can only have one mother and father, but may have multiple siblings or children. Parents are also older and thus more likely to be dead or ill (and unable to participate) than siblings or children. However, in examining participation rates by relative status, rates were comparable by generation (40% in parents, 49% in siblings, and 41% in children). Higher risk to siblings than to off-spring could suggest a recessive or X-linked basis for transmission (22), but would require more formal testing to prove or disprove this. One might argue that children are younger and may not have yet developed IBS symptoms yet, although this explanation does not hold true for parents.
When we examined the role of gender of the proband and the relative, we did not observe any gender effect on familial aggregation. Familial clustering appeared to be as strong in female probands as in male probands. This is an important observation as IBS has been reported to be more common in women than men (23). From a household or genetic standpoint, however, both genders appear at risk for developing IBS. Examining the role of gender from the relative-perspective, and despite expectations that the strength of familial aggregation would be stronger in female relatives, we did not observe this trend in relationship comparisons. Rather, we observed that the strength of the association was stronger in brothers compared with sisters (OR 4.21 vs. 3.08) and in sons compared with daughters (OR 3.24 vs. 1.97), and only weaker in fathers compared with mothers (OR 1.69 vs. 2.01). Reassuringly, as spouses are not blood-related to probands, we observed that case-spouses were not at higher risk for IBS than control-spouses.
There are several potential limitations and weaknesses of our quantitative estimate of familial aggregation that merit further discussion. First, our estimate was not derived from a population-based sample. However, the clinic and population-based study of Olmsted County residents conducted by Kalantar et al. (24) showed a similar OR of 2.7 (95% CI: 1.2–6.3) to our OR of 2.75, although their overall prevalence of IBS in case-relatives and control-relatives was lower than ours, perhaps explained by exclusion of children from the study as they were also related to the case-spouses who were used as controls. Furthermore, although a major strength of our study was that the estimate was based on relative self-report of formal Rome criteria-based symptoms, our aggregation estimate may have been affected by bias by those who participated and those who chose not to participate. Although it did appear that participating relatives were slightly more likely to have IBS than nonparticipating relatives, this difference was relatively small (21% of case-relative participants vs. 16% of nonparticipants, 5% of control-relatives participants vs. 3% of nonparticipants). Furthermore, we recalculated aggregation estimates using proband-provided information for missing relative data, and found that in general, conclusions remained unchanged. One population-based study of bowel symptoms conducted in Olmsted County found that individuals with IBS-like symptoms were more likely to report having FDRs with abdominal pain or bowel problems than individuals without IBS-type symptoms with an OR of 2.3 (95% CI: 1.3–3.9)—a figure comparable to our own (25). Another study using billing data in a large health maintenance organization also showed that children of parents with IBS were twice as likely to present with gastrointestinal problems including abdominal pain and diarrhea—but not constipation—compared with children of parents without IBS (26). Thus, we feel that our estimate of an OR of 2.75 for a family history of IBS is reasonably accurate. Nonetheless, we acknowledge that this estimate may not be applicable to other patient populations, and future studies are needed to prove or disprove our findings. Although our estimates of aggregation were similar whether we analyzed our entire case-sample (of individuals with a clinical IBS diagnosis) or we restricted the analysis to only those meeting Rome criteria for IBS, further work should certainly be performed to determine whether it is appropriate to lump together IBS and other functional bowel disorders in family studies.
Although we have confirmed familial aggregation of IBS, and the strength of the aggregation could be consistent with a genetic basis (27), nonetheless, this analysis alone cannot determine the underlying mechanism for aggregation of IBS. Familial clustering of IBS could be explained by (i) shared disease susceptibility genes for IBS among family member; (ii) shared disease susceptibility genes for another entity that increases risk of IBS (e.g., lactose intolerance, depression or anxiety, somatization, or an immune system that increases risk of infection); (iii) shared household exposures, shared lifestyle behaviors, or shared household experiences; or (iv) a combination of the above. If there is a genetic basis for IBS—whether for IBS specifically or for another trait associated or linked with IBS—complex segregation analysis would be the next analytical step to determine whether the transmission of IBS through family members appears consistent with a Mendelian disorder (21). The higher frequency of food sensitivity, psychiatric comorbidity, and intestinal infections in our cases and our affected relatives compared with controls and unaffected relatives suggest that indeed there may several genetic and molecular mechanisms underlying IBS. We attempted to collect information on active depression and anxiety symptoms using validated questionnaires and to collect data regarding past diagnoses of depression, abuse, and other psychiatric disorders (using unvalidated questionnaire items), but our methodology did not take into account the psychological status of individuals with nonactive psychiatric symptoms, those who did not seek medical attention for their mood disorder, or did not recall or report past psychiatric diagnoses. As to shared household exposures, we did not specifically assess lifestyle factors such as general diet, exercise, tobacco use, or illness behavior. However, it does appear that higher degrees of somatization—that explained away the reports of abuse by cases and affected relatives—may in part explain familial clustering of IBS. We did not measure the role sexual abuse, which itself may result in greater abdominal focus, in this survey but are in the process of collecting this information in an ongoing study exploring the role of early life trauma in the development of IBS in these families.
In summary, we conducted a large, family case–control study of nearly 500 case-families with IBS and nearly 300 control-families by contacting or attempting to contact and collect Rome-criteria based bowel symptoms from all FDRs. Our extensive efforts have confirmed that IBS aggregates strongly in families. Future studies are needed to determine whether this clustering in family members is due to shared genes or environment, or both.
Study Highlights
What is current knowledge
Family history is a known risk factor/predictor of irritable bowel syndrome (IBS).
Family studies may be helpful in elucidating the genetic and environmental contributors to the development of IBS.
However, family studies depending on proxy reporting of IBS in other family members are not reliable, as shown in a pilot study by our group.
What is new here
This large family study, which collected bowel habits directly from cases, controls, and their first-degree relatives to construct pedigrees accurately, showed relatives of a family member with IBS are 2- to 3-fold at higher risk for IBS than control patient relatives.
Although diarrhea, constipation, and mixed bowel habits did aggregate specifically based on the case's predominant bowel habit, considerable overlap of bowel habits was observed in families.
Somatization explained away abuse as a risk factor by cases and affected relatives.
Acknowledgments
We thank Lori Anderson for her assistance in preparing this article.
Financial support: National Institutes of Health (DK66271) the American Gastroenterological Association, and Solvay Pharmaceuticals.
Footnotes
Guarantor of the article: Yuri A. Saito, MD, MPH.
Specific author contributions: Saito, Petersen, de Andrade, Locke, and Talley were involved in study design, execution, and finalizing the paper. Saito was the primary author of the paper. Joseph J. Larson, Elizabeth J. Atkinson, Fridley and de Andrade assisted with data management and analyses. Zimmerman and Almazar-Elder performed all parts of the study related to recruitment, questionnaire distribution, chart abstractions, and data entry.
Potential competing interests: None.
References
- 1.Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99:409–15. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- 2.Whorwell PJ, McCallum M, Creed FH, et al. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito YA, Zimmerman JM, Harmsen WS, et al. Irritable bowel syndrome aggregates strongly in families: a family-based case–control study. Neurogastroenterol Motil. 2008;20:790–7. doi: 10.1111/j.1365-2982.2007.1077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris-Yates A, Talley NJ, Boyce PM, et al. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–7. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 5.Levy RL, Jones KR, Whitehead WE, et al. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed I, Cherkas LF, Riley SA, et al. Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol. 2005;100:1340–4. doi: 10.1111/j.1572-0241.2005.41700.x. [DOI] [PubMed] [Google Scholar]
- 7.Bengtson MB, Ronning T, Vatn MH, et al. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55:1754–9. doi: 10.1136/gut.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lembo A, Zaman M, Jones M, et al. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: a twin study. Aliment Pharmacol Ther. 2007;25:1343–50. doi: 10.1111/j.1365-2036.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 9.Saito YA, Petersen GM, Locke GR, III, et al. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:1057–65. doi: 10.1016/s1542-3565(05)00184-9. [DOI] [PubMed] [Google Scholar]
- 10.Bellentani S, Baldoni P, Petrella S, et al. A simple score for the identification of patients at high risk of organic diseases of the colon in the family doctor consulting room. The Local IBS Study Group. Fam Pract. 1990;7:307–12. doi: 10.1093/fampra/7.4.307. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WG, Creed FH, Drossman DA, et al. Functional bowel disease and functional abdominal pain. Gastrenterol Int. 1992;5:75–91. [Google Scholar]
- 12.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talley NJ, Boyce PM, Owen BK, et al. Initial validation of a bowel symptom questionnaire and measurement of chronic gastrointestinal symptoms in Australians. Aust NZ J Med. 1995;25:302–8. doi: 10.1111/j.1445-5994.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 14.Chibnall JT, Tait RC. The Psychosomatic Symptom Checklist revisited: reliability and validity in a chronic pain population. J Behav Med. 1989;12:297–307. doi: 10.1007/BF00844873. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 17.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DA, Tompson WG, Talley NJ, et al. Identification of subgroups of functional gastrointestinal disorders. Gastroenterol Int. 1990;3:159–72. [Google Scholar]
- 20.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–86. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 21.Burton PR, Tobin MD, Hopper JL. Key concepts in genetic epidemiology. Lancet. 2005;366:941–51. doi: 10.1016/S0140-6736(05)67322-9. [DOI] [PubMed] [Google Scholar]
- 22.Hopper JL, Bishop DT, Easton DF. Population-based family studies in genetic epidemiology. Lancet. 2005;366:1397–406. doi: 10.1016/S0140-6736(05)67570-8. [DOI] [PubMed] [Google Scholar]
- 23.Chial HJ, Camilleri M. Gender differences in irritable bowel syndrome. J Gend Specif Med. 2002;5:37–45. [PubMed] [Google Scholar]
- 24.Kalantar JS, Locke GR, III, Zinsmeister AR, et al. Familial aggregation of irritable bowel syndrome: a prospective study. Gut. 2003;52:1703–7. doi: 10.1136/gut.52.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke GR, III, Zinsmeister AR, Talley NJ, et al. Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–12. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 26.Levy RL, Whitehead WE, Von Korff MR, et al. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451–6. doi: 10.1111/j.1572-0241.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 27.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–7. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]