Abstract
Fluctuating neurosteroid levels during the ovarian cycle modulate neuronal excitability through effects on GABAA receptors (GABAARs). The large increase in progesterone-derived neurosteroids during pregnancy and their precipitous decrease at parturition may have considerable effects on GABAARs during pregnancy and postpartum. Here we show a significant decrease in tonic and phasic inhibitions in pregnant mice, mediated by a downregulation of GABAAR δ and γ2 subunits, respectively. This decrease rebounds immediately postpartum. Mice with GABAAR δ subunit deficiencies (Gabrd+/− and Gabrd−/−), in which the rapid postpartum restoration of tonic inhibition is likely to be impaired, exhibit signs of depression-like and abnormal maternal behaviors resulting in reduced pup survival, that were ameliorated in Gabrd+/− mice by a selective agonist (THIP) of GABAARs containing δ subunits. Such mice constitute a novel mouse model of postpartum depression and have great potential for evaluating potential therapeutic interventions.
The GABAARs are one of the principal targets of the neuroactive metabolites of steroid hormones called neurosteroids (Herd et al., 2007). Neurosteroids are synthesized de novo from cholesterol or converted from steroid precursors in the central nervous system (CNS) (Stoffel-Wagner, 2001). Altered neurosteroid levels are associated with debilitating psychiatric and neurological disorders, including premenstrual dysphoric disorder (PMDD), premenstrual syndrome (PMS), catamenial epilepsy, menstrual migraine, postpartum depression, and anxiety (Backstrom et al., 2003). However, the pathogenesis of these ailments remains unclear mainly because of the lack of useful animal models to study such complex disorders.
Numerous studies have investigated the changes in neurosteroid levels associated with anxiety and depression in women (for review see (Longone et al., 2008)). Decreased neurosteroid levels have been discovered in drug-naïve patients with major depression and antidepressants have been shown to increase neurosteroid levels (Romeo et al., 1998; Uzunova et al., 1998), which is thought to account for the therapeutic benefits of these compounds (Pinna et al., 2006).The GABAergic system has long been implicated in the pathophysiology of various psychiatric disorders due to the evidence of decreased benzodiazepine binding in the brains of patients with panic disorder (Bremner et al., 2000b; Malizia et al., 1998), posttraumatic stress disorder (Bremner et al., 2000a) and generalized anxiety disorder (Tiihonen et al., 1997). Selectively increasing ambient levels of GABA in the extracellular space of the CNS is therapeutic for the treatment of anxiety disorders (Pollack et al., 1998; Vermetten and Bremner, 2002; Zwanzger et al., 2001) while experimentally it increases tonic inhibition mediated by extrasynaptic δ subunit-containing GABAARs (Nusser and Mody, 2002),.
The pathophysiology of postpartum mood disorders is thought to be triggered by the rapid decline in the levels of reproductive hormones following pregnancy. However, administration and withdrawal of exogenous steroid hormones, designed to mimic the hormonal changes during pregnancy, result in symptoms of depression only in women with a history of postpartum depression (Bloch et al., 2000), suggesting that women must be predisposed to postpartum depression. The reason for the predisposition is unknown, but it is reasonable to assume that a preferred target of neurosteroids, the δ subunit-containing GABAARs, might be involved in this disorder.
Alterations in these GABAARs occur as hormone levels fluctuate during the ovarian cycle (Griffiths and Lovick, 2005; Maguire et al., 2005) and may also take place during pregnancy and postpartum. Indeed, changes in GABAergic function during pregnancy have been inferred from binding (Concas et al., 1998; Majewska et al., 1989) and GABAAR mRNA studies (Biggio et al., 1998; Concas et al., 1998; Follesa et al., 1998). However, the functional consequences of these changes remain unclear. The objective of our study was to identify functional changes in GABAARs during pregnancy and the postpartum period, and to find possible behavioral correlates in mice
Results
Changes in GABAAR expression during pregnancy
Potential changes in GABAARs during pregnancy were identified by Western blot analysis of total hippocampal membrane protein, since this region has previously been shown to exhibit neurosteroid-sensitive plasticity (Maguire and Mody, 2007; Maguire et al., 2005) and is thought to be involved in the presentation of mood disorders (Tsetsenis et al., 2007). GABAAR δ subunit expression in hippocampal membrane fractions was significantly decreased in mice at day 18 (d18) of pregnancy (0.28 ± 0.02 OD/100 μg of total protein) compared to virgin (0.42 ± 0.03 OD/100 μg of total protein) and postpartum mice (0.42 ± 0.05 OD/100 μg of total protein) (Fig. 1a and 1b; n=6; p<0.05, one-way ANOVA). At d18 of pregnancy, GABAAR γ2 subunit expression was also decreased (0.37 ± 0.01 OD/100 μg of total protein) compared to virgin (0.55 ± 0.01 OD/100 μg of total protein) and postpartum mice (0.55 ± 0.01 OD/100 μg of total protein) (Fig. 1a and 1b; n=6; p<0.05, one-way ANOVA). These findings indicate a considerable decrease in the expression of GABAARs underlying both tonic and phasic inhibitions during pregnancy, reverting to control levels immediately postpartum.
Figure 1. Alterations in GABAARs during pregnancy.
a, Representative Western immunoblots of total hippocampal membrane protein from virgin, pregnant (d18), and postpartum (48 hr) mice. b, Histograms of the average optical density (OD) of GABAAR δ and γ2 subunit expression in virgin, pregnant, and postpartum mice. c, Representative whole-cell patch clamp recordings of tonic inhibition from DGGCs in virgin, pregnant (d18), and postpartum (48 hr) wild type and Gabrd−/− mice before and after addition of >100μM SR95531 (black bars; to reveal the amount of tonic current). d, The average tonic current recorded in DGGCs was significantly decreased in wild type pregnant mice compared to virgin wild type and postpartum wild type mice. There were no significant changes in tonic currents between virgin Gabrd−/− mice, pregnant Gabrd−/− mice, or postpartum Gabrd−/− mice.
Changes in GABAergic inhibition during pregnancy
To determine the functional consequences of GABAAR plasticity during pregnancy, whole-cell patch clamp recordings were performed on DGGCs from wild type and Gabrd−/− mice, since the tonic inhibition in this region is predominantly mediated by δ subunit-containing GABAARs. Tonic inhibition in DGGCs was significantly decreased at d18 of pregnancy in wild type mice (19.7.0 ± 3.6 pA) compared to virgin (39.8 ± 4.4 pA) and postpartum mice (40.4 ± 7.1 pA) (Fig. 1c and 1d; n=14 mice, n=47 cells; p<0.05, one-way ANOVA). Tonic inhibition in virgin Gabrd−/− mice (17.1 ± 3.9 pA) was significantly decreased compared to virgin wild type mice (39.8 ± 4.4 pA), but Gabrd−/− mice do not exhibit changes in tonic inhibition throughout pregnancy and postpartum (virgin: 17.1 ± 3.9 pA; pregnant: 25.6 ± 3.0 pA, postpartum: 21.3 ± 3.5 pA; Fig. 1c and 1d; n=12 mice, n=36 cells; p>0.05, one-way ANOVA). The average peak amplitudes of sIPSCs were also decreased in DGGCs of wild type pregnant mice (45.4 ± 3.9 pA) compared to virgin (76.4 ± 5.5 pA) or postpartum mice (69.5 ± 6.9 pA) (Fig. 2; n=14 mice, n=41 cells; p<0.05 one-way ANOVA). The peak sIPSC amplitudes were also decreased during pregnancy in Gabrd−/− mice (45.5 ± 4.8 pA) compared to virgin Gabrd−/− mice (62.1 ± 4.6 pA) and postpartum Gabrd−/− mice (58.8 ± 8.0 pA) (Fig. 2; n=12 mice, n=48 cells; p<0.05 one-way ANOVA). There were no changes in the frequency or decay time constants of sIPSCs between any of the groups. Similar to sIPSCs, the peak amplitude of mIPSCs were significantly decreased in wild type mice at d18 pregnancycompared to control and postpartum (Fig. 2e; n=12 mice, n=36 cells; p<0.05 one-way ANOVA). Nonstationary fluctuation analysis, albeit limited by averaging across receptors at different synapses, and its peak-scaled version unable to give an accurate estimate of Po, can still be used to obtain a reasonable estimate of the average unitary conductance of the channels underlying mIPSCs (De Koninick and Mody, 1994). This analysis revealed no difference between the aggregate mean conductance of synaptic GABAARs from control (30.9 ± 2.7 pS) or pregnant mice (31.2 ± 2.2 pS; Fig. 2f; n=12 mice, n=36 cells; p<0.05 by Student's t-test), suggesting that the change in mIPSC size likely results from a reduced number of receptors, consistent with the changes in GABAAR γ2 subunit expression observed during pregnancy (Fig. 1a and b).
Figure 2. Decreased IPSCs during pregnancy.
a, Representative whole-cell patch clamp recordings from DGGC from wild type and Gabrd−/− mice. b, Superimposed averages reveal a decrease in the amplitudes of sIPSCs in both wild type and Gabrd−/− mice during pregnancy. Peak-scaled sIPSCs show no change in kinetics in wild type and Gabrd−/− mice. The decreased peak amplitude can be appreciated in the cumulative probability plots of an equal number of representative sIPSCs from wild type and Gabrd−/− virgin, pregnant (d18) mice, and postpartum mice. c, The average amplitudes of sIPSCs is decreased in both pregnant wild type and Gabrd−/− mice. d, The average mIPSCs amplitude is decreased in pregnant wild type mice compared to virgin mice. There is no change in the unitary conductance of receptors, as determinde by nonstationary fluctuation analysis, between wild type pregnant and virgin mice.
Abnormal postpartum behavior in mice with deficiencies in δ subunit-containing GABAARs
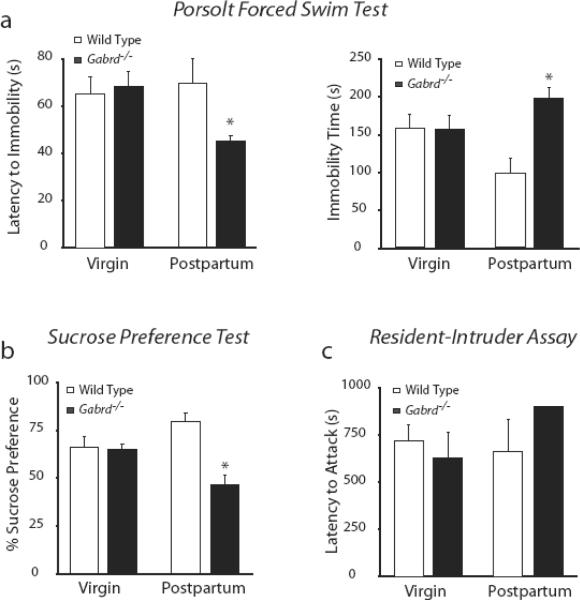
To determine the behavioral impact of GABAAR regulation during pregnancy and postpartum, postpartum behaviors were analyzed in mice deficient in GABAAR δ subunits (Gabrd−/− and Gabrd+/− mice). We used the Porsolt forced swim test to assess behaviors sensitive to antidepressants that can thus be categorized in mice as “depression-like”. Consistent with and increase in such behavior, Gabrd−/− mice exhibited a decreased latency to immobility (45.3 ± 2.5 s) and an increased amount of total time spent immobile during the 6 min test (197.8 ± 9.1 s) compared to virgin Gabrd−/− mice (latency: 68.1 ± 6.3 s; immobility time: 156.4 ± 18.0 s) and virgin (latency: 65.3 ± 7.0 s; immobility time: 157.1 ± 18.5 s) and postpartum wild type mice (latency: 69.8 ± 10.1 s; immobility time: 98.0 ± 9.0 s; n = 4–8 mice per experimental group; p<0.05 one-way ANOVA) (Fig. 3a). We next performed the sucrose preference test to assess anhedonia, another indication of depression-like behavior in mice. Mice were given access to two water bottles, one containing water and one containing a 2% sucrose solution, and the amount of each consumed over a 48 hour period was measured in virgin and postpartum mice of each genotype. Virgin mice consumed equivalent volumes of water 23.3 ± 0.5 ml (wild type) and 23.0 ± 1.7 ml (Gabrd−/−)over a 48 hr period, and both exhibited an equal preference for the 2% sucrose solution compared to water. Of the total volume consumed (100%), sucrose solution constituted 66.4 ± 5.2 % (wild type) and 65.2 ± 2.7 % (Gabrd−/−) (Fig. 3b). The postpartum mice drank more fluid than virgin mice: 32.8 ± 7.5 ml (wild type) and 33.0 ± 6.2 ml (Gabrd−/−). As virgin mice, the postpartum wild type mice exhibited a preference for the 2% sucrose solution, consuming an increased fraction of the total fluid volume (79.4 ± 4.4 %) (Fig. 3b). In contrast, postpartum Gabrd−/− mice did not preferthe 2% sucrose solution, consuming nearly equivalent amounts of water (53.6 ± 5.2 %) and sucrose (46.4 ± 5.2 %) (Fig. 3b; n = 4–6 mice per experimental group; p<0.05 one-way ANOVA), suggesting Gabrd−/− mice exhibit anhedonia only in the postpartum period.
Figure 3. Behavioral abnormalities in Gabrd+/− and Gabrd−/− mice postpartum.
a, Gabrd−/− mice exhibit a decreased latency to immobility and an increase in the total time immobile during a 6 min Porsolt forced swim test. b, Over a 48 hour period, virgin wild type, postpartum wild type, and virgin Gabrd−/− mice consumed more 2% sucrose solution than water in the sucrose preference test. Postpartum Gabrd−/− mice did not prefer the 2% sucrose solution. c, Virgin and postpartum wild type and Gabrd−/− mice had a similar latency to attack in the resident-intruder assay. Gabrd−/− mice exhibited more anxious behaviors postpartum in response to the intruder when compared to virgin or postpartum wild type mice.
To assess whether Gabrd−/− mice exhibit signs of aggression postpartum, we performed the resident-intruder assay. Virgin and postpartum wild type and Gabrd−/− mice were housed individually beginning at day 12 of pregnancy until 48 hours postpartum or for 2 consecutive weeks in virgin mice then an intruder mouse was introduced into the homecage of the resident. Aggression levels were assessed in the residents over 15 min (900 s) by measuring the latency to attack the intruder for wild type virgin (720.6 ± 81.9 s), wild type postpartum (663.0 ± 167.3 s), Gabrd−/− virgin (628.6 ± 134.9 s) and Gabrd−/− postpartum mice (900.0 ± 0.0 s; n = 4–8 for each experimental group; p<0.05 one-way ANOVA; Fig. 3c), According to these findings, Gabrd−/− mice are less aggressive postpartum. In response to the stress of the presence of the intruder, , we also observed more digging, burrowing, and circling, considered to reflect anxiety (Hebb et al., 2004; Maldonado and Navarro, 2001; Njung'e and Handley, 1991), in Gabrd−/− postpartum mice during the 15 min tests (456.0 ± 49.7 s) than in either virgin Gabrd−/− mice (249.3 ± 64.2 s) or virgin wild type (13 ± 9.9 s) or postpartum wild type mice (66.5 ± 29.9 s; p<0.05; one-way ANOVA; n = 4 for each group; Fig. 3c). Taken together, all behavioral tests are consistent with Gabrd−/− mice exhibiting depression-like and anxiety-like behaviors during the postpartum period.
Abnormal maternal behavior during the postpartum period in mice with deficiencies in δ subunit-containing GABAARs
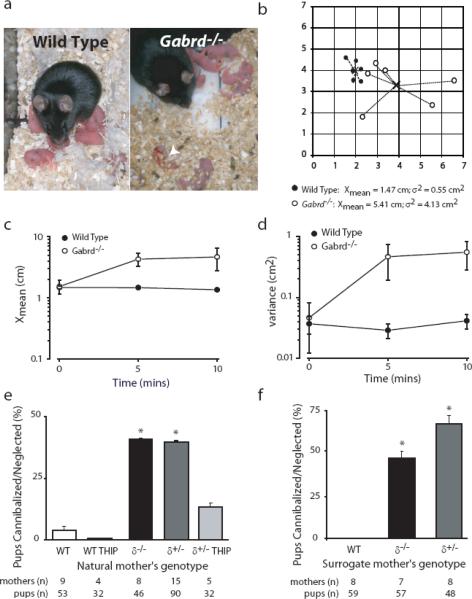
To further investigate the impact of these behavioral deficits observed in Gabrd−/− postpartum mice, we analyzed the maternal behavior of postpartum wild type and Gabrd−/− mice. Compared to wild type dams, Gabrd−/− mothers exhibited abnormal postpartum behaviors, such as the inability to build a proper nest, evidenced by the lack of a prominent wall surrounding the nest and the lack of bedding between the pups and the cage floor (Fig. 4a), and keeping an increased distance from their pups (Fig. 4a – d). The latter was measured by removing the mother from the homecage for 10 s, then replacing her into the homecage and measuring the distance of the pups from the mother over the following 10 min. The scatter of the pups was quantified as the mean distance (Xmean) of each pup from the litter centroid over time and the variance of the distance (σ2) from the centroid. An example of the pup scatter for representative wild type and Gabrd−/− litters 10 min after returning the mother to the homecage is shown in Fig. 4b, demonstrating that the pups from Gabrd−/− mothers are more dispersed throughout the cage with an average Xmean for Gabrd−/− pups of 1.53 ± 0.38 cm, 4.29 ± 1.04 cm, 4.67 ± 1.91 cm at 0, 5, and 10 min respectively and 1.48 ± 0.08, 1.47 ± 0.08 cm, 1.35 ± 0.08 cm for wild type pups (Fig. 4c). The variance of this distance is profoundly increased in Gabrd−/− litters 0.45 ± 0.33 cm2, 4.44 ± 2.61 cm2, 5.27 ± 2.59 cm2 at 0, 5, and 10 mins, respectively, compared to wild type litters 0.36 ± 0.11 cm2 0.28 ± 0.07 cm2, 0.40 ± 0.11 cm2 (Fig. 4d; n = 4 for each group; p<0.05 by one-way ANOVA), suggesting that the pups are scattered randomly throughout the cage in Gabrd−/− litters.
Figure 4. Abnormal maternal behaviors in Gabrd−/− mice.
a, Representative photographs of nests built by wild type and Gabrd−/−mothers. Gabrd−/− mothers fail to build a proper nest and keep the pups further away (arrows) than the wild type mother. A neglected/cannibalized pup is indicated by an arrowhead. b, Dispersion of the pups by their respective mothers is shown as the average distance of the pups from the centroid (Xmean) and the variance of the distance (σ2) from the centroid for wild type and Gabrd−/− pups. The values are shown for a representative wild type (black circles) and Gabrd−/− litter (white circles) after 10 min. The summarized data for Xmean and σ2 for all the wild type litters and Gabrd−/− litters over a 10 min period are shown in c and d, respectively. e, Decreased survival of pups born to Gabrd+/− and Gabrd−/− mice is reduced by enhancing GABAAR δ-subunit-mediated inhibition by including THIP in the drinking water. f, Pups born to Gabrd+/− and Gabrd−/− mice but reared by a surrogate wild type mother immediately following birth had an increase in survival compared to wild type pups reared by Gabrd+/− and Gabrd−/− surrogate mothers immediately following birth.
We also noted a significant decrease in the survival rate of pups born to Gabrd−/− and Gabrd+/− mothers compared to wild type. The mean litter size was equivalent at the time of delivery for wild type (5.89 ± 0.08 pups; n = 9 mothers; n = 53 pups), Gabrd+/− (6.00 ± 0.06 pups; n = 15 mothers; n = 90 pups), and Gabrd−/− mothers (5.75 ± 0.11 pups; n = 8 mothers; n = 46 pups). However, the percentage of pup survival per litter born to Gabrd−/− mothers (58.7 ± 2.6%; n = 8 mothers; n = 46 pups) or Gabrd+/− mothers (60.0 ± 2.0%; n = 15 mothers; n = 90 pups), was significantly decreased compared to litter of wild type mothers (96.2 ± 0.9%; n = 9 mothers; n = 53 pups; p<0.05 one way ANOVA). An increased percentage of pups born to Gabrd−/− (41.3 ± 0.3%; n = 8 mothers; n = 46 pups) and Gabrd+/− (40.0 ± 0.3%; n = 15 mothers; n = 90 pups) mothers died due to neglect or cannibalism compared to pups born to wild type mothers (3.8 ± 0.9%; n = 9 mothers; n = 53 pups; Fig. 4e; p<0.05 one way ANOVA). Therefore, the decreased mean litter size originally reported in the first study describing Gabrd−/− mice (Mihalek et al., 1999) was probably due to cannibalism of the pups which may have gone unnoticed if not observed immediately after delivery, as most of the cannibalism occurred within the first 24 hours.
To rule out the possibility that increased mortality of pups born to Gabrd−/− and Gabrd+/− mothers may have been triggered by an inherent defect in Gabrd−/− pups, we performed cross-fostering experiments. Pup mortality was increased in wild type pups cross-fostered by surrogate Gabrd−/− (41.4 ± 1.2%; n = 7 litters; n = 57 pups)or Gabrd+/− mothers (66.7 ± 1.5%; n = 8 litters; n = 48 pups) due to neglect or cannibalism compared to cross-fostered wild type pups reared by surrogate wild type mothers (0.0 ± 0.0%; n = 9 litters; n = 53 pups; Fig. 4f; p<0.05 one-way ANOVA), suggesting that the abnormal maternal behavior underlies the increased mortality of pups born to Gabrd−/− and Gabrd+/− mothers rather than some deficit in the pups. These data demonstrate that mice with defects in GABAAR δ subunit expression exhibit behavioral abnormalities in the postpartum period. Thus, restoring GABAAR δ subunit function may ameliorate these behavioral deficits in the postpartum period. We did this by administering a non-sedative dose of THIP (a GABAAR δ subunit preferring agonist) in the drinking water of wild type and Gabrd+/− mice resulted in a dose of 82.8 ± 4.6 mg/kg over 24 hrs, equivalent to the range of the 4–6 mg one-time administration employed in previous studies (Winsky-Sommerer et al., 2007). THIP treatment did not sedate or otherwise affect the maternal behaviors of wild type mothers as evident from the low pup mortality rates (0.0 ± 0.0%; n=4 litters; 32 offspring). However, this dose of THIP given to Gabrd+/− mothers significantly decreased pup mortality from maternal neglect or cannibalism (15.6 ± 0.6%; n=5 litters; 32 offspring) compared to untreated mothers (40.0 ± 0.3%; n=15 litters; 90 offspring) (Fig. 4e; p<0.05 one-way ANOVA). Therefore, the defects in tonic GABAergic inhibition mediated by δ subunit-containing GABAARs must play an important role in the behavioral deficits in Gabrd−/− and Gabrd+/− postpartum mice. Pharmacologically enhancing the inhibition mediated by these receptors may be a novel therapeutic approach to alleviating abnormal postpartum behaviors.
Discussion
Our study identified a mouse model, GABAAR δ subunit deficient mice , as exhibiting depression-like and anxiety-like behaviors during the postpartum period, associated with abnormal pup-care and decreased pup survival, consistent with comorbidity of anxiety (Ross et al., 2003), but not aggression, in the human condition of postpartum depression. The onset of depression-like behaviors in Gabrd−/− mice is restricted to the postpartum period (Fig. 5), suggesting that this is not merely a model of major depression but rather a specific model of postpartum depression. An in-depth search of the published literature does not reveal any mouse phenotypes with abnormal postpartum behavior associated with depression-like behaviors. For example, pup survival is decreased in oxytocin knockout mice due to abnormal milk ejection (Nishimori et al., 1996). Estrogen receptor-alpha knockout mice show an increased aggressive behavior and exhibit deficits in pup-induced maternal behavior in nonparturient mice unrelated to depression-like behaviors (Ogawa et al., 1998). 5HT1B receptor knockout mice exhibit abnormal maternal behavior which has been attributed to defects in ultrasonic vocalizations by the pups unrelated to depression (Weller et al., 2003). In addition, a search of the Jackson Laboratory's Mouse Behavioral Database resulted in 29 strains exhibiting “abnormal maternal behavior”; however, none of these strains exhibit depression-like behaviors as an associated phenotype. Similarly, mouse strains exhibiting depression-like phenotypes do notassociate with abnormal postpartum behavior, suggesting that Gabrd−/− and Gabrd+/− mice may represent the first mouse model exhibiting depression-like behaviors restricted to the postpartum period associated with abnormal maternal behavior. The mild epileptic phenotype of Gabrd−/− mice does not account for the maternal behavior abnormalities since Gabrd+/− mice with no overt epileptic phenotype still display the abnormal postpartum behaviors similar to Gabrd−/− mice. Moreover, numerous other mice with epileptic phenotypes do not exhibit behavioral abnormalities exclusive to the postpartum period such as those observed in Gabrd−/− and Gabrd+/− mice.
Our study reveals a potential functional mechanism for abnormal postpartum behavior resulting from dysfunction in GABAAR regulation during pregnancy and postpartum. As neurosteroid levels increase tremendously during pregnancy, brain mechanisms must have evolved to decrease the sensitivity of gravid mammals to neurosteroids. The quick postpartum reversion of GABAAR expression and inhibition to control levels in wild type mice indicates that the rapid return of neurosteroids to pre-pregnancy levels after parturition is followed by a commensurate adjustment in the number of functional GABAARs. Inability to properly regulate GABAARs during pregnancy and postpartum, as in Gabrd−/− and Gabrd+/− mice, is associated with depression-like and abnormal maternal behaviors, which may be highly relevant to the human condition of postpartum depression. During the highly vulnerable postpartum period, failure of tonic inhibition to match the lowered neurosteroid levels after parturition, as in Gabrd−/− and Gabrd+/− mice, may precipitate mood disorders associated with the postpartum period. Our data are consistent with the idea that the pathophysiology of postpartum depression may be related to the inability to properly regulate GABAergic inhibition during pregnancy and postpartum. Since defects in other GABAAR subunit genes, such as in Gabrg2+/−, Gabra1−/−, Gabra4−/−, and Gabra6−/− mice do not seem to be associated with abnormal postpartum behaviors, the plasticity of δ subunit-containing GABAARs may be particularly important in regulating neuronal excitability. This idea is further supported by the beneficial effect of alleviating abnormal postpartum behaviors and increasin pup survival in Gabrd+/− mice by specifically enhancing the function of δ subunit-containing GABAARs by pharmacologica means using THIP.,. The GABAAR δ subunit may be a novel target for therapeutical intervention in the treatment of postpartum depression.
Our study provides important clues for the pathogenesis of postpartum depression and provides the first mouse model for the disease. This model will foster further insights into the mechanisms of postpartum depression and will provide much needed therapeutic potential for the large number of women suffering from mood disorders during early motherhood.
Materials and Methods
Western Blot Analysis
Western blot analysis was carried out as previously described (Maguire et al., 2005). 100μg of total hippocampal membrane protein was subjected to SDS-PAGE, transferred to a nitrocellulose membrane (Amersham), blocked in 10% non-fat milk, and probed with a polyclonal anti-GABAAR δ (1:5,000, a gift from Dr. W. Sieghart) or anti- GABAAR γ2 (1:10,000, NOVUS). The blots were incubated with peroxidase labeled anti-rabbit IgG (1:2,000, Vector Laboratories) and immunoreactive proteins were visualized using enhanced chemiluminescence (Amersham). Optical density measurements were determined using the NIH Image J software.
Whole-Cell Recordings
Whole-cell patch clamp recordings were performed on DGGCs at approximately 34 °C as previously described (Maguire et al., 2005). The slices were perfused with normal ACSF (nACSF) (126mM NaCl, 2.5mM KCl, 2mM CaCl2, 1–2mM MgCl2, 1.25mM NaHPO4, 26mM NaHCO3, and 10–25mM D-glucose, bubbled with 95% O2 and 5% CO2 (pH=7.3–7.4)) containing 3 mM kynurenic acid and 5 μM GABA (Sigma, St. Louis, MO). Intracellular recording solution containing 140mM CsCl, 1mM MgCl2, 10mM HEPES, and 4mM Na-ATP (pH=7.25, 280–290mosmol) and electrodes with DC resistance of 2–5MΩ were used for all recordings. mIPSCs were recorded in ACSF containing 50μM CdCl2. Data analysis was performed as previously described (Maguire et al., 2005; Stell et al., 2003).
Nonstationary Noise Analysis
mIPSCs were low-pass filtered at 1 kHz and sampled at 10 kHz. Large amplitude events with a rapid 10–90% rise times were selected to ensure that the mIPSCs were from a population of synapses with similar kinetics (De Koninick and Mody, 1994). Traces containing multiple events were discarded. The selected events were peak aligned and the average variance of the current around Im was determined. The variance (σ2) was plotted against Im, binned (25 bins), and the relationship σ2 = iIm − Im/N was fit (least-squares simplex method) to the data points to obtain i, the unitary current, and N, the number of channels responsible for generating the mIPSC.
Animal Handling, Breeding, THIP administration, and Cross-Fostering
Wild type (C57Bl6), Gabrd−/−, and Gabrd+/− mice on C57Bl6 background were bred with like stud males for 24 hrs and subsequently isolated in separate cages for timed pregnancies. THIP (10 mg of THIP in 50 ml of water) was administered in the drinking water after establishing that wild type and Gabrd+/− mothers drank the same amount of water during after parturition (wt: 9.6 ± 2.1 ml/day ; Gabrd+/−: 10.4 ± 1.6 ml/day). The drinking water was replaced without any disruption of the home cage. A separate set of experiments was carried out in which a surrogate Gabrd−/− or Gabrd+/− mother (which gave birth to her own pups within the past 24 hours) was placed in the home cage of pups immediately born to a wild type mother. The wild type mother then became the surrogate to the Gabrd−/− or Gabrd+/− pups. The mothers were swapped rather than the pups to limit the disruption of the pups from their homecage. The number of surviving pups and the cause of death (neglect vs. cannibalism) was documented.
Porsolt Forced Swim Test
The Porsolt forced swim test was carried out by placing each mouse individually in a glass cylinder (21cm × 12cm), filled to 9 cm with room temperature water (22 – 25°C). The latency to immobility was recorded and the total duration of immobility throughout the 6 min forced swimming test was measured. The mouse was considered immobile when it ceased swimming and remained floating motionless, except for infrequent movements to maintain afloat.
Aggression and Anxiety-Related Behavior Assay
Forty-eight hours after parturition,aggressive behavior was monitored during 15-min exposures to wild type male intruder mouse that had been group-housed (four mice per cage) and matched with resident mice for body weight. Briefly, an intruder male was introduced into the homecage of the isolated female for 15 mins and the latency to attack and the total number of attacks was quantified. In addition, the time spent doing anxiety-related behaviors such as digging (dig, kick dig, push dig), burrowing, and circling were measured for both groups. The pups were removed from the cage 3 min before the onset of testing to avoid the possibility of injury to the pups, which does not alter the aggression of the mother (Svare et al., 1981).
Pup Dispersal Test
Three days after giving birth, the lactating female was removed from the homecage briefly (about 10 s) and then reintroduced to the homecage. The pups remained undisturbed. The behavior of the mother was videotaped and the location of the pups identified throughout the 10 min recording. Subsequently, pup location was determined at times 0, 5 min, and 10 min by calculating the x, y coordinates for each pup. The centroid (mean of all x and all y values) was determined for each litter at each time point. The mean distance from the centroid of each pup (Xmean) and the variance (σ2 was calculated for each litter at each timepoint and the averages were compared between genotypes.
Statistics
Statistical significance was set at p<0.05. The Student's t-test was used for comparison between two experimental groups. A one-way ANOVA was used for comparison of data sets with more than two groups.
Acknowledgements
We would like to thank Reyes Main Lazaro for playing an integral part in the postpartum behavioral studies and Guido Faas for help with data analysis. We thank Dr. W. Sieghart (University of Vienna, Austria) for the generous gift of the antibody used in this study. This work was supported by NIH Grant MH076994, and the Coelho Endowment to I.M. J.M was supported by a post-doctoral fellowship from the American Epilepsy Foundation and the Named New Investigator Award from the Center for the Neurobiology of Stress, UCLA.
Reference List
- Backstrom T, Andersson A, Andree L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Stromberg J, Sundstrom-Poromaa I, Turkmen S, Wahlstrom G, Wang MD, Wohlback AC, Zhu D, Zingmark E. Pathogenesis in menstrual cycle-linked CNS disorders. Steroids and the Nervous System. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Biggio G, Barbaccia ML, Follesa P, Purdy RH, Concas A. Neurosteroids modulate GABA(A) receptor structure and function during pregnancy and delivery in the rat brain. European Journal of Neuroscience. 1998;10:104. [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am. J. Psychiatry. 2000a;157:1120–1126. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, White T, Fujita M, Silbersweig D, Goddard AW, Staib L, Stern E, Cappiello A, Woods S, Baldwin R, Charney DS. SPECT [I-123]Iomazenil measurement of the benzodiazepine receptor in panic disorder. Biological Psychiatry. 2000b;47:96–106. doi: 10.1016/s0006-3223(99)00188-2. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De,Koninick Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J. Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABA(A) receptor complex during pregnancy and after delivery in the rat brain. European Journal of Neuroscience. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Lovick T. Withdrawal from progesterone increases expression of alpha4, beta1, and delta GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J. Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur. J. Neurosci. 2004;20:2415–2429. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol. Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Longone P, Rupprecht R, Manieri GA, Bernardi G, Romeo E, Pasini A. The complex roles of neurosteroids in depression and anxiety disorders. Neurochem. Int. 2008;52:596–601. doi: 10.1016/j.neuint.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J. Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005;8(6):797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Fordrice F, Falkay G. Pregnancy-Induced Alterations of Gabaa Receptor Sensitivity in Maternal Brain - An Antecedent of Post-Partum Blues. Brain Research. 1989;482:397–401. doi: 10.1016/0006-8993(89)91208-0. [DOI] [PubMed] [Google Scholar]
- Maldonado E, Navarro JF. MDMA (“ecstasy”) exhibits an anxiogenic-like activity in social encounters between male mice. Pharmacol. Res. 2001;44:27–31. doi: 10.1006/phrs.2001.0824. [DOI] [PubMed] [Google Scholar]
- Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder - Preliminary results from a quantitative PET study. Archives of General Psychiatry. 1998;55:715–720. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li ZW, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br. J. Pharmacol. 1991;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. Journal of Neurophysiology. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. American Journal of Psychiatry. 1998;155:992–993. doi: 10.1176/ajp.155.7.992. [DOI] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di MF, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Ross LE, Gilbert Evans SE, Sellers EM, Romach MK. Measurement issues in postpartum depression part 1: anxiety as a feature of postpartum depression. Arch. Womens Ment. Health. 2003;6:51–57. doi: 10.1007/s00737-002-0155-1. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA(A) receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiol Behav. 1981;26:253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Rasanen P, Lepola U, Koponen H, Liuska A, Lehmusvaara A, Vainio P, Kononen M, Bergstrom K, Yu M, Kinnunen I, Akerman K, Karhu J. Cerebral benzodiazepine receptor binding and distribution in generalized anxiety disorder: a fractal analysis. Molecular Psychiatry. 1997;2:463–471. doi: 10.1038/sj.mp.4000329. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo IL, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat. Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depression and Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- Weller A, Leguisamo AC, Towns L, Ramboz S, Bagiella E, Hofer M, Hen R, Brunner D. Maternal effects in infant and adult phenotypes of 5HT1A and 5HT1B receptor knockout mice. Dev. Psychobiol. 2003;42:194–205. doi: 10.1002/dev.10079. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta-subunit-containing receptors. Eur. J. Neurosci. 2007;25(6):1893–9. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, Schwarz M, Moller HJ, Rupprecht R. Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers. Neuropsychopharmacology. 2001;25:699–703. doi: 10.1016/S0893-133X(01)00266-4. [DOI] [PubMed] [Google Scholar]