Abstract
Affective pictures drive the activity of brain networks and impact behavior. We showed previously that viewing unpleasant pictures interfered in the performance of a basic non-emotional visual detection task. In the present study, we employed functional magnetic resonance imaging to test the hypothesis that behavioral interference may result from the interaction between negatively valenced and motor-related signals in the brain. As in our previous study, subjects performed a simple target-detection task that followed the presentation of unpleasant or neutral pictures. Our results revealed that an unpleasant emotional context modulated evoked responses in several regions engaged by the simple target-detection task. In particular, the midcingulate cortex was recruited when participants performed target-detection trials during the unpleasant context and signal responses in this region closely mirrored the pattern of behavioral interference (as revealed via reaction time). Our findings suggest that the midcingulate cortex may be an important site for the interaction between negatively valenced and motor signals in the brain, and that it may be involved in the implementation of defensive responses, such as freezing.
INTRODUCTION
There is a large body of studies demonstrating that emotion impacts perception and behavior. On the one hand, beneficial effects have been reported, including findings that threat stimuli enhance contrast sensitivity (Phelps, Ling, & Carrasco, 2006), low spatial-frequency sensitivity (Bocanegra & Zeelenberg, 2009), and search efficiency for task-relevant objects (Becker, 2009). On the other hand, the deleterious effects of emotional content are also well documented. In particular, unpleasant stimuli compete effectively for visual processing resources and often impair behavioral performance when their processing is irrelevant for the task at hand. For example, determining the orientation of a target visual stimulus was slowed down following emotional pictures (Hartikainen, Ogawa, & Knight, 2000), and the presence of a central unpleasant picture increased RT when participants discriminated peripheral target letters (Tipples & Sharma, 2000), or the orientation of bars (Erthal et al., 2005). These effects of emotional stimuli on performance are commonly thought to be mediated by attention (Pessoa, Kastner, & Ungerleider, 2002; Vuilleumier, 2005). In particular, it is believed that interference is due to the initial consumption of resources by emotional items or possibly to an increased difficulty in disengaging from emotional information (Bradley, Cuthbert, & Lang, 1996; Koster, Crombez, Verschuere, & De, 2004). This attention-like competitive advantage of emotional stimuli appears to be mediated by the amygdala (Anderson & Phelps, 2001).
Viewing unpleasant stimuli also generates defensive reactions (Azevedo et al., 2005; Bradley, Codispoti, Cuthbert, & Lang, 2001) and prepares participants for action (Hajcak et al., 2007). Darwin argued that emotions are adaptive insofar as they prompt actions that are beneficial to the organism (Darwin, 1872) . Contemporary theories of emotion are based on the belief that in order to survive, animals should be capable of identifying threat signals that allow them to avoid threats to their body envelope. From this perspective it would be expected that the processing of emotional items would modulate signals in motor-related areas. Indeed, accumulating evidence has demonstrated the involvement of motor-related cortical areas during threatening contexts in the monkey (Graziano & Cooke, 2006). This notion is also consistent with studies in humans that have reported increased motor cortex excitability during emotional processing, as assessed via the combination of electromyography and transcranial magnetic stimulation (Baumgartner, Willi, & Jancke, 2007; Hajcak et al., 2007; Oliveri et al., 2003). Other neuroimaging studies have reported that activity in primary motor cortex and putamen is robustly modulated during experimentally-induced states of fear (Butler et al., 2007; Phelps et al., 2001), consistent with the idea that aversive contexts engage motor circuits. In a recent neuroimaging study (Morrison, Peelen, & Downing, 2007), activity in cingulate areas, such as midcingulate cortex, increased during unpleasant trials and the activation depended on whether or not the participant made an overt motor response to the event. The findings concerning the midcingulate cortex are of particular interest because these medial regions have been characterized as medial premotor areas (Koski & Paus, 2000) and are known to project topographically to primary motor cortex, supplementary motor areas and putamen, in addition to having direct connections to the spinal cord (Morecraft, Louie, Schroeder, & Avramov, 1997). Importantly, the midcingulate cortex receives widespread inputs, both directly and indirectly, from emotion-related brain regions (Paus, 2001) and may be a pivotal node of emotional and motor integration (Morecraft & Van Hoesen, 1998).
As previously stated, the impact of affective stimuli on attention has been explored as an important component underlying emotional modulation (Vuilleumier, 2005). However, the potential role of interactions between affective processing and motor processes in behavioral interference has been less explored. In a recent study, we reported a long–lasting interference effect produced by unpleasant picture viewing (Pereira et al., 2006). Specifically, participants exhibited a marked slowing down of reaction time when performing a simple target-detection task during unpleasant relative to neutral blocks. Because sustained behavioral interference was observed only during the blocked presentation of stimuli, we suggested that viewing negative pictures may have caused the induction of an emotional state that was linked to the activation of defensive responses. We further suggested that the triggering of these defensive, “freezing”-like motor patterns, might have contributed to the sustained interference observed in our behavioral study.
The goal of the present study was to probe the neural underpinning of sustained behavioral interference. Specifically, we were interested in probing how behavioral interference during unpleasant picture viewing was related to motor-related evoked responses. We hypothesized that generating a motor response to a neutral target in an emotional context was associated with modulation of activity in a network of brain regions involved in motor execution. To address this question, we employed a paradigm analogous to the one used in our previous study in which sustained behavioral interference was observed (Pereira et al., 2004; Pereira et al., 2006). We further reasoned that if the interaction of affective and motor-related signals underlies behavioral interference effects, evoked responses due to unpleasant picture viewing would be correlated with evoked brain responses during motor action. Finally, we anticipated that the slow-down in reaction time (RT) during unpleasant blocks would be tied to responses in brain regions involved in the integration of emotion and motor signals, such as the cingulate cortex.
METHODS
Participants
Eleven right-handed male subjects (18–32 years old, mean = 24.8) participated in the study. All participants had normal or corrected-to-normal vision, reported no psychiatric or neurologic problems, and were not under medication with central nervous system action. The experiment was approved by the IRB of the Federal University of Rio de Janeiro, and participants gave written, informed consent.
Stimuli
Sixty pictures, 30 neutral and 30 unpleasant, were employed. Twenty-two pictures (6 neutral and 16 unpleasant) were obtained from the International Affective Picture System (IAPS), and the remaining ones were obtained from the World-Wide Web or photographed by the authors (additional images were obtained because those available in the IAPS set were not sufficient). All pictures were of the same size (640 × 480 pixels). Neutral pictures consisted of photographs of people, and unpleasant pictures consisted of photographs of mutilated bodies. We attempted to match unpleasant and neutral stimuli both in terms of color content and complexity (e.g., number of faces, body parts, etc.). Following the protocol developed by Lang and colleagues (1997), pictures were rated on a 1–9 scale in terms of valence (from negative to positive) and arousal (from low to high) by a separate group of participants (N = 40) using the paper-and-pencil version of the Self-Assessment Manikin (Bradley & Lang, 1994). Overall, pictures in the neutral category had a mean valence rating of 4.9 ± 0.3 (mean plus standard deviation) and mean arousal rating of 3.3 ± 0.6; pictures in the unpleasant category had a mean valence rating of 2.2 ± 0.4 and mean arousal rating of 6.3 ± 0.6. An additional set of 6 neutral pictures comprised of household objects from the IAPS dataset was selected for use during a practice block.
Design and procedure
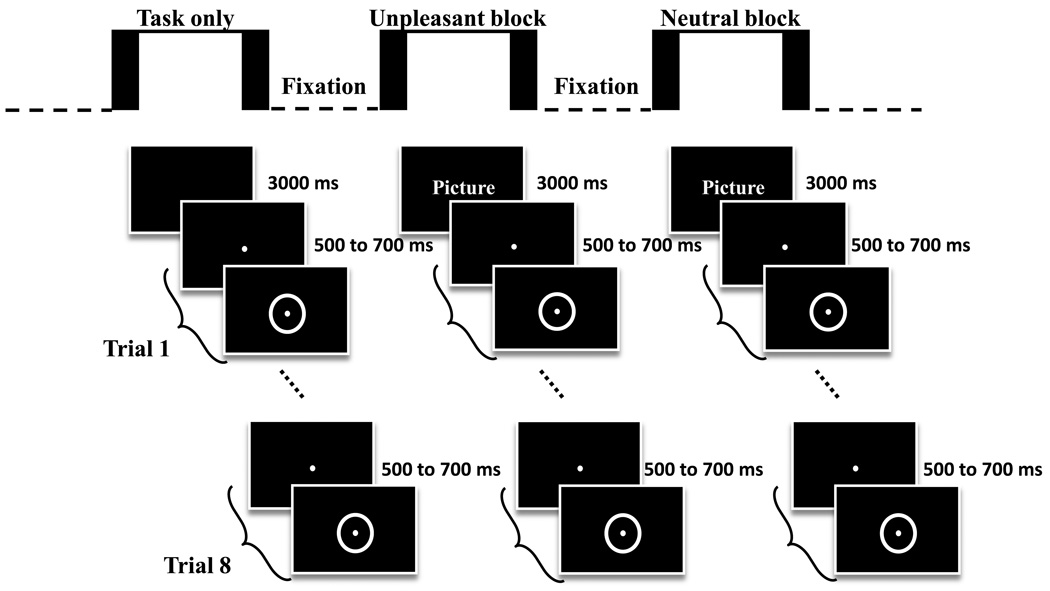
Visual stimuli were displayed by means of magnetically shielded LCD goggles (Resonance Technologies, Inc., Northridge, CA). The experimental session was divided into two runs. Each run consisted of two detection-only blocks, one unpleasant block, and one neutral block. Unpleasant and neutral blocks lasted 225 s each while detection-only blocks lasted 60 s. All blocks were followed by a 12-s fixation baseline. During the unpleasant and neutral blocks, each picture was presented for 3 seconds and immediately followed by a sequence of eight target-detections trials (Figure 1). Picture-containing blocks consisted of a single type of stimulus, unpleasant or neutral, and block order was counterbalanced across participants. Within unpleasant and neutral blocks, the order of presentation of the 15 pictures was randomized for each participant. Participants were instructed to attend to each picture for as long as it was displayed. Each target-detection trial consisted of a fixation spot presented at the center of the screen 500–700 ms prior to target onset. The target was a small annulus that appeared around the fixation spot; both remained on until the participant’s response (or until 1500 ms). Both the fixation spot and the target were presented in white on a black background. Participants were required to press a button with the right index finger as quickly as possible after target onset. A new target-detection began 500 ms following the participant’s response. After eight target-detections trials, a new picture appeared on the screen. Target-detections trials with RTs shorter than 100 ms or longer than 1000 ms were considered errors and were discarded from the RT analysis. Detection-only blocks were identical to picture-containing blocks, except that a 3-s black screen preceded the sequence of target-detections trials. At the beginning of the session, during anatomical scanning, participants performed a practice block, which was similar in structure to the neutral block, except that all images involved photographs of neutral objects, such as tools and furniture, instead of people.
Figure 1.
Experimental design. Each experimental run consisted of two detection-only blocks, one unpleasant block, and one neutral block. During the unpleasant and neutral blocks, pictures were presented for 3 seconds and immediately followed by a sequence of eight target-detection trials. Participants were instructed to attend to each picture for as long as it was displayed and to press a button with the right index finger as quickly as possible after target onset. After eight target-detection trials, a new picture appeared on the screen. In the detection-only blocks, a 3-s black screen preceded the sequence of target-detection trials. Stimuli are not drawn to scale.
Image acquisition
Functional MRI data were collected using a 1.5 Tesla MRI scanner (Magnetom Vision Plus, Siemens, Erlangen, Germany). Functional images were acquired using a gradient echo echo-planar imaging sequence (TR = 3 s; TE = 60 ms; FOV = 240; flip angle = 90°; 64 × 64 matrix). Whole-brain coverage was obtained with 25 axial slices (thickness = 4 mm; in-plane resolution = 3.75 × 3.75 mm). Echo-planar images were co-registered to a high-resolution structural T1-weighted image obtained during the same session (TR/TE =9.7/4.0 ms; flip angle = 12°; 128 sagittal slices; thickness = 1.25 mm; 256 × 256 matrix, FOV=256 mm). Head movements were restrained by foam padding. Two-hundred thirty-four functional volumes were acquired during each of two runs, each of which lasted approximately 12 minutes. Stimulus presentation was synchronized with MRI data acquisition.
Reaction time data analysis
Two participants were excluded from RT analysis due to excessive errors (>15%, mean error rate was 1.3%). As the resulting sample size (N=9) was relatively small, to increase statistical power, we ran the exact same experiment with an additional sample (N=22, all male, mean age = 24.8) outside of the scanner. The RT for successive target-detection trials 1 and 2, 3 and 4, 5 and 6, and 7 and 8 were averaged together, resulting in four mean RTs (from here on referred to as target-detection trials 1–4). Data from both groups were included in a repeated-measures ANOVA with within-participant factors valence (unpleasant, neutral) and target-detection trial (1–4), and between-participant factor group (inside, outside). Note that the RTs from trials 1–8 were not averaged together into a single value because we were interested in evaluating the time course of the interference effect – e.g., whether it was sustained or not. We employed “averaged trials” to diminish the number of levels in the above ANOVA from eight to four (as done in our previous behavioral study; Pereira et al., 2006).
fMRI data analysis
The statistical parametric mapping software package (SPM2, Wellcome Department of Cognitive Neurology, London, UK) was used for preprocessing and statistical analyses. The first three functional volumes of each run were removed to eliminate nonequilibrium effects of magnetization. The remaining images were corrected for head movement by realigning all the images to the first image via rigid body transformations. The images were then corrected for differences in slice acquisition time. For each participant, functional and structural images were coregistered. Structural data were normalized by matching them to the standardized MNI template, and the transformation parameters estimated in this step were applied to all functional images. Functional images were spatially smoothed with an 8-mm full width at half maximum Gaussian kernel prior to statistical analysis.
Data analysis was performed according to the general linear model (GLM) framework, as implemented in SPM2 (Friston, Holmes, Worsley, Poline, & Frith, 1995). Data obtained from the eleven subjects were analyzed. The first (fixed) level involved determining regression coefficients of variables of interest, which modeled the effects of each experimental condition: detection only, viewing neutral pictures, viewing unpleasant pictures, detection after neutral pictures, and detection after unpleasant pictures. Before estimation via multiple regression, regressors of interest were convolved with a canonical hemodynamic response function. Because the target-detection phase of picture-containing blocks followed the pictures immediately, detection-related regressors only modeled target-detection trials 3–8 (thus excluding the first two target-detection trials). We adopted this fairly conservative strategy to minimize any “spill over” from the picture-viewing phase into the detection phase, a notion that was supported by the absence of emotional modulation during the target-detection phase in visual cortex (as indicated by the contrast unpleasant-detection vs. neutral-detection). Note that specific phases of picture-containing blocks were contrasted to each other (e.g., unpleasant-viewing vs. neutral-viewing) but not to detection-only blocks. The latter were contrasted to the baseline condition in order to delineate the basic circuit engaged by the target-detection task.
Second-level, group analyses were conducted by means of repeated-measures ANOVAs and t tests. As random-effects analyses may be fairly conservative in the context of fMRI data (Worsley et al., 2002), we employed a threshold of P < .001 (uncorrected), as commonly employed in the literature.
Additional region-of-interest (ROI) analyses were also performed. In each case, a voxelwise contrast (e.g., detection-only vs. baseline) was first employed to determine a set of candidate clusters that were further interrogated via additional contrasts (e.g., unpleasant-detection vs. neutral-detection). The local peaks of the activation of the voxelwise contrasts were used as the center of an 10-mm radius sphere around the peak voxel, and voxels within the sphere were further considered if they were statistically significant (a threshold of p < .005, uncorrected, was employed, except for the contrast of all-conditions vs. baseline, which employed p < .001, uncorrected, given the strength of this contrast). Mean regression coefficients were then determined for each region. ROI construction and signal extraction were performed using the MarsBar toolbox for SPM2. Note that all of our ROI analyses avoided circularity in that the statistical test at the ROI level was independent from the selection criterion used to determine the ROI (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009; Vul, Harris, Winkielman, & Pashler, 2009).
Overall, three main types of inferential ROI analysis were performed. Here, we briefly describe them and the rationale for carrying them out. In the first, a set of ROIs defined via the contrast detection-only vs. baseline was probed with the contrast unpleasant-detection vs. neutral-detection (see Table 1 for a list of the ROIs). The objective of this analysis was to probe the basic circuit involved in target detection for modulations based on stimulus category (unpleasant vs. neutral).
Table 1.
| Brain region | MNI coordinates |
cluster size |
t value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Detection-only vs. baseline | ||||||
| Lingual gyrus | R | 24 | −88 | −10 | 58 | 6.25 |
| Middle occipital gyrus | L | −34 | −84 | 2 | 59 | 6.47 |
| Middle occipital gyrus | R | 30 | −80 | 10 | 53 | 7.53 |
| Precentral gyrus | L | −54 | −2 | 46 | 19 | 5.83 |
| Middle frontal gyrus | L | −24 | 8 | 40 | 4 | 4.22 |
| Middle frontal gyrus | R | 30 | 20 | 40 | 10 | 5.05 |
| Putamen | L | −24 | 2 | 4 | 131 | 6.56 |
| Putamen | R | 24 | −4 | 4 | 36 | 5.22 |
| Unpleasant-viewing vs. neutral-viewing | ||||||
| Middle occipital gyrus | L | −38 | −84 | 8 | 123 | 5.80 |
| Middle insula | L | −32 | −14 | 16 | 19 | 6.96 |
| Amygdala* | L | −26 | −6 | −14 | 133 | 2.33 |
| Amygdala* | R | 32 | −2 | −18 | 24 | 2.22 |
| Unpleasant-detection vs. neutral-detection | ||||||
| Inferior parietal gyrus | L | −40 | −40 | 52 | 31 | 5.53 |
| Precentral gyrus | L | −26 | −20 | 68 | 51 | 6.27 |
| Postcentral gyrus | L | −58 | −4 | 42 | 6 | 5.49 |
| Superior frontal gyrus | L | −18 | −2 | 56 | 10 | 4.99 |
| Midcingulate cortex | L | −4 | 8 | 46 | 9 | 4.44 |
| Putamen | L | −18 | 4 | 12 | 11 | 5.43 |
All regions at p < .001, uncorrected, except the amygdala at p < .05 (see asterisk)
L = left; R = right;
x, y, z = MNI coordinates of the maximally active voxel; t = maximum t value
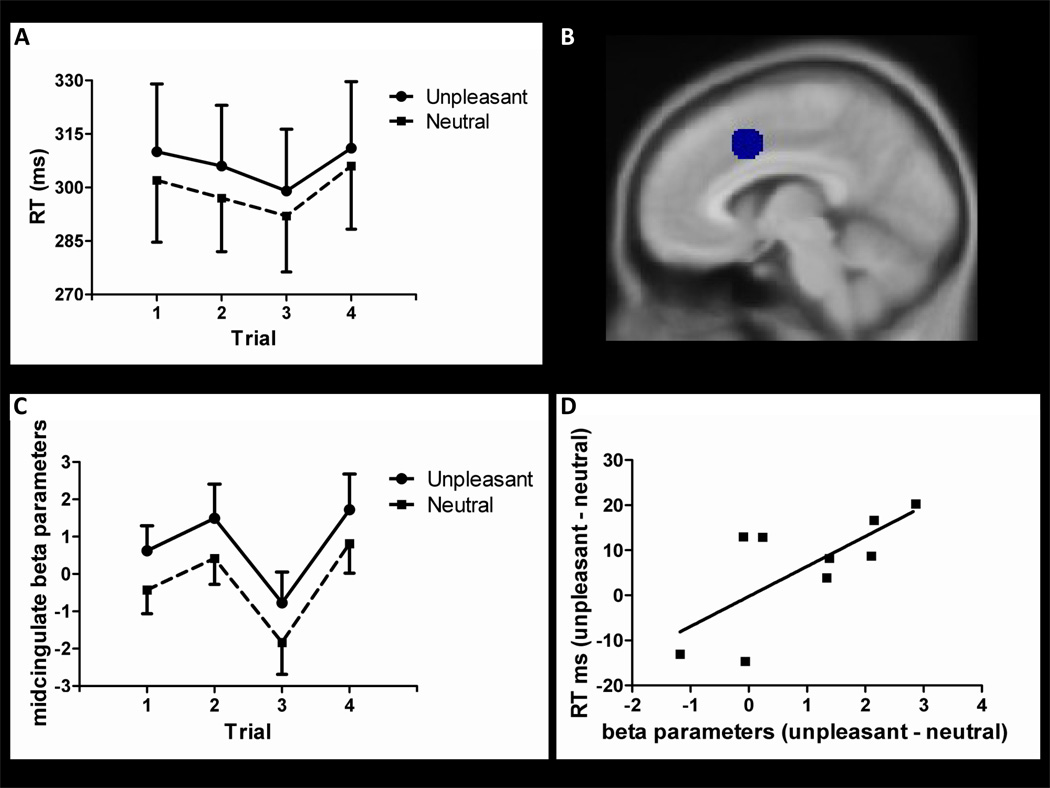
In a second ROI analysis (see Fig. 6), midcingulate cortex was identified via the contrast all-conditions vs. baseline (note that the contrast detection-only vs. baseline does not engage the midcingulate cortex; an emotionally evocative stimulus appears to be necessary). The goal of this analysis was to investigate effects of both stimulus category and target-detection trial, which was accomplished via a 2 valence (unpleasant, neutral) × 4 target-detection trial (1–4) repeated-measures ANOVA – as in the behavioral analysis, the latter factor was employed to probe the temporal unfolding of the interference effect.
Figure 6.
Midcingulate cortex responses and behavior. (A) RT data are shown as a function of pooled target-detection trial number and viewing condition. (B) Illustration of the midcingulate ROI. (C) Midcingulate cortex ROI responses (MNI coordinates: −8, 12, 46) are shown as a function of pooled target-detection trial number and viewing condition. (D) Scatter plot illustrating the linear relationship between behavioral and fMRI data. Error bars in (A) and C) indicate the standard error of the mean.
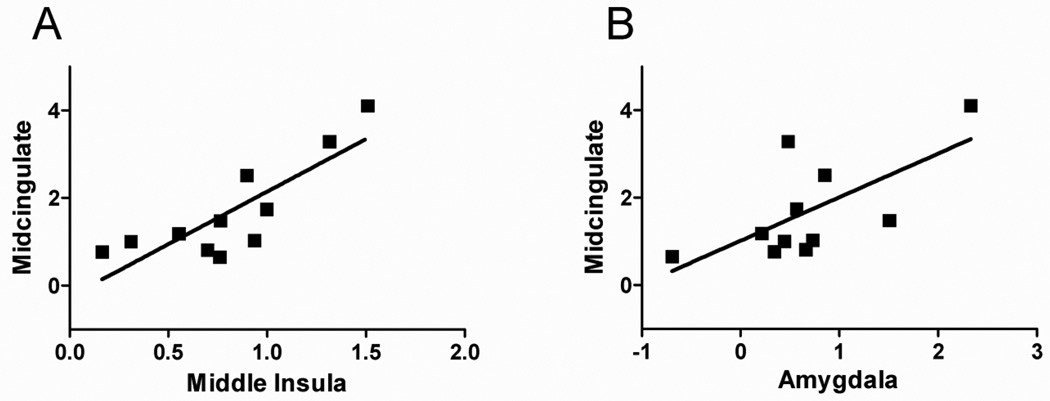
In a third ROI analysis (see Fig. 7), we focused on regions that exhibited increased responses to unpleasant-viewing vs. neutral-viewing and investigated how they were correlated with midcingulate signals. Accordingly, we tested the three regions differentially recruited by this contrast, namely middle occipital gyrus, middle insula and the amygdala. Signals from these regions were then correlated with responses observed in the midcingulate cortex. The latter was defined via the contrast unpleasant-detection vs. neutral-detection because we were interested in relating midcingulate responses during detection to those in the structures above during stimulus viewing. In other words, we were interested in testing a link between responses during viewing and detection that would be consistent with a causal relationship (naturally, “consistent with” does not imply causation).
Figure 7.
A) Scatter plot illustrating the linear relationship between responses in the middle insula (unpleasant-viewing vs. neutral-viewing) and midcingulate cortex (detection-unpleasant vs. neutral-detection). B) Scatter plot illustrating the linear relationship between responses in the amygdala (unpleasant-viewing vs. neutral-viewing) and midcingulate cortex (detection-unpleasant vs. neutral-detection).
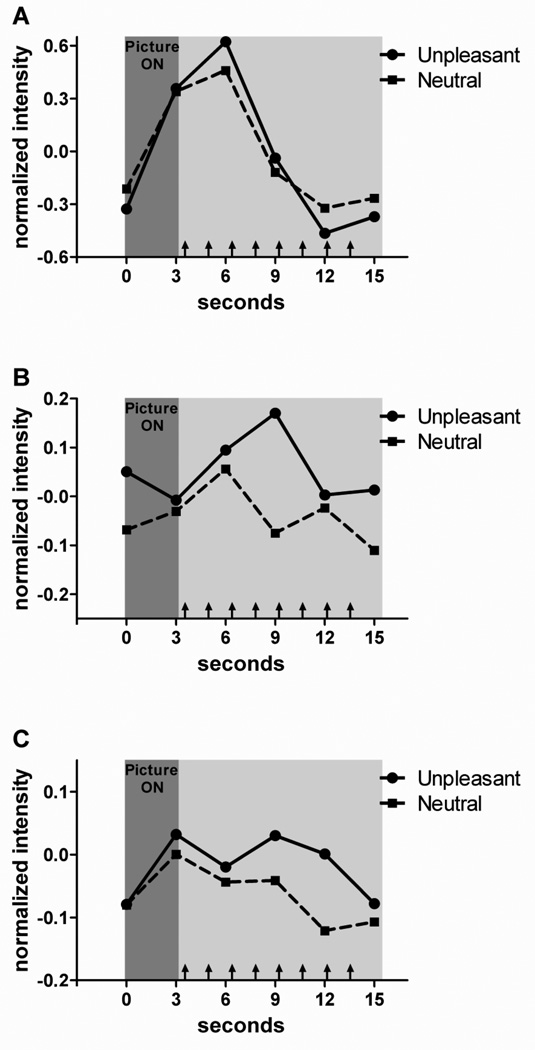
Finally, Fig. 4 plots average fMRI responses in the middle occipital gyrus, middle insula, and midcingulate cortex (all regions were defined as described above for the third ROI analysis). To do so, the activity of voxels within an ROI was normalized (z score) with respect to the mean activity of each run and trial averaging was used to generate the time courses shown. Our objective here was to illustrate how transient or sustained the responses were in these regions (no statistical inferences were performed).
Figure 4.
Mean normalized signal during unpleasant and neutral blocks. The dark-gray shaded area indicates the picture viewing phase, and each arrow in the light-gray area approximately indicates the presentation of a detection target. Responses are shown for the left middle occipital gyrus (A), left middle insula (B), and left midcingulate cortex (C). Whereas the responses in visual cortex where fairly transient and driven by picture viewing, those in the middle insula and midcingulate cortex were more directly linked to the target-detection phase.
RESULTS
Behavioral Performance
Reaction time data were analyzed according to a 2 valence (unpleasant, neutral) × 4 target-detection trial (1–4) × 2 group (inside, outside) repeated-measures ANOVA. Note that the factor target-detection trial allowed us to evaluate the time course of the interference effect (e.g., transient vs. sustained). The analysis revealed a significant main effect of valence [F(1,29) = 7.17, p = .01] only. In particular, no interactions involving valence were statistically significant [valence × target-detection trial: F(3, 87) = .81, p=.5; valence × group: F(1, 29) = .12, p = .73; valence × target-detection trial × group: F(3, 87) = 1.1, p = .3]. Post-hoc comparisons revealed that all target-detection trials performed during unpleasant blocks were significantly slower than those performed during neutral blocks (all ps < .05). This sustained effect over target-detection trials is depicted in Figure 6A, where mean RTs during the unpleasant and neutral blocks are shown for the participants of the fMRI sample. This plot clearly illustrates the same general pattern of long-lasting interference that we have reported in previous investigations (Pereira et al., 2006; Pereira et al., 2004). In fact, post-hoc comparisons of the data from the fMRI sample (N = 9) revealed that the RTs of target-detection trials performed during unpleasant blocks were slower than during neutral ones (all ps ≤ .05, except for target-detection trial 4 for which p = 0.29).
Brain Functional Activity
Simple target-detection task circuit
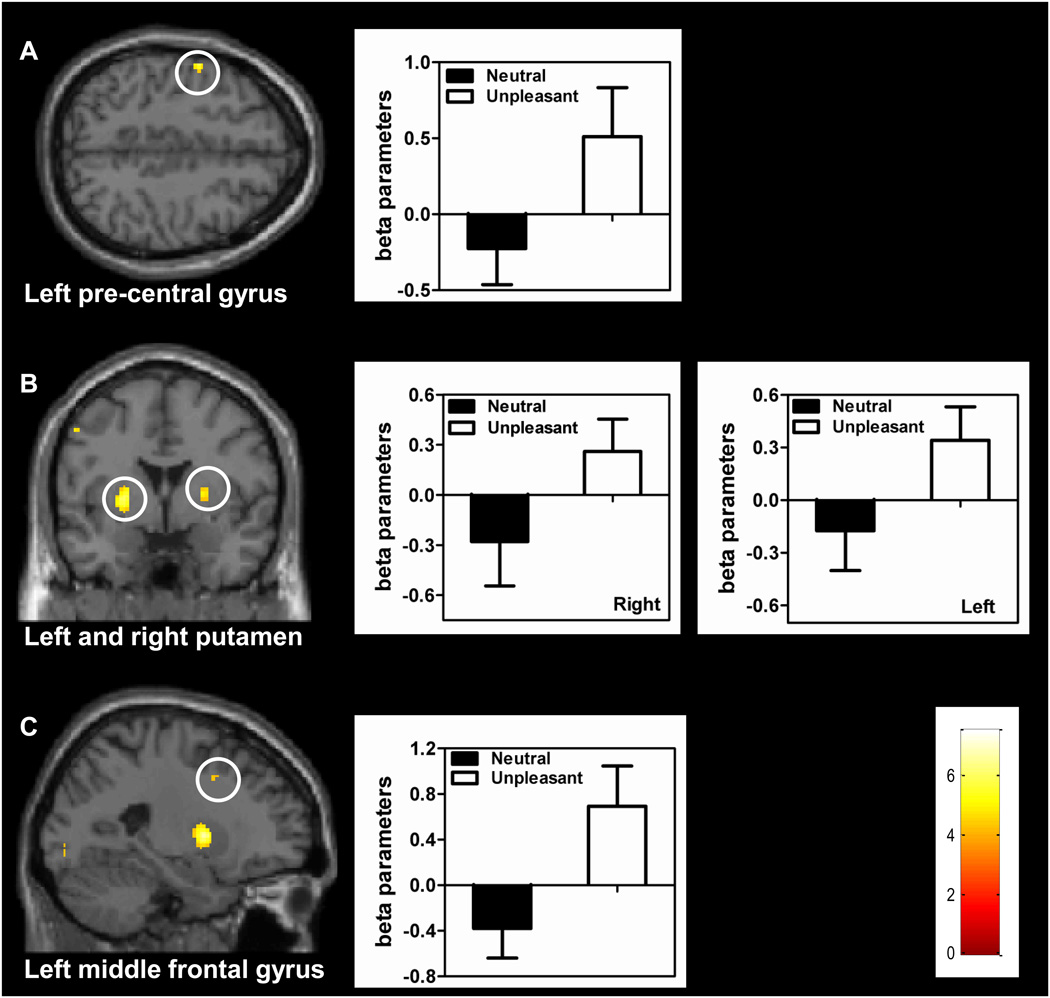
To identify the set of brain regions that was engaged by the target-detection task, the detection-only condition was contrasted to the fixation baseline, which revealed increased responses in the left precentral gyrus, right middle frontal gyrus, left middle frontal gyrus, and, subcortically, bilateral putamen (Figure 2 and Table 1).
Figure 2.
Voxelwise, group maps on the left illustrate the results of the contrast detection-only vs. baseline. These regions were further interrogated based on the contrast unpleasant-detection vs. neutral-detection, as shown in the bar plots on the right. Error bars indicate the standard error of the mean. The color scale represents z values.
Brain areas activated by viewing mutilation pictures
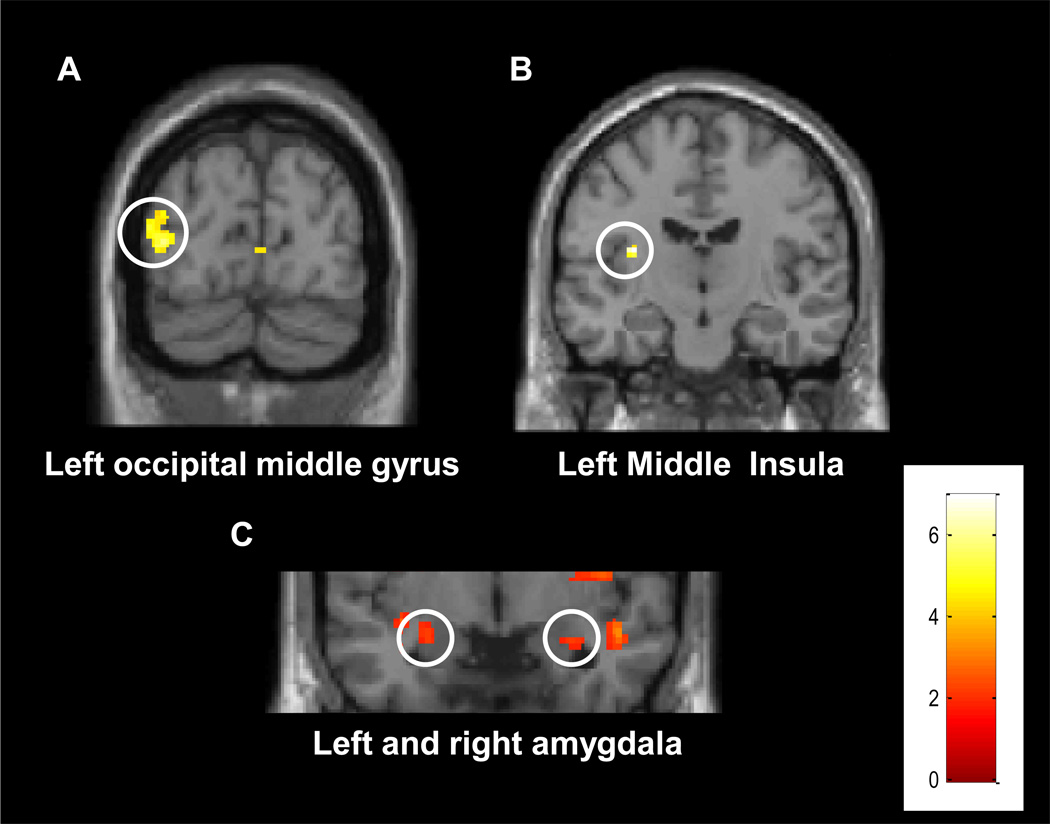
To identify the set of brain regions that was engaged by mutilation stimuli, we contrasted unpleasant vs. neutral picture viewing, which revealed enhanced activation in the occipital cortex and the middle insula (Figure 3A and B). Note that while the responses in the former were relatively transient, those in the latter were less so (Figure 4; see Methods). Given the large literature showing the involvement of the amygdala in similar conditions, a more lenient threshold for statistical significance was adopted (p < .05, uncorrected), which revealed differential responses in the amygdala bilaterally (Figure 3C).
Figure 3.
Voxelwise, group maps illustrating the contrast unpleasant-viewing vs. neutral-viewing. A) Visual cortex (MNI coordinates: −38,−84,8). B) Middle insula (MNI coordinates: −32,−14,16). C) Amygdala left (MNI coordinates: −26,−4,−14) and right (MNI coordinates: 32, −4, −18). The color scale represents z values.
Emotional modulation of the circuit involved in target detection
The behavioral results revealed a long-lasting slow down of target detection when performed during the unpleasant condition. We thus interrogated motor-related regions identified via the contrast of detection-only vs. baseline for potential effects of emotional picture viewing. Specifically, a set of ROIs defined via the contrast detection-only vs. baseline (see Table 1) was probed with the contrast unpleasant-detection vs. neutral-detection, which revealed increased responses in the left precentral gyrus, left middle frontal gyrus, and bilateral putamen (Figure 2). This analysis thus revealed that most of the neural circuit engaged by the target-detection task exhibited increased responses when target detection was performed following the viewing of unpleasant pictures.
Areas recruited during task performance in the unpleasant block
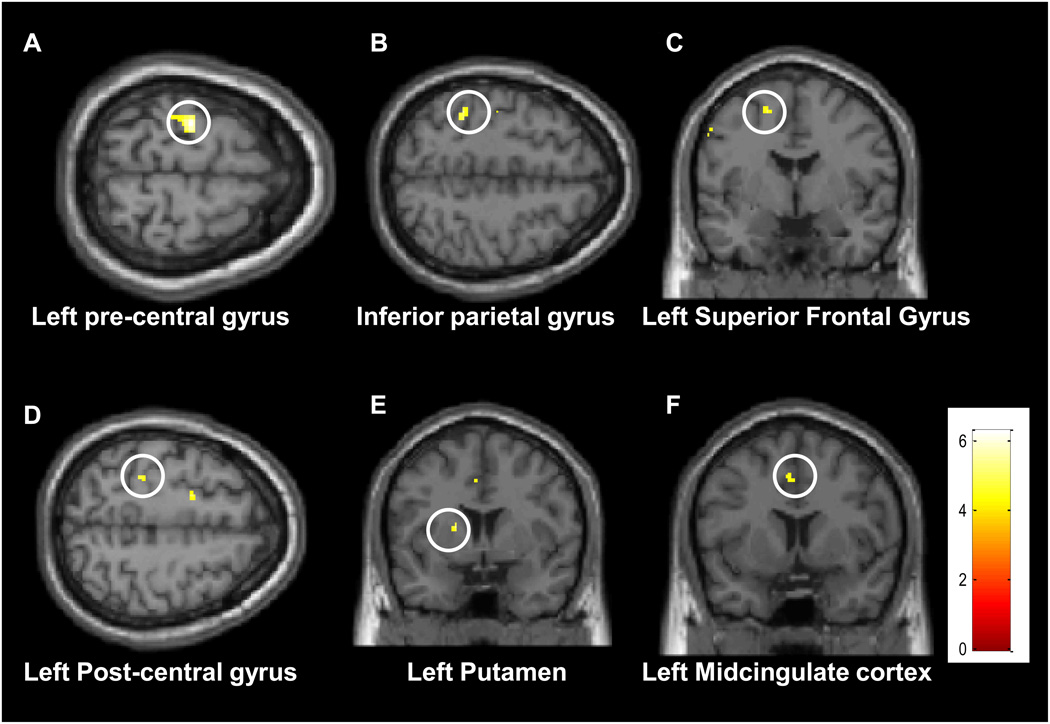
Were additional brain regions recruited during target detection within the unpleasant block? To answer this question, we conducted a whole-brain analysis contrasting the unpleasant-detection and neutral-detection conditions (Figure 5). Increased activation during the unpleasant-detection condition was observed in the inferior parietal gyrus, precentral gyrus, postcentral gyrus, midcingulate cortex, superior frontal gyrus, and putamen, all in the left hemisphere. Note that not all areas observed in the ROI analysis above were identified here because they did not reach the statistical threshold of the voxelwise analysis.
Figure 5.
Voxelwise, group maps illustrating the contrast unpleasant-detection vs. neutral-detection. See table 1 for MNI coordinates of each cluster.
Signal modulation in the midcingulate cortex parallels behavioral interference
The recruitment of the midcingulate cortex when participants performed the target-detection task during an aversive context was particularly interesting considering that this area has been suggested as a critical node in the interaction between emotion and motor systems (Morecraft et al., 1998; Morrison et al., 2007; Paus, 2001). To investigate whether signal responses in this region were related to the behavioral RT interference, we analyzed midcingulate fMRI data in a manner analogous to that done with the behavioral data – specifically, by considering responses for average target-detection trials 1 through 4 (i.e., original trials 1–2, 3–4, 5–6, and 7–8 were averaged producing “average trials” 1–4). A cluster of activation in the midcingulate cortex identified via the contrast all-conditions vs. baseline was used to create an ROI for this region (peak voxel: x= −8, y=12, z= 46; Figure 6B). A 2 valence (unpleasant, neutral) × 4 target-detection trials (1– 4) repeated-measures ANOVA revealed a statistically significant main effect of valence [F(1,10) = 4.87 p = .05)] only. The estimated regression coefficients during the unpleasant and neutral conditions are illustrated in Figure 6C, suggesting that midcingulate cortex responses paralleled the behavioral interference results. The relatively sustained increase in activity during the unpleasant block when participants were performing target-detection trials are further illustrated in Figure 4C.
To further explore the relationship between midcingulate responses and behavioral interference, we tested whether subjects with stronger evoked responses in the midcingulate ROI also exhibited the largest behavioral interference effects. Indeed, when differences in RT (unpleasant – neutral) were regressed on regression coefficients (unpleasant – neutral), a linear trend approached significance (r = .62; p = .07) (Figure 6D).
Correlations between mid-insula responses during picture viewing and midcingulate responses during target detection
Finally, we tested whether the emotional modulation observed in the midcingulate cortex during target-detection performance was linked to responses evoked during the viewing of unpleasant stimuli. We reasoned that an area that responded more strongly during unpleasant picture viewing would possibly convey the information about the aversiveness of the context to the midcingulate cortex. To probe this, we conducted a correlation analysis between the clusters activated by the contrast unpleasant-viewing vs. neutral-viewing (see Table 1) and the cluster of evoked activity in the midcingulate cortex as defined via the contrast unpleasant-detection vs. neutral-detection. Significant correlations were observed between the middle insula and the left midcingulate cortex (r = .83; p < .005) and between the left amygdala and the left midcingulate cortex (r = .67; p < .05) (Figure 7). No significant correlation was observed between early visual cortex, which was also more strongly engaged during unpleasant viewing, and the midcingulate cortex (r < 0.2).
DISCUSSION
The objective of the present study was to investigate interactions between affective and motor processes. As we were interested in the potential emotional modulation of output systems, we chose a simple detection task not involving additional processing stages, such as those involving discrimination and/or response choice. The behavioral data replicated our previous study (Pereira et al., 2006) and revealed a sustained interference effect when a simple target-detection task was executed following the viewing of unpleasant pictures. Brain activity was modulated by the emotional context across most regions engaged by the target-detection task. Interestingly, modulation of activity in the midcingulate cortex during target detection seemed to mirror the pattern of behavioral interference. Differential (unpleasant vs. neutral) evoked activity in this structure was relatively sustained during the detection phase and was correlated with the magnitude of the behavioral interference. Furthermore, emotional modulation in the midcingulate cortex during target detection was correlated with emotional modulation of mid-insula activity during picture viewing. Taken together, our findings suggest that the cingulate cortex is an important site for the interaction between negatively valenced and motor information.
Brain areas modulated by unpleasant picture viewing
The contrast of unpleasant vs. neutral viewing revealed increased responses in visual cortex, amygdala, and middle insula. The ability of emotional stimuli to modulate ongoing visual processing is well documented and all of occipito-temporal cortex appears to be robustly modulated by stimulus valence (Bradley et al., 2003; Ishai, Pessoa, Bikle, & Ungerleider, 2004; Lane, Chua, & Dolan, 1999; Lang et al., 1998; Mourao-Miranda et al., 2003; Pessoa et al., 2002). Increased responses in the amygdala during unpleasant vs. neutral picture viewing is, of course, consistent with a considerable body of data that has documented how this structure is involved in affective processing (for review, see (Phan, Wager, Taylor, & Liberzon, 2002)) – note, however, that amygdala activation was weak and only detected at less stringent thresholds (see section “Limitations of the present study” below).
Finally, the middle insula has been associated with a range of functions, including viscerosensation (Craig, 2003) and pain perception (Coghill, Sang, Maisog, & Iadarola, 1999; Peyron, Laurent, & Garcia-Larrea, 2000). Indeed, it has been recently suggested that the middle insula integrates homeostatic representations with activity that is linked with emotionally salient information (Craig, 2009). In general, the insula may serve to monitor the ongoing internal emotional state of the organism and to integrate sensory information with motivational salience to guide behavioral responses (Craig, 2009; Damasio, 1999).
Midcingulate cortex as a pivotal node of emotional and motor interaction
The contrast of unpleasant-detection vs. neutral-detection revealed an activation site in the midcingulate cortex. In addition, differential midcingulate activity appeared to be sustained during the target-detection phase, a pattern similar to that observed behaviorally (RT data). These findings suggest that, in the present paradigm, the midcingulate may have integrated emotional and motor signals, an interpretation consistent with a growing body of work, as discussed below. Here, the focus of activation observed during the unpleasant-detection condition (vs. neutral) was located at the aMCC/pMCC border (i.e., extending anteriorly to the aMCC), two subdivisions of the midcingulate cortex proposed by Vogt (for a review see Vogt, 2005). Both subdivisions are important sites observed in pain studies (Strigo, Duncan, Boivin, & Bushnell, 2003; Svensson, Minoshima, Beydoun, Morrow, & Casey, 1997; Vogt, Berger, & Derbyshire, 2003). Responses in the aMCC are enhanced by fear, too (Vogt et al., 2003). Vogt suggests that the “aMCC coordinates fear and avoidance with skeletomotor activity through the rostral cingulate motor areas” (Vogt et al., 2003). More generally, the cingulate motor cortex receives widespread emotion-related input, including signals from the orbitofrontal cortex and insula (Morecraft et al., 1998). Indeed, the importance of the cingulate motor area as a pivotal point where emotion-related signals influence the voluntary motor system was also advanced by Morecraft and Van Hoesen (1998), see also (Paus, 2001)) .
Interestingly, in the present study, mid-insula activity during the viewing of unpleasant pictures was positively correlated with midcingulate responses when participants performed the target-detection task within the same block. If we consider responses in the middle insula as an index of the emotional impact of picture viewing, the observed correlation suggests that such emotional impact might be predictive of the subsequent behavioral interference effect. We thus suggest that the middle insula might have been involved in mapping homeostatic and negatively valenced representations, and that it conveyed such information to “motivational drive” representations in the midcingulate cortex and other cingulate sites (Craig, 2003; Craig, 2009; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). Although tentative, this interpretation may be further assessed with network-related analysis techniques, such as dynamic causal modeling (Friston, Harrison, & Penny, 2003), and may profit from larger sample sizes (see also Taylor, Seminowicz, & Davis, 2008), for evidence of functional interactions between the middle insula and midcingulate cortex). It should be pointed out that the middle insula also exhibited activation during the target-detection phase when a more lenient statistical threshold was adopted (p < .005, uncorrected. Indeed, it appears that responses in this structure were also somewhat sustained following the picture-viewing phase, and extended into the target-detection phase (Figure 4). See also Wendt, Lotze, Weike, Hosten, & Hamm (2008), for evidence of sustained activation of the insula during blocked presentation of emotional stimuli.
Performing the target-detection task during the unpleasant block engaged additional regions of the motor network, including the primary motor cortex and bilateral putamen. As the cingulate motor cortex projects to these and other motor structures (Morecraft et al., 1997; Morecraft & Van Hoesen, 1992), one may hypothesize that, in our experiment, the midcingulate conveyed affective information to other components of the voluntary motor system network during a negative context.
Interference effects induced by emotional stimuli have been often interpreted in terms of attention (Vuilleumier, 2005). Accordingly, an alternative explanation for the present behavioral effects by unpleasant pictures is that they captured attention, reducing the resources available for the subsequent target-detection task. Interference effects observed for the very first target-detection trial are indeed consistent with the timing associated with attentional processes, but attention likely does not account for the longer-lasting interference effect described here. In general, selective attention effects are relatively fast and transient – e.g., attentional dwell time is suggested to last on the order of 500 ms or slightly longer (600–800 ms) (Muller, Teder-Salejarvi, & Hillyard, 1998). Thus we favor the interpretation that the origin of this longer-lasting interference effect is not attentional – at least in terms of a traditional attentional role – but instead associated with the induction of an emotional state that is possibly linked to the activation of defensive responses, as outlined next.
Midcingulate cortex and the implementation of defensive responses
Our findings of the modulation of midcingulate responses by negatively valenced information during the target-detection task also deserve to be discussed in the context of recent studies that indicate that this region is involved in the implementation of defensive responses. For instance, Morrison et al. (Morrison et al., 2007) asked participants to execute or suppress a motor response after viewing short animations depicting a noxious implement (e.g., a sharp knife) or an innocuous implement (e.g., a butter knife) striking a person’s hand. The combination of the implement’s noxiousness and whether it contacted the hand strongly affected reaction times. Responses in the midcingulate cortex mirrored this behavioral interaction pattern, leading the authors to suggest that distinct areas within the cingulate gyrus, which were also engaged, may work together during unpleasant contexts to recognize the aversive nature of the event, to mount an appropriate motor response, and to modulate this response according to current task constraints. In this context, it should be noted that the unpleasant stimulus category employed here involved mutilation pictures, which may be among the most emotionally evocative (Bradley et al., 2001; Sarlo, Buodo, Poli, & Palomba, 2005).
More direct evidence of the involvement of the cingulate cortex with the expression of defensive responses was reported in a study by Kalin et al. (2005). In this study, freezing behavior was investigated in monkeys, and it was found that responses in the dorsal ACC were positively correlated with freezing duration in aversive contexts. Another study addressed the role of the cingulate cortex in the expression of fear responses in humans (Milad et al., 2007). Increased activation that included the midcingulate cortex by a conditioned fear stimulus was reported and these signals were positively correlated with increased fear responses (as indexed via skin conductance responses), suggesting that the cingulate cortex might be involved in the expression of fear responses in general. Indeed, in a recent compilation of activation sites observed in conditioning studies, we have shown that cingulate sites, including the midcingulate cortex, are frequently engaged during the processing of aversive stimuli (Engelmann, Damaraju, Padmala, & Pessoa, 2009; Pessoa, 2009)– an observation that, together with the findings of the present study and those reviewed here (see also Sommer, Hajak, Dohnel, Meinhardt, & Muller, 2008), challenges the popular notion that more anterior and posterior portions of the anterior cingulate cortex are only specialized for affective and cognitive information, respectively (Bush, Luu, & Posner, 2000). Taken together, these studies suggest that the cingulate cortex, including the midcingulate cortex observed in the present study, likely also has an important role in the implementation of defensive behaviors, which involve the integration of negatively valenced and motor information.
In conclusion, we can hypothesize that during the unpleasant block, the midcingulate cortex participated in the implementation of a defensive response, possibly a freezing-like response. Viewing an injured individual (as present in the mutilation pictures) may signal a potential life threat in the environment. Thus, it is possible that emitting a motor response (as during the target-detection task) that was unrelated to this defensive context involved increased activation in motor-related areas and produced behavioral interference. Evidence for the induction of a freezing-like response in humans by mutilation pictures was recently reported (Azevedo et al., 2005). Posturographic and electrocardiographic recordings revealed immobility, rigidity, and bradycardia during the viewing of mutilation pictures (see also Facchinetti, Imbiriba, Azevedo, Vargas, & Volchan, 2006).
In this context, an additional alternative hypothesis for the findings reported here is worth discussing. Specifically, the increased midcingulate activity during the unpleasant condition may have reflected response conflict. According to this idea, midcingulate responses may reflect the detection of the conflict between the action required by the imperative stimulus (i.e., a motor response) and an avoidance response, possibly freezing, evoked by the aversive context. Hence, increased midcingulate cortex signals might reflect the conflict generated by the co-activation of these two “responses”. It is well known that the anterior cingulate cortex (ACC) is engaged during the monitoring and/or detection of conflict (Barch, Braver, Sabb, & Noll, 2000; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 2000; Casey et al., 2000). It is noteworthy, however, that according to Pickard and Strick (2001), on average, the sites of conflict-related activations at the time were located 24 mm ± 7 mm (mean ± standard deviation) anterior to the level of the anterior commissure. It thus appears that the present activation site of the midcingulate cortex is posterior to those observed during conflict tasks (see (Picard & Strick, 2001)).
Limitations of the present study
A limitation of the present study refers to the extent to which the present results generalize to other types of emotional stimuli. In the present study as in our previous one (Pereira et al., 2006), we choose mutilation pictures because we intended to evoke strong aversive reactions. Naturally, a disadvantage of our design is that the results cannot be generalized to other classes of stimuli. We note, however, that in a previous study that used a very similar paradigm, pleasant pictures of babies and of family interactions actually produced an acceleration of RT (instead of a slow-down) when stimuli were presented in a blocked fashion as here (Pereira et al., 2006). These findings lend credence to the notion that the present results are valence-specific, although such suggestion is tentative at present.
A second important limitation of the present study is that some of the results concerning the amygdala – a critical structure in the processing of affective stimuli – were only observed at lenient statistical thresholds. Although we do not advocate the usage of different statistical thresholds in general, in the present study we considered this strategy acceptable given that a vast literature has identified amygdala responses in similar conditions to those probed here. In other words, given the extant literature, it is unlikely that the observed responses in the amygdala reflect a gross Type I statistical error.
Implications for affective disorders
Understanding how negative emotion impairs behavior and the underlying neural circuits has important implications for affective disorders, especially depression, which is characterized by persistent negative mood and selective cognitive and behavioral disturbances. For example, depressed patients show a failure to inhibit negative information (Goeleven, De, Baert, & Koster, 2006), which might be related to decreased behavioral adjustments. Our findings revealed that the midcingulate cortex was more strongly engaged during the unpleasant context and activation of this region has been shown to be involved in the ability to adaptively regulate freezing as a function of a changing context (Kalin et al., 2005). Interestingly, it has been recently suggested that the anterior midcingulate cortex might play a protective role in the pathogenesis of depression, as individuals with reduced gray matter volume and functional activation of this region might be predisposed to greater severity of depression symptoms (Chen et al., 2007). Given that the midcingulate cortex might be involved in adaptively regulating freezing, it would be potentially valuable to test whether these patients exhibit abnormalities in the engagement of motor circuits during emotion-laden contexts, such as studied here. It is conceivable, for instance, that depressed patients exhibit an enhanced freezing-like state (in comparison to non-clinical individuals).
Given the high co-morbidity between depression and anxiety disorders, our findings may also be of interest in the context of the latter condition. Recent studies have investigated the role of the ACC in generalized anxiety disorder and have reported that heightened activity in this region may be “adaptive” (Nitschke et al., 2009; Whalen et al., 2008). For instance, patients with increased ACC activity exhibited greater reductions in anxiety and worry symptoms after pharmacological treatment. As suggested by Nitschke et al (2009), increased activity in the ACC may be an indication of unimpaired top-down regulation in patients with more favorable outcomes. It is important to bear in mind, however, that the cingulate cortex findings observed here were posterior to the ACC, although the two regions are strongly interconnected (Morecraft et al., 1998).
Concluding remarks
As shown previously, the sight of mutilation pictures leads to strong physiological reactions and evaluative reports of high arousal and unpleasantness (Bradley et al., 2001). We might conjecture that, in the present study, these alterations of body physiology were represented by the middle insula and that these negatively valenced signals were complemented by a paralleled motivational drive for action triggered by the midcingulate cortex. An appropriate response in this context is the installation of a defense pattern, probably freezing, as discussed above. In our task, the immobility aspect of the defense pattern probably competed with the requirement to emit a motor response, possibly resulting in an increased recruitment of the motor circuits necessary to execute the task. Our results suggest that, besides the privileged capture of attention and consumption of processing resources (Pessoa et al., 2002; Vuilleumier, 2005), viewing unpleasant stimuli also modulates output systems. Thus, emotional modulation by negative stimuli, and particularly behavioral interference, might be partially a reflection of the prompting of defensive responses and how they are related to ongoing behaviors.
Acknowledgments
We thank the anonymous reviewers for their careful feedback and Rita de Cássia Soares Alves for collecting the behavioral data of subjects who performed the task outside the scanner. This work was supported by funds from federal and state Brazilian research agencies (CNPq, CAPES, PROCAD, PRONEX/FAPERJ, FAPERJ and IBN-NET FINEP). L.P. was supported in part by the National Institute of Mental Health (MH071589).
Reference List
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Azevedo TM, Volchan E, Imbiriba LA, Rodrigues EC, Oliveira JM, Oliveira LF, et al. A freezing-like posture to pictures of mutilation. Psychophysiology. 2005;42:255–260. doi: 10.1111/j.1469-8986.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoring of response conflict: Evidence from an fMRI study of overt verb generation. Journal of Cognitive Neuroscience. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Willi M, Jancke L. Modulation of corticospinal activity by strong emotions evoked by pictures and classical music: a transcranial magnetic stimulation study. Neuroreport. 2007;18:261–265. doi: 10.1097/WNR.0b013e328012272e. [DOI] [PubMed] [Google Scholar]
- Becker MW. Panic Search: Fear Produces Efficient Visual Search for Nonthreatening Objects. Psychological Science. 2009;20:435–437. doi: 10.1111/j.1467-9280.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion Improves and Impairs Early Vision. Psychological Science. 2009;20:707–713. doi: 10.1111/j.1467-9280.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: effects of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J.Behav.Ther.Exp.Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butler T, Pan H, Tuescher O, Engelien A, Goldstein M, Epstein J, et al. Human fear-related motor neurocircuitry. Neuroscience. 2007;150:1–7. doi: 10.1016/j.neuroscience.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biological Psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. Journal of Neurophysiology. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Review Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. The feelings of what happens: Body and emotion in the making of consciousness. 1 ed. New York: Harcourt Brace; 1999. [Google Scholar]
- Darwin C. The expression of emotions in man and animals. London: John Murray; 1872. [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009 doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erthal FS, de OL, Mocaiber I, Pereira MG, hado-Pinheiro W, Volchan E, et al. Load-dependent modulation of affective picture processing. Cogn Affective Behavioral Neuroscience. 2005;5:388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Facchinetti LD, Imbiriba LA, Azevedo TM, Vargas CD, Volchan E. Postural modulation induced by pictures depicting prosocial or dangerous contexts. Neuroscience Letters. 2006;410:52–56. doi: 10.1016/j.neulet.2006.09.063. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Goeleven E, De RR, Baert S, Koster EH. Deficient inhibition of emotional information in depression. Journal of Affective Disorders. 2006;93:149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:2621–2635. doi: 10.1016/j.neuropsychologia.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, Nahas Z. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44:91–97. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia. 2000;38:1576–1580. doi: 10.1016/s0028-3932(00)00072-5. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Science U.S.A. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Experimental Brain Research. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Verschuere B, De HJ. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behavioral Research Therapy. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. Gainesville FL: Center for study of emotion and attention NIMH; 1997. [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Schroeder CM, Avramov K. Segregated parallel inputs to the brachial spinal cord from the cingulate motor cortex in the monkey. Neuroreport. 1997;8:3933–3938. [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. Journal of Comparative Neurology. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Research Bulletin. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- Morrison I, Peelen MV, Downing PE. The sight of others’ pain modulates motor processing in human cingulate cortex. Cerebral Cortex. 2007;17:2214–2222. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Volchan E, Moll J, de Oliveira-Souza R, Oliveira L, Bramati I, et al. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20:1955–1963. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Muller MM, Teder-Salejarvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nature Neuroscience. 1998;1:631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory Activation in the Amygdala and Anterior Cingulate in Generalized Anxiety Disorder and Prediction of Treatment Response. American Journal of Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, et al. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Experimental Brain Research. 2003;149:214–221. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Review Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pereira MG, Volchan E, de Souza GG, Oliveira L, Campagnoli RR, Pinheiro WM, et al. Sustained and transient modulation of performance induced by emotional picture viewing. Emotion. 2006;6:622–634. doi: 10.1037/1528-3542.6.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MG, Volchan E, Oliveira L, hado-Pinheiro W, Rodrigues JA, Nepomuceno FV, et al. Behavioral modulation by mutilation pictures in women. Brazilian Journal of Medical and Biological Research. 2004;37:353–362. doi: 10.1590/s0100-879x2004000300011. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cognitive Science. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Research Cognitive Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiologie Clinique. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Current Opinion in Neurobiology. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Buodo G, Poli S, Palomba D. Changes in EEG alpha power to different disgust elicitors: the specificity of mutilations. Neuroscience Letters. 2005;382:291–296. doi: 10.1016/j.neulet.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Sommer M, Hajak G, Dohnel K, Meinhardt J, Muller JL. Emotion-dependent modulation of interference processes: an fMRI study. Acta Neurobiologiae Experimentalis (Wars.) 2008;68:193–203. doi: 10.55782/ane-2008-1688. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. Journal of Neurophysiology. 2003;89:3294–3303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
- Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans. Journal of Neurophysiology. 1997;78:450–460. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2008 doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipples J, Sharma D. Orienting to exogenous cues and attentional bias to affective pictures reflect separate processes. British Journal Psychology. 2000;91(Pt 1):87–97. doi: 10.1348/000712600161691. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Review Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cognitive Science. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives in Psychological Science. doi: 10.1111/j.1745-6924.2009.01125.x. (in press) [DOI] [PubMed] [Google Scholar]
- Wendt J, Lotze M, Weike AI, Hosten N, Hamm AO. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45:205–215. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biological Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]