Abstract
Fatty liver is commonly associated with alcohol ingestion and abuse. While the molecular pathogenesis of these fatty changes is well understood, the histochemical and pharmacological mechanisms by which ethanol stimulates these molecular changes remain unknown. During ethanol metabolism, adenosine is generated by the enzyme ecto-5′-nucleotidase, and adenosine production and adenosine receptor activation are known to play critical roles in the development of hepatic fibrosis. We therefore investigated whether adenosine and its receptors play a role in the development of alcohol-induced fatty liver. WT mice fed ethanol on the Lieber-DeCarli diet developed hepatic steatosis, including increased hepatic triglyceride content, while mice lacking ecto-5-nucleotidase or adenosine A1 or A2B receptors were protected from developing fatty liver. Similar protection was also seen in WT mice treated with either an adenosine A1 or A2B receptor antagonist. Steatotic livers demonstrated increased expression of genes involved in fatty acid synthesis, which was prevented by blockade of adenosine A1 receptors, and decreased expression of genes involved in fatty acid metabolism, which was prevented by blockade of adenosine A2B receptors. In vitro studies supported roles for adenosine A1 receptors in promoting fatty acid synthesis and for A2B receptors in decreasing fatty acid metabolism. These results indicate that adenosine generated by ethanol metabolism plays an important role in ethanol-induced hepatic steatosis via both A1 and A2B receptors and suggest that targeting adenosine receptors may be effective in the prevention of alcohol-induced fatty liver.
Keywords: Adenosine, CD39, CD73, ethanol, extonucleotidase, fibrosis, fructose, hepatic stellate cell, inflammation, insulin resistance, liver, knockout, liver fibrosis, NAFLD, NASH, nucleotide, PPAR, receptor, steatosis, T cell
New approaches and therapies are needed to both dampen hepatic inflammation and to prevent progression to cirrhosis in patients with fatty liver and other chronic liver diseases [1].
In this manuscript and prior studies, Peng et al. [2,3], note that heightened extracellular adenosine levels, induced as a result of ethanol or fructose metabolism via interactions with two of the P1-adenosinergic receptors, significantly contribute to ethanol-induced fatty liver disease. Adenosine and other purinergic mediator effects on the liver are closely regulated by cell surface ectonucleotidases (i.e., ecto-AMPase/CD73, ecto-ADPases and ecto-ATPases, mainly CD39). These ecto-enzymes hydrolyze extracellular nucleotides in a tandem manner, ultimately generating adenosine [4]. Extracellular nucleotides are considered a major source of adenosine while membrane transporters that serve as scavengers also regulate concentrations of these mediators [5]. Adenosine often exerts opposing effects to extracellular nucleotides (ATP, ADP, UTP, and UDP) that bind type-2 purinergic/pyrimidinergic (P2Y G-protein coupled) receptors and P2X ATP-gated cation channels. Adenosine and extracellular nucleotides are recognized by a variety of P1-adenosine and P2-receptors on hepatocytes, platelets, endothelium, and vascular smooth muscle, hepatic stellate and immune cells (reviewed in [4]). Thus, all hepatic cells express components of the purinergic signaling apparatus that could impact metabolic and associated inflammatory responses, including glucose release, protein and glutathione synthesis, hepatic regulation of renal Na+/water excretion, and portal blood flow [4,6].
Here, mice were fed an alcohol containing liquid diet, Lieber-deCarli, to investigate whether ethanol-induced adenosine release stimulates hepatic steatosis. The underlying mechanisms were then studied. The development of fatty liver was almost completely blocked by adenosine A1 or A2B receptor antagonists. Further evidence was derived from studies using mice deficient in either of these adenosine receptors or ecto-5’-nucleotidase that were protected from alcohol-induced hepatic steatosis.
In the liver, as in other tissues, ethanol (or fructose) ingestion interfered with adenosine uptake and also resulted in an ecto-enzyme dependent increase in adenosine concentrations and mRNA for the A1, A2B and A2A receptors. Adenosine was further shown to impact expression of key enzymes in lipid metabolism, and thereby promotes hepatic steatosis. Chronic ethanol ingestion increased mRNA levels of SREBP1 and PPARγ, and downstream expression of ACL, and FAS in livers from WT, A2B null, and enprofylline (A2B receptor antagonist) treated mice. In parallel, adenosine A1 receptor deletion or blockade using DPCPX prevented the ethanol-induced increase in expression of these genes. In contrast, ethanol ingestion reduced mRNA expression of PPARα, CPTL, and ACCA in the livers of WT, A1 null, and DPCPX-treated mice, while blockade or deletion of A2B receptors abrogated these ethanol-induced decreases. Alcohol ingestion was also shown to diminish AMP-kinase (AMPK) phosphorylation in mouse liver, an effect shown to be A2B receptor-dependent.
Studies carried out in vitro on a hepatocytic cell line provided additional mechanistic data. Adenosine A1 receptor activation stimulated SREBP1 translocation to the nucleus, thereby promoting fatty acid synthesis and accumulation, whereas A2B receptor activation diminished phosphorylation and activation of AMPK and downregulated nuclear PPARα, thereby inhibiting fatty acid utilization and transport. The net result of increased fatty acid synthesis and diminished utilization clearly explain the pathogenesis of steatosis in alcoholic (and likely nonalcoholic) hepatic steatosis (Fig. 1).
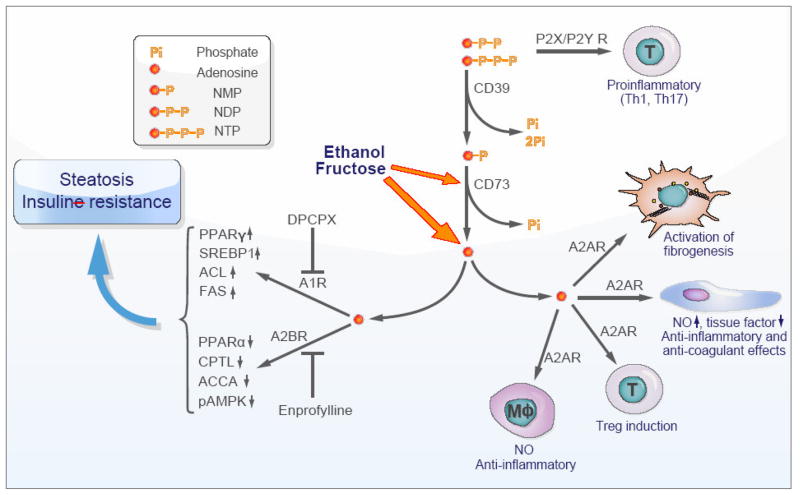
Fig. 1. Scheme depicting the possible role of ectonucleotidases and purinergic receptors in the evolution of hepatic steatosis, inflammation and fibrosis.
Extracellular nucleotides (chiefly di- and triphosphate nucleosides) bind type 2 purinergic receptors on vascular cells, platelets, and immune cells to induce T helper (Th)1 and Th17 cells. These purinergic mediators are scavenged and converted to adenosine via the actions of CD39 and CD73 ectonucleotidases. With chronic exposure to ethanol or fructose, excess extracellular adenosine seems to be generated by the liver in a CD73-dependent manner from AMP (the product of CD39 phosphohydrolysis of NTP and NDP). Adenosine binds to several type 1 purinergic receptors to often exert opposing effects to those seen with extracellular nucleotides. Liver enzymes involved in fatty acid syntheses are upregulated and those of lipid degradation downregulated via signaling of A1 and A2B receptors, respectively, which in turn induces hepatic steatosis. Furthermore, chronic exposure of hepatic stellate cells/myofibroblasts to adenosine drives fibrogenic activation via A2A receptors. Adenosine has other opposing effects in that this nucleoside dampens acute inflammatory responses in endothelial cells or macrophages via A2A receptors and also induces immunosuppressive regulatory T cells (Treg).
NO, nitric oxide; Mφ, macrophage. For the other abbreviations refer to the text.
Furthermore, adenosine, acting via A2A receptors, is already known to promote hepatic fibrosis in response to toxins like thioacetamide and carbon tetrachloride [3,7]. Somewhat dualistically, adenosine stimulates the fibrogenic potential of hepatic stellate cells, the major producers of the fibrotic extracellular matrix, by upregulating production of procollagen I, and fibrogenic growth factors such as CTGF and TGF-β but also provides a proliferative “stop” signal to these activated cells [8,9]. Many of the effects of methotrexate, known to cause hepatic and pulmonary fibrosis, may be associated with enhanced release of adenosine [10].
These data further suggest that adenosine receptor antagonism may provide a novel approach for the treatment and prevention of alcoholic and, possibly, nonalcoholic fatty liver disease. Of note, chronic use of caffeine, a nonselective adenosine receptor antagonist, has been associated with decreased hepatic fibrosis [11].
The major ectonucleotidases expressed by hepatic vascular endothelium, vascular pericytes, and leukocytes belong to the CD39 family, or are alkaline phosphatases and ecto-5’-nucleotidases such as CD73 [4]. Livers from CD73-deficient mice fed ethanol or fructose release significantly less adenosine than wild type mice and are partly protected from developing fatty liver disease and toxin-induced hepatic fibrosis [2,3,7]. Of note, CD39, which is upstream of ecto-5’-nucleotidase/CD73 in the ultimate generation of adenosine, has comparable effects (to CD73) in promoting both experimental pancreatic [12] and liver fibrosis (unpublished observations).
In contrast to these deleterious manifestations associated with adenosine as shown here, vascular CD39, CD73, and soluble adenosine have salutary effects in dampening acute inflammation, immune responses, and in limiting vascular injury and platelet activation seen in the setting of organ ischemia and reperfusion [4,13]. As noted above, Peng et al. also suggest that their findings may be applicable to forms of non-alcoholic hepatic steatosis and steatohepatitis (NASH), as linked with diabetes and hyperlipidemia. Curiously, it is known that deletion of CD39 (that would limit adenosine formation) results in insulin resistance [14], with several features of the metabolic syndrome and a tendency towards heightened vascular inflammation.
How such divergent salutary and deleterious effects are mediated over time and at different tissue sites remains currently unclear. It is feasible that experimental outcomes may be quite different when tissues are exposed to rapid, acute vs. slow, chronic increases in extracellular levels of nucleotides and the resulting nucleoside fluxes.
A possible take home message is that adenosine can be protective in the acute inflammatory setting but also participates in a final common pathway leading to development of hepatic steatosis, fibrosis, and ultimately to cirrhosis. Some of the divergent elements of the purinergic response are susceptible to intervention, especially with the availability of specific receptor antagonists. These pharmacological tools and animal models will permit to determine how the temporospatial effects of adenosine are regulated in liver diseases and likely lead to novel targeted therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 2.Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L, Blackburn MR, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, Cronstein BN. Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. Nucleosides Nucleotides Nucleic Acids. 2008;27:821–824. doi: 10.1080/15257770802146403. [DOI] [PubMed] [Google Scholar]
- 4.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 6.Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology. 1999;116:964–979. doi: 10.1016/s0016-5085(99)70081-8. [DOI] [PubMed] [Google Scholar]
- 7.Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, Cronstein BN. Ecto-5′-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis. Faseb J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 8.Che J, Chan ES, Cronstein BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.038760. [DOI] [PubMed] [Google Scholar]
- 9.Hashmi AZ, Hakim W, Kruglov EA, Watanabe A, Watkins W, Dranoff JA, Mehal WZ. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G395–401. doi: 10.1152/ajpgi.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 51:201–209. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunzli BM, Nuhn P, Enjyoji K, Banz Y, Smith RN, Csizmadia E, Schuppan D, et al. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology. 2008;134:292–305. doi: 10.1053/j.gastro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153 (Suppl 1):S457–464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enjyoji K, Kotani K, Thukral C, Blumel B, Sun X, Wu Y, Imai M, et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]