Abstract
Background
We have previously shown in a model of pressure overload hypertrophy that there is increased cardiomyocyte apoptosis during the transition from peak hypertrophy to ventricular decompensation. Electron transport chain dysfunction is believed to play a role in this process through the production of excessive reactive oxygen species. In this study we sought to determine electron transport chain function in pressure overload hypertrophy and the role of oxidative stress in myocyte apoptosis.
Methods and Results
Neonatal rabbits underwent thoracic aortic banding at 10 days of age. Compensated hypertrophy (4wks of age), decompensated hypertrophy (6wks of age), and age-matched controls (n=4–8/group) as identified by serial echocardiography were studied. ETC complex activities were determined by spectophotometry in isolated mitochondria. Complex I was significantly decreased (p=0.005) at 4wks and further decreased at 6 wks (p=0.001). Complex II was significantly decreased at both time points (4wks p=0.003; 6wks p=0.009). However, H202 production, measured in isolated mitochondria by fluorescence spectroscopy, was significantly decreased at 4wks of age in banded animals compared to controls (p=0.038), and mitochondrial DNA oxidative damage (measurement of 8-OHdG by ELISA) was also significantly decreased at 4wks of age (p=.031). Mitochondrial activated apoptosis was determined by Bax/Bcl-2 ratios (immunoblotting). Bax/Bcl-2 levels were significantly increased in banded animals at 6wks.
Conclusions
In pressure overload hypertrophy, the transition from compensated LVH to failure and cardiomyocyte apoptosis is preceded by mitochondrial complex I and II dysfunction followed by an increase in Bax/Bcl-2 ratios. The mechanism of apoptosis initiation is independent of increased oxidative stress.
Ultramini Abstract
In neonatal pressure overload hypertrophy of the LV, electron transport chain dysfunction characterized by decreased activity of electron transport chain complexes I and II worsens with ventricular decompensation. The impaired electron transport chain function is associated with decreased oxidative stress and activation of the mitochondrial apoptosis pathway.
Introduction
Pressure overload hypertrophy is the ventricular response to chronically increased systolic stress in an attempt to normalize wall stress and maintain contractility. Left ventricular hypertrophy (LVH) is a risk factor for sudden death and progression to decompensated hypertrophy or heart failure and its associated morbidity and mortality.1 Cardiomyocyte loss through apoptosis is believed to play a role in the transition from compensated LV hypertrophy to decompensated LV hypertrophy. We have previously shown in a model of pressure overload hypertrophy that there is increased cardiomyocyte loss through apoptosis during the transition from peak hypertrophy to ventricular decompensation and dilation.2, 3 No clear mechanism has been identified but mitochondria present an attractive target for investigation because of their gatekeeper role in apoptosis as well as mounting evidence for electron transport chain (ETC)dysfunction in various models of heart failure.
Oxidative phosphorylation occurs via the electron transport chain located on the inner mitochondrial membrane. The ETC is composed of four electron transport protein complexes and the ATP-syntase which harnesses the proton motive force to generate ATP. Mitochondria isolated from failing myocardium have shown impaired capacity for respiration, various defects in ETC complexes, and decreased oxidative phosphorylation.4, 5
A by-product of oxidative phosphorylation is the generation of reactive oxygen species (ROS). The single electron reduction of molecular oxygen at various sites in the ETC results in superoxide formation.6, 7 Approximately 2% of all oxygen consumed in oxidative phosphorylation results in ROS.8 The superoxide anion generated at the ETC complexes is converted to H202 by the enzyme manganese superoxide dismutase located in the mitochondrial matrix. H202 can be further detoxified to H2O by other endogenous scavenging mechanisms such as catalase and glutathione peroxidase. There is evidence that low concentrations of superoxide can function ”physiologically” as an intracellular messenger in various redox sensitive signaling pathways including HIF-1a and PI-3Kinase/AKT.9, 10 Over production of ROS and/or inadequate endogenous scavenging mechanisms can result in mitochondrial and cellular damage ultimately leading to apoptosis.11
In ischemia-reperfusion injury, as well as models of acute myocardial infarction, mitochondrial dysfunction leads to increased ROS generation and resultant peroxidation of lipids, proteins, and DNA.12 Mitochondrial peroxidative damage results in release of pro-apoptotic factors and initiation of apoptosis. However, in the pressure loaded ventricle the relationship and possible mechanism between mitochondrial dysfunction and apoptosis remains unclear. We hypothesize that the ETC chain impairment is the cause of mitochondrial dysfunction in hypertrophied myocardium and subsequent myocyte apoptosis. In this study we sought to also determine whether ROS production plays a role in the mechanism of apoptosis.
Methods
Left Ventricular Hypertrophy Model
Pressure-Overload Hypertrophy was achieved by placing a silk ligature around the descending thoracic aorta of 10 day-old New Zealand White rabbits (Millbrook Farms; Amherst, Mass). The morphologic (heart weight and heart weight/body weight ratios) and the echocardiographic performance over time of this model has been previously described and validated in more detail by our group.2, 13, 14 Implanting a fixed constriction in an immature animal and allowing it to grow induced pressure-overload hypertrophy by 3–4 weeks of age in this model. The progression of LV hypertrophy was determined by weekly transthoracic echocardiography. During these procedures, the animals remained unsedated to avoid the influences of anesthetics on the results. Transthoracic echocardiography was used measure LV wall thickness and cavity volume as previously described.13, 15 To document the development of hypertrophy and progression to failure LV mass/volume ratios were used as previously described.14 As a measure of cardiac performance, shortening fraction was determined with the formula (diastolic diameter-systolic diameter)/diastolic diameter and is expressed as percentage.
Animals (n=4–8/ group of banded and age matched controls) were euthanized with anesthetic overdose (ketamine/Xylazine) and hearts were excised and placed on a Langendorff perfusion apparatus. After perfusion with Krebs-Henseleit solution for 5 minutes to wash out residual blood, the LV was frozen in liquid nitrogen and stored at −80° C for future use.
Mitochondria Isolation
Mitochondria were isolated from LV samples as previously described.16 Briefly, frozen LV samples were pulverized in liquid nitrogen and suspended in isolation media containing 300 mM sucrose, 10 mM K+-HEPES buffer, pH 7.2, and 1 mM K+-EGTA, pH 8.0. Samples were homogenized with glass grinding vessel for approximately 30 seconds. Nagarse (0.2mg/ml) was added to the homogenate which was incubated on ice for 10 min and then centrifuged for 5 min at 750 g at 4°C. The supernatant was saved and 1 mg/ml BSA was added. The supernatant was then recentrifuged for 4 min at 750 g at 4°C, and the pellet was discarded. The resulting supernatant was then centrifuged at 9,000 g for 10 min at 4°C. The mitochondrial pellet was then twice resuspended in ice-cold isolation media containing 1 mg/ml BSA and recentrifuged at 9,000 g at 4°C. The final pellet was suspended in 0.5–1ml of isolation media (no BSA). Mitochondrial protein concentrations were determined using the bicinchoninic acid method.
Immunoblotting
Protein levels of representative subunits of Complex I and II, Bax, and Bcl2, were determined by immunoblotting. The total tissue protein extracts were separated by gel electrophoresis with SDS-PAGE gels. Equal protein loading was confirmed by coomassie blue staining of the gels. Proteins were electrophorectically transferred to nitrocellulose membranes, incubated in 5% nonfat dry milk in TBST (10 mmol/L Tris HCl pH 7.4, 100mmol/L NaCl, 0.1% Tween 20) for 1 hour and then incubated overnight with primary antibody against complex I (Iron-sulfur protein,3 and ND6 protein from Invitrogen), complex II (SDHA flavoprotein from Invitrogen), Bax and Bcl-2 (Upstate). This was followed by incubation with horseradish peroxidase-conjugated secondary antibody with primary antibody at a dilution of 1:1000. The bound antibody was detected by enhanced chemiluminescence method according to the manufacturer’s protocol (GE Healthcare Live Sciences). After exposure on film, quantitative protein analysis was conducted using laser densitometry. Data are expressed as arbitrary densitometry units.
Electron Transport Chain Activity
Mitochondria ETC complex I, II, and III activities were measured by spectophotometry in isolated mitochondria as previously described.17 For complex I, mitochondrial (20μg/ml) underwent three cycles of freeze-thawing in hypotonic media (25mM potassium phosphate and 5mM MgCl2) and were then added to buffer containing 25mM KH2PO4, 5mM MgCl2, 2mM KCN, 2.5mg/ml BSA, 2μg/ml of antimycin A, and 0.13 mM NADH and the decrease in absorbance due to the oxidation of NADH was measured at 345nm. Rotenone (2μg/ml) was added and rotenone sensitive activity was recorded. Complex II activity was measured in mitochondrial after three cycles of freeze-thawing in hypotonic media (25mM potassium phosphate and 5mM MgCl2) and then preincubated in buffer containing 25mM KH2PO4, 5mM MgCl2, and 20mM succina te for 10mins at 30° C followed by addition of antimycin A (2μg/ml), rotenone (2μg/ml), 2mM KCN, and 50μM dichlorophenolindophenol. The reaction was catalyzed by addition of 65μM ubiquinone and the reduction of dichlorophenolindophenol was measured at 600nm for 2–3 mins. Complex III activity was determined by measuring the increasing reduction of cytochrome c at 550nm. Mitochondrial were added to buffer containing 25mM KH2PO4, 5 mM MgCl2, 2mM KCN, 2 μg/ml rotenone, and 15 μM cytochrome c followed by the addition of ubiquinol (35μM). Complex IV activity was measured using cytochrome c oxidase kit (CYTOCOX1) from Sigma-Alrich.
Determination of ROS Production
ROS production by mitochondria was determined by measuring the generation of H202 using fluorescence spectroscopy with Amplex Red Hydrogen Peroxide/Peroxidase Assay from Invitrogen in isolated mitochondria using 5mM glutamate and 5mM Malate without the addition of ADP as the substrate for respiration. Mitochondria isolated from frozen tissue had 17–26% lower activity than mitochondria from fresh tissue, but the rate of the reaction was within an acceptable range. ROS mediated DNA damage was assessed by measuring 8-hydroxydeoxyguanosine (8-OHdG) in both nuclear and mitochondrial DNA by specific ELISA (8-OHdG Check Ultrasensitive ELISA, BioVendor). Isolation of nuclear DNA was performed by digestion with proteinase K and then extracted in chloroform/isoamyl alcohol and phenol as previously described.18 Mitochondrial DNA was obtained by treating isolated mitochondria with Proteinase K(10mg/ml) in SDS (54 mg/ml) followed by chloroform/isoamyl alcohol and phenol extraction and cold ethanol precipitation.18
Determination of Apoptosis by TUNEL
Cardiolmyocyte apoptosis was quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining. Left ventricular muscle paraffin sections from the midsection of the heart were deparaffinized, and then rehydrated with xylene and graded alcohol series. The sections were stained using the FragEL DNA fragmentation detection kit (EMD; Biosciences Inc, San Diego, Calif) per the manufacturer’s instructions. Briefly, the sections were incubated with terminal deoxynucleotidyl transferase and fluorescein-labeled dUTP. To identify the cardiomyocytes, sections were incubated with mouse desmin monoclonal antibody (Sigma-Aldrich, St. Louis, Mo), followed by incubation with a secondary anti-mouse immunoreagent conjugated to the red-fluorescent Alexa-594™ fluorophore at a concentration of 1:200 (Molecular Probes, Eugene Ore). Finally, to identify all nuclei (nonapoptotic and apoptotic), sections were stained with blue fluorescent DAPI nucleic acid stain (Molecular Probes). Cover-slips were applied to the sections with fluorescent mounting medium (Dako Corporation, Carpinteria, Calif). Slides were visualized using an Axiovert 35 Microscope with a Nikon 10x objective, NA=10x/0.25. Cardiomyocyte nuclei were determined by automatic counting with the computer software/image analyzer using the MetaMorph® Imaging System software (Universal Imaging Corporation, West Chester, Pa). Apoptotic nuclei were identified manually to determine that only apoptotic cardiomyocyte nuclei were included. Data are expressed as apoptotic nuclei per 1000 nuclei.
Animal Care
All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 86–23, revised 1996). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Children’s Hospital Boston.
Statistical Analysis
Data were analyzed using SPSS software package (version 15.0 SPSS Inc., Chicago, IL) and are reported as mean ± standard error of the mean (SEM). A two-tailed unpaired Student’s t-test was used for comparison between groups: at 4 weeks (control versus banded) and at 6 weeks (control versus banded) if normality was passedANOVA was used for analysis of the TUNEL staining where three groups were analysed (control, 4wks banded, and 6wks banded. A p-value of ≤0.05 was considered statistically significant.
Statement of Responsibility
The authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Results
Left Ventricular Hypertrophy Model
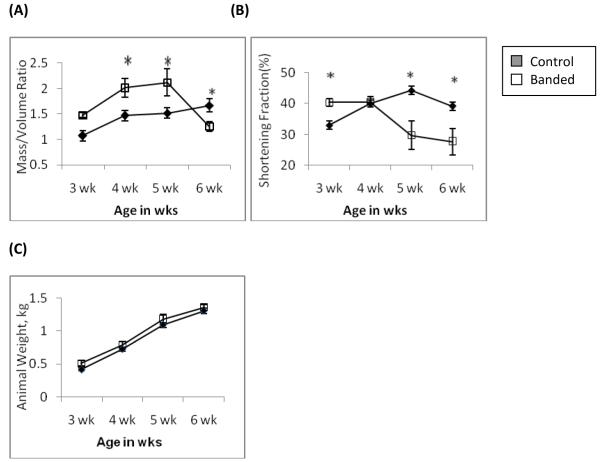
After aortic banding, animals developed peak LV hypertrophy at 4–5 weeks of age with a significantly increased mass/volume ratio. See Figure 1. Cardiac performance, as measured by fractional shortening, was preserved at 4 weeks of age. By 6 weeks of age the mass/volume ratio had decreased significantly indicating ventricular dilation and was accompanied by contractile dysfunction evidenced by a decreased fractional shortening. These animals had no clinical signs of failure such as ascites or pleural effusions. Given these findings animals were then studied at 4weeks of age to evaluate compensated hypertrophy and 6 weeks of age to evaluate decompensated hypertrophy.
Figure 1.
Transthoracic echocardiography showing development of LV hypertrophy and later decompensation. (A) Increase in Mass/Volume (M/V) ratio indicating peak hypertrophy in the banded animals occurred at 4–5 wks of age followed by ventricular dilatation at 6wks of age evidenced by a decrease in M/V ratio. (B) Contractile performance was assessed by fractional shortening (FS). The FS was preserved during hypertrophy at 4wks of age and decreased as the ventricle dilated at 6wks (n=6–11, * = p<0.05). (C) There was no significant difference in animal body weights. Banded animals at 6wks of age had no clinical signs of heart failure such as ascites or pleural effusions.
ETC Complex Activity and Protein Expression
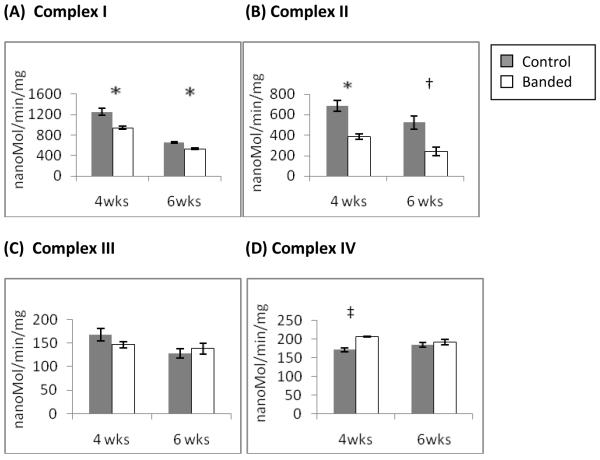
To identify the defect in oxidative phosphoryation we measured the ETC complex activities by spectophotometry in isolated mitochondria at 4 and 6 weeks of age. Complex I (figure 3a) showed significant impairment in the banded animals in hypertrophy. Complex II (3b) also showed significant impairment). There was further impairment of both complexes with ventricular decompensation at 6wks of age. Complex III activity (3c) was unchanged in both hypertrophy and decompensated hypertrophy. Complex IV activity (3d) was slightly increased in the banded animals during LV hypertrophy but normalized to control levels with ventricular dilation.
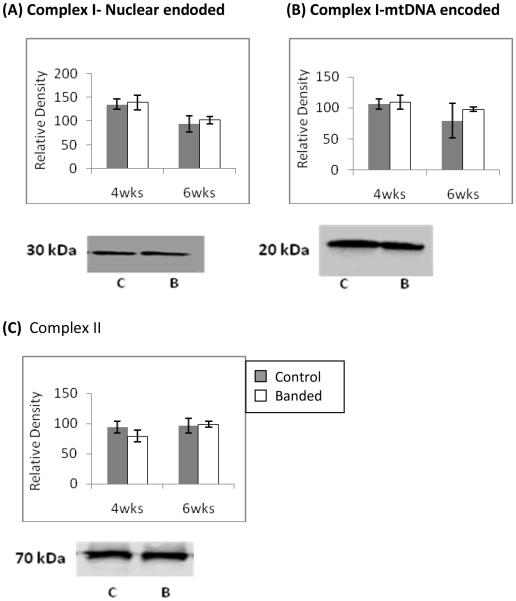
Figure 3.
Western blot analysis of ETC complex subunit protein levels at 4wks and 6wks of age. Representative blots are shown. Nuclear encoded, 30kDa Iron-Sulfur Protein 3 (A) and the mitochondrial encoded 20 kDa, ND6 subunits (B) of complex I are unchanged at both time points. (C) The protein level of the complex II 70 kDa flavoprotein is unchanged change as well. Representative immunobots are shown and bar graphs depict cumulative data which are expressed are as means ± SEM (n=4 *p≤0.05).
To determine if the decrease in complex I and II activities was attributable to a decrease in complex subunit expression we measured the protein levels of representative subunits by immunoblotting. Figure 4a shows the nuclear encoded, 30 kDa Iron-sulfur protein 3 subunit of complex I was unchanged versus controls at both 4 and 6 weeks of age. Figure 4b shows the 20 kDa, ND6 subunit of complex I which is encoded by mitochondrial DNA was unchanged at both time points as well. Figure 4c shows the 70 kDa flavoprotein subunit of complex II which is the only ETC complex entirely encoded by nuclear DNA was unchanged in both LVH and in decompensated LVH.
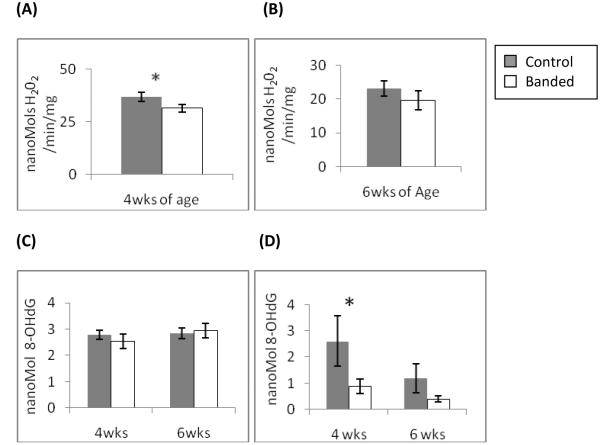
Figure 4.
Determination of ROS generation and oxidative damage. Production of H202 was determined by fluorescence spectroscopy using the Amplex Red reaction in isolated mitochondria. (A) mitochondria at 4wks of age (hypertrophy) showed significantly decreased H202 production. (B)mitochondria at 6wks of age (decompenated hypertrophy) show decreased, but not significantly, production of H202. 8-OHdG, as a marker for oxidative DNA damage was measured in digested nuclear and mitochondrial DNA. There was no difference in 8-OHdG levels in nuclear DNA (C) at either time point. In mtDNA (D) 8-OHdG levels were significantly decreased at 4 wks of age (Data expressed are as means ± SEM, n=4-6, *p≤0.05).
Determination of ROS generation and Peroxidative Damage
To determine if complex I and II dysfunction results in altered ROS generation we measured H202 production in isolated mitochondrial using 5mM glutamate and 5 mM malate as substrates for oxidative phosphorylation. Figure 5 shows ROS production was significantly decreased in LV hypertrophy at 4 weeks of age and remained decreased in decompensated hypertrophy at 6 weeks of age. To confirm the decrease in ROS generation we measured 8-hydroxydeoxyguanosine (8-OHdG) which is a marker for free radical mediated DNA damage. There was no significant difference in the amount of oxidative damage in nuclear DNA (figure 6a). However, mtDNA DNA contained significantly less oxidative damage in the hypertrophied LV at 4 weeks of age than controls (figure 6b). At 6 weeks of age the amount of 8-OHdG was decreased but not significantly in decompensated LV. These results are consistent with the findings of decreased H202 production in isolated mitochondria from hypertrophied LV.
Figure 5.

Summarized results of TUNEL staining for cardiomyocyte apoptosis at 4wks and 6wks of age in aortic banded and control animals. There was a nonsignificant increase in cardiomyocyte apoptosis at 4wks of age compared to controls and a significant increase in banded animals at 6wks of age compared to controls. Results are expressed as means + std (n=3) of TUNEL positive cardiomyocyte nuclei per 1000 nuclei, * p =.025.
Figure 6.
Western blot analysis of Bax and Bcl-2 protein levels at 6wks of age when cardiomycyte apoptosis is known to be significantly increased. (A) Significantly increased Bax protein levels in banded animals and (B) significantly increased Bax/Bcl-2 ratio. Representative immunobots are shown and bar graphs depict cumulative data which are expressed are as means ± SEM (n=4 *p≤0.05).
Activation of Apoptosis
The presence of cardiomyocyte apoptosis was determined by TUNEL staining. See figure 5. There was a nonsignificant increase in cardiomyocyte apoptosis at 4wks of age compared to controls and a significant increase in banded animals at 6wks of age compared to controls. To investigate whether the mitochondrial dysfunction contributed to cardiomyocyte loss through activation of the intrinsic mitochondrial apoptotic pathway we measured the pro and anti-apoptotic proteins Bax and Bcl-2. In the decompensated LV at 6 weeks of age there was a significant increase in the amount of Bax present (see figure 6a) and also in the ratio of Bax/Bcl-2 (figure 6b) which is indicative of a pro-apoptotic state of the mitochondria which can to lead to activation of the mitochondrial apoptosis pathway.
Discussion
The major findings of the present study are that (1) electron transport chain dysfunction, specifically of complex I and II, occurs in early hypertrophy and worsens with ventricular decompensation; (2) there is no change in protein content of complex I and II subunits; (3) ETC dysfunction is associated with decreased ROS generation; and (4) the mitochondrial dysfunction is associated with the activation of the mitochondrial apoptosis pathway, as indicated by Bax/Bcl-2 ratios. Collectively these findings implicate electron transport chain dysfunction as a potential cause of cardiomyocyte loss through apoptosis but through means other than excessive ROS production.
Our findings in a model of a pressure loaded ventricle show that complex I and II dysfunction precedes the onset of contractile dysfunction and ventricular dilatation indicating that initially the impairment is not sufficient to inhibit the production of adequate amounts of ATP for basal contractile function. The impairment could explain the reported finding of impaired ATP production at high work states in hypertrophied myocardium. 19 Worsening complex dysfunction coincides with the onset of ventricular dilation and worsening contractile function. This data is consistent with the decreased amounts of ATP in failing myocardium reported in multiple models of heart failure.20 It must be noted that the impairment of ETC complex activities causing decreased oxidative phosphorylation has not been firmly established in heart failure and requires further investigation.
There was a significant decrease in activity in both complex I and II in control animals from 4wks to 6wks of age. Because little is known about mitochondrial changes in the neonatal period, the usage of a developing animal may confound the results seen in the control group, i.e. decreases in activity of complex I and II may occur with maturation of the animal. In spite of the decreases in the activities of complex I and II in the control animals, banded animals had significantly worse complex activities.
The decrease in complex activity is not attributable to a decrease in complex subunit proteins as we found that representative subunits from complex I and II were not decreased. Complex I is composed of 45 proteins, 7 of which are encoded by the mitochondrial genome. Complex II is the only complex entirely encoded by nuclear DNA and is composed of 7 subunits. The subunits we elected to quantify have been reported as key subunits of each impaired complex. The 30 kDA, nuclear encoded, Irons-sulfur protein 3 subunit and mitochondrial encoded ND6 subunit of complex I are both a part of the core electron transfer apparatus which is mostly likely to affect complex function if decreased in quantity.21, 22 The SDHA subunit of complex II is a 70 kDa flavoprotein involved in the electron transfer process.23 Other possibilities exist that could explain the decreased function. Improper assembly of complexes by their constituent subunits or even post-translation modifications could lead to impaired functioning as well.24 Differential expression of ETC subunit isoforms that alters their activities has been described in yeast in under hypoxic conditions raising the possibility that isoform switching may occur secondary to the metabolic stress involved in the chronically pressure loaded ventricle.25
The mechanism of impairment of ETC complex activity is not well understood. Recent studies have focused on possible post-translational modifications that might function to regulate oxidative phosphorylation. Possible mechanisms include reversible processes such phosphorylation, acetylation, palmytoylation, and glycosylation, and/or irreversible process involving cleavage or degradation of the subunits. Many of these post translational modifications have been identified in complex I but remain under investigation.26, 27
We found that the impairment of complex I and II activity resulted in decreased production of H202 in isolated mitochondria from hypertrophied myocardium compared to age-matched controls. The decreased production of mitochondrial ROS was confirmed by the decreased amounts of 8-OHdG, a marker of ROS induced DNA damage in mtDNA. Because of its proximity to the ETC, the lack of negatively charged histone proteins, and the less efficient DNA repair mechanisms, mtDNA is particularly susceptible to ROS mediated damage. Complex I and II impairment decreases production of ROS by limiting the flux through the ETC. As ROS are a by-product of oxidative phosphorylation, a decrease in oxidative phosphorylation results in a proportional decrease in ROS generation.
The lack of nuclear DNA damage in hypertrophy and decompensated hypertrophy indicates that the ROS generated from all sources are sufficiently detoxified preventing nuclear DNA damage. This is an important finding because other sources of ROS are reportedly active in the hypertrophied LV. The cytosolic NADPH oxidase enzyme has been found to play a role in LV hypertrophy through ROS dependant activation of MAP kinase pathways in angiotensin-II and endothelin induced models of hypertrophy.7, 28 Elevated levels of ROS generated in a NADPH oxidase may be sufficient to induce hypertrophic signaling but insufficient to incur oxidative damage.
Our findings of decreased oxidative stress in pressure overload hypertrophy are in contrast to the documented role ROS play in heart failure models from ischemia-reperfusion or acute myocardial infarction. In our model, the aortic band is placed in a non-constricting fashion such that the pressure load gradually develops as do the structural and metabolic changes seen in LVH. This contrasts significantly with the models of ischemia-reperfusion and acute MI where a relatively healthy ventricle sustains an acute insult that leads to rapid development of multiple metabolic and structural abnormalities including cardiomyocyte apoptosis. However, the mechanism by which apoptosis is induced likely varies between the different models. Elevated ROS induce lipid and protein peroxidation in the inner mitochondrial which in sufficient quantity can promote release of cytochrome c into the cytosol and activate apoptosis. Therefore efforts in these models aim to decrease ROS generation or peroxidative damage through free radical scavenging12.
Our findings in a pressure loaded ventricle show ETC impairment precedes the onset of cardiomyocyte apoptosis which was found to be significantly increased at 6wks of age.3 We document the activation of the mitochondrial apoptotic pathway through an increase in the Bax/Bcl-2 ratio. Homodimerazation of Bax and incorporation into the outer mitochondrial membranes (which is prevented by Bcl-2) results in loss of mitochondrial membrane potential and leakage of pro-apoptotic proteins into the cytosol and ultimately activating downstream effector caspases. We demonstrated increased levels of Bax in decompensated hypertrophy hearts as well as an increased Bax/Bcl-2 ratio independent of increased oxidative stress. The increased Bax/Bcl-2 ratio at 6wks of age corresponds to the time when cardiomyocyte apoptosis is significantly increased in our model.3 The mechanism is by which Bax levels are increased is not clear. However, increased oxidative stress does not appear to play a role. An important contributor may be the lack of appropriate signaling through the Hypoxia Inducible Factor-1 (HIF-1α) pathway which is a key regulator of cell survival.29, 30 We have previously found in our model that HIF-1 α fails to translocate into the nucleus in hypertrophied hearts when subjected to hypoxia indicating a defect in HIF-1 α signaling.31 Key to the nuclear translocation of HIF-1 α is stabilization by ROS preventing its degradation. If decreased ROS production limits the stabilization and subsequent translocation of HIF-1 α then the compensatory response would be inadequate to mitigate the effects of hypoxia or cellular stress.
ETC dysfunction with a resultant decrease in ROS production is a key finding in the pathogenesis of LV hypertrophy and its progression to failure. The magnitude of its contribution to cardiomyocyte apoptosis, either through impaired oxidative phosphorylation and energy production or altered ROS metabolism requires further study. To better define the cause of ETC complex dysfunction, it will be necessary to take a proteomics approach to assess complex protein composition and post-translational modifications. Understanding the specific mechanisms of the ETC dysfunction is essential to design appropriate interventions to alter the progression of hypertrophy and development of cardiac failure.
Figure 2.
ETC Complex Activities measured by spectophotometry in isolated mitochondria from hypertrophied (4wks) and decompensated hypertrophy (6wks) LV myocardium. Complex I (A) and Complex II (B) activities were significantly decreased at 4 wks of age and further decreased significantly at 6wks of age. (c) Complex III activity was unchanged. (D) Complex IV activity was significantly increased at 4wks of age but normalizes at 6wks of age.(n =4,*p<0.005, †p=0.009, ‡ p= 0.03)
Acknowledgments
Special thanks to Dr. Moritz Wyler von Ballmoos and Dr. Huamei He for their technical assistance.
Funding Sources
This work was supported by grants from National Heart, Lung and Blood institute HL-075430 (to I. Friehs) and HL-063095 (to P.J. del Nido), HL-074734 and HL-0066186 (to F. X. McGowan). Dr. Griffiths was supported by the Harvard-Longwood Research Training in Vascular Surgery: T32 HL 007734 to FW Logerfo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annual review of physiology. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y-HMA, Colan SD, Gonnella P, Takeuchi K, Friehs I, Cowan DB, del Nido PJ, McGowan FX. Myocyte apoptosis occurs early during the development of pressure-overload hypertrophy in infant myocardium. J Thorac Cardiovasc Surg. 2008 doi: 10.1016/j.jtcvs.2008.12.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friehs I, Barillas R, Vasilyev NV, Roy N, McGowan FX, del Nido PJ. Vascular endothelial growth factor prevents apoptosis and preserves contractile function in hypertrophied infant heart. Circulation. 2006;114(1 Suppl):I290–295. doi: 10.1161/CIRCULATIONAHA.105.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological reviews. 2005;85(3):1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 5.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. Journal of the American College of Cardiology. 2002;40(7):1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. The American journal of pathology. 2006;168(5):1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsutsui H, Ide T, Kinugawa S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxidants & redox signaling. 2006;8(9-10):1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- 8.Lee HC, Wei YH. Role of Mitochondria in Human Aging. Journal of biomedical science. 1997;4(6):319–326. doi: 10.1007/BF02258357. [DOI] [PubMed] [Google Scholar]
- 9.Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxidants & redox signaling. 2006;8(11-12):2111–2124. doi: 10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- 10.Tu VC, Bahl JJ, Chen QM. Signals of oxidant-induced cardiomyocyte hypertrophy: key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. The Journal of pharmacology and experimental therapeutics. 2002;300(3):1101–1110. doi: 10.1124/jpet.300.3.1101. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Jugdutt BI. Apoptosis and oxidants in the heart. The Journal of laboratory and clinical medicine. 2003;142(5):288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294(2):C460–466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 13.Friehs I, Cao-Danh H, Nathan M, McGowan FX, del Nido PJ. Impaired insulin-signaling in hypertrophied hearts contributes to ischemic injury. Biochemical and biophysical research communications. 2005;331(1):15–22. doi: 10.1016/j.bbrc.2005.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran AM, Friehs I, Takeuchi K, Stamm C, Hammer PE, McGowan FX, del Nido PJ, Colan SD. Noninvasive serial evaluation of myocardial mechanics in pressure overload hypertrophy of rabbit myocardium. Herz. 2003;28(1):52–62. doi: 10.1007/s00059-003-2392-0. [DOI] [PubMed] [Google Scholar]
- 15.Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, del Nido PJ. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. The Annals of thoracic surgery. 2004;77(6):2004–2010. doi: 10.1016/j.athoracsur.2003.11.003. discussion 2011. [DOI] [PubMed] [Google Scholar]
- 16.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Opening of mitochondrial KATP channels enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium accumulation, and respiration. American journal of physiology. 2004;287(5):H1967–1976. doi: 10.1152/ajpheart.00338.2004. [DOI] [PubMed] [Google Scholar]
- 17.Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM. An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochemical medicine and metabolic biology. 1994;51(1):35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- 18.Ausubel FMBR, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Short Protocols in Molecular Biology. 5th ed Wiley; 2002. [Google Scholar]
- 19.Bache RJ, Zhang J, Murakami Y, Zhang Y, Cho YK, Merkle H, Gong G, From AH, Ugurbil K. Myocardial oxygenation at high workstates in hearts with left ventricular hypertrophy. Cardiovascular research. 1999;42(3):616–626. doi: 10.1016/s0008-6363(98)00332-0. [DOI] [PubMed] [Google Scholar]
- 20.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovascular research. 2008 doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porcelli AM, Angelin A, Ghelli A, Mariani E, Martinuzzi A, Carelli V, Petronilli V, Bernardi P, Rugolo M. Respiratory complex I dysfunction due to mitochondrial DNA mutations shifts the voltage threshold for opening of the permeability transition pore toward resting levels. The Journal of biological chemistry. 2008 doi: 10.1074/jbc.M807321200. [DOI] [PubMed] [Google Scholar]
- 22.Hirst J, Carroll J, Fearnley IM, Shannon RJ, Walker JE. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochimica et biophysica acta. 2003;1604(3):135–150. doi: 10.1016/s0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 23.Rustin P, Rotig A. Inborn errors of complex II--unusual human mitochondrial diseases. Biochimica et biophysica acta. 2002;1553(1-2):117–122. doi: 10.1016/s0005-2728(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 24.Fontanesi F, Soto IC, Barrientos A. Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB life. 2008;60(9):557–568. doi: 10.1002/iub.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 26.Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. Journal of molecular and cellular cardiology. 2009 doi: 10.1016/j.yjmcc.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Molecular cell. 2006;23(4):607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40(4):477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 29.Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell death and differentiation. 2008;15(4):686–690. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- 30.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. Journal of the American College of Cardiology. 2005;46(11):2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 31.Friehs IBR, Roy N, Vasilyev NV, Martinez JF, McGowan FX, del Nido PJ. Deferoxamine mediated activation of hypoxia inducible factor-1α (HIF-1α) upregulates target genes for protection of hypertrophied myocardium. Circulation (Suppl II) 2005;112(17):II–350. [Google Scholar]