Abstract
Background & Aims
Endotoxemia from sepsis can injure the GI tract through mechanisms that have not been fully elucidated. We have shown that lipopolysaccharide (LPS) induces an increase in gastric permeability in parallel with the luminal appearance of secretory phospholipase A2 (sPLA2) and its product, lyso-phosphatidylcholine (lyso-PC). We proposed that sPLA2 acted on the gastric hydrophobic barrier, composed primarily of PC, to degrade it and produce lyso-PC, an agent that is damaging to the mucosa. In the present study we have tested whether lyso-PC and/or sPLA2 have direct damaging effects on the hydrophobic barriers of synthetic and mucosal surfaces.
Methods
Rats were administered LPS (5 mg/kg, ip), and gastric contents were collected 5 h later for analysis of sPLA2 and lyso-PC content. Using these measured concentrations, direct effects of sPLA2 and lyso-PC were determined on: 1) surface hydrophobicity as detected with an artificial PC surface and with intact gastric mucosa (contact angle analysis); and 2) cell membrane disruption of gastric epithelial cells (AGS).
Results
Both lyso-PC and sPLA2 increased significantly in the collected gastric juice of LPS-treated rats. Using similar concentrations to the levels in gastric juice, the contact angle of PC-coated slides declined after incubation with either pancreatic sPLA2 or lyso-PC. Similarly, gastric contact angles seen in control rats were significantly decreased in sPLA2 and lyso-PC treated rats. Additionally, we observed dose-dependent injurious effects of both lyso-PC and sPLA2 in gastric AGS cells.
Conclusions
An LPS-induced increase in sPLA2 activity in the gastric lumen, and its product lyso-PC, are capable of directly disrupting the gastric hydrophobic layer and may contribute to gastric barrier disruption and subsequent inflammation.
Keywords: phosphatidylcholine, endotoxin, hydrophobicity, gastric mucosa, epithelium
INTRODUCTION
Acute endotoxemia is associated with shock and injury to the gastrointestinal (GI) tract. The occurrence of ileus due to endotoxic shock is accompanied by production of inflammatory mediators such as TNFα and other cytokines or chemokines that are thought to be responsible for much of the injury (1–3). However, other mechanisms could also be a part of the process. The use of bacterial lipopolysaccharide (LPS) in rodents as a model of endotoxemia has allowed a closer examination of other factors that may be involved in this shock-induced GI injury and dysfunction.
LPS has been shown to inhibit gastric acid secretion and also produce a gastroparesis, allowing the stomach to fill with an alkaline fluid (4–5). In addition, our group has reported that LPS induces a duodenogastric reflux of intestinal contents, including bile acid, into the gastric lumen, which may also contribute to gastric alkalinization (6). We have further shown that LPS induces an increase in gastric permeability to a fluorescent probe of substantial size (4000 molecular weight), without overt disruption to the mucosal architecture (7). Concurrently, there is an increase in the gastric luminal appearance of secretory phospholipase A2 (sPLA2), as well as the product of this enzyme, lyso-phosphatidylcholine (lyso-PC). The source of this gastric luminal sPLA2 is unclear, but at least part of it is likely to have derived from intestinal reflux material of pancreatic origins. Regardless of its source, sPLA2 within the gastric lumen, at an appropriate pH, may be capable of degrading its primary substrate, phosphatidylcholine (PC).
Our group and several others have shown that a portion of the gastric barrier to acid is due to the presence of a hydrophobic layer of phospholipid, mainly PC, which overlies the mucus gel layer on the mucosa (8–10). Disruption of the hydrophobic layer is associated with injury and disease states such as ulcer disease (11–12), colitis (13–14) and NSAID toxicity (15–16). LPS was shown to reduce surface hydrophobicity in a time and dose-dependent manner (6). We have proposed that LPS acts indirectly on this barrier by increasing bile acid content and sPLA2 activity in the gastric lumen, and these agents are capable of degrading the PC layer. In the current paper, we have focused on sPLA2 activity and how it may act to disrupt the hydrophobic barrier.
METHODS
Animals
Male Sprague-Dawley rats (175–250 g) were obtained from Harlan Sprague-Dawley (Indianapolis, IN). All animals were fasted overnight before use to ensure an empty stomach. Institutional Animal Welfare Committee approval was obtained prior to all studies.
LPS effect on gastric contents
Saline (control) or LPS (5 mg/kg, ip) were administered to rats and gastric contents were collected five hours later after euthanasia. The determination of sPLA2 activity, as well as lipid extraction and thin layer chromatography to separate lyso-PC was performed on the gastric fluid as previously described (7).
PC coated slides
Well-cleaned glass slides were coated with a monolayer of PC (dipalmitoyl phosphatidylcholine) using a Langmuir trough and a modification of the Blodgett and Gaines techniques as previously described (17). The slides were then incubated in normal saline or saline containing various test agents, including pancreatic sPLA2 (10 units) or lyso-PC (0.1, 0.5, 1, or 5 mM) at 37 °C for two hours with gentle stirring. The slides were rinsed, air-dried and contact angle analysis (17) was performed with a Rame-Hart goniometer (Hicksville, NY).
Effect of sPLA2 and lyso-PC on gastric hydrophobicity
Rats were anesthetized with isoflurane and underwent a pylorus ligation. The animals were then dosed by oral gavage with 1 ml of either vehicle, sPLA2 (10 units), or lyso-PC (2 mM). The agents were dissolved in 100 mM HEPES buffer at pH 7.4 to maintain gastric pH above 6.5 for the duration of the experiment. After 60 minutes, the rats were euthanized and gastric tissue was collected for contact angle analysis as previously described (17).
Cell culture
AGS cells are human gastric epithelial cells originally derived from an adenocarcinoma (18). They were maintained in Ham's F12K media plus 10% fetal bovine serum and penicillin/streptomycin. Cells were plated into 24-well culture plates at 2×105 cells per well, and were exposed to test agents lyso-PC (0.01 to 0.2 mM for 3h) or bovine pancreatic PLA2 (0.1 to 10 U/ml for 24h), after which the media was collected for lactate dehydrogenase assay (LDH) as a measure of cell membrane injury and the cell number was analyzed by the thiazolyl blue tetrazolium bromide (MTT) assay as previously described (19). For comparison to other forms of phospholipase, sPLA2 from bee venom and snake venom (Naja naja), and phospholipase C (PLC) from Clostridium perfringens were also tested. For comparison to another epithelial cell type, selected phospholipase studies were performed on IEC-6 cells which are derived from normal rat intestinal crypt epithelial cells (20). IEC-6 cells were maintained in Dulbecco's modified Eagle medium plus 10% fetal bovine serum, penicillin/streptomycin, and insulin (0.1 units/ml).
Materials
LPS (E. coli 0111:B4), all phospholipases, all lipids and a kit (TOX-7) for LDH assay were obtained from Sigma Chemical Co. (St. Louis, MO). The PC used in the monolayer slide studies was dipalmitoyl phosphatidylcholine. AGS cells were obtained from the Texas Medical Center Digestive Diseases Center (Houston, TX). IEC-6 cells were obtained from The University of Texas Trauma Research Center (Houston, TX).
Statistics
Differences among treatment groups were analyzed by analysis of variance and the Fisher LSD test. The Student t-test was used to compare differences between two groups. The level for significance was set at p<0.05.
RESULTS
Lyso-PC and sPLA2 after LPS
The gastric contents of control rats contained small, but detectable amounts of lyso-PC measured at 0.08 ± 0.02 mM. At five hours after the systemic administration of LPS at a dose of 5 mg/kg, that value increased significantly at least 60-fold to 1.29 ± 0.17 mM (p<0.001). Similarly, the gastric contents of control animals had a total of 0.6 ± 0.06 units of sPLA2 activity that increased ~5 fold to 3.2 ± 0.56 units in LPS-treated rats (p<0.05). A unit of sPLA2 activity is the amount that will hydrolyze 1.0 μmole of PC to lyso-PC producing a free fatty acid per minute at pH 9 and 37°C.
PC coated slides
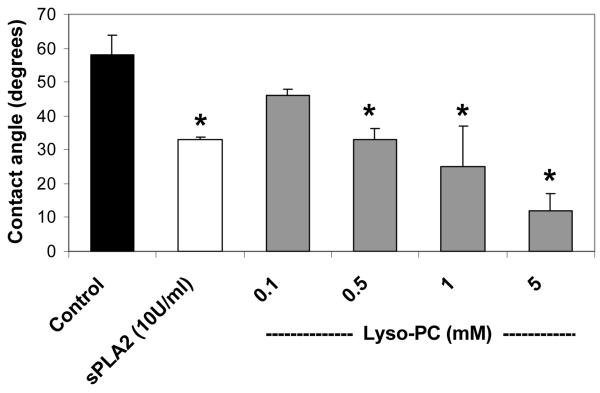
The mean contact angle for PC-coated slides incubated for 2 h in saline was 58° (n=9). PC coated slides incubated in either 10 units/ml of pancreatic sPLA2 or 0.1 to 5 mM lyso-PC showed significant reductions in contact angles, as shown in Figure 1.
Figure 1.
Effect of sPLA2 and lyso-PC in vitro. PC coated slides were incubated for 2 h with sPLA2 (10 U/ml) or lyso-PC (0.1 to 5 mM), followed by contact angle readings. Values are expressed as the mean contact angle in degrees ± standard deviation for N = 4/group. * p<0.05 versus Control.
Effect of sPLA2 and lyso-PC on rat gastric hydrophobicity
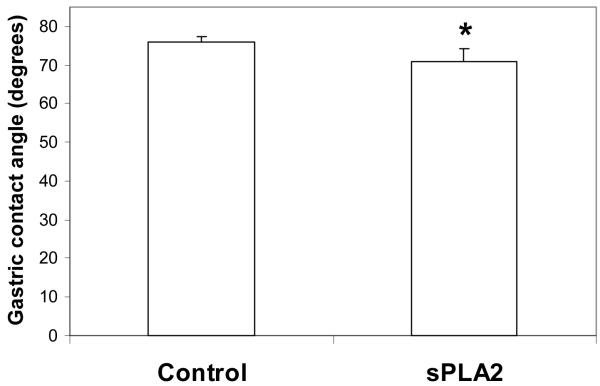
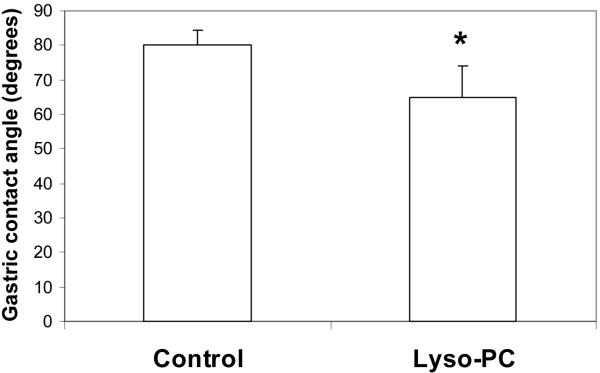
Separate in vivo studies were performed with each test agent as seen in Figure 2A and B. In the study testing sPLA2, the treated rats showed a significant 7% reduction in gastric hydrophobicity over control animals (p<0.05). In the study testing lyso-PC, treated rats had a significant 18% reduction in gastric hydrophobicity over control values (p<0.02).
Figure 2.
Effect of sPLA2 and lyso-PC in vivo. Rat stomachs were exposed to: A) pancreatic sPLA2 or B) lyso-PC, for 1 h and the gastric contact angles were then measured. Values are expressed as the mean contact angle in degrees ± standard deviation for N = 4–6/group. * p<0.05 versus Control.
Effect of lyso-PC and sPLA2 on cultured cells
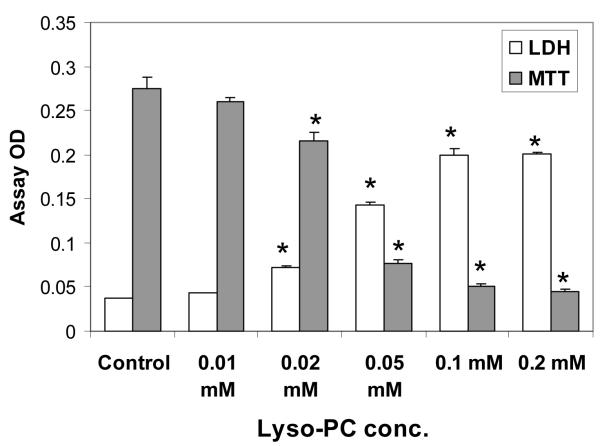
Gastric cells incubated for 3 h with lyso-PC showed a dose-dependent increase in LDH release, indicative of membrane injury (Figure 3). At the same time, the number of viable cells, as detected by the MTT assay, was correspondingly decreased. The ED50 for both measures was estimated to be about 30 to 40 μM.
Figure 3.
Effect of lyso-PC on gastric AGS cells. Cells were incubated for 3 h with varying concentrations of lyso-PC. LDH was measured in the media and MTT was assayed in the cells. Values are expressed as the mean optical density (OD) of the assay ± standard error of the mean. The study was performed three times. * p<0.05 versus Control.
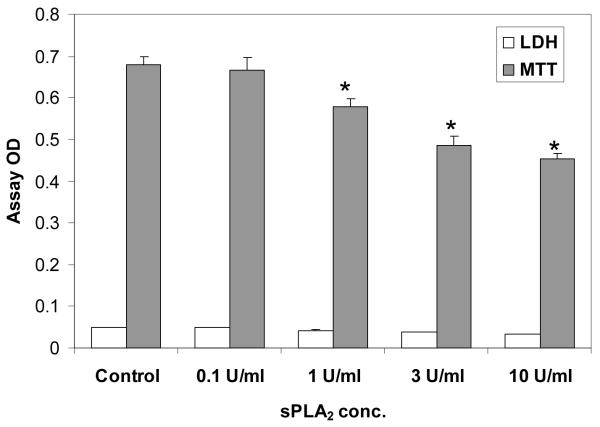
When AGS cells were initially incubated with pancreatic sPLA2 for 3 h, there were no observable changes in cell number, so the incubation time was lengthened to 24 h. At 24 h (Figure 4), it was found that LDH was not increased by sPLA2 treatment, while MTT indicated a slight, but significant, dose-dependent sPLA2-induced reduction in cell numbers. It should be noted that all cell assays of LDH activity were accompanied by a positive control (cells treated with deoxycholate for complete cell lysis) to verify that the assay was working properly.
Figure 4.
Effect of sPLA2 on gastric AGS cells. Cells were incubated for 24 h with varying concentrations of pancreatic sPLA2. LDH was measured in the media and MTT was assayed in the cells. Values are expressed as the mean OD of the assay ± standard error of the mean. The study was performed three times. * p<0.05 versus Control.
To determine the specificity of the sPLA2 effect, two other types of sPLA2 were tested on AGS cells. Neither bee venom nor snake venom sPLA2 (Table 1) had an effect on LDH or MTT of AGS cells after a 24 h incubation. In contrast, phospholipase C (PLC) was clearly injurious to the cells at ≥0.1 U/ml. Another epithelial cell type, IEC-6 cells, showed a similar lack of sensitivity to the sPLA2 enzymes (Table 1), and were slightly responsive to pancreatic sPLA2 (10 U/ml = 92 ± 1% of control for MTT (not significant) and 150 ± 4% of control for LDH (p<0.05 vs control)). IEC-6 cells were equally injured by PLC as the AGS cells.
Table 1.
Phospholipase-induced injury to gastrointestinal cells.
| AGS cells |
IEC-6 cells |
|||
|---|---|---|---|---|
| Phospholipase |
LDH |
MTT |
LDH |
MTT |
| Bee venom PLA2 | ||||
| 0.1 U/ml | 100 ± 3 | 106 ± 2 | 104 ± 3 | 111 ± 2 |
| 1 U/ml | 107 ± 3 | 113 ± 2 | 97 ± 8 | 115 ± 3 |
| 10 U/ml | 100 ± 1 | 106 ± 2 | 100 ± 2 | 111 ± 1 |
| Snake venom PLA2 | ||||
| 0.1 U/ml | 98 ± 1 | 97 ± 3 | 100 ± 3 | 109 ± 5 |
| 1 U/ml | 102 ± 2 | 101 ± 2 | 95 ± 10 | 121 ± 7 |
| 10 U/ml | 102 ± 5 | 111 ± 2 | 140 ± 26 | 94 ± 7 |
| PLC | ||||
| 0.1 U/ml | 149 ± 5* | 79 ± 3* | 292 ± 20* | 69 ± 1 |
| 1 U/ml | 423 ± 5* | 12 ± 0.1* | 464 ± 2* | 17 ± 1* |
| 10 U/ml | 446 ± 44* | 12 ± 0.1* | 473 ± 7* | 15 ± 0.1* |
AGS or IEC-6 cells were incubated for 24 h with 0.1, 1 or 10 U/ml of the indicated phospholipases. LDH and MTT were measured as described in the Methods. Values are expressed as the percent of control ± standard error of the mean for four separate studies.
= p<0.05 versus Control
DISCUSSION
Using a combination of in vitro and in vivo techniques, we have shown in the current studies that LPS induces the appearance of sPLA2 and lyso-PC in the gastric lumen, and these factors are capable of directly disrupting the gastric hydrophobic barrier.
The dose of LPS is one that we have used previously (6). Internally, the 5 mg/kg dose of LPS induces gastroparesis and accumulation of alkaline fluid within the stomach. At five hours after dosing with LPS, the accumulated load of lyso-PC within the gastric lumen was clearly elevated by at least 60-fold and measured about 1.3 mM. Similarly, we measured the accumulated level of sPLA2 activity and found an average total of about 3.2 units per stomach in LPS-treated rats, which was 5-fold higher than observed in control stomachs.
Concentrations of lyso-PC and sPLA2 similar to those obtained experimentally in rats were then tested in an in vitro monolayer system. Slides that had been coated with PC to mimic the stomach's hydrophobic barrier were exposed to test agents for only two hours, in comparison to the five hours of the in vivo study. This time was a conservative estimate since in the in vivo study, the rat stomach was exposed to an increasing amount of agent over a five hour period. We have previously shown that both sPLA2 and lyso-PC are beginning to be elevated intraluminally in the stomach by 3 h after a dose of LPS, and this amount is further increased by 5 h (7). Both sPLA2 and lyso-PC reduced the slide contact angle in confirmation of their ability to degrade or solubilize PC, respectively.
To verify that sPLA2 and lyso-PC were capable of causing a loss of gastric barrier hydrophobicity in an animal, these agents were incubated in a closed stomach preparation at levels used in vitro and measured in vivo after LPS. In fact, these in vivo data on lyso-PC agree with numerous reports on the toxicity of lyso-PC in the stomach (21–24) and the concentrations which induce injury (1–10 mM). In addition, we were careful to maintain the intragastric pH with buffer at no lower than pH 6.8 in these studies, as that is similar to the gastric pH measured following LPS administration (38), and it is also needed for enzymatic activity of sPLA2, which is most active in an alkaline range (pH 8–9). At pH 6–7, sPLA2 will still be active. Both agents caused a significant reduction in surface hydrophobicity, confirming their in vivo activity. The extent of contact angle reduction in these studies was relatively small (7% and 18%), compared to other animal studies where reported reductions due to disease (25) or chemical insults (26–28) could be 50% or more. However, the present studies were performed in pylorus-ligated rats where a single agent at a physiologically relevant concentration was exposed to the gastric surface. In our previous studies involving contact angle measurements, the studies were performed in conscious, intact animals and were the net result of multiple factors such as acid, bile/enzyme reflux, motility dysfunction, or high dose of damaging agent, as opposed to the single agent tested here. It also should be noted that our relative reductions in contact angle in the current study are comparable to changes seen in clinical studies involving gastritis or infection with the pathogen Helicobacter pylori where reductions of 8 to 19% were consistently reported (29–30). We have also shown previously in isolated canine gastric mucosa that there is a strong relationship between extent of reduction in gastric contact angle and reduction in electrical potential difference which is inversely related to gastric injury (26). Thus it is reasonable to conclude from our data that these individual agents (sPLA2 and lyso-PC) have the ability to directly affect a meaningful reduction in contact angle.
Finally, cultured gastric cells were exposed to concentrations of lyso-PC and sPLA2 at or below those that had been used in the studies above. The effect of lyso-PC on AGS cells was to induce cell membrane damage and release the cytosolic enzyme, LDH. Results with the MTT assay were consistent with that interpretation of loss of cell number due to injury. It was further noted that the gastric cells were quite sensitive to lyso-PC, with toxicity found at 10 to100-fold lower concentrations than were observed to be injurious in intact animals or the other in vitro system. Clearly, lyso-PC is particularly damaging to gastric epithelial cells, and the levels of lyso-PC that were detected in vivo after LPS treatment are capable of directly disrupting both the extracellular hydrophobic layer and mucosal epithelial cells.
In contrast to lyso-PC, the inability of pancreatic sPLA2 to induce cell membrane damage, as seen by no LDH release, was unexpected. One explanation for the lack of an injurious effect may be related to the absence or more limited nature of an extracellular hydrophobic PC layer on these cultured cells, and the related inability of extracellular sPLA2 to act on the extracellular PC to produce lyso-PC. In that regard, we attempted to add extracellular PC to cells incubated with sPLA2 in order to promote lyso-PC formation, but no injury was detected (not shown). It is likely that any PC added to this cell system will be protective, as we have shown for other cell cultures (31). Yet we would have expected that sPLA2 would attack membrane PC and it apparently did not. There was a small effect of sPLA2 to reduce numbers of AGS cells as seen by a decreased MTT assay, an effect that was apparently independent of cell membrane injury. To investigate whether the pancreatic sPLA2 effect was peculiar to AGS cells, we also tested another epithelial cell line, that of IEC-6 cells. This culture, too, was unresponsive by LDH and also MTT assay, to the sPLA2, supporting the possibility of a general effect. Although we cannot locate any references that report on effects of pancreatic sPLA2 on AGS or other human gastric cells, there is one report of damaging actions of sPLA2 on primary isolated rat gastric cells (32) in which bee venom PLA2 was used. This latter sPLA2 has similar substrate preferences to pancreatic sPLA2, but is associated with a different gene (Pla2 versus PLA2G1B) and has a different pH optimum. Those investigators found injury after short-term incubation of bee venom PLA2 with rat cells and also found a similar injurious sensitivity to lyso-PC as us (10–100 μM). Whether a difference in cell isolation or species of origin may explain these differences in sensitivity to sPLA2 is not known. Another explanation for our lack of effect with sPLA2 is that, in contrast to other types of sPLA2, the pancreatic form (1B) which we used is reported to also act through specific receptors (33–37), although its physiological role is unclear. Thus, there may be non-enzymatic actions of pancreatic sPLA2 on the gastric mucosa that explain our results.
To investigate the specificity of the pancreatic sPLA2 effect, we tested sPLA2 from bee venom and snake venom. Neither of these latter sPLA2s were active against AGS or IEC-6 cells by the LDH or MTT assays. This would support the uniqueness of the limited pancreatic sPLA2 effect on gastric cells. In addition, PLC clearly had a toxic effect on the AGS and IEC-6 cells, as would be expected from this membrane surface-acting enzyme. Further studies will be necessary to fully elucidate the effects of pancreatic sPLA2 on AGS cells.
Regardless of the direct effects of sPLA2 on gastric cells, our other results are fully consistent with the ability of sPLA2 to degrade the extracellular protective PC surface layer, and along with lyso-PC, to disrupt the PC layer, as well as cell membranes. Thus, circumstances under which sPLA2 appears in the gastric lumen, such as during endotoxic shock produced here or other duodenogastric reflux-inducing situations, can lead to disruption of the protective PC barrier and exposure of the underlying epithelium to potentially injurious luminal contents such as lyso-PC. Methods to counter the loss of surface PC, such as addition of exogenous PC which we have previously shown to be effective at preventing an increase in LPS-induced gastric permeability (38), offer a means to prevent local, as well as systemic inflammatory conditions. We hypothesize that the additional PC may form non-injurious liposomes with lyso-PC, lessening the free lyso-PC concentration.
Our preclinical findings reported here have potential clinical relevance. We have recently reported that the gastric fluid of patients following a traumatic shock is alkaline and contains intestinal reflux material such as bile acid (39). Other critically ill patients have also been reported to acquire bile and reflux material in their stomachs (40–41). Thus, among critically ill patients, there are circumstances where bile acids and sPLA2 from the intestines may be present in the stomach and could contribute to gastric barrier disruption by PC degradation. Based on our findings in rats reported here, we speculate that some of the altered GI permeability in critically ill patients may be due to exposure of the gastric lumen to sPLA2 and appearance of the potent damaging agent lyso-PC. Future clinical studies are warranted to address this possibility.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants P50 GM038529 (to EJD) and DK056338 which funds the Texas Medical Center Digestive Diseases Center.
Funding: This work was supported in part by NIH grants P50 GM038529 (to EJD) and DK056338 which funds the Texas Medical Center Digestive Diseases Center.
REFERENCES
- 1.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 2.Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL. Role of tumor necrosis factor-α in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Martich GD, danner RI, Ceska M, Suffredini AF. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of anti-inflammatory agents. J Exp Med. 1991;173:1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercer DW, Castaneda AA, Denning JW, Chang L, Russell DH. Effects of endotoxin on gastric injury from luminal irritants in rats: potential roles of nitric oxide. Am J Physiol. 275:G449–G459. doi: 10.1152/ajpgi.1998.275.3.G449. [DOI] [PubMed] [Google Scholar]
- 5.Helmer KS, West SD, Vilela R, Chang L, Cui Y, Kone BC, Mercer DW. Lipopolysaccharide-induced changes in rat gastric H/K-ATPase expression. Ann Surg. 2004;239:501–509. doi: 10.1097/01.sla.0000118750.54830.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dial EJ, Romero JJ, Villa X, Mercer DW, Lichtenberger LM. Lipopolysaccharide-induced gastrointestinal injury in rats: Role of surface hydrophobicity and bile salts. Shock. 2002;17:77–80. doi: 10.1097/00024382-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Zayat M, Lichtenberger LM, Dial EJ. Pathophysiology of LPS-induced gastrointestinal injury in the rat: Role of secretory phospholipase A2. Shock. 2008;30:206–211. doi: 10.1097/shk.0b013e318160f47f. [DOI] [PubMed] [Google Scholar]
- 8.Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- 9.Goddard PJ, Kao YJ, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98:361–370. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- 10.Hills BA, Kirkwood CA. Gastric mucosal barrier to hydrogen ions imparted by gastric surfactant in vitro. Gut. 1992;33:1039–1041. doi: 10.1136/gut.33.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spychal RT, Goggin PM, Marrero JM, Saverymuttu SH, Yu CW, Corbishley CM, Maxwell JD, Northfield TC. Surface hydrophobicity of gastric mucosa in peptic ulcer disease. Gastroenterology. 1990;98:1250–1254. doi: 10.1016/0016-5085(90)90341-w. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberger LM, Dial EJ, Ottlecz A, Romero JJ, Lechago J, Fox JG. Attenuation of hydrophobic phospholipid barrier is an early event in Helicobacter felis-induced gastritis in mice. Dig Dis Sci. 1999;44:108–115. doi: 10.1023/a:1026610418663. [DOI] [PubMed] [Google Scholar]
- 13.Tatsumi Y, Lichtenberger LM. Molecular association of trinitrobenzenesulfonic acid and surface phospholipids in the development of colitis in rats. Gastroenterology. 1996;110:780–789. doi: 10.1053/gast.1996.v110.pm8608888. [DOI] [PubMed] [Google Scholar]
- 14.Ehehalt R, Wagenblast J, Erben G, Lehmann W-D, Hinz U, Merle U, Stremmel W. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A Quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39:737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenberger LM, Wang Z-M, Romero JJ, Ulloa C, Perez JC, Giraud M-N, Barreto JC. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: Insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nature Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- 16.Lugea A, Antolin M, Mourelle M, Guarner F, Malagelada J-R. Deranged hydrophobic barrier of the rat gastroduodenal mucosa after parenteral nonsteroidal anti-inflammatory drugs. Gastroenterology. 1997;112:1931–1939. doi: 10.1053/gast.1997.v112.pm9178685. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenberger LM, Romero JJ, Kao Y-CJ, Dial EJ. Gastric protective activity of mixtures of saturated polar and neutral lipids in rats. Gastroenterology. 1990;99:311–326. doi: 10.1016/0016-5085(90)91011-t. [DOI] [PubMed] [Google Scholar]
- 18.Barranco SC, Townsend CM, Jr, Casartelli C, Macik BG, Burger NL, Boerwinkle WR, Gourley WK. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 1983;43:1703–1709. [PubMed] [Google Scholar]
- 19.Dial EJ, Doyen JR, Lichtenberger LM. Phosphatidylcholine-associated nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit DNA synthesis and the growth of colon cancer cells in vitro. Cancer Chemother Pharmacol. 2006;57:295–300. doi: 10.1007/s00280-005-0048-x. [DOI] [PubMed] [Google Scholar]
- 20.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epitheloid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kivilaakso E, Ehnholm C, Kalima TV, Lempinin M. Duodenogastric reflux of lysolecithin in the pathogenesis of experimental porcine stress ulceration. Surgery. 1976;79:65–69. [PubMed] [Google Scholar]
- 22.Kivilaakso E, Fromm D, Silen W. Effects of lysolecithin on isolated gastric mucosa. Surgery. 1978;84:616–621. [PubMed] [Google Scholar]
- 23.Maksem J, Jacobson N, Neiderhiser DH. Lysophosphatidylcholine-induced gastric injury and ulceration in the guinea pig. Am J Pathol. 1984;115:288–295. [PMC free article] [PubMed] [Google Scholar]
- 24.Karlqvist P-A, Franzen L, Sjodahl R, Tagesson C. A study of the permeability of rat stomach to larger molecules. Influence of lysophosphatidylcholine. Acta Chir Scand. 1986;152:279–284. [PubMed] [Google Scholar]
- 25.Lichtenberger LM, Dial EJ, Ottlecz A, Romero JJ, Lechago J, Fox JG. Attenuation of hydrophobic phospholipid barrier is an early event in Helicobacter felis-induced gastritis in mice. Dig Dis Sci. 1999;44:108–115. doi: 10.1023/a:1026610418663. [DOI] [PubMed] [Google Scholar]
- 26.Goddard PJ, Hills BA, Lichtenberger LM. Does aspirin damage canine gastric mucosa by reducing its surface hydrophobicity? Am J Physiol. 1987;252:G421–G430. doi: 10.1152/ajpgi.1987.252.3.G421. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenberger LM, Romero JJ, Kao YC-J, Dial EJ. Gastric protective activity of mixtures of saturated polar and neutral lipids in rats. Gastroenterology. 1990;99:311–326. doi: 10.1016/0016-5085(90)91011-t. [DOI] [PubMed] [Google Scholar]
- 28.Giraud M-N, Motta C, Romero JJ, Bommelaer G, Lichtenberger LM. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: A possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs) Biochem Pharmacol. 1999;57:247–254. doi: 10.1016/s0006-2952(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 29.Spychal RT, Goggin PM, Marrero JM, Saverymuttu SH, Yu CW, Corbishley MC, Maxwell JD, Northfield TC. Surface hydrophobicity of gastric mucosa in peptic ulcer disease. Relationship to gastritis and Campylobacter pylori infection. Gastroenterology. 1990;98:1250–1254. doi: 10.1016/0016-5085(90)90341-w. [DOI] [PubMed] [Google Scholar]
- 30.Goggin PM, Marrero JM, Spychal RT, Jackson PA, Corbishley CM, Northfield TC. Surface hydrophobicity of gastric mucosa in Helicobacter pylori infection: effect of clearance and eradication. Gastroenterology. 1992;103:1486–1490. doi: 10.1016/0016-5085(92)91168-4. [DOI] [PubMed] [Google Scholar]
- 31.Dial EJ, Rooijakkers SHM, Darling RL, Romero JJ, Lichtenberger LM. Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol. 2007;23:430–436. doi: 10.1111/j.1440-1746.2007.05153.x. [DOI] [PubMed] [Google Scholar]
- 32.Tepperman BL, Soper BD. The role of phospholipase A2 in calcium-ionophore-mediated injury to rat gastric mucosal cells. Dig Dis Sci. 1999;44:494–502. doi: 10.1023/a:1026688819939. [DOI] [PubMed] [Google Scholar]
- 33.Arita H, Hanasaki K, Nakano T, Oka S, Teraoka H, Matsumoto K. Novel proliferative effect of phospholipase A2 in Swiss 3T3 cells via specific binding site. J Biol Chem. 1991;266:19139–19141. [PubMed] [Google Scholar]
- 34.Hanasaki K, Arita H. Characterization of a high affinity binding site for pancreatic-type phospholipase A2 in the rat. J Biol Chem. 1992;267:6414–6420. [PubMed] [Google Scholar]
- 35.Kishino J, Kawamoto K, Ishizaki J, Verheij HM, Ohara O, Arita H. Pancreatic-type phospholipase A2 activates prostaglandin E2 production in rat mesangial cells by receptor binding reaction. J Biochem. 1995;117:420–424. doi: 10.1093/jb/117.2.420. [DOI] [PubMed] [Google Scholar]
- 36.Kundu GC, Mukherjee AB. Evidence that porcine pancreatic phospholipase A2 via its high affinity receptor stimulates extracellular matrix invasion by normal and cancer cells. J Biol Chem. 1997;272:2346–2353. [PubMed] [Google Scholar]
- 37.Mandal AK, Zhang Z, Chou JY, Mukherjee AB. Pancreatic phospholipase A2 via its receptor regulates the expression of key enzymes of phospholipid an sphingolipid metabolism. FASEB J. 2001;15:1834–1836. doi: 10.1096/fj.00-0831fje. [DOI] [PubMed] [Google Scholar]
- 38.Dial EJ, Zayat M, Lopez-Storey M, Tran D, Lichtenberger LM. Oral phosphatidylcholine preserves the gastrointestinal mucosal barrier during LPS-induced inflammation. Shock. 2008;30:729–733. doi: 10.1097/SHK.0b013e318173e8d4. [DOI] [PubMed] [Google Scholar]
- 39.Dial E, Lopez-Storey M, Adams S, Lichtenberger L, Gonzalez E, McKinley B, Moore F, Mercer D. A report on associations among gastric pH, bleeding, duodenogastric reflux, and outcomes after trauma. J Trauma. 2008;64:105–110. doi: 10.1097/TA.0b013e31815ebd99. [DOI] [PubMed] [Google Scholar]
- 40.Schindlbeck NE, Lippert M, Heinrich C, Muller-Lissner SA. Intragastric bile acid concentrations in critically ill artificially ventilated patients. Amer J Gastroenterol. 1989;84:624–628. [PubMed] [Google Scholar]
- 41.Wilmer A, Tack J, Frans E, Dits H, Vanderschueren S, Givers A, Bobbaers H. Duodenogastroesophageal reflux and esophageal mucosal injury in mechanically ventilated patients. Gastroenterology. 1999;116:1293–1299. doi: 10.1016/s0016-5085(99)70492-0. [DOI] [PubMed] [Google Scholar]