Abstract
Objective
Osteopontin (OPN) is a multifunctional phosphoprotein with an important but poorly understood role in non-small cell lung cancer (NSCLC) pathogenesis. We hypothesize that OPN isoforms (OPNa, OPNb, and OPNc) have divergent roles in NSCLC angiogenesis and divergent impact on vascular endothelial growth factor (VEGF) secretion.
Methods
We examined mRNA expression using RT-PCR primers for three OPN isoforms in NSCLC and immortalized bronchial epithelial cell lines, and correlated expression with OPN secretion into media detected by ELISA. Angiogenic properties conferred by OPN isoforms were evaluated by transfecting cDNA plasmids specific to each isoform and controls into NSCLC cell lines, H153 and H358 (low endogenous OPN) and A549 and H460 (high endogenous OPN) and analyzing conditioned media on a bovine capillary endothelial (BCE) platform and measuring VEGF levels by ELISA.
Results
OPNa mRNA expression correlated with OPN secretion in cell lines (r=0.912,p=0.0006). OPNa overexpression significantly increased tubule length compared to controls, OPNb had a similar, but less pronounced effect, while OPNc significantly decreased tubule length compared to controls in each cell line. OPNa overexpression was associated with significant increases in VEGF secretion, while OPNb had no effect and OPNc overexpression was associated with significant decreases in VEGF compared to controls in each cell line.
Conclusion
We demonstrated divergent effects of OPN isoforms on NSCLC angiogenesis and VEGF secretion. OPNa overexpression was associated with increased BCE tubule length and VEGF secretion, while OPNc is associated with decreases in both. These findings may lead to therapeutic strategies for selective isoform inhibition in NSCLC.
ULTRA-MINI ABSTRACT
Osteopontin is a multifunctional phosphoprotein with an important but poorly understood role in non-small cell lung cancer pathogenesis. The role of the three human isoforms has not been previously reported. We demonstrate that individual osteopontin isoforms have divergent effects on non-small cell lung cancer angiogenesis with associated changes in VEGF.
INTRODUCTION
Osteopontin (OPN) is a multifunctional phosphoprotein with a significant role in the pathogenesis of non-small cell lung cancer (NSCLC). In NSCLC, elevated OPN levels in tumors and serum are associated with poor patient prognosis and an increased risk for recurrence.1 NSCLC cell lines that natively express OPN have greater metastatic potential and invasive behavior,2 but the molecular pathways for OPN tumorigenicity are incompletely understood. Three human mRNA splice variants of OPN have been identified. Osteopontin-a (OPNa) represents the full-length cDNA, osteopontin-b (OPNb) is defined by a deletion at exon-5, and osteopontin-c (OPNc) by a deletion at exon-4. Expression and function of the individual isoforms in clinical NSCLC samples or tumor model systems have not been reported.
Increased angiogenesis is essential for growth and metastasis of solid tumors. In NSCLC, an important relationship exists between microvessel developement and progression to invasive disease.3-5 Tumor microvessel formation is dependent on adhesive interactions between tumor cells and the extracellular matrix, and is essential to establish conduits for nutrient delivery . Increased microvessel development has also been directly correlated with tumor maturation, and is an independent risk factor for the development of metastatic disease.5, 6
Vascular endothelial growth factor (VEGF) is a potent angiogenic and mitogenic protein that plays a central role in tumor neovascularization. In NSCLC, VEGF levels correlate directly with clinical outcome.7, 8 VEGF also has an established role in the progression of tumor invasion due to receptor stimulated capillary ingrowth and microvessel formation.4, 9 Binding of VEGF to endothelial cell surface receptors increases migration and proliferation. VEGF further supports the growth and stabilization of new blood vessels, which facilitates tumor expansion and formation of metastasis.10 Interruption of this pathway via VEGF inhibition results in endothelial cell apoptosis and restricted tumor progression.10
VEGF and OPN are frequently expressed simultaneously during angiogenesis; both by tumor cells and activated macrophages associated with ischemia and necrosis.11 Co-expression of VEGF and OPN is associated with enhanced tumor microvessel development and is a marker for poor prognosis in early stage NSCLC12. VEGF-mediated angiogenesis is significantly increased by concurrent OPN stimulation of endothelial cells.10 The aim of this investigation is to evaluate the effect of individual OPN isoforms (OPNa, OPNb, and OPNc) on tumor-associated angiogenic properties of NSCLC, and to determine if concurrent VEGF expression and secretion is affected in an isoform specific manner.
METHODS
Seven human lung cancer cell lines (A549, H153, H157, H1299, H460 and Calu-3) were obtained from American Type Tissue Collection (Manassas, VA). Two human immortalized bronchial endothelial cell lines, Beas2 B and HKT3 were received as gifts (Dr John Minna, University Texas Southwestern). Cell lines were maintained in culture in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, Grand Island, NY) with 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY) at 37°, 5% CO2.
RT-PCR primers were designed based upon the three NCBI GeneBank sequences to be inclusive of the regions of exon-4 and exon-5 at the N-terminus which are deleted from OPNc and OPNb respectively. The resulting primers, 5′TTGCAGTGATTTGCTTTTGC-3′ (sense) and 5′GTCAATGGAGTCCTGGCTGT-3′ (antisense), amplify a 305 bp product from OPNa, a 263 bp product from OPNb, and a 224 bp product from OPNc in a single reaction, which are separated by electrophoresis.
Using these primers we examined mRNA expression of OPN by RT-PCR and correlated that with OPN secretion into media, detected by ELISA (IBL, Gunma, Japan) in the series of nine cell lines. Conditioned media was collected from cultures after 72 hours in serum free conditions and mRNA was isolated from the cell lines using the RNEasy™ mini kit (QIAGEN, Valencia, CA). PCR amplification was carried out in a total volume of 20 ul using the standard Invitrogen PCR buffer system (Invitrogen, Carlsbad, CA) and optimized concentrations of MgCl2. The reactions were performed with denaturing for 15 seconds at 94°C, annealing for 30 seconds at 58°C and elongation for 30 seconds at 70°C for a total of 40 cycles; the final extension at 72°C for 5 minutes. RT-PCR products were analyzed by Tris-acetate EDTA agarose (2% w/v) gel electrophoresis. The amplification of peptidylprolyl isomerase A (PPIA) with primers 5′-TCTGAGCACTGGAGAGAAAGG-3′ (sense) and 5′GGAAAACATGGAACCC-3′ (antisense) served as controls for equal loading and integrity of all PCR reactions.
We evaluated the functional impact of each OPN isoform by transfecting cDNA plasmids specific to each isoform (Origene, Rockville, MD) into four NSCLC cell lines (H153, H358, A549 and H460). H153 and H358 cell lines each have low endogenous OPN expression, while A549 and H460 have high endogenous OPN expression. Plasmids were derived from single colony E.coli culture and purified by silica chromatography. The protein coding sequence of each clone matched the published NCBI GeneBank NM references for each OPN isoform. NSCLC cell lines were transfected using Lipofectamine2000 (Invitrogen, Carlsbad, CA) with either OPN isoform plasmid construct or pCMV empty vector, which served as a control. A pooled population of cells was used for RT-PCR and functional analysis.
Angiogenic properties were measured using an in vitro bovine capillary endothelial (BCE) platform. BCE cells (Cell Applications Inc, San Diego, CA) were maintained on gelatin-coated 10cm plastic dishes in 10 ml of endothelial growth media (EGM) (Cell Applications, Inc, San Diego, CA), supplemented with 10% FBS, 5 U/ml penicillin, and 5 mg/ml streptomycin at 37°, 5% CO2. Cells were subcultured for 9-13 passages prior to commencing experiments.
The BCE angiogenesis platform was performed as previously described.13, 14 In brief, BCE cells were trypsinized, washed in phosphate buffered saline (PBS), and re-suspended in conditioned media harvested from OPN isoform transfections and control cell lines at a concentration of 16,000 cells/100ml. Diluted 1:100 Matrigel™ (Becton Dickinson, Bedford, MA) was used to coat 96 well plates. Plates were incubated at room temperature for 1 hour and washed with PBS. BCE cells in conditioned media were suspended on the Matrigel™ platform in triplicates. DMEM, DMEM + fibroblast growth factor (1ug/100ml) (R&D Systems, Minneapolis, MN), and DMEM + VEGF (2ug/100ml) (R&D Systems, Minneapolis, MN) were used as controls. BCE cells were incubated with conditioned media on the Matrigel™ platform for 18 hours at 37°, 5% CO2, and then stained with .05% crystal violet. Tubule length was measured under 10x magnification utilizing a 9 grid measuring platform. The sum of all tubules within an 889 μ2 area were measured in triplicate and compared between experimental cell lines and controls. Tubules with a clear origin from an endothelial cell nucleus were included in the measurement. Tubule length is reported as a mean of total microns(μ) ± standard deviation.
We evaluated the impact of individual OPN isoforms on VEGF by measuring protein secretion into media from the OPN isoform transfected cell lines. Human VEGF ELISA Kits (R&D, Minneapolis, MN) were used to quantify VEGF secretion into media (pg/ml). Changes in OPN protein secretion as a result of isoform overexpression were simultaneously evaluated by ELISA (described above).
To explore the relationship between VEGF secretion and OPN induced mediators of endothelial cell proliferation, we utilized an Affymetrix Gene Chip™ (Santa Clara, CA) U133 Plus2 platform to evaluate gene expression profiles in A549 (high endogenous OPN) with overexpression of OPNa, OPNc, and pCMV controls, mRNA was utilized from the same population of cells used in the BCE angiogenesis assays. Differences in VEGF expression identified in gene array were validated in all four cell lines by RT-PCR. VEGF primers, 5′CCTTGCTGCTCTACCTCCAC-3′(sense) and 5′ATCTGCATGGTGATGTTGGA-3′(antisense), amplify a 280 bp product. The RT-PCR protocol was performed as previously described.
A two-sided Student’s t-test was used to evaluate differences in BCE tubule length and VEGF secretion between cell lines transfected with the individual OPN isoforms and empty vector controls, and regarded as significant at p ≤ 0.05. Statistics were performed using the Statistical Package for the Social Sciences version 11.5 (SPSS, Inc., Chicago, IL). Data are presented as mean ± standard deviation.
RESULTS
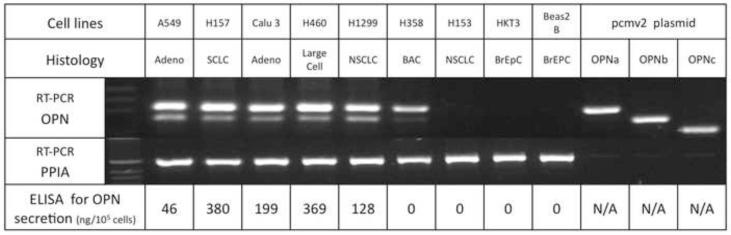
We noted a correlation between OPN mRNA expression and OPN secretion in the series of lung cancer and bronchial epithelial cell lines (r=0.9120, p=0.0006, CI: 0.6286 to 0.9816). Five cell lines (A549, H460, H157, H1299, and Calu-3) had high endogenous OPN mRNA expression and OPN secretion detected in conditioned media. Two NSCLC cell lines (H153 and H358) and the two bronchial epithelial cells lines (Beas2 Bs and HKT3) had minimal endogenous OPN mRNA expression and no detectable OPN secretion into media. OPNa, the full length isoform, was dominantly expressed in OPN secreting cell lines. Concurrent OPNb expression was noted in the same cell lines, but to a lesser extent than OPNa. OPNc was not noted to be endogenously expressed in these cell lines. (Fig. 1)
Figure 1.
OPN mRNA expression in human lung cancer and immortalized bronchial epithelial cells. Primers amplify each of the three isoforms which are then separated by electrophoresis on 2% agarose gel. OPNa produces the 305 bp dominant band seen at the top. OPNb amplifies a 263 bp fragment seen below the dominant band in A549, H157, Calu-3, H460, and H1299. OPNc amplifies a 224 bp fragment that migrates below the OPNb and is not seen in these cell lines. Amplification of pcmv2 plasmid specific to each isoform serves as positive controls and amplification of PPIA serve as control for equal loading. OPN expression correlates with OPN secretion as detected by ELISA after 72 hours in serum-free media. (BrEpC: immortalized bronchial epithelial cells, adeno: adenocarcinoma, SCLC: small cell NSCLC, NSCLC: non-small cell NSCLC, PPIA:peptidylprolyl isomerase A).
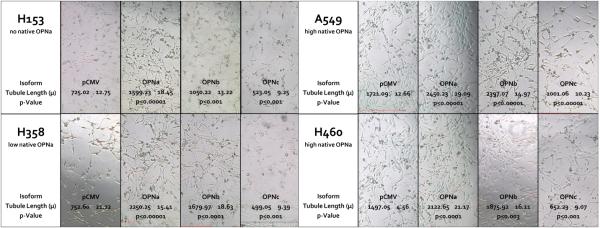
Validation of plasmid expression in each cell line is demonstrated in Fig. 2. Initial angiogenic experiments focused on overexpression of the individual OPN isoforms in H153, a NSCLC cell line with low endogenous OPN expression. Conditioned media harvested from H153 cells with forced overexpression of OPNa resulted in a significant increase in BCE tubule length (1599.23μ±18.45) compared to empty vector controls (725.02μ±12.75, p<0.0001). OPNb overexpression had a similar effect on tubule length (1050.22μ±13.22, p<0.0001). Conversely, media from cells that overexpressed OPNc resulted in a significant decrease in tubule length compared to controls (523.05μ±9.25, p<0.0001). (Fig. 3)
Figure 2.
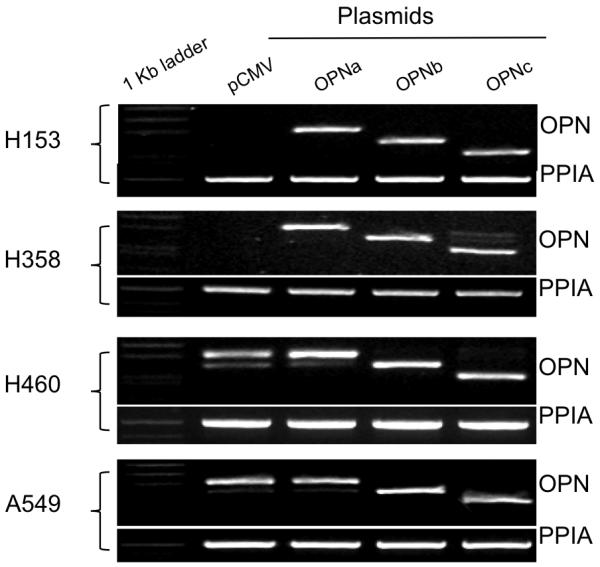
RT-PCR demonstrating forced expression of individual OPN isoforms in four NSCLC cell lines. Pooled populations of transfected cells were used in RT-PCR and angiogenesis assay. H153 and H358 have low native OPN expression. A549 and H60 have high native OPN expression.
Figure 3.
Representative sections showing tubule formation and mean tubule lengths for BCE’s plated on Matrigel™ and cultured in conditioned media collected from NSCLC cell lines transfected with the individual OPN isoforms and pCMV empty vector controls. H153 and H358 have low native OPN expression, and A549 and H460 have high native OPN expression. OPNa overexpression in all cell lines resulted in significantly more tubule formation compared to controls. OPNb overexpression also significantly increased tubule length in all cell lines compared to controls, but to a lesser extent then OPNa. OPNc overexpression resulted in a significant decrease in tubule formation compared to controls in all four NSCLC cell lines.
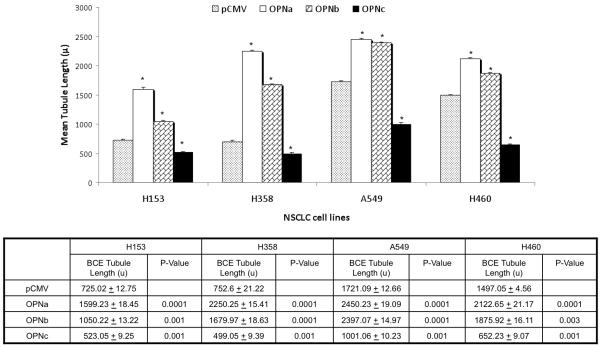
This divergent pattern of behavior with overexpression of the individual OPN isoforms was validated in three additional NSCLC cell lines, H358 with low endogenous OPN expression, and A549 and H460, both with high endogenous OPN expression. In all four cell lines the pattern of effect on tubule length was consistent: overexpression of OPNa significantly increased BCE tubule length compared to controls, overexpression of OPNb also significantly increased tubule length but to a lesser extent than OPNa, while overexpression of OPNc significantly decreased BCE tubule length compared to controls. (Fig. 3) Empty vector controls from A549 and H460 formed significantly longer tubules compared to controls with low endogenous OPN (p<0.0001), most clearly demonstrated in bar graft depiction of results from BCE assay. (Fig 4a)
Figure 4a.
Bar graft depicting the effect of the overexpression of individual OPN isoforms compared to pCMV empty vector controls on mean BCE tubule length (μ) in four NSCLC cell lines. Asterisk denotes a significant difference between OPN isoform overexpression in cell lines and pCMV controls. Mean tubule length ± standard deviation and p-value are listed below bar graft. H153 and H358 wild type cells had significantly shorter BCE tubule lengths than A549 and H460, which have high native OPN expression. Tubule formation was significantly increased in all cell lines with forced overexpression of OPNa and OPNb compared to controls. Conversely, overexpression of OPNc resulted in significantly fewer tubules formed compared to controls in all cell lines.
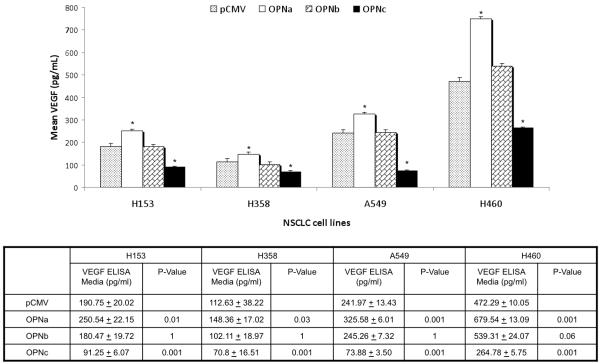
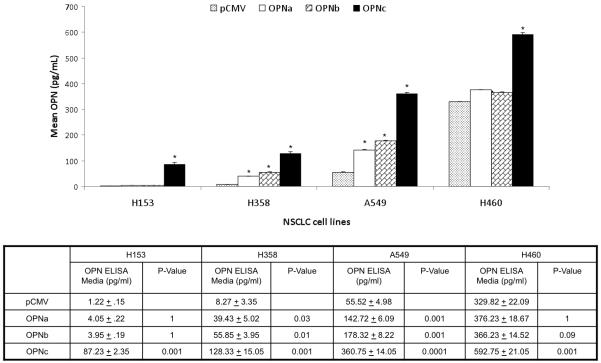
Overexpression of the individual OPN isoforms was also associated with a divergent effect on VEGF secretion. OPNa overexpression was associated with a significant increased in VEGF secretion compared to controls in all four cell lines. OPNc overexpression was associated with a significant decrease in VEGF secretion in all cell lines compared to controls. However, OPNb overexpression was not associated with significant alterations in VEGF secretion compared to controls in any of the cell lines. (Fig. 4b)
Figure 4b.
Bar graft depicting associated changes in VEGF secretion (pg/ml) with overexpression of individual OPN isoforms compared to pCMV empty vector controls on in four NSCLC cell lines. Asterisk denotes a significant difference between OPN isoform and pCMV controls. Mean VEGF concentrations ± standard deviation and p-value are listed below bar graft. H153 and H358 have low native OPN expression and A549 and H460 have high native OPN expression. Significant increases in VEGF secretion were associated with OPNa overexpression compared to controls. Conversely, OPNc overexpression was associated with in significant decreases in VEGF secretion compared to controls in all cell lines. OPNb overexpression was associated with no significant change in VEGF secretion in any of the four cell lines.
Overexpression of the individual OPN isoforms was also associated with a divergent effect on OPN secretion. OPNa and OPNb overexpression were associated with very small increases in the concentration of OPN protein in conditioned media, but OPNc overexpression was associated with a significant increase in OPN protein secretion in all four cell lines. (Fig. 4c)
Figure 4c.
Bar graft depicting the effect of the overexpression of individual OPN isoforms compared to pCMV empty vector controls on OPN secretion (pg/ml) in four NSCLC cell lines. Asterisk denotes a significant difference between OPN isoform overexpression in cell lines and pCMV controls. Mean OPN concentrations ± standard deviation and p-value are listed below bar graft. In H153, H358 and H460 overexpression of OPNa and OPNb had no significant impact on OPN protein concentrations in conditioned media. In A549 overexpression of OPNa and OPNb caused significant step-up in OPN protein secretion. Overexpression of OPNc caused a significant increase in OPN secretion in all cell lines.OPN protein is measured by ELISA which is not isoform specific.
Significant differences in downstream patterns of regulation were noted on expression profiles of A549 overexpressing OPNa and OPNc. Relative expression of VEGF was increased two-fold compared to controls with overexpression of OPNa, and decreased 40% with overexpression of OPNc. This pattern of divergent VEGF expression was validated by RT-PCR in all four cell lines. We noted that control cell lines with high endogenous OPN (A549 and H460), had appreciably higher VEGF mRNA expression than control cell lines with low endogenous OPN expression (H153 and H358). In all cell lines, overexpression of OPNa was associated with increased VEGF mRNA expression most appreciably in cell lines with low endogenous OPN, and OPNc with decreased VEGF expression compared to controls, most appreciably in cell lines with high endogenous OPN. OPNb overexpression, which was not evaluated by gene array, appears to have a less consistent impact on VEGF expression. (Fig. 5)
Figure 5.
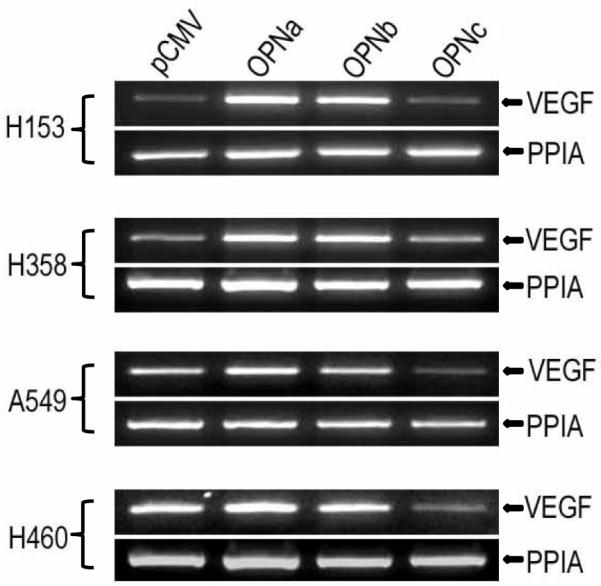
Gene array analysis demonstrated OPNa overexpression was associated with a two-fold increase in relative expression of VEGF while OPNc overexpression was associated with a 40% decrease in VEGF expression compared to controls. RT-PCR validation of gene array with regard to divergent VEGF expression with overexpression of the OPN isoforms is demonstrated here. Overexpression of OPNa in all four cell lines resulted in elevated VEGF mRNA expression, while OPNc overexpression decreased VEGF expression in all cell lines compared to wild type cells. Elevated VEGF mRNA expression was appreciated in control cell lines with high native OPN (A549 and H460) compared to cell lines with low native OPN expression (H153 and H358).
DISCUSSION
OPN has been identified in a remarkable range of normal and pathologic contexts.15 It is an important adhesive bone matrix protein, and plays a key role in immune cell recruitment, wound healing, and tissue remodeling.16, 17 Overexpression of OPN is also recognized in pathologic conditions that involve inflammation, ischemia, and malignancy. The importance of OPN in tumor pathogenesis is highlighted in gene transfer experiments, where transfection of OPN increases the malignant phenotype,18 and OPN knock-out experiments with antisense oligonucleotides which decrease malignant potential.19, 20 OPN’s principle functions are related to cell migration and adhesion, and are mediated by integrin binding to its central and well conserved RGD (arginine-glycine-aspatate) domain. OPN is overexpressed by immunohistochemistry in human NSCLC tumors compared to surrounding normal lung paranchyma.2 In vitro experiments demonstrate increased migration and invasion by NSCLC cell lines which overexpress OPN.2 These data suggest an important role for OPN in determining the metastatic potential of NSCLC.
Increased angiogenesis is a key feature of malignant transformation which allows for tumor growth and facilitates tumor cell entry into the circulation.21 VEGF is a crucial intermediate in this process, which acts via endothelial cell activation, facilitating mitogenic and vascular permeability-enhancing activities.3, 4, 9 7 Inhibition of VEGF decreases tumor neovascularization and substantially inhibits primary tumor growth.22 OPN has an established relationship with VEGF in the angiogenic development of early stage NSCLC,10, 12 and VEGF mediated angiogenesis is enhanced by concurrent up-regulation of OPN10.
OPN and VEGF are known to acts synergistically via αvβ3 integrin on the endothelial cell surface. Exposure to VEGF increases αvβ3 expression, allowing for increased endothelial cell migration in the presence of OPN.11 The αvβ3 integrin is up regulated in NSCLC tumors compared to normal tissue, and antibody mediated inhibition of this integrin results in endothelial cell apoptosis and restricted invasion.23, 24 This common pathway for OPN and VEGF could serve as an important target for the regulation of malignant behavior in NSCLC.10
We have demonstrated on an in vitro angiogenesis platform that overexpression of the individual OPN isoforms has a divergent impact on angiogenic properties in NSCLC. OPNa increase BCE tubule formation, while OPNc reduces tubule length. We have also demonstrated that OPNa overexpression is associated with increased VEGF secretion and expression, while OPNc is associated with decreased VEGF secretion and expression, particularly in cell lines with high endogenous OPN. These findings suggest that some of the angiogenic properties conferred by OPN may be due to modulation of VEGF production by tumor cells. Studies that initially described the BCE platform used in our experiments noted a relationship between VEGF activity and increased tubule formation.25 Enhanced capillary tubule formation with simultaneous increases in VEGF secretion by NSCLC cells that overexpress OPNa in our experiments is consistent with these observations. We also noted that wild type NSCLC cell lines with high endogenous OPN (A549 and H460) had higher endogenous VEGF secretion and greater angiogenic potential measured by BCE tubule formation compared to wild type cell lines with low endogenous OPN (H153 and H358). However, OPNb overexpression increased BCE tubule length without associated changes in VEGF secretion or expression, indicating that additional pathways are likely important, and further demonstrating that each isoform has a unique effect in this complex process. Senger demonstrated OPN’s ability to increase endothelial cell migration via binding to the αvβ3 integrin. It is reasonable to assume that OPNb has the ability to activate endothelial receptors via binding to αvβ3 integrin, which would result in increased tubule formation observed in these experiments, but it does so without a concomitant augmentation of VEGF secretion or expression by tumor cells. This ‘two-hit’ effect on angiogenesis is likely exclusive to the full length protein.
The relationship between overexpression of OPNa and increased tubule formation was not linear. This is best appreciated in H460 (high endogenous OPN), where overexpression of OPNa significantly increased tubule formation compared to controls, but to a smaller extent than in H358 (low endogenous OPN) (Fig. 4) VEGF and OPN are known to act synergistically via αvβ3 integrin to increase angiogenesis in NSCLC. A similar non-linear increase in endothelial cell migration has been noted with exposure of increasing concentrations of the two mediators.11 Cell lines with low endogenous OPN expression demonstrated a larger percentage increase in tubule length with overexpression of OPNa, but a smaller increase in VEGF secretion, compared to cell lines with high native OPN. This may indicate that the introduction of OPNa into a system without endogenous OPN may have a greater impact on endothelial cell activation than increasing expression in the presence of already high levels of OPNa.
The impact of OPNc overexpression, which decreased VEGF secretion and tubule length, appeared more linear. Cell lines with high endogenous OPN had relatively large decreases in tubule length and VEGF secretion compared to cell lines with low endogenous OPN in the presence of the c-isoform. OPNc appears to act as a dominant negative in this system, overriding the effects of the native OPN isoforms.
Our data suggests that OPN’s effect on tumor angiogenesis is multi factorial and that each OPN isoform has a unique effect. OPNa, the full length protein and the dominant isoform expressed in NSCLC cell lines, increases angiogenesis by activating endothelial cells likely via binding to the αvβ3 integrin, with associated increases in VEGF secretion by the tumor cells. OPNb, which has a deletion at exon-5, appears to activate endothelial cells, but does not increase VEGF secretion by tumor cells. Neither, OPNa or OPNb overexpression resulted in significant increases in OPN protein secretion, indicating that the effects in endothelial cell activation are not merely due to the presence of more OPN protein. OPNc, which lacks exon-4, has a dominant negative effect in the system, decreasing endothelial cell activation and VEGF secretion by the tumor cells. This supports our hypothesis that individual OPN isoforms have divergent roles with respect to tumor associated angiogenesis and VEGF production in NSCLC. The pro-angiogenic properties of OPN function in an isoform specific manner, and this phenomenon is most pronounced in the OPNa isoform. Structural similarities between OPNa and OPNc, which behave divergently, may make OPN ideal for isoform specific targeted therapies aimed at reducing the angiogenic potential of NSCLC.
Appreciable weaknesses of this study include the inability to evaluate isoform specific inhibitory effects of OPN on VEGF expression, secretion, and endothelial cell activation due to the lack of isoform specific antibodies currently available. Our lab is currently working on production of such antibodies. Future experiments include silencing RNA (shRNA) in cell lines with high endogenous OPN to further define of the role of OPN on tumor angiogenesis. The lack of quantitative PCR or gene array data from all transfected cell lines weakens our findings with respect to isoform specific mRNA expression.
CONCLUSION
These in vitro data demonstrate that OPNa confers greater angiogenic properties with increased BCE tubule formation and an associated increase in secretion of VEGF in NSCLC. OPNb, which lacks exon-5, activates endothelial cells, but has no effect on VEGF secretion. OPNc, which lacks exon-4, has a dominant negative effect, decreasing tubule length and VEGF secretion. Importantly, OPNa is the isoform preferentially up-regulated in NSCLC cell lines. An enhanced understanding of the specific behavior of these subunits may lead to therapeutic strategies that selectively inhibit OPN isoforms to alter the angiogenic and metastatic potential of NSCLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Author Harvey I. Pass has a patent pending for use of osteopontin as a biomarker for asbestos related malignancy. This Study was funded in part by U01 CA111295-03, Early Detection Research Network, NCI, NIH, and from Belluck and Fox, LLP, NY, NY.
REFERENCES
- 1.Donati V, Boldrini L, Dell’Omodarme M, et al. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res. 2005;11(18):6459–65. doi: 10.1158/1078-0432.CCR-05-0541. [DOI] [PubMed] [Google Scholar]
- 2.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005;11(13):4646–52. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 3.Lucchi M, Fontanini G, Mussi A, et al. Tumor angiogenesis and biologic markers in resected stage I NSCLC. Eur J Cardiothorac Surg. 1997;12(4):535–41. doi: 10.1016/s1010-7940(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 4.Duarte IG, Bufkin BL, Pennington MF, et al. Angiogenesis as a predictor of survival after surgical resection for stage I non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1998;115(3):652–8. doi: 10.1016/S0022-5223(98)70331-9. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 5.Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992;340(8812):145–6. doi: 10.1016/0140-6736(92)93217-b. [DOI] [PubMed] [Google Scholar]
- 6.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 7.Shibusa T, Shijubo N, Abe S. Tumor angiogenesis and vascular endothelial growth factor expression in stage I lung adenocarcinoma. Clin Cancer Res. 1998;4(6):1483–7. [PubMed] [Google Scholar]
- 8.Takanami I, Tanaka F, Hashizume T, Kodaira S. Vascular endothelial growth factor and its receptor correlate with angiogenesis and survival in pulmonary adenocarcinoma. Anticancer Res. 1997;17(4A):2811–4. [PubMed] [Google Scholar]
- 9.Fontanini G, Vignati S, Bigini D, et al. Neoangiogenesis: a putative marker of malignancy in non-small-cell lung cancer (NSCLC) development. Int J Cancer. 1996;67(5):615–9. doi: 10.1002/(SICI)1097-0215(19960904)67:5<615::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Shijubo N, Uede T, Kon S, Nagata M, Abe S. Vascular endothelial growth factor and osteopontin in tumor biology. Crit Rev Oncog. 2000;11(2):135–46. [PubMed] [Google Scholar]
- 11.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. The American journal of pathology. 1996;149(1):293–305. [PMC free article] [PubMed] [Google Scholar]
- 12.Shijubo N, Uede T, Kon S, et al. Vascular endothelial growth factor and osteopontin in stage I lung adenocarcinoma. Am J Respir Crit Care Med. 1999;160(4):1269–73. doi: 10.1164/ajrccm.160.4.9807094. [DOI] [PubMed] [Google Scholar]
- 13.Kang IC, Lee YD, Kim DS. A novel disintegrin salmosin inhibits tumor angiogenesis. Cancer Res. 1999;59(15):3754–60. [PubMed] [Google Scholar]
- 14.Vasile E, Qu H, Dvorak HF, Dvorak AM. Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J Histochem Cytochem. 1999;47(2):159–67. doi: 10.1177/002215549904700205. [DOI] [PubMed] [Google Scholar]
- 15.Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16(4):885–93. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 16.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 17.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101(7):1468–78. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. Faseb J. 1993;7(15):1475–82. [PubMed] [Google Scholar]
- 19.Behrend EI, Craig AM, Wilson SM, Denhardt DT, Chambers AF. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994;54(3):832–7. [PubMed] [Google Scholar]
- 20.Gardner HA, Berse B, Senger DR. Specific reduction in osteopontin synthesis by antisense RNA inhibits the tumorigenicity of transformed Rat1 fibroblasts. Oncogene. 1994;9(8):2321–6. [PubMed] [Google Scholar]
- 21.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 22.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 23.Brooks PC, Montgomery AM, Rosenfeld M, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79(7):1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 24.Max R, Gerritsen RR, Nooijen PT, et al. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71(3):320–4. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Goto F, Goto K, Weindel K, Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993;69(5):508–17. [PubMed] [Google Scholar]