Abstract
This study examined the effect of aerobic exercise training on vagal and sympathetic influences on the modulations of heart rate and systolic blood pressure in response to an oral glucose load in obese individuals with and without type 2 diabetes (T2D). Beat-to-beat arterial pressure and continuous electrocardiogram were measured after a 12-hour overnight fast and in response to glucose ingestion (75 g dextrose) in obese subjects with (T2D group, n=23) and without (OB group, n=36) T2D, before and after 16 weeks of aerobic exercise training at moderate intensity. Autonomic modulation was assessed using spectral analysis of systolic blood pressure variability (BPV), heart rate variability (HRV), and analysis of baroreflex sensitivity (BRS). Glucose ingestion significantly increased low frequency BPV (LFSBP), low frequency HRV (LFRRI)) and the ratio of low-to-high frequency components of HRV (LFRRI/HFRRI), and decreased the high frequency power (HFRRI), (p<0.05). Exercise training increased LFRRI and LFRRI/HFRRI responses, and reduced HFRRI and LFSBP responses to glucose ingestion in both groups (p<0.05), but increased fasted BRS in the OB group only (p<0.05); glucose intake had no effect on BRS (p>0.05). In conclusion, a 16-week exercise training program improved cardiac autonomic modulation in response to an oral glucose load in obese adults, independently of diabetes status, and in the absence of remarkable changes in body weight, body composition, fitness level, and glycemic control.
Keywords: autonomic regulation, obesity, insulin sensitivity
1. Introduction
Glucose ingestion is associated with alterations in cardiovascular autonomic regulation in lean, healthy subjects. Several authors have reported increased muscle sympathetic nervous system activity [1, 2] and plasma norepinephrine concentrations [3], and elevated ratios of low-to-high frequency components of heart rate variability (HRV) following oral glucose intake [4]. This normal response maintains arterial blood pressure compensating for splachnic vasodilation with peripheral vasoconstriction [5]. It has been suggested that the increased activity of the sympathetic nervous system following ingestion of a glucose load is insulin-mediated [4].
Previous studies suggest that cardiovascular autonomic regulation is impaired in obesity, an insulin resistant-state [4]. At rest, obesity is often characterized by cardiac sympathetic predominance [6] and high rates of muscle sympathetic firing [1]. In response to a glucose challenge, obese subjects fail to increase muscle and whole-body sympathetic activity [1, 3]. The impaired autonomic response to glucose in obesity has been previously attributed to an attenuated baroreflex response associated with chronic hyperinsulinemia and insulin resistance [4, 7].
The impaired cardiovascular autonomic regulation seen in obese and insulin resistant individuals is consistent with an increased risk of mortality and adverse cardiac events [8]. Given this cardiovascular risk, interventions to improve cardiovascular regulation in this population would be clinically significant. Exercise training lowers sympathetic nerve traffic [9], increases baroreflex sensitivity (BRS), and improves glycemic control and insulin sensitivity [8]. It has been suggested that exercise training may improve autonomic responses to glucose ingestion by decreasing insulin resistance [3]. Weight reduction programs via caloric restriction have been shown to improve sympathetic responses to glucose intake in obese subjects [10]. However, the effects of exercise training on cardiovascular autonomic modulation in response to an oral glucose intake have not been examined in obese subjects with and without type 2 diabetes (T2D). Therefore, the main purpose of this study was to investigate the effect of aerobic exercise training on cardiac autonomic modulation (HRV), BRS and sympathetic vasomotor modulation (systolic blood pressure variability, BPV) after oral glucose ingestion in asymptomatic obese subjects with and without T2D. The effects of this exercise training on body composition, central adiposity, glycemic control, insulin sensitivity, fitness level, and lipid profile were also examined. We hypothesized that a 16-week exercise training program would improve autonomic responses to an oral glucose load in obese adults with and without T2D.
2. Methods
2.1. Subjects
Sixty-two obese men and women (age 40-60 years) were recruited from the local community. Fifty-nine subjects completed all aspects of testing and training. Exclusion criteria included: smoking, participation in regular physical activity programs during the past 6-months, irregular menstrual cycles, peripheral neuropathy, overt cardiovascular disease, electrocardiogram (ECG) abnormalities, hormonal contraceptives, uncontrolled hypertension, and β-blocker therapy. Self-reports indicated that none of the subjects had retinopathy or albuminuria. All subjects had a body mass index (BMI) greater than 30 kg/m2 and were classified into two groups based on their metabolic status: obese without T2D (OB group: fasting glucose < 100 mg/dL) and obese with T2D (group with T2D: fasting glucose ≥ 126 mg/dL and glucose levels after a 2-hour oral glucose tolerance test ≥ 200 mg/dL). Table 1 presents baseline subject characteristics for each group. Participants currently treated for hypertension (n = 10 with T2D and 6 OB), hypercholesterolemia (n = 7 with T2D and 9 OB), and depression (n = 4 with T2D and 4 OB) were instructed to continue their medication throughout the duration of the study. Subjects with T2D had been diagnosed with diabetes within 4.4 ± 2.2 years before they started the intervention and were treated with oral hypoglycemic drugs; none were treated with insulin. None of the patients changed their medication or the dose of their medication, and they also took their medication the same time during the day pre- and post-training testing. Table 2 presents a detailed list of drug type and class the participants were on. All pre-menopausal female subjects were tested during the first 10 days of their menstrual cycle, and of the post–menopausal women, only 3 were on hormone therapy. The Institutional Review Boards at Syracuse University and SUNY Upstate Medical University approved the protocol and written informed consent was obtained from all subjects prior to any testing.
Table 1.
Baseline subject characteristics
| T2D (n=26) | OB (n=36) | |
|---|---|---|
| Females/Males | 13/13 | 22/14 |
| Age (yrs) | 50 ± 1 (41-59) | 49 ± 1 (40 – 59) |
| Height (cm) | 170 ± 2 (152– 194) | 168 ± 1 (154 – 188) |
| Weight (kg) | 110 ± 5 (75 – 159) | 101 ± 2 (80 – 123) |
| BMI (kg/m2) | 38 ± 1 (30 – 52) | 36 ± 1 (29 – 44) |
Values are means ± SE (range). T2D, subjects with type 2 diabetes; OB, obese subjects; BMI, body mass index
Table 2.
Type and class of medication
| *Medication | No of participants |
|---|---|
| Glucose-lowering drugs | 18 |
| Metformin | 16 |
| Thiazolidinedione | 7 |
| Sulfonamides | 2 |
| Anti-depressants | 8 |
| Benzodiazepine | 1 |
| Selective serotonin reuptake inhibitors | 4 |
| Serotonin-norepinephrine reuptake inhibitors |
1 |
| Aminoketones | 2 |
| Anti-hypertensive drugs | 16 |
| Angiotensin converting enzyme inhibitors | 8 |
| Hydrochlorothiazides | 4 |
| Angiotensin receptor blocker | 5 |
| Lipid-lowering drugs | 16 |
| HMG-CoA reductase inhibitors | 15 |
| Fibrate | 1 |
Some participants received more than one medication and may therefore be counted in more than one categories
2.2. Experimental design
The study involved 4 laboratory visits and a 16-week exercise training program. On the 1st and 2nd visits, all subjects underwent a body composition assessment, a physician-supervised exercise stress test, and assessment of their autonomic function in the fasted state and following glucose ingestion. After the 2nd visit, all subjects participated in a 16-week exercise program, and at the end of the exercise intervention, the tests performed on visits 1 and 2 were repeated to determine the effects of exercise training on autonomic function. To minimize the confounding effects of medication on beat-to-beat blood pressure and heart rate measurements each subject's medication regimen was closely monitored and did not change during the study. Subjects were encouraged to maintain their dietary habits throughout the intervention and all measurements were conducted after a 12-hour overnight fast. In addition, subjects were instructed to refrain from caffeinated products 12 hours before testing, and avoid heavy exertion and alcohol consumption for 24 hours before testing.
2.3. Experimental procedures
Height (cm) and weight (kg) were measured following standard procedures and BMI (kg/ m2) was calculated. Waist circumference (cm) was measured at the umbilicus and was used as an index of regional fat distribution [10]. Body composition was analyzed using air plethysmography (Bod Pod, Life Measurements Inc., Concord, CA). Fitness level was assessed using a physician-supervised maximal aerobic exercise test on a treadmill as previously described [6, 11]. Resting and exercise 12-lead ECG was recorded, and expired gases were collected and analyzed using a calibrated metabolic system (Cosmed Quark b2, Rome, Italy).
For the assessment of the autonomic function, subjects arrived at the laboratory after a 12 hour of overnight fast. At 07:00 AM, a venous catheter was inserted into an antecubital vein and kept patent with normal saline. Baseline blood samples were drawn for glucose, insulin, and lipid levels. After 20 minutes of quiet rest in the supine position, resting ECG (modified CM5, sampling rate: 1000 Hz; Biopac Santa Barbara, CA) and beat-to-beat arterial pressure (sampling rate: 200 Hz; Portapres, TNO Biomedical Instrumentation, Amsterdam, The Netherlands) were collected for 5 minutes. The subjects were instructed to pace their breathing rate at 0.2 Hz (12 breaths per minute) during the recordings. All subjects practiced with a metronome before the tests began to ensure that they breathe comfortably using the experimental pace, without hyperventilating or substantially changing the depth of their breathing. Upon completion of the fasting cardiovascular recordings, a 75 g glucose drink (NERL Diagnostics, East Providence, RI) was consumed and 5 mL blood samples were drawn every 30 minutes for 4 hours. Five-minute continuous ECG and beat-to-beat arterial pressure were recorded a second time when the glucose concentrations were at their peak, which was 1 hour after the glucose ingestion for the group with T2D and 30 minutes after the glucose ingestion for the OB group [12, 13].
2.4. Exercise intervention
Subjects participated in a supervised/home-based aerobic exercise program for 16 weeks, 4days per week at 65% of their maximal oxygen consumption (VO2peak). The initial training workload was based upon a continuous recording of their VO2 using a calibrated metabolic system on the first training day. All subjects were instructed to walk on a treadmill or outdoors for 30 minutes per day (4 days per week) for the first 8 weeks. One day per week the subjects were required to walk in a one-on-one supervised setting where they were instructed on how to monitor their workload. The intensity of all training sessions was controlled and monitored using heart rate and ratings of perceived exertion. After the completion of the first 8 weeks, the duration of exercise increased gradually to 45 minutes so that for the last 6 weeks subjects walked for 45 minutes per day, 4 days per week. Subjects completed exercise logs and their exercise progress was discussed with them weekly.
2.5. Data analyses
2.5.1. Heart rate variability
Heart rate variability was analyzed using the Heart Signal software (Oulu, Finland) as previously described [6, 11]. The continuous ECG signal was filtered with visual and automatic editing and any R-R interval that deviated more than 30% from the previous interval was considered premature and was eliminated. As described by Huikuri et al. [14], an average of accepted R-R intervals in the local neighborhood was computed and used as the new value for the premature R-R intervals. This filtering technique has been suggested to make the data more stationary because it removes abrupt temporary changes in R-R interval sequence [14]. Only recordings with less than 2% of filtered beats were included in the analysis. An autoregressive model (order of 10) was used to estimate the power spectral densities of the R-R interval variability. Spectral power was expressed as the integrated areas in low (LFRRI: 0.05-0.15 Hz), high (HFRRI: 0.15-0.4 Hz) and total (TPRRI: 0.05-0.4 Hz) frequency ranges. The high frequency (HFRRI) power is a marker of the vagal influences on the modulations of heart rate, whereas the low frequency (LFRRI) power is jointly mediated by both sympathetic and parasympathetic influences [15, 16]. The ratio between low- and high-frequency spectra (LFRRI/HFRRI) was calculated [17] and was used as an estimation of the interaction between vagal and sympathetic influences on the cardiac pacemaker. High frequency and low frequency power spectral densities were calculated in both absolute (msec2) and normalized units (nu). Normalized units were calculated by dividing the power of a given component by the total power and multiplying by 100. All data analyses were carried out according to the standards set by the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology [17].
2.5.2. Blood pressure variability and baroreflex sensitivity
Systolic BPV and cardiovagal BRS were calculated from the systolic arterial pressure and R-R interval time series as previously described (WinCPRS, Absolute Aliens Oy, Turku, Finland) [18]. For the BPV analysis, the non-equidistant waveforms were re-sampled at 5 Hz and passed through a low-pass filter with a cut-off frequency of 0.5 Hz. The spectrum of each signal was calculated using Fast Fourier transformation. Spectral power was expressed as the integrated areas in low (LFSBP: 0.05-0.15 Hz), high (0.15-0.4 Hz) and total (0.05-0.4 Hz) frequency ranges. The LFSBP (mm Hg2) was used as an index of vasomotor sympathetic modulation [19]. For the BRS analysis, we determined the coupling between fluctuations in R-R intervals and systolic arterial pressure using the sequence technique [20]. Baroreflex sequences were selected from the changes in systolic arterial pressure if R-R intervals concurrently changed in the same direction with the arterial pressure for 3 or more consecutive beats for at least 4 msec. The slope of the regression line between systolic arterial pressure and R-R intervals was used to calculate BRS (ms/mm Hg). Only sequences with correlations equal or greater than 0.80 were accepted [6].
2.5.3. Blood analyses
Whole blood was used to determine total cholesterol, high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), and triglycerides (TRG) via a point-of-care testing (POCT) device (Cholestech instruments, Hayward, CA) following the manufacturers guidelines.
Whole blood glucose concentrations were measured using the glucose oxidase method with the YSI 2300 Stat (Yellow Springs Instruments, Yellow Springs, OH). Insulin concentrations were measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA). The intra- and inter-assay coefficients of variation for these assays were 7.6% and 8.9%, respectively. Hemoglobin A1c was measured using kits from Diabetes Technologies (Thomasville, GA). Whole-body insulin sensitivity was calculated using data from the oral glucose tolerance test using the following equation [21]: Whole-body insulin sensitivity index = 10,000 / √(FPG × FPI) × (mean PG × mean PI). FPI is fasting plasma insulin (in mU/L), FPG is fasting plasma glucose (in mg/dL), and mean values are the average of concentrations at times 0, 30, 60, 90, and 120 minutes. This index reflects insulin sensitivity in the fasted state and after the ingestion of a glucose load, and reflects both hepatic and peripheral tissue insulin sensitivity [3]. The whole-body insulin sensitivity index (ISI) has been validated in groups of subjects with different degrees of obesity and glucose tolerance [21, 22] and correlates well with the rate of whole-body glucose disposal during the euglycemic insulin clamp (r=0.73, p<0.0001) [23].
2.6. Statistical Analysis
The distributions of the following variables were significantly skewed: TPRRI (msec2), LFRRI/HFRRI, LFSBP and BRS. To satisfy the assumption of normality, we first log transformed each value and then used parametric statistics. The insulin-related variables were also not normally distributed and log transformation was used.
Analysis of variance (ANOVA) with repeated measures in a mixed model (between subject factor: group, OB vs. T2D; within subject factor: time, pre-training vs. post-training) was used to examine main effects and interactions for anthropometric and metabolic variables. In order to determine main effects and interactions for the HRV, BPV, and BRS we used a 3-way ANOVA with repeated measures (group (OB vs. T2D) × metabolic status (fasted vs. glucose loaded) × time (pre-training vs. post-training)). If significant interactions were found, we performed post hoc analyses (Tukey test and Bonferroni corrections) examining the data across group or metabolic status. Pearson correlations were used to assess the relationship between HRV and LDL-c, HDL-c, total cholesterol, as well as the relationship between insulin-related variables and waist circumference. All results are presented as means ± SE. The level of statistical significance was set at α=0.05.
3. Results
Anthropometric, body composition and fitness characteristics of the two experimental groups are shown in Table 3. At baseline, there were no significant differences in weight, BMI, %body fat, and fitness level between the two groups, but the obese subjects with T2D had greater waist circumference than the OB subjects (mean difference, OB vs. T2D ± SE: 10.5 ± 3.2 cm, p = 0.002). The exercise intervention reduced body weight (mean difference, pre- vs. post-training: 0.82 ± 0.32 kg, p = 0.015), BMI (mean difference, pre- vs. post-training: 0.3 ± 0.1 kg/ m2, p = 0.009), and waist circumference (mean difference, pre- vs. post-training: 2.9 ± 0.9 cm, p = 0.002). Fitness level, expressed in both absolute (L/min) and relative units (ml/kg/min), increased in response to the exercise training program (VO2peak, L/min: mean difference, pre- vs. post-training: 0.17 ± 0.03, p = 0.000; VO2peak, ml/kg/min: mean difference pre- vs. post-training: 1.8 ± 0.4 cm, p = 0.000). There was no group by time effect for any of the above variables.
Table 3.
Anthropometrics, body composition, fitness level, and blood pressure and heart rate pre- and post-exercise intervention
| T2D | OB | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Anthropometrics | ||||
| Weight (kg) | 108.0 ± 3.5 | 107.3 ± 3.6 | 100.9 ± 2.7 | 100.0 ± 2.8* |
| Waist (cm) | 120.7 ± 2.7 | 117.1 ± 2.6 | 109.4 ± 2.1 | 107.3 ± 2.0*† |
| Body Composition | ||||
| %body fat | 42.1 ± 1.8 | 41.9 ± 1.8 | 41.6 ± 1.4 | 41.3 ± 1.3 |
| BMI (kg/m2) | 37.5 ± 1.0 | 37.3 ± 1.0 | 35.7 ± 0.8 | 35.4 ± 0.8* |
| Fitness | ||||
| VO2peak (L/min) | 2.3 ± 0.1 | 2.5 ± 0.2 | 2.3 ± 0.1 | 2.5 ± 0.1* |
| VO2peak (ml/kg/min) | 22.4 ± 1.1 | 24.5 ± 1.2 | 23.2 ± 0.8 | 24.7 ± 0.9* |
| Blood Pressure & Heart Rate | ||||
| SBP (mm Hg) | 124.2 ± 2.9 | 122.7 ± 3.0 | 121.7 ± 2.5 | 117.1 ± 2.5 |
| DBP (mm Hg) | 61.5 ± 1.6 | 70.7 ± 1.9 | 62.6 ± 1.3 | 68.1 ± 1.6* |
| HR (bpm) | 70.0 ± 2.0 | 69.4 ± 2.0 | 68.6 ± 1.7 | 65.9 ± 1.7 |
Values are means ± SE.
p<0.05, time effect (pre-training vs. post-training);
p<0.05, group effect (T2D vs. OB).
T2D, subjects with type 2 diabetes; OB, obese subjects; BMI, body mass index; VO2peak, maximum oxygen consumption; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
3.1. Metabolic status
As expected, the group with T2D had higher fasting glucose (mean difference: 2.02 ± 0.29 mmol/L, p=0.000), glucose AUC (mean difference: 1033 ± 108 min.mmol/L, p=0.000), and hemoglobin A1c (mean difference: 1.7 ± 0.2, p=0.000) than the OB group (see Table 4). Fasting insulin levels were greater (p=0.019), whereas the whole-body insulin sensitivity index was lower (p=0.005) in the group with T2D compared to the OB group. The exercise training program did not affect glycemic control, but it significantly decreased the insulin AUC in the OB group (p=0.000). The insulin-related variables were not normally distributed and therefore, their log transformed values were used in the statistical analysis. For clarity, however, the back-transformed values are presented in Table 4. The OB group had greater LDL-c levels than the group with T2D (p=0.036) but there were no group differences in total cholesterol, HDL-c, and TRG concentrations. In the OB group, LDL-c levels decreased following the exercise training program (p=0.011), whereas there was no change in the group with T2D, which remained lower than the OB group.
Table 4.
Glycemic control, insulin profile and lipid profile pre- and post-exercise intervention
| T2D | OB | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Glycemic control | ||||
| Fasting glucose (mmol/L) | 7.2 ± 0.2 | 6.9 ± 0.3 | 5.0 ± 0.2 | 5.0 ± 0.2† |
| Glucose AUC (min·mmol/L) | 2469 ± 94 | 2374 ± 92 | 1403 ± 77 | 1374 ± 75† |
| HbA1c (%) | 7.2 ± 0.3 | 7.1 ± 0.2 | 5.7 ± 0.2 | 5.3 ± 0.1† |
| Insulin profile | ||||
| Fasting insulin (pmol/L) | 78.3 ± 1.2 | 84.9 ± 1.2 | 58.9 ± 1.1 | 42.5 ± 1.2† |
| Insulin AUC (min·pmol/L) | 51880 ± 1 | 58884 ± 1 | 60953 ± 1 | 50582 ± 1‡ |
| ISI | 3.3 ± 1.2 | 2.7 ± 1.2 | 3.9 ± 1.1 | 5.4 ± 1.1†‡ |
| Lipid profile | ||||
| Total cholesterol (mg/dL) | 188 ± 10 | 193 ± 9 | 222 ± 8 | 203 ± 7‡ |
| LDL-c (mg/dL) | 108 ± 9 | 117 ± 8 | 142 ± 7 | 125 ± 7†‡ |
| HDL-c (mg/dL) | 43 ± 13 | 44 ± 14 | 51 ± 15 | 48 ± 14 |
| TGL (mg/dL) | 183 ± 83 | 164 ± 71 | 159 ± 53 | 145 ± 65 |
Values are means ± SE. The insulin-related variables were not normally distributed and therefore, they were log transformed for the statistical analysis and were back-transformed for tabular presentation.
p<0.05, group effect (T2D vs. OB);
p<0.05, time (pre- vs. post-training) by group (T2D vs. OB) effect.
T2D, subjects with type 2 diabetes; OB, obese subjects; AUC, area under the curve; ISI, insulin sensitivity index; LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol; TRG, triglycerides
3.2. Heart rate variability, blood pressure variability, and baroreflex sensitivity
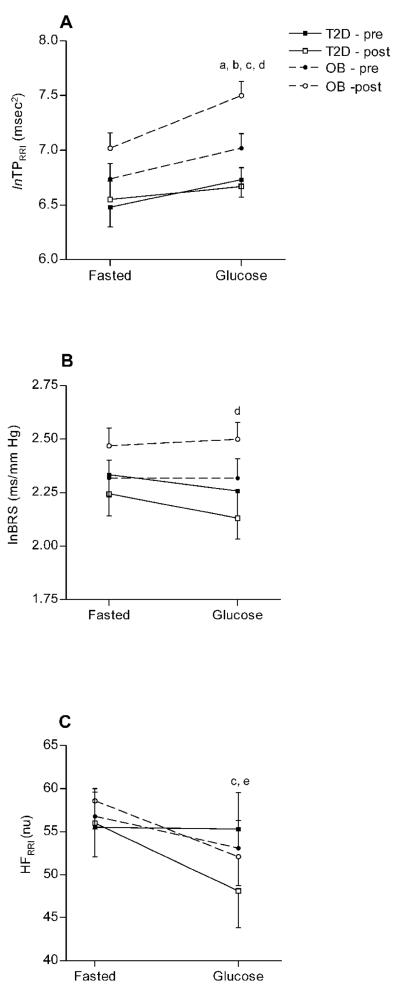
The HRV (TPRRI, LFRRI/HFRRI) and BPV (LFSBP) measures that were not normally distributed were transformed into natural logarithms (ln) before any statistical analysis. The log transformed data are presented in Figures 1-2 and the non-transformed data are presented in Table 5. LFRRI (nu) and HFRRI (nu) were normally distributed. The OB group had significantly greater fasted lnTPRRI compared to the group with T2D (p = 0.013, Figure 1A). The exercise training program significantly increased fasted lnTPRRI (p=0.033, Figure 1A) and BRS (p = 0.027, Figure 1B) in the OB subjects but not in those with T2D.
Figure 1A-C.
Total power (lnTPRRI, Figure 1A), baroreflex sensitivity (lnBRS, Figure 1B), and high frequency (HFRRI, Figure 1C) responses (means ± SE) to glucose ingestion before and after exercise training. All values are presented as natural logarithms (ln). ap<0.05, pre- vs. post training; bp<0.05, OB vs. T2D; cp<0.05, fasted vs. glucose loaded; dp<0.05, time by group interaction; ep<0.05, time by metabolic status interaction
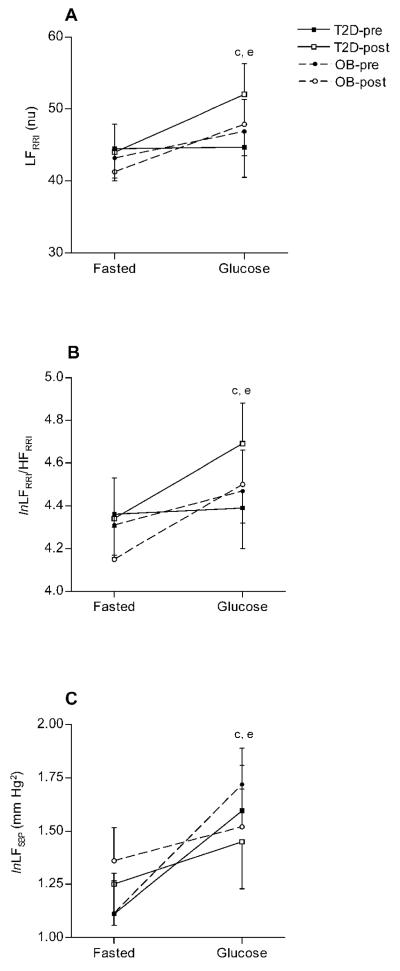
Figure 2A-C.
Low frequency power of HRV (LFRRI, Figure 2A), low frequency to high frequency ratio (lnLFRRI/HFRRI, Figure 2B) and low frequency (lnLFSBP, Figure 2C) responses (means ± SE) to glucose ingestion before and after exercise training. All values are presented as natural logarithms (ln). ap<0.05, pre- vs. post training; bp<0.05, OB vs. T2D; cp<0.05, fasted vs. glucose loaded; dp<0.05, time by group interaction; ep<0.05, time by metabolic status interaction
Table 5.
Non-transformed baroreflex sensitivity, heart rate and blood pressure variability
| T2D | OB | ||||
|---|---|---|---|---|---|
| Fasted | Glucose Load | Fasted | Glucose Load | ||
| TPRRI (msec2) | Pre Post |
902 ± 238 927 ± 253 |
1002 ± 370 1098 ± 388 |
1267 ± 190 1574 ± 202 |
1794 ± 296 2658 ± 310 |
| LFRRI/HFRRI | Pre Post |
91 ± 16 80 ± 12 |
87 ± 17 112 ± 22 |
85 ± 13 75 ± 10 |
97 ± 14 114 ± 18 |
| LFSBP (mm Hg2) | Pre Post |
3.8 ± 1.9 4.4 ± 1.4 |
8.0 ± 2.7 5.2 ± 3.3 |
5.5 ± 1.5 6.3 ± 1.1 |
9.1 ± 2.2 9.8 ± 2.7 |
| Baroreflex Sensitivity (ms/mm Hg) |
Pre Post |
11.4 ± 1.3 10.2 ± 1.5 |
10.2 ± 1.4 9.1 ± 1.3 |
11.0 ± 1.0 12.5 ± 1.2 |
11.4 ± 1.1 12.8 ± 1.0 |
Values are mean ± SE. Non-transformed baroreflex sensitivity (BRS), heart rate (HRV) and blood pressure variability (BPV) parameters. TPRRI, total power of HRV; LFRRI, low frequency power of HRV; HFRRI, high frequency power of HRV; LFRRI/HFRRI, low to high frequency ratio; LFSBP, low frequency power of BPV; nu, normalized units
A reduction in HFRRI (p = 0.005, Figure 1C) and an increase in lnTPRRI (p = 0.000, Figure 1A), LFRRI (p = 0.005, Figure 2A), lnLFRRI/HFRRI (p = 0.004, Figure 2B) and lnLFSBP (p = 0.001, Figure 2C) were observed after the glucose load. Conversely, BRS did not change following oral glucose administration in any of the groups (Figure 1B). Both groups responded with a greater increase in LFRRI (p = 0.040) and lnLFRRI/HFRRI (p = 0.043), a smaller increase in lnLFSBP (p = 0.020), and a greater reduction in HFRRI (p = 0.040) in response to a glucose load following a 16-week exercise program.
3.3. Correlations
The improved fasted lnTPRRI, as measured following the exercise intervention, was correlated with total cholesterol (r=−0.487, p = 0.005, n=31) and LDL-c (r=−0.464, p = 0.009, n=31) in the OB group but not in the group with T2D. There were no significant correlations between pre-training fasted lnTPRRI or fasted lnLFRRI/HFRRI and fitness level, or any of the anthropometric, metabolic and lipid measures. Insulin AUC (r=−0.345, p = 0.039, n = 36), whole body insulin sensitivity index (r=−0.334, p = 0.047, n=36) and BRS (r = −0.416, p=0.012, n=36) correlated with waist circumference in the OB subjects only.
4. Discussion
The main findings of this study showed that a 16-week home-based, supervised exercise training program resulted in increased sympathetic modulation and reduced vagal modulation in response to an oral glucose load in obese adults. These alterations were seen in both obese groups, independently of diabetes status, and in the absence of remarkable changes in body weight, body composition, fitness level, and glycemic control. Further, the subjects with T2D showed changes in both cardiac and vasomotor vagal and sympathetic modulation in response to glucose ingestion, in the absence of any changes in fasted autonomic and insulin sensitivity measures.
4.1. Exercise training and autonomic modulation
Recently, Strazincky et al. [24] reported that a 3-month hypocaloric diet with or without an exercise program reduced body weight (8 kg) and reversed blunted sympathetic responsiveness to glucose ingestion in insulin resistant subjects with the metabolic syndrome. Our findings further suggest that a 4-month exercise program with minor weight loss (less than 1 kg) may also reverse the blunted autonomic responses to glucose intake in obese subjects. Both groups showed a greater increase in LFRRI and LFRRI/HFRRI, and a greater reduction in HFRRI in response to a glucose load following the exercise intervention. These changes in the spectral components of HRV reflect changes in cardiac vagal and sympathetic modulation [25]. Previous reports demonstrated a significant relationship between LFRRI and plasma norepinephrine concentrations during increased muscle sympathetic activity, whereas there was no association between resting LFRRI and resting muscle sympathetic activity or plasma norepinephrine concentrations [26]. Subsequent studies suggested that an increase in LFRRI from a resting to a stressed state represents an increase in sympathetic modulation of heart rate, as long as respiratory rate remains unchanged and HFRRI decreases or does not change [27-29]. Since in this study cardiac vagal modulation (HFRRI) in response to glucose ingestion was reduced after training without any change in respiratory rate and LFRRI was increased, we can speculate that the increase in LFRRI and LFRRI/HFRRI responses to a glucose load indicate an increase in cardiac sympathetic modulation. It is also possible that the exercise training program altered the sympathetic modulation during hyperglycemia, without any change in sympathetic tone per se [25]. In a similar fashion, the reduction in the high frequency component of HRV may reflect either reduced cardiovagal modulation [15] or diminished ability of the parasympathetic nervous system to respond to its regulatory mechanisms [25].
Data from previous investigations suggest that obese and insulin resistant subjects have an impaired sympathetic neural response to oral glucose intake [1, 4]. Conversely, we have recently shown that obese subjects with and without T2D preserve normal sympathetic responses to orthostatic stress [6], isometric muscle contraction and the cold pressor test [11], when compared with lean age-matched subjects. Others have demonstrated normal sympathetic responses to a Valsalva maneuver in this population [1]. This suggests that the efferent sympathetic pathways of obese subjects can respond appropriately to deactivation of the baroreflex and stimulation of cutaneous afferents, but not to insulin-mediated sympathoexcitation [1].
In our study, the OB group had improvements in fasted autonomic modulation and autonomic responses to glucose associated with an increase in whole-body insulin sensitivity and insulin AUC. If the improved autonomic responses to glucose ingestion are related to changes in insulin sensitivity, one would have expected the group with T2D to also demonstrate improvements in insulin sensitivity. This was not observed, possibly due to their ingestion of a variety of oral glycemic control medications. It should also be mentioned that the index of insulin sensitivity used in this study reflects whole-body insulin sensitivity and does not distinguish between peripheral and central neural actions of insulin. It has been shown that insulin can stimulate the sympathetic nervous system via hypothalamic regulation [30, 31] or indirectly via baroreflex-mediated sympathetic stimulation in response to insulin-induced vasodilation [3]. Also, individuals with insulin resistance exhibit an imbalance between insulin-induced vasodilation and insulin-mediated cardiovascular sympathetic nerve activity [32, 33] . Hence, the improvements in autonomic function responses to glucose load seen in the group with T2D following an exercise intervention may reflect a selective influence of exercise upon insulin-mediated pathways. However, this hypothesis was not examined in the present investigation. More research is needed to assess the effects of exercise on the differential effects of insulin on autonomic nervous system activity in T2D.
In agreement with previous studies [34], we also found that exercise training improved autonomic responsiveness to physiological stressors in obese subjects, even in the absence of improvements in baseline (fasted) autonomic function. Indeed, the obese subjects with T2D, who showed no improvements in fasted HRV and BRS, improved their autonomic responses to an oral glucose load after the exercise intervention. Interestingly, before the exercise training program, the OB group had an 18% increase in LFRRI/HFRRI in response to oral glucose intake, whereas the group with T2D had a smaller (2.5%) increase. Following the exercise intervention, these responses increased up to 42% for both groups, suggesting a greater improvement in cardiac sympathetic responsiveness to glucose challenge in the obese subjects with T2D, even in the absence of autonomic improvements in the fasted state.
In addition to improving the HRV responses to glucose intake, a 16-week exercise training program increased fasted total power of HRV by 34% only in the obese subjects without T2D and not in the group with T2D. This is in agreement with previous studies that found no changes in fasted HRV following a 6-month [34] or a 12-month [8] aerobic exercise training program in patients with T2D. Our data also support that exercise training accentuated fasted BRS, in addition to having beneficial effects on atherogenic lipid levels and insulin responses to a glucose challenge in the OB group. These improvements were not seen in the T2D subjects. Diabetes is characterized by vascular alterations such as endothelial dysfunction and reduced arterial elasticity [35, 36] that may diminish the cardiovagal barororeflex gain. Therefore, changes in both mechanical and neurogenic mechanisms may be necessary to enhance the baroreflex function. Consequently, it is possible that the length of our intervention could not improve BRS in subjects with T2D because of structural and functional vascular changes associated with both diabetes and hypertension. In support of this notion, previous investigations showed improvements in BRS following 12-month of exercise training [8] but they showed no changes after 5-month of training in a cohort of patients with T2D [37].
In this study, LFSBP increased in response to glucose intake reflecting an elevated sympathetic modulation of vasomotor tone [19]. After 16 weeks of aerobic exercise training this response was smaller in both obese groups by 40-60%. It has been shown that insulin-induced vasodilation is impaired in obesity and T2D [1, 38, 39] due to endothelial dysfunction [40]. A reduction in sympathetic vasomotor modulation may reflect alterations in sympathetic-mediated vasoconstriction in response to impaired vasodilation, but since we did not measure muscle blood flow and endothelial function, this question is unanswered. Given that an increase in LFRRI/HFRRI reflects elevated cardiac sympathetic modulation and LFSBP is an index of sympathetic vasomotor modulation, our findings suggest that exercise training may have differential effects on sympathetic responses, increasing cardiac modulation and diminishing modulation of vasomotor tone in response to glucose loading.
It is noteworthy that in this study we found no differences in baseline HRV and BPV between the two groups. Type 2 diabetes is often accompanied by autonomic dysfunction and therefore, the subjects with T2D were expected to show greater alterations in autonomic function compared to the obese subjects without T2D. The absence of peripheral neuropathy in the cohort of individuals with diabetes tested in this study may partially explain the lack of differences in baseline HRV and BPV between groups [6]. Previous investigations demonstrated a positive relationship between autonomic dysfunction and severity of neuropathy with subjects having severe neuropathy exhibiting blunted HRV responses to physiological stressors, whereas those without neuropathy having normal HRV responses [41]. In addition, most subjects with T2D had a good metabolic control as indicated by their low HbA1c levels and fasting glucose levels. Further, sixteen patients with T2D were using metformin, which has been shown to improve both insulin resistance and cardiovascular autonomic function [42].
4.2. Lipid profile, central adiposity, and autonomic modulation
The OB subjects had elevated levels of LDL-c at baseline but these levels were reduced after the exercise intervention. The use of lipid-lowering agents, however, diminished our ability to detect changes in lipid levels by exercise alone. Nevertheless, the improved LDL-c levels represent a reduction in cardiovascular risk and it may be one of many factors contributing to the improved autonomic profile found in the OB group, as manifested by the significant relationship between the reduced atherogenic cholesterol and the improved total HRV. Although the LDL-c levels were reduced in the OB group after the exercise intervention, they remained higher than those in the group with T2D. This may have been due to more aggressive lipid-lowering therapy in those with T2D. Exercise training did not improve HDL-c levels in any of the groups. This may be explained by the fact that both groups had pre-exercise HDL-c levels close to normal (mean value>43 mg/dL) due to the use of lipid-lowering medication. Other exercise training studies have also found improved HRV in obese patients without any improvements in HDL-c levels [34]. Greater exercise intensity and duration that induce weight loss may be necessary in order to obtain improvements in HDL-c concentrations.
In the OB group, but not the group with T2D, the increase in fasted BRS was associated with a reduction in waist circumference and an increase in insulin sensitivity following the exercise training program. This suggests that the beneficial effects of exercise training on fasted autonomic modulation in obesity are due to improvements in insulin action and cardiovagal baroreflex. The group with T2D had a 3 cm reduction in waist circumference after the training program but this was still 10 cm greater than the OB group and approximately 15 cm greater than the optimal values [43]. It is possible that a greater reduction in central adiposity is necessary to affect basal autonomic modulation in this population.
4.3. Limitations
In this study, autonomic regulation was solely assessed with HRV and BPV spectral analyses. Spectral analysis of HRV provides information about the sympathetic and parasympathetic modulation but does not give us insight into the status of the tonic stimulus. Therefore, our conclusions are limited to the effects of exercise training on vagal and sympathetic influences on the modulations of heart rate and arterial pressure, and inferences cannot be made regarding the tone of the two arms of the autonomic nervous system in response to glucose load and exercise training in obese patients. Future studies assessing autonomic tone along with HRV and BPV are necessary in order to provide a more complete assessment of the autonomic function in response to glucose intake in obese patients with and without T2D.
The lack of a time control group (non-exercising group) in this study is a limitation. We have previously, however, shown that HRV is a stable a reliable measure in this population, at rest and during a perturbation such as hand grip exercise [11]. We have also shown that HRV does not change, is very stable and highly reliable in the absence of exercise training [44]. Furthermore, we have shown that HRV increases with resistance training, but decreases with detraining [45]. In addition, data from our lab conducted 7 days apart showed high intra-class correlation coefficients for the LF and HF measurements (ICC>0.9) [46]. These previous data clearly show that HRV does not change in the absence of exercise training, decreases with de-training and has satisfactory reproducibility for repeated measures. Thus, the current findings are likely due to the exercise training, and it is unlikely that our findings are a function of the Hawthorne effect.
In conclusion, the findings of this study demonstrated that an aerobic exercise program of moderate intensity is adequate to alter autonomic modulation during hyperglycemia in obese subjects with and without T2D. This is the first report to suggest that autonomic alterations occur without remarkable changes in body weight and insulin sensitivity. It is noteworthy that the subjects in this study successfully followed a generally recommended exercise regimen (30 minutes per day, ~ 4 days per week) exercising 3 days per week on their own. The exercise training program elicited improvements in fitness level in both groups but these improvements did not explain the changes in autonomic responsiveness. In addition, all medicated participants kept taking their medication throughout their participation in the study. This experimental approach reflects the effects of exercise training on autonomic function in a realistic setting.
Acknowledgements
The authors thank the subjects for their tremendous amount of time and effort and acknowledge Rose Kingsbury, RN, NP for her dedication to the study and placing all of the catheters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors had a financial or personal conflict of interest.
Supported by a National Institutes of Health grant R21 DK063179
REFERENCES
- 1.Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest. 1994;93(6):2365–71. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berne C, Fagius J, Niklasson F. Sympathetic response to oral carbohydrate administration. Evidence from microelectrode nerve recordings. J Clin Invest. 1989;84(5):1403–9. doi: 10.1172/JCI114313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straznicky NE, Lambert GW, Masuo K, Dawood T, Eikelis N, Nestel PJ, et al. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am J Clin Nutr. 2009;89(1):27–36. doi: 10.3945/ajcn.2008.26299. [DOI] [PubMed] [Google Scholar]
- 4.Paolisso G, Manzella D, Rizzo MR, Barbieri M, Varricchio G, Gambardella A, et al. Effects of insulin on the cardiac autonomic nervous system in insulin-resistant states. Clin Sci (Lond) 2000;98(2):129–36. doi: 10.1042/cs0980129. [DOI] [PubMed] [Google Scholar]
- 5.Fagius J. Sympathetic nerve activity in metabolic control--some basic concepts. Acta Physiol Scand. 2003;177(3):337–43. doi: 10.1046/j.1365-201X.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanaley JA, Baynard T, Franklin RM, Weinstock RS, Goulopoulou S, Carhart R, Jr., et al. The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus. Metabolism. 2007;56(6):778–85. doi: 10.1016/j.metabol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muscelli E, Emdin M, Natali A, Pratali L, Camastra S, Gastaldelli A, et al. Autonomic and hemodynamic responses to insulin in lean and obese humans. J Clin Endocrinol Metab. 1998;83(6):2084–90. doi: 10.1210/jcem.83.6.4878. [DOI] [PubMed] [Google Scholar]
- 8.Loimaala A, Huikuri HV, Koobi T, Rinne M, Nenonen A, Vuori I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes. 2003;52(7):1837–42. doi: 10.2337/diabetes.52.7.1837. [DOI] [PubMed] [Google Scholar]
- 9.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, et al. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension. 1991;18(5):575–82. doi: 10.1161/01.hyp.18.5.575. [DOI] [PubMed] [Google Scholar]
- 10.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90(11):5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 11.Franklin RM, Baynard T, Weinstock RS, Goulopoulou S, Carhart R, Jr., Ploutz-Snyder R, et al. Autonomic responses to physiological stressors in women with type 2 diabetes. Clin Auton Res. 2008;18(2):66–73. doi: 10.1007/s10286-008-0461-4. [DOI] [PubMed] [Google Scholar]
- 12.Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54(7):866–75. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Baynard T, Franklin RM, Goulopoulou S, Carhart R, Jr., Kanaley JA. Effect of a single vs multiple bouts of exercise on glucose control in women with type 2 diabetes. Metabolism. 2005;54(8):989–94. doi: 10.1016/j.metabol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Huikuri HV, Linnaluoto MK, Seppanen T, Airaksinen KE, Kessler KM, Takkunen JT, et al. Circadian rhythm of heart rate variability in survivors of cardiac arrest. Am J Cardiol. 1992;70(6):610–5. doi: 10.1016/0002-9149(92)90200-i. [DOI] [PubMed] [Google Scholar]
- 15.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 16.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248(1 Pt 2):H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 17.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 18.Goulopoulou S, Fernhall B, Kanaley JA. Hemodynamic responses and linear and nonlinear dynamics of cardiovascular autonomic regulation following supramaximal exercise. Eur J Appl Physiol. 2009;105(4):525–31. doi: 10.1007/s00421-008-0930-4. [DOI] [PubMed] [Google Scholar]
- 19.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249(4 Pt 2):H867–75. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch JM, Eckberg DL, Graves LD, Wallin BG. Arterial pressure ramps provoke linear increases of heart period in humans. Am J Physiol. 1986;251(6 Pt 2):R1086–90. doi: 10.1152/ajpregu.1986.251.6.R1086. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Mannucci E, Bardini G, Ognibene A, Rotella CM. Comparison between 2 insulin sensitivity indexes in obese patients. Diabetes Care. 2000;23(7):1042–3. doi: 10.2337/diacare.23.7.1042. [DOI] [PubMed] [Google Scholar]
- 23.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096–101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 24.Straznicky NE, Lambert GW, McGrane MT, Masuo K, Dawood T, Nestel PJ, et al. Weight Loss May Reverse Blunted Sympathetic Neural Responsiveness to Glucose Ingestion in Obese Metabolic Syndrome Subjects. Diabetes. 2009 doi: 10.2337/db08-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik M, Camm AJ. Components of heart rate variability--what they really mean and what we really measure. Am J Cardiol. 1993;72(11):821–2. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 26.Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol. 1990;258(3 Pt 2):H713–21. doi: 10.1152/ajpheart.1990.258.3.H713. [DOI] [PubMed] [Google Scholar]
- 27.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261(4 Pt 2):H1231–45. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- 28.Weise F, Heydenreich F, Runge U. Contributions of sympathetic and vagal mechanisms to the genesis of heart rate fluctuations during orthostatic load: a spectral analysis. J Auton Nerv Syst. 1987;21(2-3):127–34. doi: 10.1016/0165-1838(87)90015-4. [DOI] [PubMed] [Google Scholar]
- 29.Weise F, Baltrusch K, Heydenreich F. Effect of low-dose atropine on heart rate fluctuations during orthostatic load: a spectral analysis. J Auton Nerv Syst. 1989;26(3):223–30. doi: 10.1016/0165-1838(89)90171-9. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334(6):374–81. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins DF, Williams G. Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet Med. 1997;14(12):1044–50. doi: 10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Takagi M, Tanaka Y, Yamasaki Y, Yamamoto M, Hori M, Nakaniwa T, et al. Responsiveness of insulin-induced cardiac sympathetic nerve activation associates with blood pressure regulation in diabetics. Am J Physiol Endocrinol Metab. 2003;284(5):E1022–6. doi: 10.1152/ajpendo.00169.2002. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita J, Tanaka Y, Niwa M, Yoshii H, Takagi M, Kawamori R. Impairment of insulin-induced vasodilation is associated with muscle insulin resistance in type 2 diabetes. Diabetes Res Clin Pract. 2000;47(3):185–90. doi: 10.1016/s0168-8227(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 34.Zoppini G, Cacciatori V, Gemma ML, Moghetti P, Targher G, Zamboni C, et al. Effect of moderate aerobic exercise on sympatho-vagal balance in Type 2 diabetic patients. Diabet Med. 2007;24(4):370–6. doi: 10.1111/j.1464-5491.2007.02076.x. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45(5):623–34. doi: 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- 36.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loimaala A, Huikuri H, Oja P, Pasanen M, Vuori I. Controlled 5-mo aerobic training improves heart rate but not heart rate variability or baroreflex sensitivity. J Appl Physiol. 2000;89(5):1825–9. doi: 10.1152/jappl.2000.89.5.1825. [DOI] [PubMed] [Google Scholar]
- 38.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41(9):1076–83. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 39.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85(6):1844–52. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanikawa T, Abe H, Tanaka Y, Nakashima Y. Cardiac autonomic balance and QT dispersion during head-up tilt testing in diabetics with and without sensory neuropathy. Clin Exp Hypertens. 2004;26(2):137–44. doi: 10.1081/ceh-120028551. [DOI] [PubMed] [Google Scholar]
- 42.Manzella D, Grella R, Esposito K, Giugliano D, Barbagallo M, Paolisso G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens. 2004;17(3):223–7. doi: 10.1016/j.amjhyper.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1-253. [PubMed] [Google Scholar]
- 44.Heffernan KS, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Heart rate recovery after exercise is associated with resting QTc interval in young men. Clin Auton Res. 2007;17(6):356–63. doi: 10.1007/s10286-007-0450-z. [DOI] [PubMed] [Google Scholar]
- 45.Heffernan KS, Jae SY, Vieira VJ, Iwamoto GA, Wilund KR, Woods JA, Fernhall B. C-reactive protein and cardiac vagal activity following resistance exercise training in young African-American and white men. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1098–105. doi: 10.1152/ajpregu.90936.2008. [DOI] [PubMed] [Google Scholar]
- 46.Goulopoulou S, Heffernan KS, Fernhall B, Yates G, Baxter-Jones AD, Unnithan VB. Heart rate variability during recovery from a Wingate test in adolescent males. Med Sci Sports Exerc. 2006;38(5):875–81. doi: 10.1249/01.mss.0000218126.46242.2e. [DOI] [PubMed] [Google Scholar]