Abstract
Background
Neonatal abstinence syndrome (NAS) expression is widely variable among affected infants and the reasons for this variability are largely unknown; mechanisms that predispose infants to NAS expression are not understood. It has been postulated that the regulatory problems of prenatally drug exposed infants are manifested in dysfunctional vagal regulation of autonomic processes. The current study examines whether cardiac vagal tone, an indicator of parasympathetic neuroregulation, provides a marker for autonomic dysregulation subsequently expressed as NAS in prenatally opioid-exposed newborns.
Methods
Heart period (HP) and cardiac vagal tone (V) were derived from electrocardiogram data collected from 64 methadone-exposed infants on postnatal days 1 and 3. The postpartum NAS course was assessed serially.
Results
Infants with lower V on day 1 had significantly higher NAS symptomatology on day 3. Boys had more severe NAS symptoms than girls through the first 4 days of life and, among infants receiving pharmacologic treatment for NAS, boys required longer treatment course and hospitalizations. Greater poly-drug exposure, detected through toxicology screening throughout pregnancy, and cocaine use in particular, were associated with lower V and shorter HP (faster heart rate) in newborns. Multiple regression models accounted for 25 to 35% of the variance in NAS symptoms and duration of hospitalization in methadone-exposed infants. Significant predictors included infant sex, SSRI/SNRI use, and cigarette smoking.
Conclusions
Results support the hypothesis of a biologic vulnerability of autonomic regulatory functioning in methadone-exposed infants and greater male infant vulnerability to maternal methadone use.
Keywords: vagal tone, infant, methadone, neonatal drug exposure, neonatal abstinence syndrome
1. Introduction
Internationally, the diversion and abuse of narcotic-containing pharmaceuticals, which constitutes a serious health risk, has increased. This has lead to the tripling in the global consumption of methadone over the past decade, with countries in North America and Europe reporting the highest levels of use (International Narcotics Control Board, 2008). Opioid use during pregnancy continues to be a major public health problem in the US, and there is evidence that use of opioid drugs, especially misuse of opioid prescription drugs by women of childbearing age, is increasing (SAMSHA, 2008). Opioid dependent pregnant women comprise a special population due to the multiple considerations of the mother, the pregnancy, and the fetus. Management of opioid dependence with methadone has been used for decades in this population, and its use offers significant health benefits to the mother-infant dyad (Kaltenbach, 1998). However, a major consequence of maternal opioid/methadone use is a constellation of withdrawal symptoms in the newborn known as neonatal abstinence syndrome (NAS), which occurs in between 48 and 94% of exposed newborns (Osborn, 2005) .
Infants with NAS typically express dysfunction of respiratory, gastrointestinal, and/or nervous system regulation. Symptoms generally commence within 48-72 hours of birth and include, but are not limited to hypertonicity and tremors, irritability, hyperthermia, tachypnea, vomiting and poor feeding (American Academy of Pediatrics, Committee on Drugs, 1998). NAS is generally scored and treated empirically, that is, treatment is typically based on the number and severity of symptoms. The Finnegan Scale Scoring System (Finnegan, 1975), or a variant of this scale, is often used to assess NAS in affected newborns and, in clinical settings, often provides the basis for a treatment algorithm. Standard treatment involves the use of non-pharmacotherapeutic interventions, in combination with the use of an opiate replacement drug such as morphine when symptoms become more severe.
NAS expression is widely variable among individual infants, and while almost all opioid-exposed infants express some withdrawal symptoms, only a subset of infants manifest severe enough symptomatology to require pharmacotherapy; the reasons for this variability are largely unknown (Jansson, 2007) and myriad factors are likely to affect expression (Seligman, 2008; Burns, 2007). The relationship between maternal methadone dose and NAS severity has been disputed, with most studies finding that the degree of methadone exposure during pregnancy is unrelated to NAS expression (McCarthy et al., 2005; Berghella et al., 2003; Mack et al., 1991; Jansson et al., 2007). Currently, there is no specific identifiable maternal or infant factor linked conclusively to the severity of NAS displayed by the newborn. The pathophysiology of this disorder, costly to the infants affected, their families, the health care system and society, remains unknown.
Drug-exposed infants exhibit significant impairment in their ability to regulate internal homeostatic processes and to organize behavioral and physiologic responses (Bard, 2000). It has been postulated that the regulatory problems of prenatally drug exposed infants are manifested in dysfunctional vagal regulation of autonomic processes (Porges & Greenspan, 1991). The autonomic nervous system is a crucial regulator of neural homeostasis and physiological adaptability, by dynamically controlling the body's responses to external and internal stimuli (Porges, 2007). A well-established indicator of autonomic function in developmental research is the degree of spontaneous variability in heart rate (Bernston et al.,1997). Since heart rate variability is subject to both autonomic and non-autonomic influences, efforts to isolate the parasympathetic input have relied on quantifying the magnitude of the respiratory sinus arrhythmia which is mediated by the vagus. Measures of vagal tone have been applied to examination of neonatal adaptability to extra uterine life in both full-term and preterm infants; results indicate that lower vagal tone is associated with greater perinatal risk (DiPietro et al., 1994; Doussard-Roosevelt et al., 1997). Studies of the effects of prenatal drug exposure on vagal tone in infants have been limited to cocaine exposure. A dose-dependent effect of cocaine on respiratory sinus arrhythmia has been reported (Scheutze & Eiden, 2006). Prenatal methadone exposure is associated with disruption to autonomic functioning in the developing fetus expressed as decreased fetal heart rate variability (Ramirez-Cacho, et al., 2006; Navaneethakrishnan et al., 2006; Jansson et al., 2005) and with disruption to maternal autonomic functioning and subsequent NAS expression in the infant (Jansson et al., 2007).
Male sex provides a vulnerability to developmental deficits throughout infancy and childhood (Nagy et al., 2001). Sex differences in neurobehavior among healthy, term infants have been described, with male infants displaying poorer levels of functioning on the Brazelton neonatal behavioral assessment scale (Lundqvist & Sabel, 2000). Male infant vulnerability to affective regulatory (Weinberg et al., 1999), developmental (Nagy et al, 2001) and health (Morse et al., 2006; McGregor et al., 1992) dysfunction has long been established. Male infants have been found to be more susceptible to behavioral deficits secondary to teratogenic drug exposures (Riese, 1989) and more vulnerable to maternal depressive symptoms (Weinberg et al., 2006) than female infants, suggesting differential vulnerability of the developing nervous system. Female infants demonstrate greater activation of the autonomic nervous system than males during the challenge of adaptation to extra uterine life (Bernardes, 2009). In animal models, prenatal morphine exposure induces long-term alternations in adult rat brain and behaviors in both males and females, but these alterations differ between sexes (Vathy, 2002). Greater sensitivity to methadone in the neonatal brain of male offspring has been shown in rats (Hou et al., 2004). Among methadone-exposed infants, boys are predisposed to more severe NAS expression as neonates (Jansson et al., 2007) and increased vulnerability to adverse environmental conditions as infants (Johnson & Rosen, 1982).
We have previously shown that among methadone-maintained women who were otherwise drug free in the last trimester of pregnancy, maternal autonomic nervous system functioning is correlated with newborn NAS severity. Specifically, offspring of women who reacted to methadone administration with greater change in vagal tone, regardless of whether methadone had a suppressive or augmenting effect, had more severe NAS expression (Jansson et al., 2007). This association was stronger for boys than girls. The current study was designed to further examine the relationships among prenatal methadone exposure, infant sex, and NAS expression by evaluating vagal tone in opioid-exposed offspring. We predicted that opioid-exposed infants with lower vagal tone will have greater difficulties with functional regulation during the early neonatal period, which will be expressed in terms of more severe NAS course than infants with higher vagal tone, and that male infants prenatally exposed to methadone will have more severe NAS expression.
2. Methods
2.1 Participants
Participants were infants of women enrolled in comprehensive, multidisciplinary treatment at the Center for Addiction and Pregnancy in Baltimore, Maryland. The program, described fully elsewhere (Jansson et al., 1996), provides an array of services, including substance abuse treatment, psychiatric consultation, obstetric care and methadone maintenance when warranted to pregnant women with drug dependency living in Maryland. Participants were opioid-dependent women meeting federal criteria for methadone maintenance, delivering a singleton infant at term and free of significant pregnancy or pre-existing medical conditions. Participants were enrolled for study participation upon the birth of their infant. Alcohol dependent women were ineligible. Eligible program participants were approached for enrollment following consecutive deliveries between December 2006 and August 2008. Of the 66 women offered enrollment, one declined, resulting in 65 subjects. All women provided written informed consent for the participation of their infants and the study was approved by the governing Institutional Review Board.
Maternal participants were mature (M age = 29.1 years, sd = 5.95), mostly unmarried (86.2%) and had less than a high school education (M years of education = 11.13; sd = 1.42). Subjects were principally Caucasian (72.3%) and African-American (24.6%). Most (70.8%) delivered vaginally. Maternal medical, substance use, and drug treatment history was obtained via self report from participants and maternal chart review upon the infant's birth. At the time of delivery, 21 women (32.3%) were maintained on psychotropic medications, primarily for depression. Of these, 13 were maintained on selective serotonin or serotonin-norepinephrine reuptake inhibitor (SSRI/SNRI) medications (duloxetine, citalopram, fluoxetine, sertraline and escitalopram). Almost all (96.9%) smoked cigarettes (M cigarettes daily = 11.1, sd 6.1). Cocaine was the single most frequently reported drug used in addition to heroin during pregnancy, with reported use by 36 (56.3%) participants at any point during gestation. Maternal urine toxicology was obtained at the time of delivery and was available for all but one participant; 84.6% were negative for illicit substances. Of the 9 positive samples, detected substances included heroin (5), non-prescribed benzodiazepines (2), cocaine (1) and another opioid (1). Maternal drug abuse history and methadone treatment parameters are presented in Table 1. Twenty eight women (43.1%) were methadone-maintained for all three trimesters, 27 (41.5%) were methadone-maintained for the 2nd and 3rd trimesters and 10 (15.4%) for the 3rd trimester only.
Table 1.
Maternal drug use and methadone maintenance histories (n = 65)
| Mean | sd | Range | |
|---|---|---|---|
| Age first drug use (years) | 15.75 | 4.36 | 11-29 |
| Years of regular (>3x/week) drug use (years) | 8.81 | 5.38 | 1-21 |
| Length of time in treatment (days) | 147.17 | 63.32 | 17-247 |
| Number of urine toxicologies obtained | 18.23 | 9.29 | 1-36 |
| Positive urine toxicologies during pregnancy (%)† | 23.85 | 29.66 | 0-100 |
| Methadone dose (mg) at delivery | 76.17 | 21.09 | 10-130 |
| Number of gravid days methadone received | 154.02 | 76.17 | 19-277 |
| Total methadone dose received during gestation (mg) | 10,962.17 | 6,580.96 | 1,090-28,010 |
Note:
indicates illicit drug use or licit drug misuse
Infants were hospitalized for a minimum of four full days for observation for signs/symptoms of NAS per standard operating procedure of the birth hospital. Infants not requiring NAS treatment were discharged on hospital day 5. All infants received NAS scoring every 3 to 4 hours for their entire hospitalization beginning at birth, using a modification of the Finnegan Neonatal Scoring System (Finnegan et al., 1975) which provides a weighted ranking of symptoms to assess NAS severity. Scoring was done by the clinical nursing staff which is highly experienced in the treatment of drug-exposed neonates. Opiate replacement treatment (i.e. morphine sulfate) was provided based on a symptom-based algorithm that has been previously described (Jansson et al., 2009). Pharmacotherapy for the treatment of NAS began when two consecutively obtained scores were greater than 8. Increasing doses of mediation were provided for escalating NAS scores until the infant achieved a plateau of NAS scores of 8 or less. The infant was stabilized on this dose of medication for 48 hours, and then gradually weaned from medication using standardized protocols for weaning and re-escalation as needed. Infant birth and medical data, including NAS symptomatology and treatment, were collected from the infant's medical chart shortly after discharge.
2.2 Procedures
Ten minutes of infant electrocardiogram (ECG) were recorded on days 1 and 3 of life, defined as the first and third 24-hour periods after birth. Since circumcision can affect vagal tone (Porter et al., 1988), this procedure was delayed until after the day 3 recording. Data were collected using a 3-lead electrocardiogram while the infant was in a sleep state in a bassinette. State was determined by criteria based on the NICU Network Neurobehavioral Scale (NNNS; Lester, 2004) by one of two examiners (LMJ or MV) who are certified in the use of this scale. Infant sleep state was recorded for each minute of the recording, and corresponds to NNNS states 1 (quiet sleep) or 2 (active sleep). Three disposable Ag-AgCL electrodes were triangulated on the infant's trunk, the signal was amplified (PhysioControl, Model Lifepak 5, Plainview, NY), digitized, and recorded on a computer. R-wave detection, editing for artifact, and timing of sequential heart periods proceeded off-line using MXedit software (Delta-Biometrics, Inc., Bethesda, MD). Extracted variables include heart period (HP) quantified via inter-beat intervals in msec and a measure of vagal tone (V) calculated using the analytic method developed by Porges (Porges 1985). Briefly, this procedure uses a 21-point polynomial to detrend sequential HP data and a band-pass filter to extract the variance within the frequency band consistent with respiration within this age group (i.e., .24 to 1.04 Hz). The estimate of V, which corresponds to the respiratory sinus arrhythmia, was calculated as the natural log of the extracted variance. Mean values of the 30 s epochs for both cardiac measures were computed.
2.3 Data analysis
Data analysis relied on repeated measures analysis of variance to characterize change over time in both NAS symptomatology (4 repeated measures) and cardiac variables (2 repeated measures) and testing for main effects of treatment condition and infant sex. Correlation coefficients, computed for the entire group and separately by sex, were used to evaluate linear associations among methadone dosing history, NAS outcomes, and cardiac measures. Effects of other substances on infant measures were explored using correlations and t-tests as appropriate. Multiple linear regression was used to evaluate the cumulative and independent effects of predictor measures on neonatal outcomes.
3. Results
3.1 Neonatal Abstinence Syndrome and treatment
Descriptive infant data and NAS course information is presented in Table 2. Seventy-five percent of infants required treatment for NAS (n = 48); Table 2 provides information stratified by the need for treatment. One male infant developed a prolonged NAS course requiring treatment with multiple medications and was hospitalized for nearly 2 months; his data were removed from analyses, leaving a total of 64 infants. Infants who did not require treatment were discharged on the 5th postpartum day per hospital policy with the exception of one untreated infant who was hospitalized for an extra day (i.e. 6 days) due to concerns regarding weight gain. For treated infants, the mean days of treatment was 11.4 (sd = 7.0) with mean total opiate (i.e., morphine sulfate) therapy dosage of 7.19 mg (sd = 8.30). Half (n = 32) of the infants were boys.
Table 2.
Infant characteristics (n=64)
| Mean(sd) | |||
|---|---|---|---|
| All (n=64) | Treated (n=48) | Untreated (n=16) | |
| Gestational age (weeks) | 39.16 (1.23) | 39.22 (1.27) | 38.96 (1.12) |
| Birth weight (grams) | 3085.00 (495.01) | 3135.94 (514.51) | 2932.19 (408.05) |
| Birth length (cm) | 49.36 (2.52) | 49.50 (2.70) | 48.94 (1.89) |
| Birth head circumference (cm) | 33.27 (1.53) | 33.34 (1.53) | 33.06 (1.56) |
| 1 minute Apgar | 8.22 (1.18) | 8.15 (1.26) | 8.44 (0.89) |
| 5 minute Apgar | 9.00 (.18) | 9.00 (.21) | 9.00 (0.00) |
| Mean NAS score day 1 | 4.36 (2.14) | 4.51 (2.26) | 3.93 (1.69) |
| day 2 | 6.96 (2.09) | 7.63 (1.82) | 4.93 (1.44) |
| day 3 | 6.22 (1.76) | 6.79 (1.52) | 4.54 (1.34) |
| day 4 | 5.65 (1.72) | 6.14 (1.48) | 4.17 (1.55) |
| Total days hospitalized | 11.84 (6.97) | 14.10 (6.66) | 5.06 (0.25) |
NAS symptom scores peaked on day 2 and subsequently declined over the next 2 days, F (3,186) = 15.06, p < .001, in both treated and untreated groups although neither attained day 1 levels by the conclusion of data collection. As expected, there was a main effect for treatment such that treated infants had significantly higher scores overall, F (1, 62) = 35.49, p < .0001. However, the NAS symptoms by time interaction was significant, F (3, 186) = 4.06, p < .01; post hoc analyses indicated that the differences between the two groups began after the first day of life.
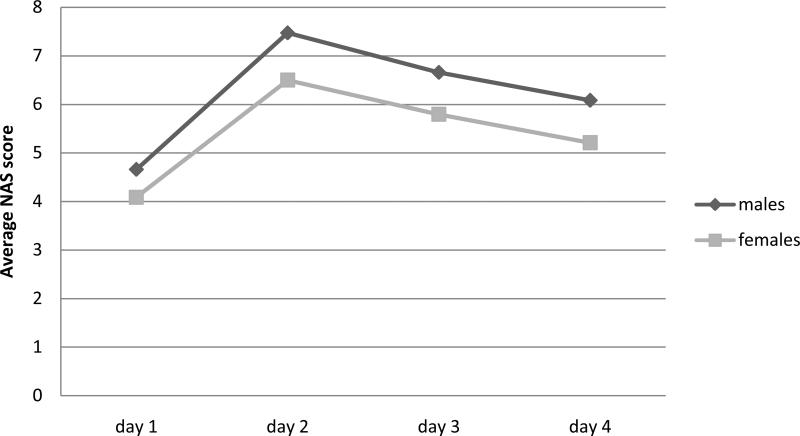
Boys displayed significantly higher NAS scores on each day than girls, although their pattern of change over time was similar, F (1,62) = 6.03, p < .05 (Figure 1). Although boys were not significantly more likely to be treated for NAS, (81% vs. 69%), when they were treated, their treatment duration (13.4 vs. 9.0 days; t (46) = 2.25, p < .05) and their hospital stay (15.9 vs. 12.0, t (46) = 2.09, p < 05) were longer.
Figure 1.
NAS symptoms on the first 4 days of life, stratified by infant sex.
Correlation coefficients computed between maternal methadone pregnancy dosing history (total days on methadone, total cumulative dose during pregnancy, and daily methadone dose at the time of delivery) were generally unrelated to NAS outcomes and treatment with one exception: infants of women with higher total cumulative methadone exposure during gestation had higher NAS scores on days 3 and 4, rs (62) = .26, p < .05, both days).
3.2 Cardiac outcomes
Data generated from 3 heart rate recordings on day 3 (2 boys, 1 girl) were too noisy to use in the cardiac analyses; so day 3 analyses are based on 61 cases. Two potential methodological covariates were examined in relation to the cardiac pattern data: infant state and postpartum age at the time of the recording. All infants were recorded in a sleep state but comparisons were conducted between values generated by those in a predominantly quiet sleep (ns = 10 on day 1 and 4 on day 3) as compared to the majority of infants recorded in active sleep. Neither heart period (HP) nor vagal tone (V) differed by sleep state. Postpartum age at recording on day 1 ranged from 2 to 23 hours after birth (M = 14.0 hours, sd = 5.8) and from 49 to 71 hours on day 3 (M = 60.6 hours, sd = 6.1). Correlation coefficients revealed no significant associations between testing time on either day and either cardiac measure.
Heart period values were: day 1, M = 493.54, sd = 52.20; day 2, M = 477.43, sd = 61.68. V values were: day 1, M = 2.72, sd = 1.24; day 2, M = 3.39, sd = 1.16. Both HP and V increased significantly from day 1 to 3, F (1, 59) = 5.95, p < .05 and F (1,59) = 19.88, p < .0001, respectively. There were no sex differences in either measure. Maternal methadone dosing history during pregnancy was unrelated to either cardiac measure on day 1 or 3 for the full sample or male and female subsamples analyzed separately. None of the infants who received opiate treatment for NAS were treated prior to the day 1 ECG recording, although most (n = 40) had begun treatment by the second data collection on day 3.
There were no significant concurrent associations between day 1 NAS scores and day 1 cardiac measures, or between day 3 NAS scores and day 3 cardiac measures. However, there was a consistent lagged pattern of significant associations between both cardiac measures collected on day 1 and NAS scores on day 3. Table 3 presents the correlations for each day of life to illustrate the pattern of findings and variation in the strength of the association by infant sex. Lower HP (i.e., faster heart rate) and lower V on day 1 were associated with more severe, (i.e. higher) NAS scores on day 3. This association was particularly true for boys. In an effort to better understand this pattern of findings, an additional set of correlation coefficients was computed for those infants who were untreated on day 3 (i.e., never treated or began morphine sulfate treatment after day 3 recording). For this subset of infants, there was an initial positive association between heart period and NAS scores on day 1 and the strength of the negative associations with day 3 NAS scores was somewhat larger that for the full sample.
Table 3.
Associations between NAS symptoms during the first 4 days of life and cardiac measures collected on day 1 of life
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| All infants (n = 64) | ||||
| HP | .09 | −.03 | −.29* | −.17 |
| V | .01 | −.14 | −.41*** | −.19 |
| Boys (n = 32) | ||||
| HP | .03 | .07 | −.38* | −.28 |
| V | −.03 | −.12 | −.51** | −.48** |
| Girls (n = 32) | ||||
| HP | .16 | −.08 | −.17 | −.03 |
| V | .07 | −.13 | −.28 | .13 |
| Untreated by day 3 (n = 24) | ||||
| HP | .46* | .21 | −.54** | −.31 |
| V | .27 | .04 | −.61** | −.18 |
p < .05
p < .01
p < .001.
3.3 Influence of other substances
Exploratory analyses were conducted on co-exposures that have been independently implicated in the literature as influences on either NAS, V, or both. These include cigarette smoking, SSRI/SNRI use, and cocaine exposure. In addition, the percent of times women tested positive for other illicit substances in urine tests while in treatment was analyzed as a marker of general compliance to program targets of drug abstinence.
3.3.1. Cigarettes
Because almost all women smoked, analyses were conducted based on number of reported cigarette use per day, which ranged from 0 to 1 pack (20 cigarettes). Daily cigarette use was unrelated to any NAS treatment or cardiac measure. However, unexpectedly, infants exposed to higher doses of prenatal cigarette use had lower NAS scores on days 1 and 2 of life (rs (62) = -33, p < .01 and -.28, p < .05, respectively); the associations were in the same direction but at a trend level of significance (p < .10) on days 3 and 4.
3.3.2. SSRI/SNRIs
T-tests comparing offspring of the 13 women who were prescribed SSRI/SNRI mediations during pregnancy versus those who were not revealed no differences on NAS scores. Although SSRI/SNRI-exposed infants were not more likely to be treated for NAS, when they were treated they received significantly higher opiate dosing (10.4 mg morphine vs. 4.1, t (62) = 2.73, p < .01). HP or V did not differ significantly based on SSRI/SNRI exposure on either day.
3.3.3. Cocaine
Slightly over half (56.3 %) of women reported using some cocaine during pregnancy. Analysis revealed no associations between cocaine use and NAS scores or treatment using either a categorical (any cocaine use) or a linear one (number of days of reported cocaine use). However, repeated measures analysis of variance (days 1 and 3) revealed that infants of women who used cocaine had lower HP, F (1,59) = 6.92, p < .01, and lower V, F(1,59) = 5.67, p < .05. In addition, the more frequently (days of use) women reported using cocaine, the lower the HP and V values although these were significant only on day 1, r (62) = -.28 for HP and r (62) = -.27 for V, ps < .05.
3.3.4. Positive urine toxicologies during treatment
Women were administered an average of 18.3 (sd = 9.3) urine toxicology tests during their course of prenatal substance abuse treatment. The percent of positive urine toxicologies for other substances ranged from 0 to 100% (M = 23.9%), although 31% of participants had no positive tests. The number of tests administered was unrelated to the percent detected positive (r (62) = -.05). Substances tested for included amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine, methadone, propoxyphene and ethanol. Because individual substance exposures other than cocaine were too low to subject to group analyses, the percent of positive tests, independent of drug detected, was analyzed as a general indicator of maternal treatment program compliance. Participants were stratified into three groups: those with no positive screens during treatment (n = 20), those within the next tertile (n = 21, actual values ranged from 2.8% to 16.7% positive tests), and the remaining (n = 23; actual values ranged from 25 to 100% positive tests). Categories were unrelated to maternal age, infant sex or NAS symptoms or treatment. However, women who tested positive more often had infants with lower V, F (2,58) = 3.49, p < .05 (Figure 2) and faster heart rates, F (2,58) = 5.01, p < .01 (not shown).
Figure 2.
Infant vagal tone (V) on days 1 and 3 by the percent of maternal positive urine toxicology evaluations during methadone treatment.
3.4 Prediction of NAS and treatment outcomes
Given the pattern of responses noted above, and the role of other substances on outcomes, two outcome measures were selected that reflect different facets of infant outcome for linear regression modeling: NAS symptom scores on day 3 and duration of hospitalization. Predictors included: infant sex, methadone dose (cumulative during pregnancy), prenatal SSRI/SNRI exposure, percent positive toxicology screens, number of cigarettes smoked, and both day 1 and day 3 V. Together, these measures yielded multiple R's (7, 53) = .60 and .50 for NAS symptoms and length of hospitalization, respectively. Individual predictor values are presented in Table 4. Day 1 V continued to be predictive of day 3 NAS in the regression despite adjustment for other predictors. Cigarette smoking continued to demonstrate a negative association with NAS symptoms, but the univariate relation between cumulative methadone dose did not persist. With respect to hospital duration, infant sex was the only significant predictor, with boys having long hospitalizations, and there was a marginal association with SSRI/SNRI exposure.
Table 4.
Prediction of NAS and duration of hospitalization
| NAS on day 3 of life | Duration of hospitalization | |||||
|---|---|---|---|---|---|---|
| Predictor: | β | T | Sig T | β | T | Sig T |
| Infant sex | −.17 | −1.45 | n.s. | −.25 | −2.06 | .04 |
| Prenatal exposures: | ||||||
| Methadone dose | .18 | 1.62 | n.s. | .16 | 1.29 | n.s. |
| SSRI/SNRI | .09 | .85 | n.s. | .22 | 1.82 | .07 |
| Positive toxicologies | .15 | 1.26 | n.s. | .13 | 1.00 | n.s. |
| Number of cigarettes | −.24 | −2.07 | .04 | −.12 | −.94 | n.s. |
| Postpartum vagal tone | ||||||
| Day 1 | −.38 | −2.83 | .007 | −.08 | −.55 | n.s. |
| Day 3 | .18 | 1.36 | n.s. | −.04 | −.27 | n.s. |
4. Discussion
These results provide support for both original hypotheses: 1) that indicators of autonomic regulation, indexed here in terms of heart period and vagal tone, provide a physiologic basis for NAS expression and 2) that boys are more vulnerable to prenatal opioid exposure. The results also demonstrate the multiple sources of influence on behavioral and autonomic outcomes in methadone-exposed populations. However, the observed associations were unexpected in a number of fundamental ways, starting with the time-lagged associations between the cardiac measures and NAS outcomes. The primary focus of this study was to examine whether variation in cardiac parameters indicative of autonomic regulation were useful indicators of the physiological disruption that may accompany prenatal methadone exposure. Results from both correlation analysis and linear regression indicated that infants with lower V and HP (i.e., faster heart rates) on day 1 had more severe NAS by day 3. NAS values on the third day of life are particularly salient due to the well-documented trajectory of methadone withdrawal symptoms in the newborn during the first 48 to 72 hours (The American Academy of Pediatrics, Committee on Drugs, 1998). These associations were somewhat larger when infants who received postnatal morphine sulfate treatment were excluded from the analysis, suggesting that the effects of on-going opioid exposure on either the dependent or independent variables may suppress detection of a relationship. This observation is supported by the previously known effect of morphine on vagal tone in adults (Pretorius et al., 1990).
In contrast to the autonomic predictors, variation in fetal exposure to methadone based on timing of initiation and dosage was unrelated to NAS severity, with the exception of the modest association between cumulative maternal methadone exposure and NAS scores. However, these associations did not persist when other factors were controlled in the regression. Prior reports have also failed to find associations between trimester of maternal methadone initiation or methadone dose and NAS severity in infants (McCarthy et al., 2005, Jansson et al., 2007, Berghella, 2003, Mack, 1991); others have detected dose-response relations. Maternal methadone dose (Malpas, 1995) and total dose ingested during the last 12 weeks of pregnancy (Harper, 1977) have been related to NAS severity, and maternal dose has been correlated with the duration of neonatal hospitalization and treatment for NAS (Dasche, 2002). The current findings suggest that while dosage may or may not be related directly or indirectly to NAS severity, the finding that autonomic predictors are correlated with NAS expression may be evidence of in utero programming related to methadone exposure and/or genetic predisposition. Infants with lower vagal tone may represent infants who adapt in utero to changing maternal vagal tone in response to methadone administration via alterations in autonomic functioning. While these alterations may assist the fetus in the uterine environment, they may be maladaptive in the transition to extra uterine life and beyond. This maladaptive response may be expressed as more severe NAS. Programming has been implicated in the causal pathway underlying deficits observed in alcohol exposed offspring (Zhang, 2005; Hellemans, 2009) and cocaine, methamphetamines and SSRI exposed offspring (Salisbury, 2009).
Indications of greater male vulnerability were expressed in terms of higher NAS scores across the first four days of life and the ensuing longer hospitalizations for boys. This is consistent with the literature documenting greater vulnerability of boys to biological insults in general and replicates prior work by this team in another methadone-exposed sample (Jansson et al., 2007). Although there is no single coherent theory that can account for the sex differential in the development literature, an animal model has identified brain region-dependent gender differences in opioid receptor G protein coupling, with prenatally methadone-exposed males showing greater sensitivity to methadone than females (Hou et al., 2004). It is not clear why boys were not significantly more likely to be treated for NAS in this sample, although this may be attributable to insufficient power. However, it is notable that the same inability to detect a difference in initiation of treatment but more intensive treatment necessary once treatment began was observed for both boys and SSRI/SNRI exposed offspring. This suggests that SSRI/SNRI medications may produce either a potentiation of methadone induced NAS symptoms, or contribute additional, though qualitatively different, NAS symptoms that prolong the course of NAS expression. The role of SSRI/SNRI exposure could not be fully explored given the relatively low level of exposure and the broad distribution of specific medications. SSRI/SNRI exposure has previously been implicated in causing a neonatal abstinence symptom after in utero exposure by others (Levinson-Castiel et al., 2006; Moses-Kolko et al., 2005, Gentile, 2007) and the current findings support this association.
While this study is focused on the role of prenatal methadone exposure, infants were actually poly-drug exposed, as is the case with nearly all studies evaluating infants of drug dependent women. Although alcohol dependency was an exclusion criterion, cigarette smoking was nearly universal. Heavy nicotine exposure has been found to potentiate NAS symptoms in opioid-exposed infants (Choo et al., 2004), although our previous work found no increase in NAS severity among infants of women who were moderate or light smokers (Jansson et al., 2007). The observed linear association between greater cigarette smoking and milder NAS symptoms observed in the current sample was unexpected and we have no ready explanation. However, although most women smoked, the ceiling was a pack per day, indicating a low to moderate level of use.
Although participants reported use of a variety of drugs, only cocaine was used by enough of the sample to analyze as a separate exposure. Cocaine use was unrelated to NAS expression, but was associated with lower V and faster heart rate. This result is consistent with a previous report finding no overall increase in severity of NAS expression or length of treatment required for the dually-exposed vs. methadone only-exposed neonates (Mayes & Carroll, 1996). However, we caution that these results may not be attributable to neurobiological effects of cocaine, particularly since a similar finding was generated by simply counting the number of times that women had positive drug screens during treatment, regardless of specific drug detected. Thus these associations may be indicative of a generally poorer lifestyle, which can imply less prenatal care, poorer nutrition and numerous other maternal risk factors. However, at least one other report (Scheutze & Eiden, 2006) has found lower RSA in cocaine-exposed infants.
Clinicians and investigators have been stymied by an inability to predict the neonatal consequences of prenatal opioid exposure for individual neonates since there is not a dose-response relation between methadone exposure and NAS severity. The regression analysis accounted for 35% of the variance in predicting NAS symptoms on day 3 and 25% of the variance in predicting length of hospital stay. Infant sex and day 1 vagal tone were the most prominent predictors, although they acted differently with respect to each outcome. This study has reiterated a previously demonstrated risk factor, male sex, and also identified lower V and faster heart rate on the first day of life as potential candidates in the assembly of a potential risk assessment. Prior work has also identified that infants born to women who express more physiological reactivity (i.e., the degree of suppression or activation of maternal V) to methadone administration are at greater risk for NAS expression (Jansson et al., 2007). The associations between maternal methadone exposure, changes in maternal vagal tone and severity of NAS expression documented in prior work and the associations between low vagal tone and NAS expression in methadone exposed infants detected here may represent dual aspects of fetal programming. Repeated exposures to methadone during pregnancy provides both a neurotoxic challenge to the developing fetal brain as well as instability in the maternal-fetal autonomic interface. Variability in NAS expression among methadone exposed infants may reflect differential vulnerability to these distinct consequences of exposure as the infant adapts to postnatal life.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The American Academy of Pediatrics, Committee on Drugs Neonatal drug withdrawal. Pediatrics. 1998;101:1079–1088. [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arounsal modualtion in 8-week-old infants. Dev Psychobiol. 2000;36:194–212. [PubMed] [Google Scholar]
- Berghella V, Lim P, Hill M, Cherpes J, Chennat J, Kaltenbach K. Maternal methadone dose and neonatal withdrawal. Obstet Gynecol. 2003;189:312–317. doi: 10.1067/s0002-9378(03)00520-9. [DOI] [PubMed] [Google Scholar]
- Bernardes J, Gonçalves H, Ayres-de-Campos D, Rocha AP. differences in linear and complex fetal heart rate dynamics of normal and acidemic fetuses in the minutes preceding delivery. Sex. J Perinat Med. 2001;37:168–76. doi: 10.1515/JPM.2009.024. [DOI] [PubMed] [Google Scholar]
- Bernston G, Bigger J, Eckberg D, Grossman P, Kaufmann P, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, Van Der Molen MW. Heart rate variability: Origins, methods, and interpretative caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Burns L, Mattick RP. Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev. 2007;26:487–492. doi: 10.1080/09595230701494416. [DOI] [PubMed] [Google Scholar]
- Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alc Depend. 2004;75:253–260. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Dashe JS, Sheffield JS, Olscher DA, Todd SJ, Jackson GL, Wendel GD. Relationship between maternal methadone dosage and neonatal withdrawal. Obstet Gynecol. 2002;100:1244–1249. doi: 10.1016/s0029-7844(02)02387-6. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caughy MOB, Cusson R, Fox NA. Cardiorespiratory functioning of preterm infants: Stability and risk associations for measures of heart rate variability and oxygen saturation. Dev Psychobiol. 1994;27:137–52. doi: 10.1002/dev.420270302. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Irizarry RA, Chen P, Merialdi M, Zavaleta N. Prenatal development of intrafeal and maternal-fetal synchrony. Beh Neurosci. 2006;120:687–701. doi: 10.1037/0735-7044.120.3.687. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Dev. 1997;68:173–86. [PubMed] [Google Scholar]
- Finnegan L, Connaughton J, Kron R, Emich J. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2:141–158. [PubMed] [Google Scholar]
- Gentile S. Serotonin reuptake inhibitor-induced perinatal complications. Pediatr Drugs. 2007;9:97–106. doi: 10.2165/00148581-200709020-00003. [DOI] [PubMed] [Google Scholar]
- Harper R, Solish G, Feingold E, Gersten-Woolf N, Sokal M. Maternal ingested methadone, body fluid methadone, and the neonatal withdrawal syndrome. Am J Obstet Gynecol. 1977;129:417–424. doi: 10.1016/0002-9378(77)90588-9. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Sliwowska J, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.06.004. doi:10.1016/j.neurobiorev.2009.06.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Tan Y, Belcheva MM, Clark AL, Zahm DS, Coscia CJ. Differential effects of gestational buprenorphine, naloxone, and methadone on mesolimbic mu opioid and ORL 1 receptor G protein coupling. Brain Res Dev Brain Res. 2004;151:149–157. doi: 10.1016/j.devbrainres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- International Narcotics Control Board [October 12, 2009];2008 Annual Report: http://www.incb.org/pdf/annual-report/2008/en/AR2008_Chapter_II.pdf.
- Jansson LM, Svikis D, Lee J, Paluzzi P, Rutigliano P, Hackerman F. Pregnancy and addiction: a comprehensive care model. J Subst Abuse Treat. 1996;13:321–329. doi: 10.1016/s0740-5472(96)00070-0. [DOI] [PubMed] [Google Scholar]
- Jansson LM, DiPietro JA, Elko A, Velez M. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J Matern Fetal Neonatal Med. 2007;20:677–685. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Velez M, Harrow C. The opioid exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5:47–58. [PMC free article] [PubMed] [Google Scholar]
- Jansson LM, DiPietro J, Elko A. Fetal response to maternal methadone administration. Am J Obstet Gynecol. 2005;193:611–617. doi: 10.1016/j.ajog.2005.02.075. [DOI] [PubMed] [Google Scholar]
- Johnson H, Rosen T. Prenatal methadone exposure: Effects on behavior in early infancy. Pediatr Pharmacol. 1982;2:113–120. [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. The Obstetric and Gynecology Clinics of North America. 1998;25:139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–667. [PubMed] [Google Scholar]
- Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptakeinhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160:173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Sabel K. The Brazelton Neonatal Behavioral Assessment Scale detects differences among newborn infants of optimal health. J Pediatr Psychol. 2000;25:577–582. doi: 10.1093/jpepsy/25.8.577. [DOI] [PubMed] [Google Scholar]
- Mack G, Thomas D, Giles G, Buchanan N. Methadone levels and neonatal withdrawal. J Paediatr Child Health. 1991;27:96–100. doi: 10.1111/j.1440-1754.1991.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Malpas T, Darlow B, Lennox R, Horwood L. Maternal methadone dosage and neonatal withdrawal. Australia and New Zealand Obstet Gynecol. 1995;35:175–177. doi: 10.1111/j.1479-828x.1995.tb01863.x. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Carroll KM. Neonatal withdrawal syndrome in infants exposed to cocaine and methadone. Subst Use Misuse. 1996;31:241–253. doi: 10.3109/10826089609045811. [DOI] [PubMed] [Google Scholar]
- McCarthy J, Leamon M, Parr M, Anania B. Hi-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Obstet Gynecol. 2005;193:606–610. doi: 10.1016/j.ajog.2005.03.072. [DOI] [PubMed] [Google Scholar]
- McGregor J, Leff M, Orleans M, Baron A. Fetal gender differences in preterm birth: Findings in a North America cohort. Am J Perinatol. 1992;9:43–48. doi: 10.1055/s-2007-994668. [DOI] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Dev Psychol. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Morse S, Wu S, Ma C, Ariet M, Resnick M, Roth J. Racial and gender differences in the viability of extremely low birth weight infants: A population-based study. Pediatrics. 2006;117:e106–e112. doi: 10.1542/peds.2005-1286. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- Nagy E, Loveland K, Orvos H, Molnar P. Gender-related physiologic differences in human neonates and the greater nulnerability of males to developmental brain disorders. J Gend Specif Med. 2001;4:41–49. [PubMed] [Google Scholar]
- Navaneethakrishnan R, Tutty S, Sinha C, Lindow SW. The effect of maternal methadone use on the fetal heart rate pattern: a computerised CTG analysis. BJOG. 2006;113:948–950. doi: 10.1111/j.1471-0528.2006.01020.x. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Cole MJ, Jeffrey HE. Opiate treatment for opiate withdrawal in newborn infants. The Cochrane Database of Systematic Reviews 2002. 2002;(3) doi: 10.1002/14651858.CD002059. Art. No.:CD002059.DOI:10.1002/14651858.CD002059. [DOI] [PubMed] [Google Scholar]
- Porges S, Greenspan S. Regulatory disorders II: Psychophysiologic perspectives. NIDA Res Monogr. 1991;114:173–181. [PubMed] [Google Scholar]
- Porges S. Method and Apparatus for Evaluating Rhythmic Oscillations in Aperiodic Physiological Response Systems. US Patent and Trademark Office; Washington, DC: 1985. [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FL, Porges SW, Marshall RE. Newborn pain cries and vagal tone: parallel changes in reponse to circumcision. Child Dev. 1988;59:495–505. [PubMed] [Google Scholar]
- Pretorius M, Wong C, Newlin D. Cardiovascular components of the response to morphine. National Institutes on Drug Abuse Research Monograph. 1990;105:417–418. [PubMed] [Google Scholar]
- Ramirez-Cacho WA, Flores S, Schrader RM, McKay J, Rayburn WF. Effect of chronic maternal methadone therapy on intrapartum fetal heart rate patterns. J Soc Gynecol Investig. 2006;13:108–111. doi: 10.1016/j.jsgi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Riese ML. Maternal alcohol and pentazocine abuse: Neonatal behavior and morphology in an opposite-sex twin pair. Acta Genet Med Gemellol. 1989;38:49–56. doi: 10.1017/s0001566000002828. [DOI] [PubMed] [Google Scholar]
- Salisbury A, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clin Perinatol. 2009;36:595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal cocaine use during pregnancy and physiological regulation in 4- to 8-week-old infants: An examination of possible mediators and moderators. J Pediatr Psychol. 2006;31:15–26. doi: 10.1093/jpepsy/jsj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman NS, Salva N, Hayes EJ, Dysart KC, Pequignot EC, Baxter JK. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol. 2008;199:396e1–396e7. doi: 10.1016/j.ajog.2008.06.088. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies . Results from the 2007 national survey on drug use and health: national findings. Rockville, Maryland: 2008. Rep No NSDUH Series H-34, DHHS Publication No SMA 08-4343. [Google Scholar]
- Vathy I. Prenatal opiate exposure long-term CNS consequences in the stress system of the offspring. Psychoneuroendocrinology. 2002;27:273–283. doi: 10.1016/s0306-4530(01)00049-x. [DOI] [PubMed] [Google Scholar]
- Weinberg M, Tronick E, Cohn J, Olson K. Gender differences in emotional expressivity and self-regulation during early infancy. Dev Psychol. 1999;35:175–188. doi: 10.1037//0012-1649.35.1.175. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: Effects on neuroendocrine and immune function. Exp Biol Med. 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]