Abstract
Alterations in central networks involved in regulation of arousal, attention, and cognition may be critical for IBS symptom maintenance and exacerbation. Differential sensitivities in these networks may underlie sex differences noted in IBS. The current study examined prepulse inhibition (PPI), a measure of sensorimotor gating, in male and female IBS patients. Relationships between PPI and symptom severity were examined, as well as potential menstrual status effects. Compared to healthy controls, male IBS patients had significantly reduced PPI; whereas female IBS patients (particularly naturally cycling women) had significantly enhanced PPI suggesting hypervigilance. Considering previously demonstrated sex-related differences in perceptual and brain imaging findings in IBS patients, the current findings suggest different neurobiological mechanisms underlie symptom presentation in male and female IBS patients. Compromised filtering of information in male IBS patients may be due to compromised top down (prefrontal, midcingulate) control mechanisms while increased attention to threat due to increased limbic and paralimbic circuits may be characteristic of female IBS patients.
Keywords: Irritable bowel syndrome, functional bowel disorders, acoustic startle response, prepulse inhibition of startle, sex differences, hypervigilence
1. Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by recurrent abdominal pain or discomfort combined with alterations in bowel function (Longstreth et al., 2006). Increased perceptual responses (“visceral hypersensitivity”) to physiologically occurring and to experimentally induced visceral stimuli are a hallmark factor in IBS, and these abnormalities are shared by other functional gastrointestinal (GI) and urological disorders (Azpiroz et al., 2007; Whitehead & Palsson, 1998). While a variety of putative peripheral factors may play a role in visceral hypersensitivity in IBS, there is growing evidence that central mechanisms that enhance responses to interoceptive information (“central pain amplification”) are also critical for symptom maintenance and exacerbation (Azpiroz et al., 2007; E. A. Mayer, 2000; E. A. Mayer, Tillisch, & Bradesi, 2006). Recent functional brain imaging studies have shown altered responses to the threat of an aversive visceral stimulus in limbic and prefrontal brain regions in IBS, in addition to increased responses to the actual stimulus (S. M. Berman et al., 2008). Increased startle responses to threat of aversive visceral and non-visceral stimulation has also been shown in female IBS patients (B. D. Naliboff et al., 2008). These studies suggest that IBS patients have enhanced activity in emotional arousal and pain facilitation circuits in the brain related to visceral and non-visceral stimuli. Less is known about the role of specific attentional or information processing deficits that might also play a role in altered IBS responses. We have previously demonstrated that female IBS patients show a failure of habituation of an early evoked potential response, the P50, to auditory stimuli, consistent with altered pre-attentional mechanisms associated with hypervigilance (S. M. Berman et al., 2002).
Sex differences in perceptual responses have been shown in IBS patients. Perceptual hypersensitivity to visceral stimuli is especially pronounced in female IBS patients (Chang, Naliboff et al., 2006; E. A. Mayer, Naliboff, Lee, Munakata, & Chang, 1999). In contrast, male IBS patients show less visceral hypersensitivity than female patients but have greater sympathetic nervous system responses measured by skin conductance, and decreased cardiovagal activity measured by heart rate variability (HRV) compared to female IBS patients (Tillisch et al., 2005) and male controls. These sex differences have been hypothesized to reflect differential sensitivity of prefrontal and limbic arousal circuits underlying symptom generation in male and female IBS patients respectively (Labus et al., 2008; B.D. Naliboff et al., 2003), and analogous findings have recently been reported from functional brain imaging studies in the rat (Wang et al., 2009). We speculate that in terms of basic attentional processes, male IBS patients may therefore show poorer attentional control [associated with decreased vagal tone (Thayer & Brosschot, 2005)] compared to female IBS, and that the pronounced hypervigilance in female IBS might be associated with increased cognitive modulation of attention, reflected in greater symptom-related anxiety or response bias.
The acoustic startle response (ASR) is a fast defensive response to an intense exteroceptive stimulus, measured in humans via the eyeblink reflex. A simple neural circuit is known to mediate the startle reflex (Davis, Gendelman, Tischler, & Gendelman, 1982; Koch, 1999) but the modulation of this reflex based on affective state and other information processing demands has resulted in the startle reflex being highly useful as a non-invasive measure of central mechanisms. While the brain areas that mediate sensorimotor gating are in the pons, anterior cingulate and lateral prefrontal cortices provide ‘top down’ influence on this process via the thalamus (Campbell et al., 2007). These brain regions play an important role in modulation of pain and affect. Changes in the startle reflex following brief non-startling stimuli, prepulse inhibition of startle, has been widely used as a neurophysiological measure of the early pre-attentive stages of information processing and as an operational measure of sensorimotor gating and its top down modulation (Braff & Geyer, 1990; Braff, Geyer, & Swerdlow, 2001; Cadenhead, Carasso, Swerdlow, Geyer, & Braff, 1999). Prepulse inhibition (PPI) of startle is the normal unlearned suppression of the startle reflex when the intense startling stimulus is immediately preceded (30-300 ms) by a weak nonstartling stimulus (the prepulse). PPI represents a general ability to inhibit responses to external (auditory, visual, tactile) and internal (thoughts, impulses) stimuli (Geyer, Swerdlow, Mansbach, & Braff, 1990). Sex differences and menstrual cycle effects have been repeatedly demonstrated in prepulse modulation of startle, with healthy women exhibiting less PPI than healthy men and reduced PPI in the luteal compared to the follicular phase of the menstrual cycle (Jovanovic et al., 2004; Swerdlow et al., 1993; Swerdlow, Hartman, & Auerbach, 1997).
Although earlier research using prepulse modulation of startle has focused on information processing deficits in schizophrenia, many studies have now shown deficits in PPI in patients with other psychiatric disorders (reviewed in Braff et al., 2001), notably anxiety disorders including panic disorder (Ludewig, Ludewig, Geyer, Hell, & Vollenweider, 2002) and post traumatic stress disorder (Grillon, Morgan, Southwick, Davis, & Charney, 1996) and obsessive compulsive disorder (Hoenig, Hochrein, Quednow, Maier, & Wagner, 2005). More generally, a decreased PPI has been hypothesized to occur in disorders that involve a deficit in inhibition of responding to unimportant sensory, cognitive or interoceptive input (Swerdlow, Filion, Geyer, & Braff, 1995).
Although there have been no prior studies of PPI in patients with IBS or other functional somatic symptoms, one study of healthy individuals found enhanced PPI in subjects scoring higher on the ‘Hysteria’ scale of the MMPI, a scale associated with increased functional somatic symptoms and suppression of negative affect (Swerdlow et al., 1995). This suggests that at least in healthy subjects, somatic hypervigilance may be associated with the opposite effect on PPI from anxiety and ruminative disorders. In addition, PPI has been shown to be enhanced if subjects are given instructions that increase awareness/attention to the prepulse, are under conditions of threat, or if the prepulse itself is negative valenced (Aitken, Siddle, & Lipp, 1999; Cornwell, Echiverri, Covington, & Grillon, 2008; Dawson, Hazlett, Filion, Nuechterlein, & Schell, 1993; Filion, Dawson, & Schell, 1993; Schell, Dawson, Hazlett, & Filion, 1995).
The current study was designed to test if IBS patients show a lack of sensorimotor inhibition compared to healthy controls as evidenced by a deficit in PPI, or if they show increased PPI due to generalized hypervigilance. Since sex differences have been found both in studies of PPI and in IBS, the current study also examined sex differences in these responses. The following specific hypotheses were addressed:
IBS patients would generally show decreased PPI reflecting difficulty inhibiting responses to irrelevant stimuli (including physiological visceral stimuli), a concept previously suggested by lack of P50 habituation.
In addition, male and female IBS patients would show differing PPI responses compared to their same sex controls. Specifically, female IBS patients would show less reduction or even increases in PPI relative to female controls due to increased hypervigilance, while males would show greater declines in PPI compared to male controls.
2. Methods
2.1 Participants
A sample of 86 subjects with IBS (62 female) and 61 healthy controls (36 female) were recruited by advertisement. IBS diagnosis was confirmed using Rome II criteria during a clinical exam by a gastroenterologist or nurse practitioner experienced in functional GI disorders (Thompson et al., 1999). Bowel habit predominance was based on the Rome II supportive symptoms. Inclusion criteria for all subjects were: 1) no current use of psychoactive medication; 2) no current or past psychotic, major depressive, or substance abuse disorder; 3) no major medical conditions as determined during the clinical exam (with the exception of IBS for the IBS group). All study protocols were performed after approval by the UCLA and V.A. Greater Los Angeles Health Care System Institutional Review Boards. Informed consent was obtained from all subjects.
2.2 Stimuli
The startle stimulus consisted of a 50 ms burst of white noise at 105 dB SPL with a 0 ms rise time presented binaurally through stereophonic earphones. No masking noise was used; the ambient background noise varied between 30 and 35 dB SPL. For each trial, startle stimuli were presented either alone (Startle Alone), or preceded by a 25 ms 1000 Hz tone at 75 dB SPL with 4 ms rise and fall times and onset 120 ms before the startle stimulus onset (Prepulse) or by a continuous tone for 2000 ms.
2.3 Data Acquisition
Electromyogram (EMG) activity of the orbicularis oculi was recorded from electrodes placed beneath the right eye approximately 10 mm apart edge to edge, and 8 mm below the lower lid margin. The medial electrode was placed directly under the pupil. A ground electrode was placed in the center of the forehead. The impedance level of the electrodes was 20 kOhm or less. EMG activity was recorded with a sampling rate of 1 kHz and was full-wave rectified with low and high frequency cut-off values of 30 Hz and 1kHz, respectively. A vertical electro-oculogram (EOG) was recorded from electrodes placed above and below the left eye to monitor lid position, spontaneous blinking, and vertical saccades. The vertical EOG was used to control delivery of stimuli and in data preprocessing as discussed below. Data acquisition was performed with a Grass Instruments 15RX1 isolated physiodata amplifier system interfaced with a PC computer via a National Instruments BMC 2090; Multifunction Board. Custom programming using Labview® (National Instruments, Austin, TX, USA) software controlled all aspects of data acquisition, auditory and visual stimuli presentation and experimental timing. Separate custom programs were used for data screening and analysis.
2.4 Procedure
Prior to beginning the study, subjects with IBS were asked to rate the overall severity of their usual gastrointestinal symptoms on a numerical rating scale of 0 (no symptoms) to 20 (the most intense symptoms imaginable).
Following electrode placement, subjects were seated upright in a sound attenuated room adjacent to the experimental room, interconnected via intercom and closed-circuit video camera that provided a view of the subjects from two angles. Behavioral observations permitted identification of trials involving excessive body movements or drowsiness for data inspection and rejection if necessary.
Subjects were fitted with the headphones and watched a muted movie while they received the startle stimuli and prepulses. There was no instruction either to pay attention to or to ignore the sounds. Specifically, subjects were told that from time to time, they would hear some loud sounds and soft sounds through the headphones but that they did not have to say or do anything. All subjects received the same order of startle trials based on a Latin square design. The first and last trials consisted of a startle stimulus presented alone and were not included in analyses. Following the initial trial, subjects received 12 Startle Alone trials and 12 trials in which startle stimuli were preceded by the 25 ms prepulse and 12 trials in which the 2000 ms continuous tone preceded the startle stimulus to provide a measure of prepulse facilitation. The long prepulse led to only very small facilitation and no diagnosis differences; therefore, these trials were not included in the analyses reported below.
Trials were presented with a minimal intertrial interval (ITI) of 20 sec. Following the minimal ITI, the next trial was withheld until lid position level and slope (vertical EOG) and EMG levels were within initially selected ranges. If values became unacceptable within the 200 ms preceding presentation of a prepulse or a startle stimulus, then the prepulse and/or the startle stimulus was withheld. The program then attempted to give the trial that was withheld. Therefore, most subjects received, in addition to completed trials, partial trials during which a prepulse tone was not followed by a startle stimulus. There were no significant differences in the mean ITI or number of partial trials in male and female IBS patients and controls (p's>.05). The mean ITI and standard error were 47.87 s (±1.96) for female controls; 46.05 s (±2.14) for male controls; 46.62 s (±0.8) for female IBS patients; and 46.69 s (±2.19) for male IBS patients. The average number of partial trials was (.54 ± .08) for female controls; (.47 ± .08) for male controls; (.50 ± .07) for female IBS patients; and (.52 ± .09) for male IBS patients. The general procedures for stimulus presentation and recording follow those of previous studies of prepulse modulation of startle in our laboratory (e.g. Ornitz, Guthrie, Kaplan, Lane, & Norman, 1986).
Upon completion of the experiment, subjects completed a hearing test via audiometric screening. Tones were delivered monaurally at 1 kHz, 2 kHz, 3 kHz, and 4 kHz in 5 dB increments. Subjects failed the hearing test if he/she did not hear two consecutive tones at any frequency in either ear at or below 35 dB.
2.5 Data Analysis
2.5.1 Startle blink responses
Following application of a 2 ms moving average, peak startle amplitude was identified as the highest point within a window from 20 ms to 104 ms following startle stimulus onset. Response amplitude, expressed in microvolts (μV), was the difference between the peak and baseline EMG level (mean EMG in the 200 ms prior to the startle stimulus onset). Trials during which response peaks were less than 2 standard deviations above the baseline EMG level or in which a response onset could not be found within a 20 ms to 80 ms window following startle stimulus onset were considered as zero amplitude responses and were included in the analysis (8.4% of Prepulse trials; 1.2% of Startle Alone trials). Response onsets were EMG increments above the 2 standard deviation level in the response onset window that did not drop below that level for more than 10 ms. Individual trials were rejected for the following reasons: (1) a response onset occurred prior to 20 ms following startle stimulus onset, (2) behavioral observations indicated movement or excessive drowsiness, and (3) examination of the vertical EOG recording indicated a concurrent spontaneous blink (EOG onset prior to EMG onset and/or EOG amplitude excessively large). 5.1% of all trials were rejected.
2.5.2 Data Loss
Subjects in which more than half of the trials were rejected were excluded from analyses (1 female control, 3 female IBS patients, 2 male controls, and 1 male IBS patient). Additionally, subjects in which over half of the Startle Alone trials were judged to be zero responses were considered non-responders and were excluded from analyses (1 female control, 5 female IBS patients, 2 male controls, and 2 male IBS patients). Subjects failing the audiometric screening were excluded (2 female controls, 1 male control, and 2 male IBS patients). Finally, one female control subject and 1 male IBS subject were excluded due to technical difficulties. Thus, the final analyses were based on 54 female IBS patients, 18 male IBS patients, 31 female controls and 20 male controls.
2.6 Statistical Analyses
2.6.1 Overview
The primary statistical approach was individual growth curve modeling (IGCM) for repeated measures data. IGCM has a number of advantages including missing data handling, precision of parameter estimates, and the ability to make better statistical inference compared to other repeated measures approaches (Bagiella, Sloan, & Heitjan, 2000; Kristjansson, Kircher, & Webb, 2007; Singer & Willet, 2003). IGCM are increasingly being applied to psychophysiological data as a powerful alternative to traditional analytic techniques (see a review and tutorial in Kristjansson et al., 2007). To our knowledge, this is the first study to apply this advanced analytical technique to startle data.
EMG response amplitudes were log-transformed to diminish the effect of positive skewness of their distribution. EMG response analyses were performed using the Proc Mixed procedure in SAS (SAS Institute Incorporated, Carey, NC). The dependent variable was the natural log-transformed EMG data. The within person change over time was modeled by specifying intercept and time as random effects and age of the subject was entered into the model as a nuisance variable. In all analyses, an unstructured covariance structure yielded the best fit among the commonly used variance-covariance structures as indicated by Akaike's Information Criteria.
2.6.2 Primary Analyses
Differences in startle response magnitude and habituation for the Startle Alone trials were evaluated using a Diagnosis (IBS; Control) × Sex (Male; Female) × Trial Number (1:12) mixed models analysis of variance (ANOVA). To evaluate prepulse effects, a Diagnosis (IBS; Control) × Sex (Male; Female) × Trial Type (Startle Alone; Prepulse) design was used. Prepulse inhibition was calculated as the difference between mean estimated LnEMG in Startle Alone trials and in Prepulse trials across time using contrast statements within the Proc Mixed procedure. Since the difference between log-transformed values is the logarithm of the ratio of the responses, PPI estimates were equivalent to proportional changes in amplitude. Post hoc comparisons were Sidak adjusted.
2.6.2 Covariate Analyses
Covariate analyses examined the influence of symptom severity on PPI in IBS subjects using Sex x Trial Type mixed model analyses of covariance (ANCOVAs). The slope between symptom severity and PPI was estimated for male IBS patients and for female IBS patients.
2.6.3 Menstrual Cycle Analysis
In addition to the primary Sex by Diagnosis analyses and covariate analyses, a more exploratory analysis of menstrual-related effects on PPI was performed. Naturally cycling subjects were classified as follicular or luteal based on the self-reported first day of last menses (follicular: days 0-13; luteal: days 14-30). Almost half of the female subjects were classified as not naturally menstruating for various reasons including: use of oral contraceptives (OC), peri- or post-menopausal status, hysterectomy, or unexplained amenorrhea. For OC subjects, the pill brand was recorded. Subjects used a wide variety of pill types and no differences between controls and IBS patients were noted. Two healthy control subjects could not be classified due to missing or discrepant information and were excluded from the analysis. The number of subjects within each category is provided in Table 1. A Diagnosis × Menstrual Group (Follicular; Luteal; Non-cycling) × Trial Type mixed model ANOVA was used. PPI estimates and group differences in PPI were calculated as in the primary analysis.
Table 1.
Number of female subjects in each menstrual group
| Controls | IBS | |
|---|---|---|
| Menstrual Group | ||
| Follicular | 8 | 13 |
| Luteal | 9 | 13 |
| Non-cycling | 12 | 28 |
3. Results
3.1 Subject Data
The number of patients with usable EMG data determined to be constipation predominant, diarrhea predominant, and with mixed bowel habits is shown in Table 2. Table 2 also includes the number of IBS patients rating their usual symptoms as mild (can be ignored if you don't think about it), moderate (cannot be ignored but does not affect your lifestyle), severe (affects your lifestyle), and very severe (markedly affects your lifestyle). The mean age (and standard error) was 28.9 (±1.8) for female controls (range 19-49), 33.3 (±2.2) for male controls (range 20-46), 33.8 (±1.4) for female IBS patients (range 20-61) and 38.2 (±2.4) for male IBS patients (range 21-54). There were significant main effects of sex and diagnosis on age (F(1,119)=4.95, p=.028; F(1,119)=6.15 p=.015, respectively). Female subjects were younger than male subjects and controls were younger than IBS patients. No significant interactions were found among the groups. Age was entered as a covariate in all analyses.
Table 2.
Number of IBS patients in the final sample with different predominate bowel habits and usual symptom severity ratings
| Female | Male | |
|---|---|---|
| Bowel Habit | ||
| Constipation | 23 | 3 |
| Diarrhea | 21 | 12 |
| Alternating | 10 | 3 |
| Usual Symptom Severity | ||
| Mild | 3 | 4 |
| Moderate | 25 | 7 |
| Severe | 24 | 7 |
| Very Severe | 2 | 0 |
3.2 Primary Analyses: Startle and Prepulse Modulation
3.1.1 Startle response magnitude and habituation
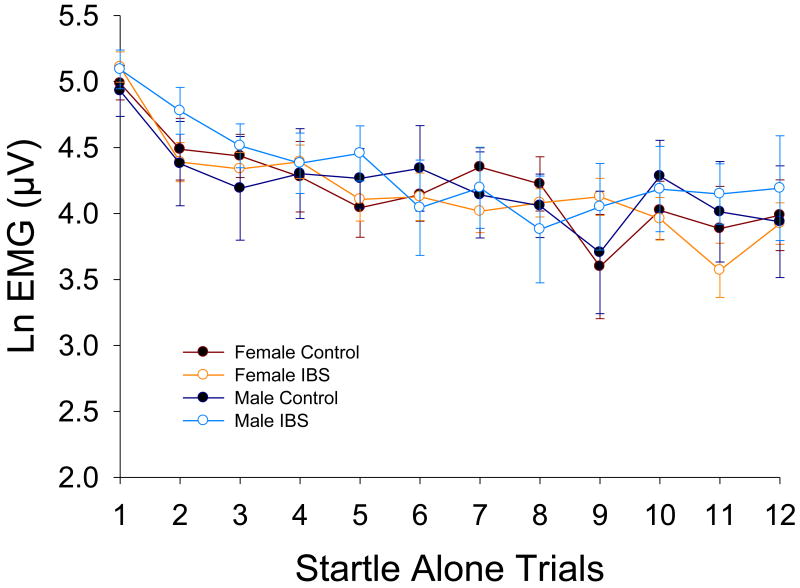
The Diagnosis × Sex × Trial Number mixed model ANOVA showed no significant group differences in response during Startle Alone trials or in habituation of this response (p's > 0.05; see Figure 1).
Figure 1.
Unmodulated acoustic startle response magnitude. Values are means (with standard error bars) for each Startle Alone trial throughout the experimental session. Ln(μV) = natural log transformed microvolts
3.1.2 Prepulse inhibition
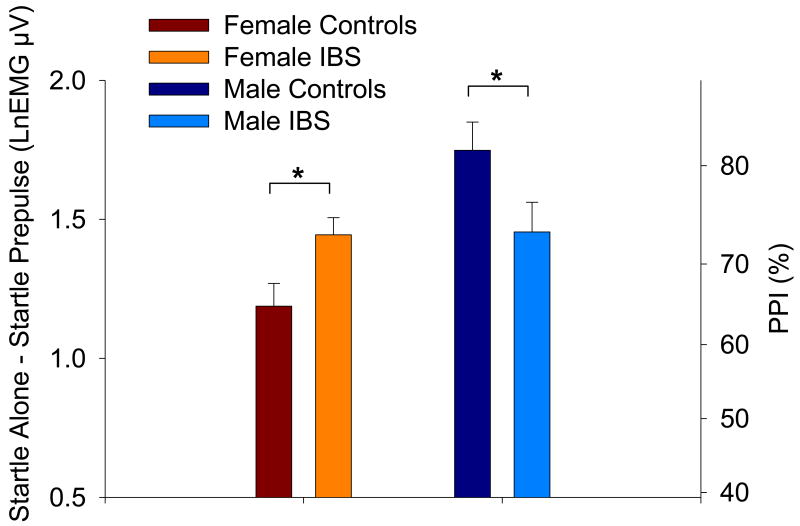
The Diagnosis × Sex × Trial Type mixed model ANOVA revealed a significant main effect of Trial Type (F(1,2540)=1052.40, p<.0001) indicating smaller startle responses during the Prepulse trials than during the Startle Alone trials. Results revealed a significant interaction between Sex and Trial Type (F(1,2540)=11.78, p=.0006) and a significant three-way interaction between Sex, Diagnosis and Trial Type (F(1,2540)=8.10, p=.0045). There were no significant main effects of Diagnosis or Sex, (p's>.05). Linear contrasts were performed to interpret the significant interactions in terms of PPI. Female IBS patients had greater PPI than healthy females (p=.0356) while male IBS patients had reduced PPI relative to healthy males (p=.0452; Figure 2). Estimated means are provided in Table 3.
Figure 2.
Prepulse inhibition of the acoustic startle response. Values are the estimated difference between mean Startle Alone and Prepulse trials (with standard error bars). Increased prepulse inhibition is indicated by an increased difference (positive axis). The right axes shows percent change derived from the log-transformed values. Significance is indicated for group differences within each sex. LnEMG μV = natural log transformed microvolts.
Table 3.
Estimated means and standard errors for Startle Alone and Prepulse trials, the difference between them (i.e. PPI), and the percent PPI derived from the difference values. Units are natural log transformed microvolts.
| Controls | IBS | |||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Startle Alone Trials | 4.62(.21) | 4.66(.26) | 4.69(.16) | 4.83(.28) |
| Prepulse Trials | 3.43(.21) | 2.92(.26) | 3.24(.16) | 3.37(.28) |
| PPI | 1.19(.08) | 1.75(.10) | 1.44(.06) | 1.45(.11) |
| %PPI | 69.58 | 82.62 | 76.31 | 76.54 |
3.2 Covariate Analyses: PPI and symptom severity
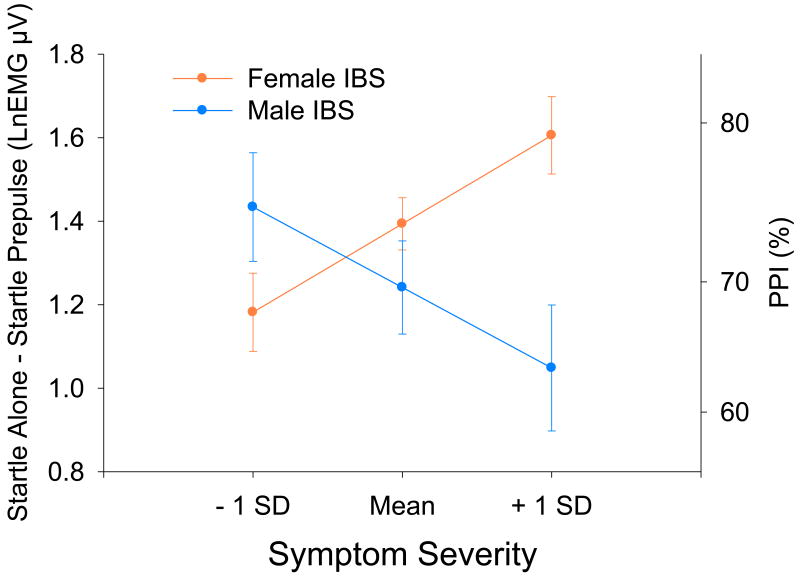
Male and female IBS patients did not differ in mean symptom ratings (10.12 (±5.24) and 10.85 (±3.66), respectively; F(1,68)=.41, p=.523).A mixed model ANCOVA revealed a significant Symptom × Sex × Trial Type interaction (F(1,1449)=13.45, p=.0003). For both male and female IBS patients, symptom severity was not related to responses during Startle alone trials (p's>.05). For female IBS patients, there was a positive relationship between symptom severity and PPI (p=.0021). For male IBS patients, there was a negative relationship between symptom severity and PPI (p=.0254; Figure 3).
Figure 3.
Estimated prepulse inhibition (and standard errors) for IBS patients within 1 standard deviation above and below the mean on the 21-point rating of the overall severity of their usual gastrointestinal symptoms. The right axes shows percent change derived from the log-transformed values. LnEMGμV = natural log transformed microvolts.
3.3 Exploratory Analysis: PPI and Menstrual Cycle effects
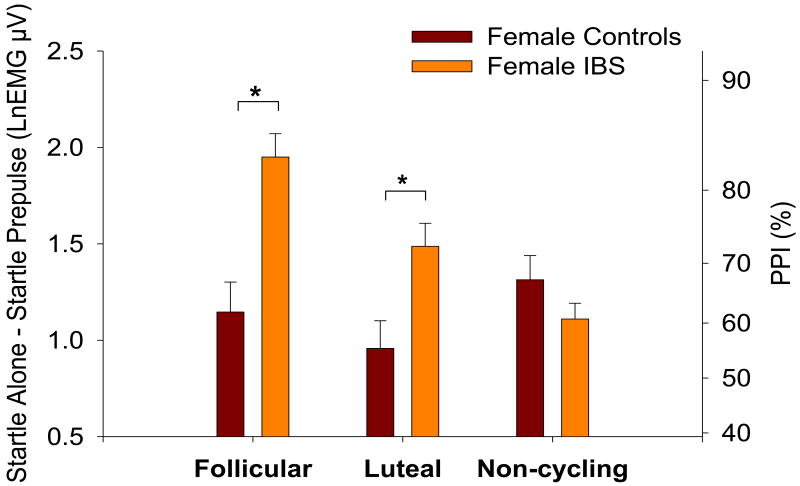
A Diagnosis × Menstrual Group × Trial Type mixed model ANOVA revealed significant interactions between Menstrual Group and Trial Type (F(1,1707)=4.22, p=.0148), Diagnosis and Trial Type (F(1,1707)=13.30, p=.0003), Menstrual Group and Diagnosis (F(2,76)=3.27, p=.0432) as well as a significant three-way interaction between Menstrual Group, Diagnosis and Trial Type (F(2,1707)=9.64, p<.0001). Naturally cycling female IBS patients (follicular and luteal) showed significantly greater PPI than naturally cycling healthy females (p<.0001; p=.0047, respectively) while non-cycling female IBS patients showed non-significantly reduced PPI relative to non-cycling healthy females (p=.1752; Figure 4). Similar results were obtained when the analysis was performed after limiting the non-cycling group to subjects on oral contraceptives (the largest subgroup within the non-cycling group). Comparisons between follicular and luteal phases within healthy controls did not reveal any significant difference in PPI (p>.05). However, female IBS patients displayed significantly reduced PPI during the luteal phase relative to the follicular phase (p=.0065).
Figure 4.
Prepulse inhibition of the acoustic startle response within each menstrual group. Values are the estimated difference between mean Startle Alone and Prepulse trials (with standard error bars). Increased prepulse inhibition is indicated by an increased difference (positive axis). The right axes shows percent change derived from the log-transformed values. Significance is indicated for diagnosis differences within each menstrual group. LnEMG μV = natural log transformed microvolts.
4. Discussion
The current study evaluated the general hypothesis that IBS patients have alterations in pre-attentive mechanisms in the form of sensorimotor gating, a process by which an organism can filter the flow of information from its internal and external environments (Geyer et al., 1990). It represents an important form of pre-attentional information processing that is critical for the maintenance of selective attention and normal cognitive functioning (Braff & Light, 2004). While the overall hypothesis of altered PPI in IBS was confirmed, the direction of the changes associated with IBS differed based on the sex of the subject. Relative to male controls, male IBS patients demonstrated significantly reduced sensorimotor gating and greater IBS symptom severity was associated with a greater reduction in PPI, analogous to previously reported findings in patients with anxiety disorders. In contrast, female IBS patients demonstrated significantly enhanced sensorimotor gating relative to female controls with greater symptom severity associated with a greater enhancement of PPI, analogous to previously reported findings of enhanced PPI associated with hypervigilance.
4.1 Sex differences in PPI in healthy control subjects
Sex differences in PPI in healthy subjects have been previously reported and, in general, the results for the healthy controls in the current study are consistent with these previous reports. As in the current study, prior studies have shown greater PPI in healthy men compared to healthy women (Aasen, Kolli, & Kumari, 2005; Kumari, Aasen, & Sharma, 2004; Swerdlow et al., 1993; Swerdlow et al., 1999). Although the absolute value of PPI varies considerably across studies, the difference in PPI between healthy men and women in previous studies is about 10-15% which is similar to the approximately 13% difference found in the current sample. Thus it is unlikely that the current findings of differences between the IBS groups and their same sex controls are due to unusual responses in our control subjects or an artifact of the methods used.
4.2 Reduced PPI in male IBS patients
We had hypothesized that IBS patients would show a deficit in PPI based on their decreased ability to inhibit responses to low intensity interoceptive visceral information, and the overlap of IBS with anxiety. Indeed, male IBS patients did show a pattern of reduced sensorimotor gating relative to male controls. The association of reduced PPI with IBS in males is further supported by the finding that this effect was more pronounced in patients with increased symptom severity. Reduced sensorimotor gating has been associated with a number of neurocognitive and affective disorders including schizophrenia, PTSD, panic disorder (reviewed in Braff et al., 2001) and premenstrual dysphoric disorder (Kask, Gulinello, Backstrom, Geyer, & Sundstrom-Poromaa, 2008). These findings have been interpreted as reflecting the impaired ability of these patient populations to properly inhibit incoming information, especially intrusive thoughts and feelings. In schizophrenia, it has been suggested that this reduction may be due to a reduction in top down inhibition of the reflex (Hazlett et al., 1998).
4.3 Increased PPI in female IBS patients
The finding of increased PPI in the female IBS patients relative to controls was unexpected. Increased PPI had not been previously associated with any specific psychiatric or neurological disorders; however, recently Gogos et al (2009) demonstrated increased PPI in women with bipolar disorder. Swerdlow et al. (1995) reported increased PPI to be associated with higher scores on a psychological scale of illness fears and concerns (the Hysteria scale of the MMPI) in healthy subjects. PPI is also greater when task demands require attention to the prepulse and less when instructions are to ignore the prepulse (Dawson et al., 1993; Filion et al., 1993; Schell et al., 1995). Although subjects were not explicitly instructed to attend to the prepulse in the current study, IBS females may have attended to the prepulse to a greater extent than controls as a result of hypervigilance. Prepulse inhibition is also enhanced by threat conditions, presumably also due to increased vigilance (Cornwell et al., 2008; Grillon & Davis, 1997). We have previously provided evidence for increased attention to threat in female IBS patients (B. D. Naliboff et al., 2008) and have implicated this finding as a mechanism contributing to greater central pain amplification in female patients. Based on this limited data we would therefore hypothesize that the increased PPI found for the female IBS patients relative to female controls is consistent with the overall greater vigilance and selective attention to threat seen in female IBS patients compared to male IBS and controls for both visceral and non-visceral stimuli (Chang, 2005; Heitkemper, Jarrett, Bond, & Chang, 2003).
Although there is limited data on sex differences in PPI for most of the patient populations studied, the literature on schizophrenia has shown sexual dimorphism. Decreased PPI appears to be more robust in men with schizophrenia compared to female patients (Kumari et al., 2004) although it has been found in women with schizophrenia under some conditions (Braff, Light, Ellwanger, Sprock, & Swerdlow, 2005). It has been hypothesized that men may have a higher fixed level of inhibition, which greatly declines with the onset or exacerbation of schizophrenia, while females are somewhat protected against this inhibitory collapse because their general inhibition level is lower and fluctuates with the menstrual cycle (Braff et al., 2001). It is intriguing to speculate that a similar interaction between sex-based differences in inhibition and illness related changes may operate in IBS in addition to the mechanisms underlying increased vigilance in women with IBS.
4.4 Possible relationship of observed sex differences in PPI with previously reported sex differences in IBS patients
Increased perception of experimental and naturally occurring visceral and somatic stimuli is greater in female IBS patients and may in fact not be a significant factor in male IBS patients (Chang, Mayer et al., 2006). On the other hand, male IBS patients show increased sympathetic and decreased cadiovagal stress responses compared to controls while female IBS patients show little or no cardio-autonomic abnormalities (Tillisch et al., 2005). Functional brain imaging studies in human subjects (S. Berman et al., 2000; S.M. Berman et al., 2006; Labus et al., 2008; B.D. Naliboff et al., 2003) and freely moving rodents (Wang et al., 2009) have demonstrated homologous sex differences in response to aversive visceral stimuli and their expectation, consistent with greater prefrontal and insula activation in males, and greater limbic and paralimbic activations in females. Furthermore, female IBS patients compared to males show greater engagement of an “emotional arousal” network (Pezawas et al., 2005; Stein et al., 2007) which includes the amygdala, rostral and subgenual cingulate regions, and this was seen primarily during the expectation of visceral discomfort. (Labus et al., 2008). Although speculative, we propose that similar to the findings for interoceptive stimuli, IBS males have reduced prefrontal cortical activation to the prepulse compared to healthy males which would result in decreased top down inhibition of PPI. In contrast, women with IBS, in an analogous fashion to the sexual dimorphism of PPI in schizophrenia, show less dysregulation of top down inhibition of the PPI compared to IBS men and in fact show increased PPI due to increased vigilance and therefore saliency of the prepulse compared to healthy women. The data from the current study therefore support a model of symptom development in IBS that is highly sexually dimorphic with a primary role for a lack of prefrontal cortical mediated pain inhibition in men and increased pain facilitation involving emotional arousal and vigilance in women (E.A. Mayer, Labus, & Berkley, 2008).
4.5 Influence of menstrual cycle on PPI in female IBS patients
The exploratory analysis indicated that menstrual cycle status appeared to moderate the effect of diagnosis on PPI. While naturally cycling female IBS patients displayed enhanced PPI, non-cycling (due to oral contraceptives, postmenopausal status, etc) female IBS patients displayed no difference (a non-significant reduction) in PPI relative to controls. Only recently have the effects of oral contraceptive pills and postmenopausal status on PPI been investigated (Borgstrom, Kask, Gulinello, Odlind, & Sundstrom-Poromaa, 2008; Kumari et al., 2008). Kumari et al (2008) found no differences in PPI between pre- and postmenopausal healthy women. Borgstrom et al (2008) found that women reporting adverse mood effects during ongoing oral contraceptive use had lower levels of PPI and were more likely to report prior premenstrual symptoms compared to asymptomatic users. Premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) affects women with IBS significantly more than women without IBS (Altman et al., 2006). Given the lack of information on the hormonal or PMS symptom status of patients in the current study is it not possible to determine if comorbid PMS and PMDD contributed to the near reversal in PPI alterations seen in non-cycling compared to naturally cycing IBS patients. Given the wide use of oral contraceptives by IBS and non-IBS women, further studies on the effect of oral contraceptives on PPI and IBS are warranted.
4.6 Limitations
Procedural differences such as the use of pure tone vs a broadband prepulse, the presence or absence of a constant background sound and the signal to noise ratio of the prepulse can influence both the amount of PPI and group differences in PPI (Franklin, Bowker, & Blumenthal, 2009). Although the current methods led to robust levels of PPI and similar sex differences typically found for healthy controls, it will be important to test the consistency of these results using other PPI parameters and signal to noise ratios. The subject groups did significantly differ in age and age may impact PPI, therefore it was entered as a covariate in all analyses. Also, enhanced PPI was found mainly in the naturally cycling subjects, for which there were no significant age differences, further suggesting that differences in ages were not driving the results in the female subjects. Previous studies in healthy women have also suggested that PPI is decreased during the luteal phase compared to the follicular phase of the menstrual cycle (Jovanovic et al., 2004; Swerdlow et al., 1997). However, in the current study, female control subjects did not significantly differ during the luteal and follicular phases although the non-significant difference was in the expected direction. This may be due to limitations in the simplistic method of determining phase implemented in the current study. Menstrual cycle phase was determined by the number of days from the self-reported first day of last menses. This method ignores variation in phase length among women and does not confirm ovulation. Future studies should use hormonal measures to better categorize menstrual phase.
4.7 Conclusions
These findings demonstrate altered pre-attentional information processing in IBS patients and emphasize the need to take sex and menstrual cycling status into account when studying sensory processing in IBS. The results support previous findings that different central mechanisms may play a role in symptom generation/maintenance in male and female IBS patients. While male patients have reduced sensorimotor gating, suggesting a decreased ability to filter information, female IBS patients have enhanced prepulse inhibition, possibly related to increased vigilance and greater attention to threat. However, female IBS patients may be heterogeneous due to such factors as oral contraceptive use and comorbities with menstrual cycle disorders. We hypothesize that the observed PPI differences between male and female IBS patients may be related to compromised top down (prefrontal, midcingulate) control mechanisms in male IBS patients, and to increased attention to threat due to increased limbic and paralimbic circuits in female IBS patients.
Acknowledgments
Supported in part by NIH grants NR007768 (BN), P50 DK64539 (EM), R24 AT002681 (EM), VA Medical Research (BN), and a gift from the Virginia Friedhofer Charitable Trust (EO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasen I, Kolli L, Kumari V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological and treatment studies. J Psychopharmacol. 2005;19(1):39–45. doi: 10.1177/0269881105048890. [DOI] [PubMed] [Google Scholar]
- Aitken CJ, Siddle DA, Lipp OV. The effects of threat and nonthreat word lead stimuli on blink modification. Psychophysiology. 1999;36(6):699–705. [PubMed] [Google Scholar]
- Altman G, Cain KC, Motzer S, Jarrett M, Burr R, Heitkemper M. Increased symptoms in female IBS patients with dysmenorrhea and PMS. Gastroenterol Nurs. 2006;29(1):4–11. doi: 10.1097/00001610-200601000-00002. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(1 Suppl):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37(1):13–20. [PubMed] [Google Scholar]
- Berman S, Munakata J, Naliboff B, Chang L, Mandelkern M, Silverman DH, et al. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Chang L, Fitzgerald L, Antolin T, Camplone A, et al. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am J Gastroenterol. 2002;97(11):2791–2797. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28(2):349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgstrom A, Kask K, Gulinello M, Odlind V, Sundstrom-Poromaa I. Patients with adverse mood effects from combined oral contraceptives have lower levels of prepulse inhibition than healthy controls. Psychoneuroendocrinology. 2008;33(4):487–496. doi: 10.1016/j.psyneuen.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47(2):181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2-3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174(1):75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biol Psychiatry. 2005;57(7):817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biol Psychiatry. 1999;45(3):360–364. doi: 10.1016/s0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, et al. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2007;26(8):2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol Clin North Am. 2005;34(2):271–279. doi: 10.1016/j.gtc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R277–284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- Chang L, Naliboff BD, Labus JS, Schmulson M, Lee OY, Olivas TI, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–R284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Echiverri AM, Covington MF, Grillon C. Modality-specific attention under imminent but not remote threat of shock: evidence from differential prepulse inhibition of startle. Psychol Sci. 2008;19(6):615–622. doi: 10.1111/j.1467-9280.2008.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Hazlett EA, Filion DL, Nuechterlein KH, Schell AM. Attention and schizophrenia: impaired modulation of the startle reflex. J Abnorm Psychol. 1993;102(4):633–641. doi: 10.1037//0021-843x.102.4.633. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: a tool for investigating early and late attentional processes. Biol Psychol. 1993;35(3):185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Franklin JC, Bowker KB, Blumenthal TD. Anxiety and prepulse inhibition of acoustic startle in a normative sample: The importance of signal-to-noise ratio. Personality and Individual Differences. 2009;46:369–373. [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25(3):485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- Gogos A, van den Buuse M, Rossell S. Gender differences in prepulse inhibition (PPI) in bipolar disorder: men have reduced PPI, women have increased PPI. Int J Neuropsychopharmacol. 2009:1–11. doi: 10.1017/S1461145709000480. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34(5):511–517. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64(3):169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, et al. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35(2):186–198. [PubMed] [Google Scholar]
- Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5(1):56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry. 2005;57(10):1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, et al. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41(3):401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kask K, Gulinello M, Backstrom T, Geyer MA, Sundstrom-Poromaa I. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283–2290. doi: 10.1038/sj.npp.1301599. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Progr Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology. 2007;44(5):728–736. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Papadopoulos A, Bojang F, Poon L, Halari R, et al. A comparison of prepulse inhibition in pre- and postmenopausal women and age-matched men. Neuropsychopharmacology. 2008;33(11):2610–2618. doi: 10.1038/sj.npp.1301670. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr Res. 2004;69(2-3):219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC, et al. ROME III: The Functional Gastrointestinal Disorders. Third. McLean, VA: Degnon Associates; 2006. Functional bowel disorders; pp. 487–556. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Depress Anxiety. 2002;15(2):55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Labus JS, Berkley KJ. Sex differences in pain. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex differences in the brain: from genes to behavior. New York: New York: Oxford University Press Inc.; 2008. pp. 371–395. [Google Scholar]
- Mayer EA, Naliboff B, Lee O, Munakata J, Chang L. Review article: gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol Ther. 1999;13 2:65–69. doi: 10.1046/j.1365-2036.1999.00008.x. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24(6):919–933. doi: 10.1111/j.1365-2036.2006.03078.x. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, et al. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Waters AM, Labus JS, Kilpatrick L, Craske MG, Chang L, et al. Increased acoustic startle responses in IBS patients during abdominal and nonabdominal threat. Psychosom Med. 2008;70(8):920–927. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Kaplan AR, Lane SJ, Norman RJ. Maturation of startle modulation. Psychophysiology. 1986;23(6):624–634. doi: 10.1111/j.1469-8986.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Schell AM, Dawson ME, Hazlett EA, Filion DL. Attentional modulation of startle in psychosis-prone college students. Psychophysiology. 1995;32(3):266–273. doi: 10.1111/j.1469-8986.1995.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Singer J, Willet J. Applied longitudinal data analysis. Oxford; Oxford University Press; 2003. [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL. Men are more inhibited than women by weak prepulses. Biol Psychiatry. 1993;34(4):253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA, Braff DL. “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biol Psychiatry. 1995;37(5):286–299. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Hartman PL, Sprock J, Auerbach PP, Cadenhead K, et al. Sex differences in sensorimotor gating of the human startle reflex: all smoke? Psychopharmacology (Berl) 1999;146(2):228–232. doi: 10.1007/s002130051111. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41(4):452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30(10):1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 2:II43–47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54(10):1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JM, Lee K, et al. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009 doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115(5):1263–1271. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]