Summary
It is generally established that active-coping strategies and greater perceived control over pain are associated with improved pain-related outcomes; however, it remains unclear whether these factors independently or interactively influence adrenocortical function in reaction to a painful stimulus. The present study examined whether active coping predicted magnitude cortisol response to acute pain, whether perceived control over pain moderated this association, and whether effects differed as a function of sex. Our findings suggest that perceived control moderates the active coping-adrenocortical relation among women but not men, such that active coping may augment the release of cortisol in response to a painful stimulus only in the presence of greater perceived control over pain. Taken together, active coping and perceived control may potentiate an adaptive neuroendocrine response to an acute painful stressor.
Keywords: Active Coping, Perceived Control, Sex Differences, Cortisol, Acute Pain, Area Under the Curve
1. Introduction
The role of cognition in shaping neuroendocrine responses to threat and challenge is receiving increasing attention (Ursin, 1998; Ursin & Erikson, 2004); however, evidence for cognition-related influences on cortisol production following experimental pain stimulation is limited. It is suggested that men and women may demonstrate divergent responses to noxious stimuli, yet studies of sex differences in HPA axis responses following stressful stimulation have revealed inconsistent patterns of results (see Zimmer, Basler, Vedder, & Lautenbacher, 2003 for review). Additional studies are needed to examine whether (and which) cognitive processes might modulate neuroendocrine responses to noxious stressors, and whether these relations vary as a function of sex.
A dynamic HPA axis response following acute noxious stimulation should be considered adaptive as the release of cortisol has widespread actions which help restore homeostasis following a stressful event. In addition to the nature of the stressor, individuals’ cognitions during the stress experience may partially determine the HPA axis response. In clinical samples, passive coping, repression, and denial of a stressful event have been shown to be related to hypocortisolism, a condition believed to be implicated in inflammatory processes that promote the development of cardiovascular disease (Heim, Ehlert, & Hellhammer, 2000). Additionally, a relation between hypercortisolism and impaired cognition (attentional and memory deficits) has been reported, particularly for indivdiuals reporting depressive symptoms (O’Brien et al., 2004). There is currently insufficient evidence describing whether adaptive cognitions, such as active coping and sense of control, may be related to adaptive cortisol responses following noxious stimulation in non-clinical samples.
In this study, we examined active coping strategies, perceived control over pain, and their interaction with cortisol response. Furthermore, we examined whether these relations varied as a function of sex. It was hypothesized that 1) perceived control over the painful stressor would interact with active coping strategies in relation to an adaptive cortisol response and 2), given previous literature suggesting sex differences in cortisol response to noxious stress, men and women would demonstrate divergent cortisol responses in relation to perceived control and coping.
2. Methods
2.1. Participants and Procedures
The present study conducted secondary analyses on self-report and neuroendocrine data from a study investigating the relations among cortisol awakening response, stress-induced cortisol reactivity, and pain reports following quantitative sensory testing (see Fabian et al., 2009 for review). A total of 80 participants were recruited from an urban university in the mid-Atlantic United States. Participants completed a screening battery to determine eligibility, and exclusion criteria included: a) age younger than 18 or older than 45, b) ongoing chronic pain, c) diagnosed with hypertension or taking medication for blood pressure, d) circulatory disorders, e) history of cardiac events, f) history of metabolic disease or neuropathy, g) pregnancy, h) currently using prescription analgesics, tranquilizers, antidepressants, or other centrally acting agents, i) use of nicotine, j) use of prescription medication (including oral contraceptives), k) diagnosed with a psychiatric disorder (e.g., depression). The protocol was approved by the university’s Institutional Review Board. All participants provided informed consent and were compensated for their participation.
To control for the diurnal rhythm of cortisol, participants attended afternoon laboratory sessions. They were presented with audio-taped instructions for the CPT, which involved a circulating cold water bath (ThermoNeslab RTE17, Portsmouth, NH) maintained at a temperature of 4°C (± 0.2°C). Participants completed one cold water immersion (dominant hand) for up to 5 minutes, and were told they could withdraw their hand from the cold water at any time. Time to hand withdrawal was recorded for each individual (i.e., pain tolerance). During the water immersion, participants used a 0–100 Numerical Rating Scale (NRS) to rate pain unpleasantness every 30 seconds until task completion. Seven salivary cortisol samples were obtained over the course of the laboratory session to assess baseline cortisol levels and capture cortisol reactivity in response to the CPT. At predetermined times (30min and 15min prior to initiation of cold water immersion, and 5min, 10min, 15min, 20min, 30min after initiation of cold water immersion) participants placed a sterile cotton pad (Sarstedt) in their mouth for 2min 30s. Cortisol obtained in this way is not influenced by saliva flow rate and is in its unbound, biologically active state (Kirschbaum & Hellhammer, 1994). Samples were stored at −80 °C until batch assayed using high sensitivity enzyme immunoassay kits (Salimetrics, State College, PA).
2.2. Measures
Data analyses were conducted on questionnaire items pertaining to pain coping strategies and perceptions of control over pain. In the current study, a situation-specific version of the Coping Strategies Questionnaire Short-Form (CSQ-SF; Rosenstiel & Keefe, 1983; Jensen et al., 2003) was administered to assess individuals’ pain coping strategies. The situation-specific CSQ-SF was administered immediately following the completion of the CPT and uses the same 14 items as the original CSQ-SF, but with modified instructions and item wording that asks participants about “the thoughts and feelings you had during the painful procedures you just experienced.” An active coping composite scale was created by averaging together the diverting attention, coping self-statements, ignoring pain, and reinterpreting pain sensations subscales (α = .75). Creation of a composite active coping scale has been previously described and is generally an accepted means for assessing active pain coping strategies (Edwards & Fillingim, 2005).
The control scale of the Survey of Pain Attitudes (SOPA; Jensen et al., 1994) was used to assess the extent to which an individual sees himself/herself as having control over his/her pain. The control scale consists of 10 items, four of which are negatively worded. After reverse coding the four negatively worded items, responses to these items are added to the six positively worded items, with higher scores indicating greater perceived control over pain (α = .87). The SOPA control scale was administered prior to the initiation of the laboratory pain task and is considered a valid assessment of individuals’ perceived ability to control their experience of pain (Jensen et al., 1994).
2.3. Data analysis
Missing data existed for salivary cortisol samples of four participants, who did not significantly differ from the full sample on sex, race, coping strategies, perceived control, or pain ratings. These data were excluded from analyses leaving a final sample N = 76 (51% women, mean age = 20.3 years old). The area under the curve (AUC) was calculated for salivary cortisol collected before, during and after the CPT immersion. AUC is a frequently used method in endocrinological research to comprise information that is contained in repeated measurements over time. As previously described by Pruessner and colleagues (2003), the area under the curve with respect to ground (i.e., AUCG) is a measure of the overall magnitude of cortisol production, which was examined as the primary outcome in our analyses.
Independent-samples t-tests were conducted to assess for significant differences among all study variables by sex. A pairwise t-test was completed to assess cortisol response from baseline to peak production. A hierarchical multiple regression analysis was performed to examine the associations of active coping, perceived positive control over pain, sex and their interaction with salivary cortisol response to the CPT. Pain tolerance and pain unpleasantness (ratings averaged across CPT immersion) were selected as control variables because they were found to relate directly to cortisol response. Specifically, greater CPT exposure related to increased salivary cortisol production (r = .24, p < .05) and greater unpleasantness ratings related to decreased salivary cortisol production (r = −.15, p = .10), which is likely due to attenuated exposure to the CPT resulting from early task termination.
3. Results
3.1. Differences by sex
The independent samples t-tests demonstrated significant differences between men and women for several key variables. Compared to women, men reported significantly greater active coping strategies (Men; M = 4.19, SD = 2.19; Women; M = 3.02, SD = 2.04; t(78) = 2.47, p < .05) and perceived control over pain (Men; M = 22.23, SD = 8.64; Women; M = 17.93, SD = 7.85; t(78) = 2.33, p < .05). Men had significantly greater exposure to the painful stimulus (i.e., CPT immersion time) than women (Men; M = 202.33, SD = 113.44; Women; M = 114.10, SD = 110.59; t(78) = 3.52, p < .01) and reported significantly less severe pain unpleasantness ratings (Men; M = 58.58, SD = 30.68; Women; M = 72.72, SD = 25.94; t(78) = 2.23, p < .05). There were no significant differences between men and women for salivary cortisol AUCG (Men; M = 9.73, SD = 6.19; Women; M = 7.41, SD = 5.70; t(74) = 1.70, p > .05).
3.2. Salivary Cortisol (AUCG)
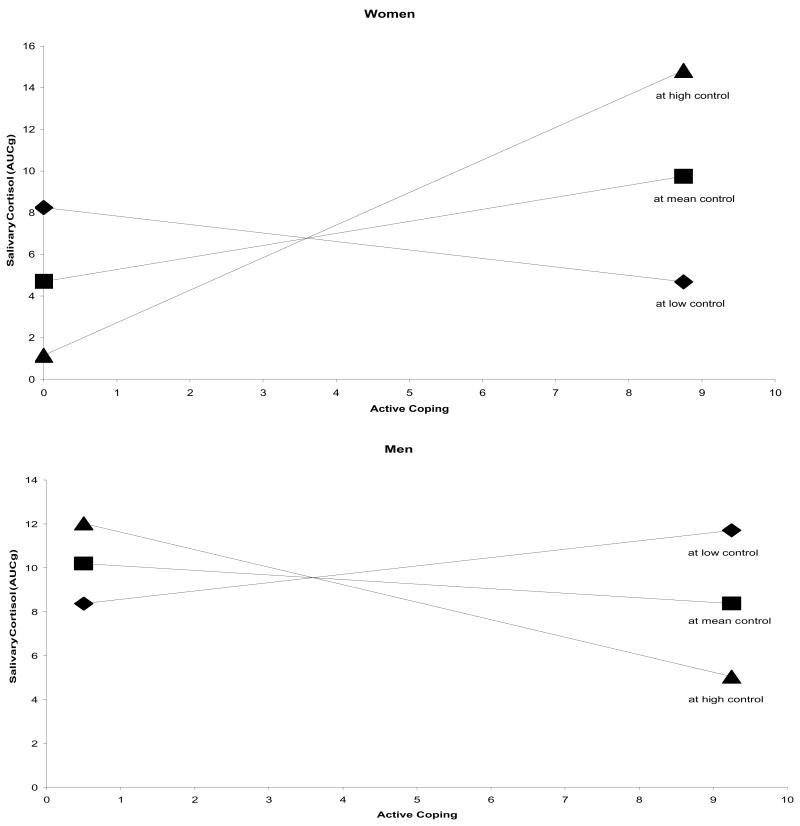
The CPT successfully produced a cortisol response for men t(39) = 3.01, p < .01 and women t(39) = 2.92, p < .01. After controlling for pain tolerance and pain unpleasantness, the active coping, perceived control, and sex main effects were non-significant. However, as predicted, the active coping X perceived control X sex interaction was significantly associated with salivary cortisol response (Table 1). For women, active coping was positively associated with salivary cortisol response at high (β = 0.541, p = .007) and intermediate (β = 0.205, p = .221) values of perceived control but was negatively associated with salivary cortisol response at low perceived control values (β = −0.132, p = .569) (Figure 1, upper panel). It should be noted that only the simple slope at high perceived control values was significant. For men, active coping was negatively associated with salivary cortisol response at high (β = −0.234, p = .379) and intermediate (β = −0.063, p = .795) values of perceived control but was positively associated with salivary cortisol response at low perceived control values (β = 0.108, p = .753) (Figure 1, lower panel). None of these simple slopes were significant. As can be seen in Figure 1, the pattern of relations between active coping and cortisol response across levels of perceived control was reversed for men relative to women. This suggests that cognitive processes may differentially engage HPA axis response contingent upon participants’ sex.
Table 1.
Results of analyses predicting magnitude cortisol response (AUCg)
| B | SEB | β | R2 | ΔR2 | ΔF | |
|---|---|---|---|---|---|---|
| Step 1 | 0.078 | --- | 3.09 | |||
| Pain Tolerance | 0.012 | 0.006 | 0.246* | |||
| Pain Unpleasantness | −0.013 | 0.025 | −0.066 | |||
| Step 2 | 0.120 | 0.042 | 1.12 | |||
| Active Coping | 0.265 | 0.401 | 0.097 | |||
| Control Scale | 0.113 | 0.104 | 0.161 | |||
| Sex | 0.965 | 1.453 | 0.081 | |||
| Step 3 | 0.179 | 0.059 | 4.96* | |||
| Active Coping x | ||||||
| Control Scale x Sex | 0.107 | 0.048 | 0.273* | |||
p < 0.05
Figure 1.
Simple regression lines for cortisol response regressed on active coping at high, intermediate, and low perceived control.
4. Discussion
Research examining cortisol response differences by sex and adaptive cognitive processes following acute noxious stimulation is scant, with mixed findings. The current study found that perceived control moderates an active coping-adrenocortical relation among women but not men. It seems that active coping strategies may potentiate the release of cortisol in response to a painful stimulus only in the presence of greater perceived control over pain, and this response is independent of the subjective experience of pain.
Current literature is replete with studies reporting associations among stress, HPA axis responses, and negative health outcomes; however, it should be considered that health is maintained through homeostatic processes that are constantly challenged by intrinsic or extrinsic adverse forces (i.e., stressors). Chrousos (1998) defined stress as a state of threatened homeostasis, which is reestablished by complex physiologic and behavioral adaptive responses. It is not until these adaptive responses become excessive or otherwise inadequate that pathology may ensue. Therefore, the HPA axis response, and the subsequent production of cortisol, is an essential component of successful adaptation because of the regulatory role cortisol plays on the basal control of HPA axis activity and on the termination of the stress response.
It is not clear why the active coping-perceived control interaction was significantly related to cortisol response for women but not men. It may be that acute noxious stimulation elicits other cognitive processes that differentially affect the cortisol response of men and women. Data from other studies support the importance of control, mastery, self-efficacy, and coping as modulators of HPA axis activity (Gaab et al., 2006). Perhaps men and women use different coping strategies that actively influence cortisol response. Future studies investigating sex differences and cortisol response should examine a broader array of cognitive processes.
In summary, the present study provides evidence that the coping strategies used by women affect their cortisol response to an acute noxious stimulus at high values of perceived control. Further examination of the influence of cognition on the HPA axis may enhance the ability to differentiate adaptive and maladaptive stress responses. Identification of cognitive processes that promote adaptive stress responses, and the reestablishment of homeostasis following a stressor, could contribute to the development of stress reduction and stress preparation techniques (Gaab et al., 2003).
Acknowledgments
We thank Ms. Kate Guilfoyle and Mr. Ray Richinelli who assisted with the data collection and project management.
Footnotes
Conflict of Interest
All authors declare that they do not have any conflicts of interest.
Contributors
Authors Bier, Goodin, and McGuire were primary contributors to the writing of the manuscript. Authors Fabian and McGuire designed the study, while authors Bier and Goodin completed all secondary data analyses. Authors Page, Goodin, and Fabian conducted salivary cortisol assays. Authors Page and Quinn proof-read and edited the final draft of the manuscript. Author Quinn prepared the citations and references. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chrousos GP. Stressors, Stress and Neuroendocrine Integration of the Adaptive Response: 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Styles of coping predict cardiovascular function following a cold pressor test. Pain Res Manag. 2005;10:219–222. doi: 10.1155/2005/216481. [DOI] [PubMed] [Google Scholar]
- Fabian LA, McGuire L, Page GG, Goodin BR, Edwards RR, Haythornthwaite J. The association of the cortisol awakening response with experimental pain ratings. Psychoneuroendocrinology. 2009;34:1247–1251. doi: 10.1016/j.psyneuen.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Blattler N, Menzi T, Pabst B, Stoyer S, Ehlert U. Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology. 2003;28:767–779. doi: 10.1016/s0306-4530(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Gaab J, Sonderegger L, Scherrer S, Ehlert U. Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting: A randomized controlled trial. Psychoneuroendocrinology. 2006;31:428–438. doi: 10.1016/j.psyneuen.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One- and two-item measures of pain beliefs and coping strategies. Pain. 2003;104:453–469. doi: 10.1016/S0304-3959(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Lawler BK. Relationship of pain specific beliefs to chronic pain adjustment. Pain. 1994;57:301–309. doi: 10.1016/0304-3959(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A Longitudinal Study of Hippocampal Volume, Cortisol Levels, and Cognition in Older Depressed Subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Ursin H. The psychology in psychoneuroendocrinology. Psychoneuroendocrinology. 1998;23:555–570. doi: 10.1016/s0306-4530(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Ursin H, Eriksen HR. The cognitive activation theory of stress. Psychoneuroendocrinology. 2004;29:567–592. doi: 10.1016/S0306-4530(03)00091-X. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Basler H, Vedder H, Lautenbacher S. Sex differences in cortisol response to noxious stress. The Clinical Journal of Pain. 2003;19:233–239. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]